Abstract
We hypothesized that aging is characterized by a reduced release of nitric oxide (NO) in response to shear stress in resistance vessels. Mesenteric arterioles and arteries of young (6 mo) and aged (24 mo) male Fischer 344 rats were isolated and cannulated. Shear stress (15 dyn/cm2)-induced dilation was significantly reduced and shear stress (1, 5, 10, and 15 dyn/cm2)-induced increases in perfusate nitrite were significantly smaller at all shear stress levels in vessels of aged rats. Inhibition of NO synthesis abolished shear stress-induced release of nitrite. Furthermore, shear stress (15 dyn/cm2)-induced release of nitrate was significantly higher and total nitrite (nitrite plus nitrate) was significantly lower in vessels of aged rats. Tiron or SOD significantly increased nitrite released from vessels of aged rats, but this was still significantly less than that in young rats. Superoxide production was increased and the activity of SOD was decreased in vessels of aged rats. There were no differences in endothelial NO synthase (eNOS) protein and basal activity or in Cu/Zn-SOD and Mn-SOD proteins in vessels of the two groups, but extracellular SOD was significantly reduced in vessels of aged rats. Maximal release of NO induced by shear stress plus ACh (10−5 M) was comparable in the two groups, but phospho-eNOS in response to shear stress (15 dyn/cm2) was significantly reduced in vessels of aged rats. These data suggest that an increased production of superoxide, a reduced activity of SOD, and an impaired shear stress-induced activation of eNOS are the causes of the decreased shear stress-induced release of NO in vessels of aged rats.
Keywords: endothelium, superoxide, superoxide dismutase
Age-related endothelial dysfunction has been characterized by reduced agonist-induced vasodilatation (33). In addition, several studies showed that flow-induced dilation, the most physiologically relevant measure of endothelium-dependent regulation of vascular tone, was reduced in healthy elderly humans and aged animals (4, 7, 25, 36) and that the sensitivity of arteriolar endothelium to fluid shear stress is decreased with advancing age (31). The reduced flow-induced dilation in vessels of aged animals is largely a result of impaired nitric oxide (NO)-mediated dilation (7, 25, 36), but a direct relation between the level of shear stress and the release of NO in blood vessels of aged animals remains unclear.
One of the broadly accepted theories of endothelial dysfunction in aging is the increase in oxidative stress. In clinical studies, administration of the antioxidant vitamin C improved vasodilatation to ACh and restored the inhibition by Nω-monomethyl-l-arginine, an inhibitor of NO synthase (NOS), of vasodilator responses, indicating that a decreased bioavailability of NO because of the presence of superoxide (O2−·) contributes to endothelial dysfunction in aging (33). Indeed, the production of O2−· within the vascular wall increases with age (7, 13). Also, O2−· formation can be further augmented, resulting in impaired endothelium-dependent dilator responses, by a reduction of superoxide dismutase (SOD) activity (10). Decline in the expression of Cu/Zn-dependent SOD was reported in arteries of skeletal muscle (35) but not in coronary vessels (7) of aged rats. On the other hand, much less is known about changes in SOD activity in vascular aging. We reported previously that scavenging O2−· only partially restored flow-induced dilation in aged vessels (7), suggesting that additional mechanisms, besides the metabolism of NO by O2−·, may also play roles in the development of endothelial dysfunction. Vascular aging is, according to some studies, also characterized by a decrease in the expression of endothelial NOS (eNOS) in small arteries (7, 35) and an increase in large conduit vessels (5, 34). Nevertheless, both the activity of eNOS and the release of NO have been suggested to be reduced in aging (1, 5, 34). Accordingly, an increase in the production of O2−·, a decrease in the activity of SOD, or a decrease in the activation of eNOS could all reduce endothelium-dependent, NO-mediated vasodilatation. However, the underlying mechanisms responsible for the changes in shear stress-activated release of NO in aging have not yet been elucidated.
The aims of the present study were, therefore, to investigate the changes in flow-induced dilation and the release of NO in response to different levels of shear stress in isolated mesenteric arteries of young and aged rats and to explore the possible mechanisms leading to endothelial dysfunction, focusing mainly on the changes in O2−· formation, and eNOS and SOD protein expression and activity.
Methods
Isolated mesenteric arteries
All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conformed to the current guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals. Six- and twenty-four-month-old male Fischer 344 rats were anesthetized with intraperitoneal injections of pentobarbital sodium (50 mg/kg). The mesentery was removed, and the superior mesenteric artery was cannulated and perfused with saline to flush blood out of the vascular bed. The mesentery was then placed in a dissecting dish containing cold (0–4°C) MOPS-buffered physiological salt solution (MOPS-PSS; pH 7.4). To assess shear stress-induced dilation, third-order mesenteric arterioles were isolated and cannulated in a vessel chamber as we described previously (32). To assess shear stress-induced NO release, full-length first-order mesenteric arteries (17 and 22 mm in 6- and 24-mo-old rats, respectively) were isolated, cannulated at both ends in a perfusion chamber, and perfused and suffused with sodium bicarbonate-5% CO2 (plus room air)-buffered PSS (37°C and pH 7.4) containing l-arginine (10−6 M). All side branches were ligated, and intraluminal pressure was maintained at 80 mmHg with a pressure-servo controller (Living Systems).
Flow-induced dilation
To avoid variation in the level of stimulation by shear stress when intraluminal flow was applied to vessels with different internal diameters, as in vessels of 6- and 24-mo-old rats, an initial shear stress (τ) of 15 dyn/cm2 [τ = 4Qη/πr3; where Q is intraluminal flow, η is the viscosity of perfusate (∼0.007 P, at 37°C), and r is the radius of the vessel] was applied to all vessels to induce a flow-induced dilation. This was obtained by applying an intraluminal flow calculated according to the basal diameter (the diameter obtained before application of shear stress) of the vessels. Flow was established by a syringe pump (model 100, KD Scientific) coupled with an inflow pressure transducer. An outflow pressure-servo controller maintained constant intravascular pressure at 80 mmHg. During intraluminal flow, intravascular pressure was maintained by lowering outflow pressure to an amount equal to the increase in inflow pressure. A period of 3–5 min of intraluminal flow was applied to obtain a stable dilation. Dilator function of a vessel in response to stimulation by flow was then evaluated by comparing the reduction of shear stress from its initial level, calculated according to the intraluminal flow and the vessel diameter after flow-induced dilation.
Application of shear stress and measurement of perfusate nitrite and nitrate
It has been shown previously that first-order mesenteric arteries of rats exhibit little myogenic constriction at 80 mmHg of intravascular pressure (32). To ensure that the diameter of the vessels is maintained constant at their maximal diameter, adenosine (10−5 M) was added to the suffusion solution. The average diameter of the vessels (334 and 400 μm at 6 and 24 mo, respectively) was calculated by measuring the diameters along the entire length of the vessel at 500-μm intervals. Wall shear stresses of 1, 5, 10, and 15 dyn/cm2 were established by increasing perfusate flow using a syringe pump (Harvard Apparatus) and were calculated based on the average diameter of each vessel. Thus, in the presence of constant intraluminal pressure and constant diameter, a desired level of wall shear stress could be obtained solely by changing the flow rate.
Two hundred microliters of perfusate in the outflow tubing were collected at each level of shear stress in control and after administration of Nω-nitro-l-arginine methyl ester (l-NAME; 10−4 M), amino-guanidine (10−5 M), SOD (120 U/ml) plus catalase (80 U/ml), or 4,5-dihydroxy-1,3-benzenedisulfonic acid (tiron; 10−4 M). The chemicals were administered both intra- and extraluminally, and vessels were incubated for 30 min before reapplication of shear stress. In one set of experiments, perfusate was collected in control (shear stress: 15 dyn/cm2) and, after the vessel was incubated with SOD plus catalase, in the presence of shear stress (15 dyn/cm2) and simultaneous administration of ACh (10−5 M) to the chamber solution. Time control experiments showed no significant difference in perfusate nitrite within 3 h after repeated application of shear stress.
Nitrite (NO2−) formation in the perfusate was assessed by a fluorometric assay (21, 22) with a spectrofluorometer (SFM25, Kontron Instruments). Nitrate (NO3−) was converted to nitrite by the action of nitrate reductase (21, 22). Standard curves of nitrite (0–640 μM) were constructed using bicarbonate-5% CO2-buffered PSS as a vehicle. Inhibitors or O2−· scavengers did not interfere with the background reading. Changes in nitrite concentration in the chamber solution in response to the increases in shear stress or before and after administration of inhibitors or scavengers were insignificant. Because vessel size was different and because the aging process may have caused changes in vessel wall structure, the luminal surface area of vessels was calculated according to the length and average luminal diameter of each vessel and final nitrite production in response to shear stress was expressed as picomoles per millimeter squared per minute.
Chemiluminescent detection of O2−· production
O2−· production of isolated first-order mesenteric arteries was assayed by the lucigenin chemiluminescence method (23, 30). Vessels were equilibrated in PSS at 37°C for 30 min and then transferred into 5 μmol/l of lucigenin, and photon counts were recorded with a scintillation counter (LS 700, Beckman Instruments). Afterward, every vessel was completely digested with 50 μl of 1 N NaOH, and total protein was assayed. Final results are expressed as counts per minute per microgram of protein.
Protein expression with Western blotting
After perfusion experiments, vessels were cut longitudinally to open the lumen and were snap frozen in liquid nitrogen. Samples (one vessel each) were solubilized in Laemmli buffer with 1% protease inhibitor cocktail (Sigma P-8340) and sonicated (2 × 60 s) before being boiled for 5 min. Proteins were separated on a SDS-PAGE gel (10% acrylamide), transferred to a polyvinylidene difluoride membrane, and probed with primary antibodies to phospho-eNOS (Ser1177, Cell Signaling), eNOS and mitochondrial SOD (Mn-SOD; BD Transduction Laboratories), intracellular SOD (Cu/Zn-SOD; Calbiochem), extracellular SOD (ECSOD) (Dr. Oury's laboratory), GAPDH (Chemicon International), and β-actin (Novus Biologicala). Secondary antibodies were conjugated to horseradish peroxidase according to the Amersham ECL-Plus protocol.
In separate experiments, to analyze shear stress-induced phosphorylation of eNOS, a shear stress of 15 dyn/cm2 was applied to mesenteric arteries of 6- and 24-mo-old rats for a period of 20 min (according to our preliminary results, phosphorylation of eNOS reached its peak between 10 and 30 min in isolated, perfused rat mesenteric arteries). Vessels were then removed from the cannulae and snap frozen in liquid nitrogen for Western blotting analysis.
NOS activity
NOS activity of mesenteric arteries was assayed by measuring the rate of conversion of radiolabeled l-[3H]citrulline from l-[3H]arginine (Amersham) using an assay kit (Cat. No. 482700, Calbiochem). Two full-length mesenteric arteries were used in each sample. Vessels were cut longitudinally to expose the endothelial surface, and incubations were carried out at 37°C for 60 min. Final activity was normalized by the protein content of each sample.
SOD activity
SOD activity of mesenteric arteries was assessed by measuring the inhibition of pyrogallol autoxidation (20). First-order mesenteric arteries were isolated and pooled. Forty micrograms of protein from each rat were used, and the reaction was monitored spectrophotometrically at room temperature for 3 min. The activity was calculated against a standard curve of SOD (0–1.6 U/ml; S-2515, Sigma).
Statistical analysis
Data are expressed as means ± SE; n refers to the number of rats from which mesenteric arteries were isolated. Statistical analysis was performed using repeated-measures ANOVA followed by the Tukey-Kramer post hoc test and Student's t-test Statistical significance was accepted at a level of P < 0.05.
Results
In 6- and 24-mo-old rats, basal diameter and passive diameter at 80 mmHg of intravascular pressure of third-order mesenteric arterioles were 77 and 94 μm (P < 0.05) and 127 and 145 μm (P < 0.05), respectively. In response to an initial shear stress of 15 dyn/cm2, flow-induced dilation in arterioles of 6-mo-old rats resulted in a significantly decreased shear stress (6.2 ± 0.3 dyn/cm2) compared with that in 24-mo-old rats (10.6 ± 0.3 dyn/cm2; Fig. 1). Inhibition of NOS with l-NAME (10−4 M) increased the level of shear stress to 10.4 ± 0.4 and 12.1 ± 0.3 dyn/cm2 in arterioles of 6- and 24-mo-old rats, respectively. Dilation to sodium nitroprusside (SNP; 10−7 M) was comparable in arterioles of 6-mo-old (24 ± 3% of passive diamter) and 24-mo-old (22 ± 4% of passive diameter) rats, respectively.
Fig. 1.
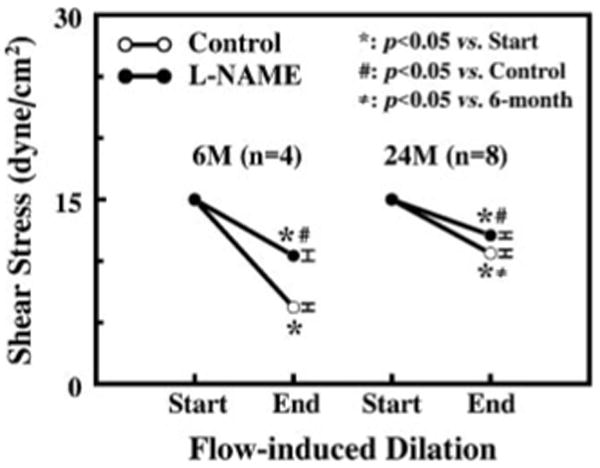
Shear stress-induced dilation in mesenteric arterioles of 6-mo-old (6M) and 24-mo-old (24M) male Fischer 344 rats before (control) and after inhibition of nitric oxide (NO) synthesis with Nω-nitro-l-arginine methyl ester (l-NAME; 10–4 M). An initial shear stress of 15 dyn/cm2 was applied to the vessels by constant intraluminal flow calculated according to their initial diameter. Reduction of shear stress due to flow-induced dilation was calculated according to the flow and the diameter of the vessels at the end of flow-induced dilation. SEs are indicated on the right of each symbol.
Perfusate nitrite release to shear stresses of 1, 5, 10, and 15 dyn/cm2 was measured in first-order mesenteric arteries of 6-and 24-mo-old rats. Figure 2 shows that the average perfusate nitrite from all controls of different experimental groups was significantly increased with increases in fluid shear stress in both groups; but at all levels of shear stress (1-15 dyn/cm2) the rate of release of nitrite was significantly attenuated in arteries of aged rats compared with those of young rats. l-NAME (10−4 M) eliminated the increase in perfusate nitrite (Fig. 3, A and B), indicating that the increased perfusate nitrite to shear stress is due to the activation of NOS and hence the released NO is decomposed in an oxygenated solution to nitrite. Aminoguanidine (10−5 M), an inhibitor of inducible NOS (iNOS), had no significant effect on shear stress-induced nitrite release in arteries of the two groups of rats (Fig. 3, C and D).
Fig. 2.
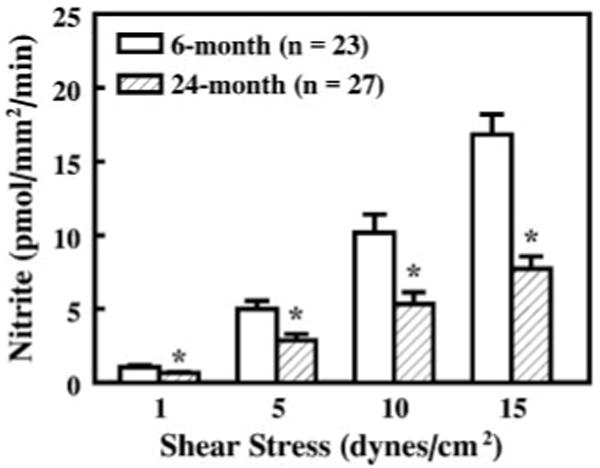
Shear stress-induced perfusate nitrite of mesenteric arteries of 6- and 24-mo-old male Fischer 344 rats. Nitrite release is expressed as picomoles per millimeter squared of the luminal surface area of each vessel per minute. *P < 0.05 compared with corresponding data of 6-mo-old rats.
Fig. 3.
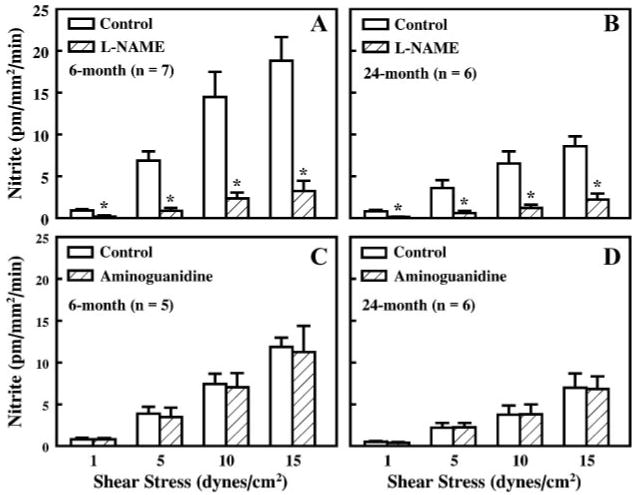
Shear stress-induced perfusate nitrite in vessels of 6-mo-old (A and C) and 24-mo-old rats (B and D) before (control) and after inhibition of NO synthesis with l-NAME (10–4 M) and inhibition of inducible NO synthase with aminoguanidine (10–5 M). *P < 0.05.
Administration of tiron, an intracellular O2−· scavenger, did not significantly affect perfusate nitrite in arteries of young rats (Fig. 4A) but significantly increased perfusate nitrite (mainly at high levels of shear stress) in arteries of aged rats (Fig. 4B). However, the enhanced perfusate nitrite, after O2−· was scavenged with tiron, was still significantly lower in arteries of aged rats than in those of young rats. For example, at a shear stress of 10 and 15 dyn/cm2, levels of perfusate nitrite were 10.6 ± 1.6 and 16.8 ± 2.0 pmol·mm−2·min−1 and 5.8 ± 1.3 and 9.9 ± 1.7 pmol·mm−2·min−1 in arteries of young and aged rats, respectively. These results indicate that in arteries of aged rats, there is an increased production of O2−· accompanied by a decreased release of NO in response to increases of shear stress. Similar results were also obtained after O2−· was scavenged with SOD plus catalase (Fig. 4, C and D). SOD was administrated intra- and extraluminally to ensure that it reached the extracellular space. SOD significantly increased nitrite production in arteries of aged rats, but the increased nitrite compared with that in arteries of young rats was still significantly less (9.9 ± 1.7 and 16.9 ± 2.2 pmol·mm−2·min−1 and 7.2 ± 1.7 and 10.5 ± 1.5 pmol·mm−2·min−1 in arteries of young and aged rats at a shear stress of 10 and 15 dyn/cm2, respectively).
Fig. 4.
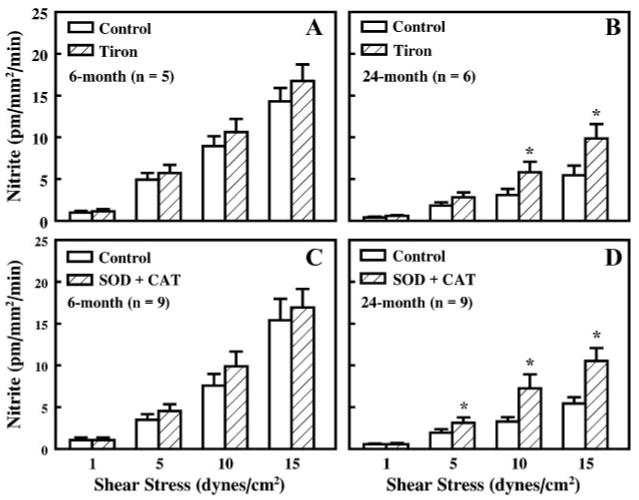
Shear stress-induced perfusate nitrite in vessels of 6-mo-old (A and C) and 24-mo-old rats (B and D) before (control) and after administration of tiron (10–4 M) and administration of SOD (120 U/ml) plus catalase (CAT; 80 U/ml). *P < 0.05.
Superoxide reacts with NO to form peroxynitrite, which undergoes rapid reduction to form nitrate in PSS (2). Figure 5A shows that in response to a shear stress of 15 dyn/cm2, perfusate nitrate is significantly higher in arteries of aged rats than in those of young rats. These results, in agreement with the results shown in Fig. 4, confirm that there is an increased O2−· production in response to shear stress in arteries of aged rats. Furthermore, with the use of the technique of lucigenin-derived chemiluminesence, basal release of O2−· in noncannulated and nonstimulated arteries was also significantly higher in vessels of aged rats than in those of young rats (Fig. 5B). Figure 5A also shows that the total perfusate nitrite (NO2− + NO3−) was significantly less in vessels of aged rats than in those of young rats. These data together with those obtained after the administration of tiron or SOD, which failed to restore the release of nitrite to shear stress in arteries of aged rats to the levels found in arteries of young rats, imply that shear stress-induced synthesis of NO is reduced in arteries of aged rats.
Fig. 5.
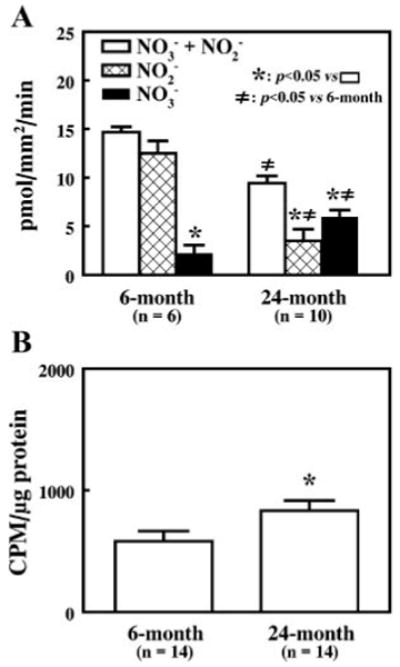
A: perfusate nitrite, nitrate, and total nitrite (NO2– + NO3–) after the conversion of nitrate to nitrite by nitrate reductase in response to 15 dyn/cm2 of shear stress in vessels from 6- and 24-mo-old rats. Perfusate nitrate was calculated by subtracting nitrite from the total nitrite. *P < 0.05 compared with total nitrite within age groups; ≠P < 0.05 compared with corresponding data obtained from vessels of 6-mo-old rats. B: basal level of superoxide formation as determined by lucigenin chemiluminescence (5 μM) in mesenteric arteries of 6- and 24-mo-old rats. CPM, counts per minute. *P < 0.05.
The role of eNOS in the mediation of shear stress-induced NO release was evaluated by assessing eNOS protein expression as well as its basal and stimulated activity. Figure 6A shows a representative Western blot of eNOS from first-order mesenteric arteries of 6- and 24-mo old rats. GAPDH was used as a loading control, and expression of eNOS was normalized to that of GAPDH. Results from four blots, summarized in Fig. 6B, indicate that there were no significant differences in eNOS protein expression in vessels of young and aged rats. Also, basal NOS activity, evaluated by the rate of conversion of l-[3H]arginine to l-[3H]citrulline, in isolated (noncannulated and nonstimulated) arteries was not different in young and aged rats (Fig. 6C). Administration of ACh (10−5 M) in the presence of 15 dyn/cm2 of shear stress only slightly increased perfusate nitrite in arteries of young rats but significantly increased nitrite in those of aged rats (Fig. 6D). As a result, the level of perfusate nitrite in arteries, stimulated simultaneously by shear stress and ACh, became comparable in young and aged rats. To further confirm that the capacity of NO synthesis may not be impaired but rather that shear stress-induced activation of eNOS is impaired in mesenteric arteries of aged rats, shear stress (15 dyn/cm2)-induced phosphorylation of eNOS was assessed in vessels of the two groups of rats. Figure 7A shows Western blotting signals of phospho-eNOS, eNOS, and GAPDH. As in Fig. 6, A and B, the densitometric ratio of eNOS to GAPDH (Fig. 7B) was not different between vessels of the two groups of rats, but the densitometric ratio of phospho-eNOS to eNOS decreased significantly in vessels of aged rats compared with those of young rats.
Fig. 6.
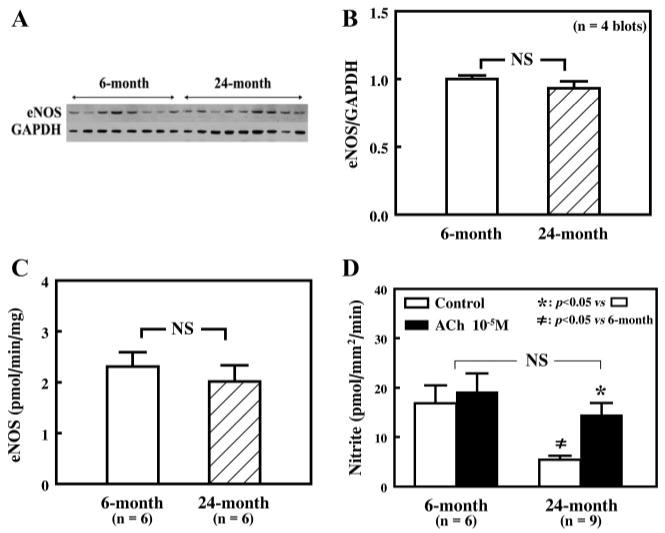
A: representative Western blot of endothelial NO synthase (eNOS) in single mesenteric arteries of 6- and 24 mo-old rats. GAPDH was used to normalize for loading variations. B: summary data of four Western blots. Data are normalized by means of densitometric ratios of eNOS and GAPDH from vessels of 6-mo-old rats. NS, not significant. C: basal activity of eNOS as determined by measuring the rate of conversion of l-[3H]citrulline from l-[3H]arginine in mesenteric arteries of 6- and 24-mo-old rats. D: perfusate nitrite in control (15 dyn/cm2 of shear stress) and in the presence of ACh (10–5 M) plus shear stress (15 dyn/cm2) in mesenteric arteries of 6- and 24 mo-old rats. *P < 0.05 vs. control; ≠P < 0.05 compared with corresponding data from 6-mo-old rats.
Fig. 7.

Shear stress (15 dyn/cm2)-induced phosphorylation of eNOS in mesenteric arteries of 6- and 24-mo-old male Fischer 344 rats. A: representative Western blots of phospho-eNOS (P-eNOS), eNOS, and GAPDH. B: summary data of densitometric ratios from the blots shown in A. *P < 0.05 vs. data from 6-mo-old rats.
SOD in first-order mesenteric arteries of 6- and 24-mo old rats was assessed by Western blotting analysis (Fig. 8A). The density of specific bands of SOD was normalized by GAPDH or (β-actin. Protein expression of Cu/Zn-SOD and Mn-SOD was not different in arteries of young and aged rats. However, EC-SOD protein was decreased significantly (by 23%) in arteries of aged rats (Fig. 8B, 4 blots). The total activity of SOD in homogenates of pooled rat mesenteric arteries was also assessed. A significant reduction of SOD activity was found in arteries of aged rats compared with those of young rats (Fig. 8C), suggesting the presence of an attenuated antioxidant capacity in vessels of aged rats, which may lead to increases in O2−· production and a reduction in the bioavailability of NO.
Fig. 8.
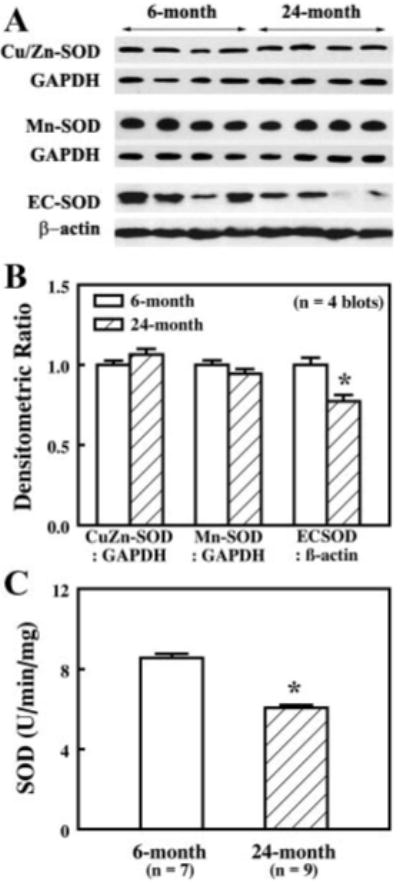
A: representative Western blots of Cu/Zn-SOD, Mn-SOD, and extra-cellular SOD (ECSOD) in single mesenteric arteries of 6- and 24 mo-old rats. GAPDH and β-actin were used to normalize for loading variations. B: summary data of four Western blots. Data are normalized by means of densitometric ratios from vessels of 6-mo-old rats. C: total SOD activity as determined by measuring the inhibition of pyrogallol autoxidation in 40 μg protein of pooled mesenteric arteries of 6- and 24-mo-old rats. *P < 0.05 vs. data from 6-mo-old rats.
Discussion
In the present study, we measured flow-induced dilation and the release of NO in rat mesenteric arterioles and arteries in response to different levels of shear stress. The results show that shear stress-induced dilation and release of NO are significantly reduced in vessels of aged rats. There are several potential mechanisms that could be responsible for this impairment. These include decreased activation of NOS, increased superoxide production, and reduced SOD expression.
A few recent studies have demonstrated that aging impairs flow-induced dilation in isolated coronary (7) and skeletal muscle arterioles (25, 36). In the present study, we used isolated third-order mesenteric arterioles to investigate flow-induced dilation in 6- and 24-mo-old rats in response to an initial shear stress of 15 dyn/cm2 (Fig. 1). Although the size (basal or passive diameters) of vessels in the two groups of rats was significantly different, all vessels were subjected to an equal level of an initial shear stress. In this condition, vessels of aged rats exhibited a decreased ability to regulate wall shear stress compared with vessels of young rats. More importantly, the reduced inhibitory role of l-NAME in vessels of aged rats and the similar dilation to SNP in vessels of young and aged rats demonstrate that the impairment in flow-induced dilation in vessels of aged rats is mainly due to a decreased endothelium-derived NO-induced dilation. Similar to third-order arterioles, in first-order arteries, shear stress-induced release of NO was also reduced in aged rats compared with young rats. To investigate the direct relationship between levels of shear stress and endothelium-derived NO release and the possible mechanisms of the impairment in the function of aged vessels, we used isolated mesenteric arteries in the rest of the experiments.
In agreement with evidence obtained previously by us (7) and others (13, 33), the present results indicate an increased formation of O2−· in nonstimulated and stimulated conditions in mesenteric arteries of aged rats. The possible sources of the increased formation of O2−· in aged vessels was not investigated in the present study. Previous studies, however, demonstrated that O2−· derived from NAD(P)H oxidase (30), xanthine oxidase (18), eNOS uncoupling (16), and other causes (3) all impaired endothelial dilator functions. Among these, NAD(P)H oxidase seems to be the major source of the excessive O2−· in vascular aging (7, 13). We (7) demonstrated previously that there is a severalfold increase in the basal formation of O2−· in coronary arteries of aged rats, which was inhibited by diphenyleneiodonium, an inhibitor of NAD(P)H oxidase. It is not clear, however, whether the increased NAD(P)H oxidase-derived O2−· measured in basal conditions accounts for the diminished vasodilatation after stimulation by increased intraluminal flow (7). In the present study, basal production of O2−· in noncannulated and nonstimulated arteries was 40% greater in vessels of aged rats compared with young rats (Fig. 5B); moreover, in aged vessels, shear stress-induced perfusate nitrate, derived from the reaction of NO and O2−· forming peroxynitrite in oxygenated PSS (2), was 300% greater than in those of young animals (Fig. 5A). Also, scavenging O2−· with tiron or SOD nearly doubled shear stress-induced perfusate nitrite in aged vessels, mostly at high shear stress levels (Fig. 4). Thus these data imply that besides the basal release of O2−· shear stress elicits an additional increase in O2−· formation, a phenomenon that only occurs in aged vessels. It has been shown previously that hemodynamic forces affect O2−· formation in certain conditions. In our previous study (15), we demonstrated that an acute high intra-arteriolar pressure increased O2−· formation and consequently reduced NO-mediated shear stress-induced dilation. Others showed that exposure of cultured endothelial cells to shear stress increased the production of O2−· (8, 14) and that the increased O2−· was due to enhanced NADH oxidase activity (8). A recent study (28) using a real-time recording technique further demonstrated a concomitant endothelial generation of O2−· and NO by shear stress (28). A shear stress-induced increase in O2−· formation in the aged vasculature may have particular clinical importance because it impairs flow-induced dilation resulting in further increases in shear stress and O2−· formation.
Similar to our previous findings, in which tiron and exogenous SOD did not fully restore flow-induced dilation in aged coronary vessels (7), in the present study, we found that tiron and SOD plus catalase failed to completely normalize shear stress-induced NO release in aged vessels (Fig. 4). Additionally, tiron and SOD had no influence on perfusate nitrite in vessels from either group of rats in response to stimulation by low shear stress, when there was significant difference in nitrite production between young and aged rats (Figs. 2 and 4). Furthermore, total perfusate nitrite (NO2− + NO3−), which represents NO and O2−·-inactivated NO, in response to 15 dyn/cm2 of shear stress was significantly less in arteries of aged rats than in those of young rats (Fig. 5A). These data suggest that the synthesis of NO in response to stimulation by shear stress is decreased in the endothelium of aged vessels. Thus both decreased activation of eNOS by shear stress and increased scavenging of NO by O2−· contribute to the reduced shear stress-induced release of NO in mesenteric arteries of aged rats.
Although several previous studies reported that eNOS mRNA (6) and eNOS protein expression (7, 35) were reduced in the aging coronary and skeletal muscle vasculatures, in the present study eNOS protein expression was not significantly different in mesenteric arteries of young and aged rats (Fig. 6, A and B). Furthermore, basal eNOS activity, measured by the conversion of l-[3H]arginine to l-[3H]citrulline in noncannulated and nonstimulated vessels, was comparable between vessels of the two age groups (Fig. 6C). More importantly, the administration of ACh (10−5 M) via a receptor-mediated, calcium-dependent enhancement of NO synthesis in the presence of 15 dyn/cm2 of shear stress increased perfusate nitrite significantly in arteries of the aged rats, leading to a nitrite level that was comparable to that released by vessels of young rats. Therefore, in mesenteric arteries of aged rats, shear stress-induced synthesis of NO is reduced, but the capacity of eNOS to synthesize NO is preserved. We interpret these paradoxical results to mean that it is the signaling cascade eventuating in the phosphorylation of eNOS that specifically mediates the response to shear stress in the endothelium of aged arteries that is likely to be impaired. This interpretation is supported by the results shown in Fig. 7 demonstrating that the level of phosphorylation of eNOS in response to a shear stress of 15 dyn/cm2 was decreased in vessels of aged rats. It is known that aging is associated with structural changes of the vascular wall, resulting in arterial stiffness (12, 24). A recent study (27) indicates that wall stiffness suppresses shear-induced phosphorylation of Akt and consequently eNOS. In this context, endothelial function and mechanosignaling of shear stress may well be altered by changes in the compliance of vascular wall during the process of aging. Some recent studies suggested that an increased nitration of proteins by peroxynitrite within the aged vascular wall (7, 34) could alter protein structure and function, including uncoupling of eNOS (16, 37), but whether this alteration plays a role in the impaired shear stress-stimulated NO release observed in the present study is not known. On the other hand, a decreased serum l-arginine has been reported to have a role in age-related NO deficiency (29), which, however, could be excluded in the present study on the basis of the maintained basal and ACh-induced increase in perfusate nitrite in aged vessels (Fig. 8D). A possible increased expression of iNOS (5, 7) is also unlikely to play a role because inhibition of iNOS had virtually no effect on perfusate nitrite in vessels of the two groups of rats.
Effects of aging on SOD expression and activity in the vascular wall are not well known, albeit a specific contribution of an attenuated activity of SOD to the impairment of endothelial function in certain pathological conditions, such as coronary artery disease (17) and heart failure (18), has been reported. In the present study, we found that expression of Cu/Zn-SOD and Mn-SOD was not reduced, but that of ECSOD was significantly reduced, paralleled by a reduced total SOD activity in vessels of aged rats (Fig. 8). These results provide evidence to support our conclusion that age-related downregulation of ECSOD contributes to the reduced NO bioavailability in response to shear stress. This is supported by the finding that ECSOD is the predominant isozyme of SOD in arteries (11, 26) and has recently been shown to be a major regulator of NO activity in arteries (9). Moreover, reduced activity of the other two isoforms of SOD, although their expression remains normal, may also play a role. Indeed, some studies demonstrated a significantly enhanced tyrosine-nitrated Mn-SOD level in aging (34) that leads to a decreased Mn-SOD activity (19), followed by impaired NO bioavailability.
In summary, downregulation of ECSOD expression and SOD activity, enhanced production of superoxide, and an impaired shear stress-dependent signaling pathway are likely to be responsible for the age-related reduction in NO release in response to shear stress in isolated mesenteric arteries. Thus a loss of a balance between oxidant and antioxidant effects, which may interfere with the function of signaling proteins or coupling of the eNOS reaction, seems to be the mechanism that underlies endothelial dysfunction characteristic of vascular senescence.
Acknowledgments
Grants: This study was supported by National Heart, Lung, and Blood Institute Grants HL-43023, HL-31069, HL-66331, HL-68813, HL-070653, and HL-63700 and American Heart Association, New York Affiliate, Grant 9830015T.
References
- 1.Amrani M, Goodwin AT, Gray CC, Yacoub MH. Ageing is associated with reduced basal and stimulated release of nitric oxide by the coronary endothelium. Acta Physiol Scand. 1996;157:79–84. doi: 10.1046/j.1365-201X.1996.451171000.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonner FT. Nitric oxide gas. Methods Enzymol. 1996;268:50–57. doi: 10.1016/s0076-6879(96)68008-2. [DOI] [PubMed] [Google Scholar]
- 3.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 5.Cernadas MR, Sanchez dM, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 6.Challah M, Nadaud S, Philippe M, Battle T, Soubrier F, Corman B, Michel JB. Circulating and cellular markers of endothelial dysfunction with aging in rats. Am J Physiol Heart Circ Physiol. 1997;273:H1941–H1948. doi: 10.1152/ajpheart.1997.273.4.H1941. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 8.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 9.Demchenko IT, Oury TD, Crapo JD, Piantadosi CA. Regulation of the brain's vascular responses to oxygen. Circ Res. 2002;91:1031–1037. doi: 10.1161/01.res.0000043500.03647.81. [DOI] [PubMed] [Google Scholar]
- 10.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 11.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 12.Gaballa MA, Jacob CT, Raya TE, Liu J, Simon B, Goldman S. Large artery remodeling during aging: biaxial passive and active stiffness. Hypertension. 1998;32:437–443. doi: 10.1161/01.hyp.32.3.437. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh HJ, Cheng CC, Wu ST, Chiu JJ, Wung BS, Wang DL. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J Cell Physiol. 1998;175:156–162. doi: 10.1002/(SICI)1097-4652(199805)175:2<156::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 15.Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress- and agonist-induced dilations. Circ Res. 1998;83:960–965. doi: 10.1161/01.res.83.9.960. [DOI] [PubMed] [Google Scholar]
- 16.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–2270. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 18.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 19.MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 20.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 21.Miles AM, Wink DA, Cook JC, Grisham MB. Determination of nitric oxide using fluorescence spectroscopy. Methods Enzymol. 1996;268:105–120. doi: 10.1016/s0076-6879(96)68013-6. [DOI] [PubMed] [Google Scholar]
- 22.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 23.Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol Heart Circ Physiol. 1994;266:H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- 24.Moreau P, d'Uscio LV, Luscher TF. Structure and reactivity of small arteries in aging. Cardiovasc Res. 1998;37:247–253. doi: 10.1016/s0008-6363(97)00225-3. [DOI] [PubMed] [Google Scholar]
- 25.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- 26.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 27.Peng X, Haldar S, Deshpande S, Irani K, Kass DA. Wall stiffness suppresses Akt/eNOS and cytoprotection in pulse-perfused endothelium. Hypertension. 2003;41:378–381. doi: 10.1161/01.hyp.0000049624.99844.3d. [DOI] [PubMed] [Google Scholar]
- 28.Qiu W, Kass DA, Hu Q, Ziegelstein RC. Determinants of shear stress-stimulated endothelial nitric oxide production assessed in real-time by 4,5-diaminofluorescein fluorescence. Biochem Biophys Res Commun. 2001;286:328–335. doi: 10.1006/bbrc.2001.5401. [DOI] [PubMed] [Google Scholar]
- 29.Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC. Changes in nitric oxide precursor, l-arginine, and metabolites, nitrate and nitrite, with aging. Life Sci. 1994;55:1895–1902. doi: 10.1016/0024-3205(94)00521-4. [DOI] [PubMed] [Google Scholar]
- 30.Rey FE, Li XC, Carretero OA, Garvin JL, Pagano PJ. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: role of gp91(phox) Circulation. 2002;106:2497–2502. doi: 10.1161/01.cir.0000038108.71560.70. [DOI] [PubMed] [Google Scholar]
- 31.Sun D, Huang A, Koller A, Kaley G. Decreased arteriolar sensitivity to shear stress in adult rats is reversed by chronic exercise activity. Microcirculation. 2002;9:91–97. doi: 10.1038/sj/mn/7800124. [DOI] [PubMed] [Google Scholar]
- 32.Sun D, Messina EJ, Kaley G, Koller A. Characteristics and origin of myogenic response in isolated mesenteric arterioles. Am J Physiol Heart Circ Physiol. 1992;263:H1486–H1491. doi: 10.1152/ajpheart.1992.263.5.H1486. [DOI] [PubMed] [Google Scholar]
- 33.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 34.Van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- 36.Woodman CR, Price EM, Laughlin MH. Selected Contribution: Aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol. 2003;95:2164–2170. doi: 10.1152/japplphysiol.01073.2002. [DOI] [PubMed] [Google Scholar]
- 37.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]


