Abstract
Serotonin and oxytocin influence aggressive and anxiety-like behaviors, though it is unclear how the two may interact. That the oxytocin receptor is expressed in the serotonergic raphe nuclei suggests a mechanism by which the two neurotransmitters may cooperatively influence behavior. We hypothesized that oxytocin acts on raphe neurons to influence serotonergically-mediated anxiety-like, aggressive and parental care behaviors. We eliminated expression of the oxytocin receptor in raphe neurons by crossing mice expressing Cre recombinase under control of the serotonin transporter promoter (Slc6a4) with our conditional oxytocin receptor knockout line. The knockout mice generated by this cross are normal across a range of behavioral measures: there are no effects for either sex on locomotion in an open-field, olfactory habituation/dishabituation or, surprisingly, anxiety-like behaviors in the elevated O and plus mazes. There was a profound deficit in male aggression: only one of 11 raphe oxytocin receptor knockouts showed any aggressive behavior, compared to eight of 11 wildtypes. In contrast, female knockouts displayed no deficits in maternal behavior or aggression. Our results show that oxytocin, via its effects on raphe neurons, is a key regulator of resident-intruder aggression in males but not maternal aggression. Furthermore, this reduction in male aggression is quite different from the effects reported previously after forebrain or total elimination of oxytocin receptors. Finally, we conclude that when constitutively eliminated, oxytocin receptors expressed by serotonin cells do not contribute to baseline anxiety-like behaviors or maternal care.
Keywords: conditional knockout, maternal aggression, serotonin, oxytocin
Introduction
Serotonin (5-HT) plays important roles in social behaviors like aggression and parental care and in the maladies of anxiety and depression (Carrillo et al., 2009, Holmes et al., 2003, Williams et al., 2011). Oxytocin (Oxt) also affects aggressive, anxiety-like, social and reproductive behaviors (Lee et al., 2009). Recent studies indicate that Oxt and 5-HT interactions control some of these behaviors (Dolen et al., 2013, Yoshida et al., 2009).
Serotonin influences aggression in both sexes. Manipulations that enhance serotonergic activity inhibit intermale offensive aggression, while interference with 5-HT signaling typically facilitates such aggression (Carrillo et al., 2009, Yanowitch & Coccaro, 2011). Knockout (KO) of the transcription factor Pet-1 leads to defective embryonic development of the 5-HT system and increased intermale aggression (Hendricks et al., 2003), and 5-HT transporter KO mice have reduced intermale (Holmes et al., 2003) and maternal aggression (Heiming et al., 2013). There is no consensus as to how 5-HT or pharmacological manipulations of 5-HT activity acutely affect maternal aggression (De Almeida & Lucion, 1994, Ieni & Thurmond, 1985). Chronic increases of synaptic 5-HT during pregnancy increase maternal aggression, decrease maternal behavior and decrease Oxt signaling (Cox et al., 2011, Johns et al., 2005).
Oxt signaling is inversely correlated with aggression in males and females (Bosch & Neumann, 2012, Lee et al., 2008). Pharmacologically decreasing Oxt signaling increases maternal aggression in rats, while administration of Oxt reduces male aggression (Calcagnoli et al., 2013, Giovenardi et al., 1998, Lubin et al., 2003). Female total Oxt KOs in a semi-natural environment show increased aggression, as do total Oxt male KOs from total Oxt KO dams and total Oxt receptor (Oxtr) male KOs (Ragnauth et al., 2005, Takayanagi et al., 2005, Winslow et al., 2000). Mice with a selective deletion of Oxtr in the forebrain do not exhibit this increased aggression (Dhakar et al., 2012), leaving the brain regions responsible for the behavioral changes unknown.
Alterations in anxiety are frequently accompanied by changes in aggression and parental care, but not consistently (Kessler et al., 2011, Neumann et al., 2010). In animal models bred for increased aggressiveness, changes in anxiety are common (Hogg et al., 2000, Nehrenberg et al., 2009, Nyberg et al., 2003). In humans, anxiety and aggression are co-morbid in borderline personality disorder (Critchfield et al., 2008), post-traumatic stress disorder (Marsee, 2008, Taft et al., 2009), and in children with autism (Maskey et al., 2013, Pugliese et al., 2013). It is unknown whether associations between anxiety, aggression and parental care have similar underlying interactive neurobiological mechanisms involving Oxt and 5-HT. In patients with autism, elevated 5-HT and reduced Oxt are found in serum samples (reviewed in (Hammock et al., 2012)). Oxt can acutely regulate serotonergic tone in the median raphe (MR) nuclei and reduce anxiety-like behavior through an interaction of Oxt and 5-HT signaling in adults (Yoshida et al., 2009). Given these results, we tested the hypotheses that removal of Oxt receptor from 5-HT neurons, regardless of sex, would increase anxiety-like and aggressive behaviors and decrease maternal behaviors.
Materials and Methods
Subjects
We previously reported the creation of a line of mice with loxP sites flanking (flox) the coding sequence of the Oxtr gene (Oxtrflox/flox) (Lee et al., 2008). The development and genotyping of the 5-HT Oxtr KO is similar to that of the forebrain Oxtr line (Lee et al., 2008): the conditional Oxtr KO line (B6.129(SJL)-Oxtrtm1.1Wsy/J) is crossed with a transgenic line expressing Cre recombinase under the control of serotonin transporter [Tg(Slc6a4-cre)ET33Gsat, originally on FVB/N background; generously provided by Charles Gerfen, NIMH; (Gong et al., 2007)]. In this line, the Slc6a4 promoter drives Cre recombinase expression in the serotonergic neurons (Fig. 1, for dorsal and median raphe expression). Both lines were back-crossed for more than 10 generations into the C57BL/6J strain (Jackson Laboratories, Bar Harbor, ME). Cre recombinase recognizes the two loxP sites flanking the Oxtr exons (floxed) and excises the Oxtr sequence resulting in progeny in which the conditional Oxtr allele is inactivated only in the 5-HT neurons. In practical terms, the Oxtrflox/flox male mice were crossed with Oxtr+/flox,cre female mice that had one transgenic allele for Cre. The offspring of this pairing yielded the following genotypes: 1) Oxtrflox/flox, 2) Oxtr+/flox 3) Oxtr+/flox,cre 4) Oxtrflox/flox,cre. The first and second are wildtype (WT), the third is heterozygous (Het) for expression of the Oxtr and Cre in the 5-HT neurons, and the fourth is a KO of the Oxtr (and het for Cre) in 5-HT neurons (hereafter referred to as 5-HT Oxtr KO). Mice were genotyped by PCR as previously described (Lee et al., 2008).
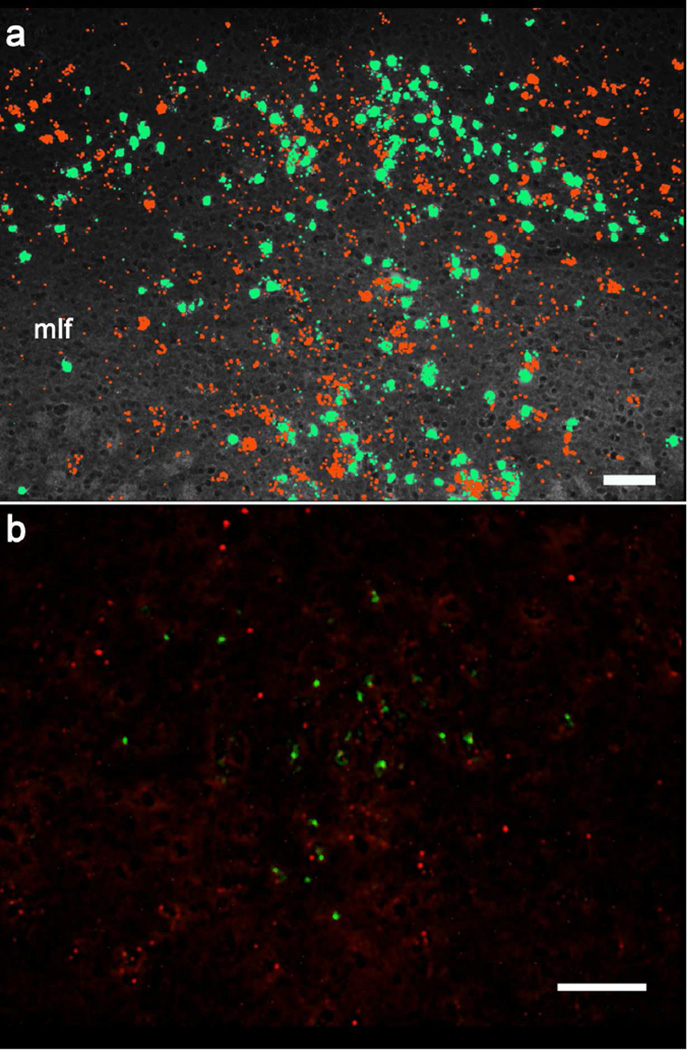
Figure 1.
Oxytocin receptor and cre recombinase transcripts were examined in the dorsal and median raphe nuclei of WT and raphe cre-expressing mice. In panel a, adjacent 16µm sections were processed for Oxtr (red signal) and cre recombinase (green signal) using the single probe RNAscope technique. Signals in the photomicrographs were pseudocolored, then the images were overlayed and aligned using tissue landmarks. Many cells would be represented in both sections but no cre-expressing cells showed signal for Oxtr, as expected. (These sections are shown separately at lower magnification in Supplementary Fig. 1.) Panel b shows a slightly higher magnification photomicrograph, from a single section containing the dorsal raphe, demonstrating cre recombinase (green signal) and Oxtr (red signal) transcripts from a 5-HT Oxtr KO mouse. Most, if not all, raphe cre-positive neurons do not express Oxtr This section was processed for the ViewRNA technique as described in the Materials and Methods and yields smaller signals than the single probe RNAscope approach. The bars in panels a and b are 100µm. mlf, medial longitudinal fasciculus.
All mice were maintained on a 12-hour light/dark cycle (lights on at 04:00) with food and water available ad libitum. Behavioral tests (excluding pup retrieval and maternal aggression) were conducted on sexually naïve animals. All animal procedures were approved by the National Institute of Mental Health Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines on the care and use of animals.
Procedural information
See Table 1 for group number, age, and litter information for each experiment.
Table 1.
Experimental Information. The number of animals used in each group from each experiment is listed. Litters indicates the number of pup litters required to collect the animals in each experiment. The age range when behavioral testing began is expressed in postnatal days.
| Wild type | Heterozygous | Knockout | Total | Litters | Age range | |
|---|---|---|---|---|---|---|
| Experiment 1 | 11 | N/A | 11 | 22 | 8 | 70–135 |
| Experiment 2 | 9 | 4 | 9 | 22 | 12 | 130–200 |
| Experiment 3 | 8 | 6 | 5 | 19 | 8 | 89–212 |
| Experiment 4 | 8 | 4 | 9 | 21 | 15 | 80–120 |
| Experiment 5 | 8 | 4 | 8 | 20 | 11 | 60–200 |
Experiment 1: Mice were tested for anxiety-like behavior on the elevated O maze (EOM), aggression, open field activity and olfactory habituation (in that order) with a minimum of 1 week between tests.
Experiment 2: Male mice were run solely on the elevated plus maze (EPM).
Experiment 3: Male mice were solely tested for aggression, extending experiment 1 to include cre recombinase-positive Hets.
Experiment 4: Female mice were tested on the EPM, various maternal behaviors (e.g., pup retrieval) approximately 3 weeks later (see Maternal Retrieval section for details), and maternal aggression two days following the final retrieval test. They were retested on the EPM a week after aggression testing ended. 2 WT and 2 KO were removed from the postpartum study due to pup death upon delivery.
Experiment 5: Female mice were tested on the EOM, open field, and olfactory habituation with a minimum of 5 days between tests.
The behavioral equipment used to (serially) test multiple mice (EPM, EOM, open field) was cleaned with 70% ethanol after each mouse. At the end of testing, mice were killed and tail-clipped to confirm genotype.
Behavioral Tests
Elevated O and Plus Mazes
Anxiety-like behavior was tested in an EOM (San Diego Instruments, San Diego, CA). Testing took place in a dark room with the open areas of the maze illuminated to 120 lux at the maze surface with soft white light. An EPM (San Diego Instruments) was used to confirm findings in the EOM. A row of LED lights controlled by a dimmer switch was set to illuminate only the open arms at an intensity of 60 lux at the maze surface. These conditions were chosen to provide a low-anxiety setting to enhance the chances of observing even subtle increases in anxiety-like behavior.
All mice were moved into an anteroom of the behavioral suite one half hour prior to lights out and assessment began one half hour after lights out. The mazes were placed in the dark main chamber of the behavioral suite with illumination only on the open arms. Mice were placed on the open arm of the EOM or center choice point of the EPM (Lee et al., 2008) facing a closed arm and were allowed to explore the maze for 5 minutes. Mice were recorded using a ceiling mounted camera connected to a Dell computer running Ethovision software (Noldus Information Technology, Leesburg, VA). Trials began as soon as the experimenter left the room. The percentage of time in the open arms [of the open plus closed arm times; or open/(open+closed)x100] is reported for the EOM and EPM. For the EPM, the numbers of open arm entries are also reported.
Open Field
The open field activity was measured as previously described (Caldwell et al., 2006) except that the inner square was 32 × 32 cm and exploration time was 10 min. Mice were brought in 30 min. prior to lights out and testing began 30 min. after lights out. Mice were placed in the center of the open field (opaque Plexiglas, (40×40 cm) illuminated at 200 lux and allowed to explore for 10 min. Recording and analysis was done with the same camera and software used for the EOM and EPM tests.
Intermale Aggression
The resident-intruder paradigm was used to assess aggression as previously reported by our lab (Pagani et al., 2014, Wersinger et al., 2007, Wersinger et al., 2002). Each mouse was given three tests with three days between each test. The aggressive encounters took place in the resident’s home cage (32×17.5×14cm) with the wire rack for food and water removed. Encounters were recorded under red lighting with a Panasonic HDC-TM700 camera and later analyzed with The Observer XT software (v.10; Noldus Information Technology) on a Dell personal computer. All experimental mice were singly housed for two weeks, with a cage change one week prior to the aggressive encounter. Stimulus mice (sexually naïve Balb/cJ males; Jackson Laboratories) were group housed and used for only a single encounter per day. They were returned to their home cages after they were used to prevent them from being contaminated with experimental mouse odors. No stimulus mouse was used for more than two aggressive episodes.
Mice were brought into an anteroom and weighed just prior to lights out, and were then transported to the behavioral room and left to sit for one hour. Weight matched intruder mice were introduced into the resident’s cage. The initiation of aggression was marked from the first instance when the resident approached and bit the intruder. Aggression was allowed to continue for 2 min. after its onset, which was defined by an attack. The latency to attack, attack duration, number of attacks, bites and tail rattles were recorded. If, after 5 min., no aggression occurred, the intruder was removed and a latency of 300 s was recorded. The latency to the first attack was calculated by summing the encounters until an attack was observed. For example, a subject that did not attack in test 1 (300 seconds maximum), but attacked after 45 seconds in test 2 and after 60 seconds in test 3 was assigned a cumulative latency of 345 seconds (Wersinger et al., 2007, Wersinger et al., 2002). For all other measures, the average across all (and only) aggressive encounters was analyzed.
Maternal Aggression
The procedure for maternal aggression was similar to resident-intruder protocol described above with two exceptions: the number of tests and the light cycle of testing. Females were monitored daily for birth, considered postpartum day 0 (PPD 0). Maternal aggression was tested only two times between PPD 5–8 with at least one day in between test sessions (i.e., PPD 5 & PPD 7 or PPD 6 & PPD 8). This represents the peak time for maternal aggression (Caughey et al., 2011). Tests were performed during the light cycle between 10:00–15:00 under 80 lux lighting conditions. Timing during the day did not affect WT maternal aggressive behavior (pilot data; not shown). Pups were removed from the home cage and placed in a warmed plastic cage during testing and returned 5 min. following the removal of the sexually naïve Balb/cJ male intruder. No mating behaviors were observed during testing.
Maternal Retrieval Behavior
Virgin females were paired with C57BL/6J males (age 80–120 days; Jackson Laboratories). Following confirmation of pregnancy by increased weight gain, males were removed and females remained singly housed for the duration of testing. Females were monitored daily for birth (PPD 0). Litters were culled to 6 pups following weighing on PPD 1. Dams were tested for pup retrieval on PPD 1 and PPD 3. Dams were brought to the testing room and allowed to habituate for 30 min. Ten minutes of undisturbed maternal-pup interactions were recorded. Following this, all pups were removed, weighed, and placed in a warmed plastic container for 5 min. Two male and two female pups were returned to the cage on opposite corners of the nest, and the interaction was recorded for 10 min. Encounters were recorded under 80 lux lighting with a Panasonic HDC-TM700 camera and later analyzed with The Observer XT software. Latency and frequency of retrieval was analyzed. Frequency and duration of pup investigation, pup grooming, self-grooming and non-pup related behaviors were quantified. Retrieval was defined as the female carrying the pup to the nest and depositing it. Pup grooming was defined as the mother licking any body part of a pup. Pup investigation was defined as the mother sniffing any part of a pup. Time ‘in nest’ was measured as the duration the female was in the nest with at least 1 pup, absent other measured maternal behaviors. All pup-directed behaviors were summed to create the category ‘Pup contact’. Non-pup directed activities included self-grooming, locomotor activity (walking/running), and rearing/climbing in the cage.
Olfactory Testing
Olfaction was tested using the habituation/dishabituation task previously used (Lee et al., 2008) and based on an established protocol (Wrenn et al., 2004). Mice were placed in a clean cage (32×17.5×14cm) containing fresh bedding between 10:00 and 14:00. Following a 1-hour habituation, water and the four odorants (diluted in distilled water) were presented for 2 min., three times each, with 1-min. inter-trial intervals. The order of odors presented was: water, lemon (1:100 in water), almond (1:100), male mouse urine (1:100) and female mouse urine (1:100). Presentation was made with a ~1cm cotton swab suspended from the cage lid approximately four centimeters from the bedding. The amount of time mice spent in proximity (1 cm or less) to the cotton tip was recorded, but any time spent chewing on the swab was not counted as exploration. Lemon and almond scents were from extracts (McCormick and Company, Inc., Spark MD). Urine samples were mixtures from 10–15 mice collected using a metabolic chamber over the course of a single day for each sex and were stored frozen (Wersinger et al., 2007).
RNA Chromogenic In Situ Hybridization Histochemistry
The RNAscope 2.0 RED kit was used to locate expression from single genes according to the manufacturer's instructions (ACD, Advanced Cell Diagnostics, Inc., Haywood CA) using C1 probes (ACD nomenclature) that target bases 1179–4568 of the mouse Oxtr mRNA (NCBI Reference Sequence: NM_001081147.1) or bases 112–1139 of the enterobacteria phage P1 cre recombinase transcript (GenBank: AB449974.1). Briefly, 16µm sections were cut from fresh-frozen 5-HT Oxtr KO and WT mouse brains and mounted onto Superfrost Plus slides (ThermoFisher, Waltham MA). The sections were fixed in 4% paraformaldehyde (PFA) for 15 min. and then dehydrated through graded (50%, 70% and 100%) ethanol washes for 5 min. each. The sections then were covered with the Pre-Treatment 3 solution (protease digestion) for 30 min. at room temperature (RT), followed by a brief rinse in water. Next, the sections were hybridized with the probe solution, without a coverslip, at 40°C for 2 hours in a humidified oven. Then, probe detection was achieved by using kit reagents: Amp 1 (30 min., 40°C), Amp 2 (15 min., 40°C), Amp 3 (30 min., 40°C), Amp 4 (15 min., 40°C), Amp 5 (30 min., RT) and Amp 6 (15 min., RT). Hybridization wash buffer steps were performed twice after each Amp step for 2 min. Signal was revealed using a solution of Fast Red A in Fast Red B at 1:60 (v/v). The tissues were washed in water, counterstained with kit DAPI, and mounted with EcoMount (Biocare Medical, Concord, CA) after overnight drying on a slide warmer.
Either RNAscope or the QuantiGene ViewRNA 2-Plex kit (Panomics-Affymetrix, Santa Clara, CA) was used to colocalize both cre recombinase and Oxtr transcripts in the same sections according to the manufacturers' instructions, targeting the same transcript sequences as listed above. For ViewRNA, the probes were mixed in a 1:1 (v/v) ratio for ViewRNA. The sections were fixed in 4% formaldehyde in PBS overnight at 4°C. They were then dehydrated through graded ethanol washes (50%, 70%, 100%; RT, 10 min. each), and then baked at 60°C for 30 min. Protease was applied (1:100 in pre-warmed PBS; 40°C, 10 min.) followed by a brief wash in PBS and another fixation for 5 min. at RT. The working probe set solutions were prepared at a 1:40 dilution in pre-warmed probe set diluent for hybridization for 2 hours at 40°C. Following incubation, slides were washed three times for 2 min. in wash buffer, with constant and vigorous agitation. The slides were then stored in storage buffer overnight. Signal Amplification was performed with kit reagents: Preamplifier Mix QT (25 min.,40°C), Amplifier Mix QT (15 min., 40°C), and probe 6-AP hybridization (1:1000 dilution in pre-warmed label probe diluent QF; 15 min, 40°C) with wash steps in between. A fast blue substrate was then applied and slides were incubated for 30 min. in the dark at RT. Quenching of the label probe 6-AP was achieved by applying the AP Stop QT (30 min., in the dark at RT). Probe 1-AP hybridization was next applied (at 1:1000; 15 min., 40°C) followed by subsequent washes. AP-Enhancer Solution was added to the tissues (5 min., RT). Detection of the fast red substrate included an incubation at 40°C for 30 min.
For RNAscope duplex ISHH, the cre recombinase (C1 probe) and Oxtr (C2 probe) probes were mixed in a 50:1 (v/v) ratio. Pretreatment of sections and probe hyrbridization were performed as described above. The probe detection process then went through Amp 1-FL (30 min., 40°C), Amp 2-FL (15 min., 40°C), Amp 3-FL (30 min., 40°C), and Amp 4-FL (15 min., 40°C), with washes after each incubation. Finally, after a DAPI counterstain and drying, a fluorescent mounting medium (Prolong Gold, Life Technologies, Grand Island, NY) was used for coverslipping.
Serotonin Levels and Metabolism
Brains from 6 adult male wildtype and 9 adult male raphe KO mice were rapidly removed and single coronal slices between bregma −0.9 and −3.0 were cut and placed on a rubber stopper. Then, dorsolateral neocortex, amygdala, hippocampus and hypothalamus were dissected from the slices with a scalpel and forceps. The tissue samples were homogenized in 100 µl of 0.1 N HClO4 and centrifuged at 12,200 x g for 15 min. Concentrations of 5-HT and its acid metabolite, 5-hydroxyindoleacetic acid (5-HIAA), were quantified in the supernatant using high-pressure liquid chromatography with electrochemical detection (HPLC-ECD). Aliquots were injected onto an HPLC column linked to a coulometric detector (ESA Model Coulochem III, Dionex Corp., Chelmsford, MA, USA). Mobile phase consisting of 50 mM sodium phosphate monobasic, 250 mM Na2EDTA, 0.03% sodium octanesulfonic acid and 25% methanol (pH 2.75) was recirculated at 0.9 ml/min. Data were acquired by an Empower software system (Waters Corp., Milford, MA, USA), where peak heights of unknowns were compared to standards. The lower limit of assay sensitivity (3 x baseline noise) was 1 pg/20 ml sample. One KO hippocampal sample was contaminated by striatum and not used and one WT hypothalamic sample was lost.
Statistics
Weight data were analyzed using one-way ANOVAs. Anxiety-like behaviors were compared with a t-test for Experiment 1 and a one-way analysis of variance (ANOVA) for Experiments 2, 4 and 5. The olfactory data were analyzed with a 2 (genotype) x 15 (odorant) mixed model ANOVA and post-hoc analysis with Fisher’s LSD. Using the Kolmogorov-Smirnov Normality test, we determined that the latency data for two of three aggression experiments were not normally distributed. Thus, latency data were analyzed using the appropriate non-parametric test (Mann-Whitney for experiments with 2 groups, Kruskal-Wallis followed by Dunn’s multiple comparisons for those with 3). An ANOVA was used for Experiment 4. One-way ANOVAs were also used for the number and duration of attacks and the number of tail rattles, except in cases when there were too few animals (<2) in a group performing the behaviors (Experiment 3). In this case a t-test was used to compare aggressive behaviors. The percentage of mice attacking was analyzed with an on-line calculator (http://vassarstats.net) that utilized the Freeman-Halton extension of Fisher’s Exact Probability test. HPLC data were analyzed using one-way ANOVAs.
Results
Selective removal of Oxtr from 5-HT cells
Oxtr mRNA is distributed throughout the DR and MR of a WT mouse (Supplemental Fig. 1a), while 5-HT Oxtr KO mice exhibit no Oxtr mRNA in the Cre recombinase-expressing neurons there (Fig. 1). This is in contrast to the co-localization of Oxtr mRNA with cre recombinase transcripts in mice not containing the floxed Oxtr allele (Supplemental Fig. 1b). The expression patterns of Oxtr and cre recombinase are similar in the raphe nuclei, but Oxtr mRNA (Yoshida et al., 2009), is also found in non-Cre-expressing (presumably non-serotonin) neurons (Supplemental Fig. 1).
Growth and development
The average litter size of the animals tested was 6±2 pups, similar to our colony-bred C57BL/6J strain. The proportions of pups with each genotype were: Oxtr+/flox: 25.7%, Oxtrflox/flox: 27.0%, Oxtr+/flox,cre: 22.9%, Oxtrflox/flox,cre: 24.2%. The proportion of males to females for each genotype did not differ (Oxtr+/flox: 50.4%, Oxtrflox/flox: 50.8%, Oxtr+/flox,cre: 50.0%, Oxtrflox/flox,cre: 52.7%). No differences were observed in the adult (70–200 days) weights of males (in grams, WT: 27.3±1, Het: 27.6±1, KO: 27.7±1; F(2,34=0.21,p=0.8)) or females (WT: 22.7±2, Het: 23.7±2, KO: 22.7±2; F(2,28=0.29, p=0.7)).
Male Mice
Anxiety-like behavior
There were no differences in performances in the EOM (time spent in open arms) or open field (times spent in the inner and outer squares or in the distance traveled) (Table 2). Experiment 2, which included Hets, assessed anxiety-like behavior in the EPM. The genotypes did not differ on risk behavior ([open/(open+closed)]) or open arm entries (See Table 3).
Table 2.
Aggressive and anxiety-like behavior in WT and 5-HT Oxtr KO male mice (Experiment 1). Mice were tested first on the EOM, then for aggression, and finally on the open field. Only one KO mouse showed aggression. Mean ± SD is indicated for each measurement. WT: wildtype, KO: 5-HT Oxtr KO.
| WT (n=11) | KO (n=11) | ||
|---|---|---|---|
| Resident-Intruder Paradigm | |||
| Aggressive Duration (s) | 29.4 ± 5.9 | 10.2 | |
| Attack Frequency (per test) | 9.1 ± 2.1 | 3 | |
| Open Field | |||
| Time in inner square (s) | 162 ± 19 | 172 ± 22 | t=0.34, p= 0.7 |
| Time in outer square (s) | 438 ± 19 | 428 ± 22 | t=0.34, p= 0.7 |
| Distance traveled (cm) | 6292 ± 1015 | 4343 ± 264 | t=1.85, p=0.07 |
| Elevated O Maze | |||
| Time in open arms [open/(open+closed)x100] |
48.9 ± 5.8 | 40.9 ± 3.7 | t=1.1, p=0.3 |
Table 3.
Aggressive and anxiety-like behavior in WT, heterozygyous cre recombinase-expressing siblings, and 5-HT Oxtr KO male mice (Experiments 2 and 3). Only one KO mouse showed aggression, therefore there are no standard deviation values for the resident-intruder KO column and t tests were used to compare WT and Het groups. Mice were tested either for anxiety or aggression. Mean ± SD is indicated for each measurement. WT, wildtype; HET, heterozygous Cre recombinase-expressing; KO, 5-HT Oxtr KO.
| WT (n=8) | Het (n=6) | KO (n=5) | ||
|---|---|---|---|---|
| Resident-Intruder Paradigm | ||||
| Aggression Duration (s) | 18.9 ± 3.9 | 17.9 ± 6.8 | 18.9 | t=0.35, p=0.7 |
| Attack Frequency (per test) | 4.3 ± 0.9 | 4.6 ± 0.9 | 4.0 | t=0.62, p=0.5 |
| Attack Latency (s) | 326.0 ± 8.0 | 328 ± 9.0 | 789 | t= 0.02, p=0.9 |
| Tail Rattles (per test) | 5.7 ± 1.1 | 4.3 ± 1.1 | 5 .00 | t=0.70, p=.49 |
| WT (n=9) | Het (n=4) | KO (n=9) | ||
| Elevated Plus Maze | ||||
| Time in open arms [open/(open+closed)x100] |
49.0 ± 11.5 | 49.6 ± 13.4 | 51.8 ± 11.2 | F(2,21)= 0.02, p=0.9 |
| Number of open arm entries | 4.9 ± 1.0 | 5.3 ± 1.7 | 4.4 ± 1.0 | F(2,21)= 0.96, p=0.4 |
Olfaction
The male WT and 5-HT Oxtr KO mice performed the same on the habituation/dishabituation task: there was no main effect or interaction with genotype (Supplemental Fig. 1). There was a main effect for odorant [F(14, 280)=7.97, p<0.001]. Post hoc analysis using Fisher’s LSD indicated significant dishabituations with almond (p<0.01) and male urine (p<0.001).
Aggression
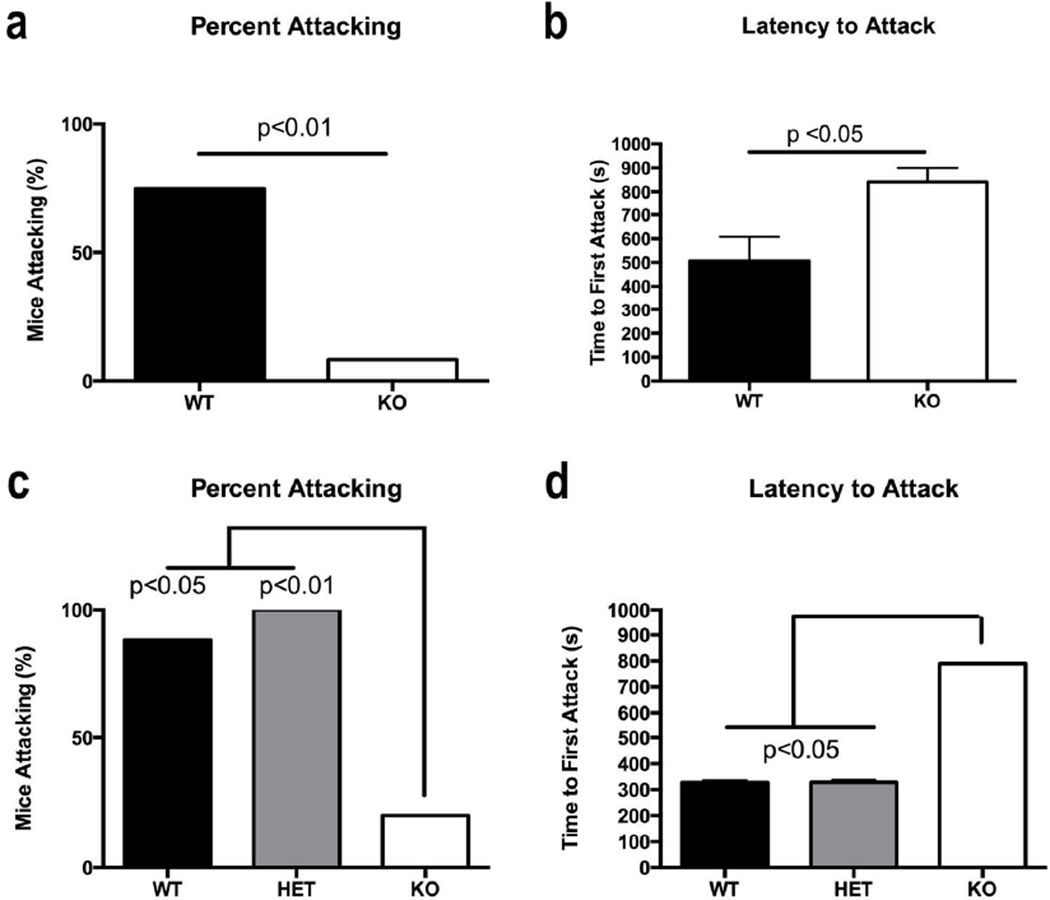
In Experiment 1 (see Fig. 2 a-b, Table 2), male 5-HT Oxtr KO mice were much less aggressive than their WT littermates: only a single KO mouse attacked (1/11), while 8 of 11 WT mice attacked (p<0.01, Fisher’s Exact Probability test). The KO group took longer to attack than their WT littermates (Mann-Whitney U=24.5, p<0.005). No other comparisons of aggression-related behaviors were performed due to the lack of variance (only one aggressor in the KO group).
Figure 2.
Male aggressive behavior. a) In Experiment 1, fewer raphe Oxtr KO mice (white bars) than wildtype (WT, black bar) exhibited attack behavior (p<0.01). b) 5-HT Oxtr KO mice had a significantly higher latency to attack than WT mice (p<0.05). c) In Experiment 3, significantly fewer KO mice exhibit attack behavior compared to WT (p<0.05) and cre recombinase-expressing raphe heterozygotes (grey bars; p<0.01). d) 5-HT Oxtr KO mice display significantly greater latency to attack compared to WTs and Hets (p<0.05). Mean ± SD is indicated for each measurement. WT, wildtype; HET, heterozygous Cre recombinase-expressing; KO, 5-HT Oxtr KO.
Aggression could have been affected by disruption of a gene or regulatory element by insertion of the Cre recombinase-containing DNA in the transgenic mouse. We examined this possibility in Experiment 3 (Fig. 2 c-d, Table 3) and observed a significant difference between the groups (p<0.01). Two 2×2 matrices, one for each comparison, showed that fewer male 5-HT Oxtr KO mice attacked than either WT (p<0.05) or Het (p<0.01) mice, and no differences were observed between the WT and Het mice.
Similar to Experiment 1, in Experiment 3 only one of the 5-HT Oxtr KO mice attacked making it difficult to quantify agonistic behavior. The KO group had a significantly higher attack latency (Kruskal-Wallis=7.9, p<0.05) than WT and Het mice. All other analyses conducted made comparisons between the Het and WT mice. In the behaviors examined, Hets performed equivalently to WTs. There were no significant differences between these genotypes on the latency to attack, the number or duration of attacks, or the number of tail rattles.
Female Mice
Anxiety-like behavior
Females did not show any significant differences between the genotypes in open arm time, frequency or distance traveled in the EPM prior to pregnancy (see Table 4). No significant difference was observed in the repeated EPM testing during the postpartum period. However, all females exhibited very low levels of open arm exploration time (Supplemental Table 1). In experiment 5, no differences were observed in locomotor activity, center duration or entries (open field) or open arm entries and durations (EOM) (Supplemental Table 1).
Table 4.
Maternal behavior and aggression in WT, heterozygyous cre recombinase-expressing siblings, and 5-HT Oxtr KO females (Experiment 4). Mice were tested in the EPM followed by maternal behavior and maternal aggression. Mean ± SD is indicated for each measurement. WT, wildtype; HET, heterozygous Cre recombinase-expressing; KO, 5-HT Oxtr KO. No differences were observed in any measurement.
| WT (n=6) | HET (n=4) | KO (n=7) | ||
|---|---|---|---|---|
| Maternal Aggression | ||||
| Attacking (%) | 66.6 (4/6) | 100 (4/4) | 71.4 (5/7) | |
| Attack latency (s) | 135 ± 168 | 316 ± 269 | 154 ± 157 | F(2,11)=0.25, p=0.8 |
| Aggression Duration (s) | 73.1 ± 55.5 | 43.3 ± 30.3 | 51.6 ± 41.8 | F(2,11)=0.51, p=0.6 |
| Attacking Frequency (per test) | 17.1 ± 11.5 | 14.5 ± 15.4 | 18.0 ± 11.4 | F(2,11)=0.09,p=0.9 |
| Maternal Behavior | ||||
| Pup retrieval latency (s) | 141 ± 30 | 262 ± 116 | 108 ± 64 | F(2,14)=0.71 p=0.5 |
| Pup grooming duration (s) | 42.5 ± 48.0 | 46.1 ± 55.9 | 39.7 ± 56.6 | F(2,14)=0.01, p=0.9 |
| Pup investigation (s) | 78.7 ± 18.5 | 126 ± 56 | 102 ± 34 | F(2,14)=2.11, p=0.1 |
| Pup contact (s) | 244 ± 66 | 235 ± 87 | 234 ± 124 | F(2,14)=0.02 p=0.9 |
| Self-grooming (s) | 35.8 ± 43.1 | 17.1 ± 27.1 | 17.4 ± 21.4 | F(2,14)=0.65, p=0.5 |
| Locomotor activity (s) | 164 ± 53 | 178 ± 40 | 164 ± 46 | F(2,14)=0.13, p=0.9 |
| Rearing/climbing (s) | 25.7 ± 17.0 | 19.3 ± 14.0 | 63.6 ± 65.3 | F(2,14)=1.73, p=0.2 |
| Pups born | 6.1 ± 2.0 | 9.0 ± 0.8 | 6.5 ± 1.8 | F(2,14)=3.82 p=0.1 |
| Pup weight (g, PND1) | 1.58 ± 0.63 | 1.37 ± 0.04 | 1.32 ± 0.20 | F(2,14)=0.73 p=0.5 |
| Pup growth (g, PND7) | 2.85 ± 0.66 | 3.16 ± 0.44 | 2.75 ± 0.88 | F(2,14)=0.41 p=0.7 |
| Male pups per litter (%) | 55.3 ± 7.7 | 46.7 ± 30.5 | 57.3 ± 7.2 | |
Olfaction
The female WT and 5-HT Oxtr KO mice performed the same on the habituation/dishabituation task (Supplemental Fig. 1). There was a main effect for odorant (F(11, 264)=5.54, p<0.001) and a main effect of genotype (F(2, 264)=4.23, p<0.05). Post hoc analysis using Fisher’s LSD indicated significant dishabituation with male urine (p<0.001) in the WT and 5-HT Oxtr KO but not in Het females.
Aggression
In experiment 4 (Table 4), WT, Het and 5-HT Oxtr KO females did not differ in any measures of maternal aggression. Specifically, there were no changes in aggression duration, number of aggressive episodes, latency to aggression, or number of tail rattles. Additionally, there were no differences in locomotor activity or anogential and facial sniffing during the aggression testing (data not shown).
Maternal Behaviors
No genotypic differences were observed in numbers of females who became pregnant; numbers of delivered pups or pup deaths; or pup weights at either PND 1 or 7 (see Table 4). There were no significant genotypic differences in retrieval latencies for the first pup or all pups on PPDs 1 or 3. Maternal care behaviors, including sniffing, mouthing, grooming, nest-building, time in nest or pup contact, were the same for all genotypes. There were no genotypic differences in non-pup directed behaviors of rearing/climbing, eating, self-grooming or exploratory behavior across groups.
Discussion
Manipulation of the Oxt and 5-HT systems affects anxiety-like behaviors and aggression (summarized in (Carrillo et al., 2009, Lee et al., 2009, Miczek et al., 2002, Neumann, 2008)). We investigated whether a constitutive localized deletion of Oxtr in the serotonergic nuclei would lead to alterations of aggressive and anxiety-like behaviors. Our experiments demonstrate that 5-HT Oxtr KO mice show normal locomotion, olfactory habituation/dishabituation and anxiety-like behaviors but almost no intermale aggression. We established that genomic insertion of the Cre recombinase-containing transgene does not lead to a decrease in aggression. Furthermore, unlike heterozygote KOs of the serotonin transporter that have intermediate levels of reduced intermale (Holmes et al., 2003) and maternal (Heiming et al., 2013) aggression compared to total KOs, our heterozygotes of the 5-HT Oxtr KO have normal aggression. This is the first time that Oxt's effects on aggression have been shown by manipulating Oxt’s influence on a single neurotransmitter system.
Presumably, Oxtr in the raphe neurons is modulating serotonergic functioning in normal adult mice, as direct infusion of Oxt into the median raphe facilitates 5-HT release there (Yoshida et al., 2009). Using HPLC to examine 5-HT/5-HIAA ratios in the neocortex cortex, amygdala, hypothalamus, and hippocampus of a subset of WT and 5-HT Oxtr KOs, we did not find a difference in basal serotonin levels (Supplemental Table 2). It is possible that, rather than affecting basal 5-HT levels, Oxtr expressed by 5-HT neurons affects 5-HT in a context-dependent manner. In the ventral striatum, 5-HT levels increase during social investigation and prior to aggressive episodes and then rapidly drop when the social stimulus is removed (Ferrari et al., 2003, Nakazato, 2013). Since the initiation of this study, research has shown that in the nucleus accumbens, presynaptic Oxtr expressed on neurons originating in the DR are necessary for socially conditioned place preference. They modulate 5-HT release and post-synaptic 5-HT1B receptor activation (Dolen et al., 2013). Our studies do not show any differences in Oxtr binding throughout the brain of 5-HT Oxtr KOs (unpublished data); however, the amount of Oxtr contributed by 5-HT axons in any given brain region may not constitute a significant enough proportion to be detected with current techniques. A more detailed and global investigation of serotonergic parameters (e.g., 5-HT receptor subtype autoradiography, microdialysis survey of 5-HT and its metabolites both basally and during intermale contact) may eventually reveal a projection site of raphe neurons important for regulation of aggression by Oxt.
Another mechanism by which 5-HT Oxtr deletion may affect aggression is through early organizational effects on other neurotransmitter systems. Oxt administered subcutaneously on postnatal day 1 causes increases aggression in adult female, but not male, mandarin voles (Jia et al., 2008). Intraperitoneal Oxt treatment increases 5-HT axon length in weanling male prairie voles in hypothalamic areas known to be involved in aggression (Eaton et al., 2012). Also, subcutaneous Oxt administration to neonatal rats causes sex-specific regional changes in 5-HT and its metabolites in adult males and females (Hashemi et al., 2013). Quantitative analyses of changes in serotonergic innervation may reveal what role Oxtr plays in serotonergic neural circuit development.
Given that Oxt infusion results in anxiolysis through a serotonin receptor, we expected an elevation of anxiety-like behavior in the 5-HT Oxtr KOs, but we saw no changes using the open field, the EOM or EPM tasks. This is perhaps explained in part by procedural differences compared to previous studies (e.g., removal of raphe Oxtr versus lateral ventricular Oxt administration (Yoshida et al., 2009)). Another possibility is that there are compensatory mechanisms that adjust for the lifelong deletion of Oxtr. Such an effect has been reported for the acquisition of fear: deletion of Oxtr did not affect fear conditioning when it was constitutive, but reduced acquisition of both context and tone fear when deletion occurred after post-natal day 21 (and limited to the forebrain excitatory neurons) (Pagani et al., 2011). Potential compensatory mechanisms in the current study could act through the raphe non-serotonergic neurons that still retain their Oxtr, such as GABAergic or glutamatergic neurons. Another option is that Oxt acting directly upon 5HT neurons does not affect anxiety-like behaviors as total knockouts of Oxtr do not affect them (Lee et al., 2008).
We hypothesized that we would observe a decrease in maternal aggression, similar to the change observed in males. 5-HT1A receptor activation in the dorsal raphe increases maternal aggression in the rat consistent with a direct role for 5-HT release in regulating maternal aggression (Da Veiga et al., 2011). Oxt administered directly into the median raphe increases 5-HT release (Yoshida et al., 2009) to act on the raphe 5-HT1A receptors. However, we observed no differences in maternal aggression. The normal maternal aggression observed in the 5-HT Oxtr KO mice might be a result, for example, of compensatory mechanisms or sex-specific differences in Oxtr’s effects on 5-HT release. It is also possible that other forms of female aggression, which do not depend on the lactation state of the female and are sensitive to Oxt signaling, perhaps in a different environment (Ragnauth et al., 2005), may be more sensitive to the Oxtr removal in 5-HT neurons.
5-HT Oxtr KO female mice behave similarly to WTs on measures of anxiety-like behavior, pup retrieval and maternal aggression. Global deletion of the Oxtr shows deficits in the initiation of maternal behaviors, with increased latencies to retrieval, and decreased crouching behaviors (Takayanagi et al., 2005). Specific deletion of Oxtr from the forebrain causes significant deficits in pup survival, but not any specific maternal behaviors (Macbeth et al., 2010). Currently, however, these studies suggest that Oxtr is interacting with a yet unidentified neurotransmitter system to optimize maternal care in the peripartum period. Oxt is known to interact with dopamine, norepinephrine and opioids in the early postpartum period to affect maternal care (Nelson & Panksepp, 1998, Rutherford et al., 2011, Shahrokh et al., 2010), but the exact locations of these interactions remain areas for future study.
We show that the deletion of Oxtr from 5-HT neurons in the hindbrain selectively reduces aggression in male mice without affecting anxiety-like behaviors. This is consistent with previous genetic disruptions of Oxtr that impact aggression without altering trait anxiety (Lee et al., 2008). In contrast, previous models of disrupted 5-HT signaling consistently report alterations in both anxiety and aggression phenotypes (monoamine oxidase, type A, KO (Popova et al., 2001); ETS oncogene family Pet1 KO (Hendricks et al., 2003, Schaefer et al., 2009); Slc6a4 KO (Holmes et al., 2003); tryptophan hydroxylase 2 KO (Mosienko et al., 2012); 5-HT receptors 1a and1b KOs (Zhuang et al., 1999); and a version of 5-HT receptor 2c (Martin et al., 2013)). These studies indicate that constitutive deletion of Oxtr may selectively affect social behaviors, whereas deletion of components of the serotonergic system more broadly affect a diverse array of behaviors. Additionally, our manipulation to reduce raphe Oxtr expression results in a decrease, not an increase (as in total Oxtr elimination), in intermale aggressive behavior without affecting maternal aggression, indicating the complexity of Oxt signaling in the control of behavior. Furthermore, we have documented a sex-specific interaction of Oxtr with 5-HT signaling, which may have implications for a variety of social and stress-related behaviors.
Supplementary Material
Acknowledgements
We greatly appreciate the technical assistance of Emily Shepard and Raquel Collier and the animal care provided by the building 49 Animal Research Facility. This research was supported by the NIMH (Z01-MH-002498).
References
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm. Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, de Boer SF, Althaus M, den Boer JA, Koolhaas JM. Antiaggressive activity of central oxytocin in male rats. Psychopharmacology (Berl.) 2013;229:639–651. doi: 10.1007/s00213-013-3124-7. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Stewart J, Wiedholz LM, Millstein RA, Iacangelo A, Holmes A, Young WS, 3rd, Wersinger SR. The acute intoxicating effects of ethanol are not dependent on the vasopressin 1a or 1b receptors. Neuropeptides. 2006;40:325–337. doi: 10.1016/j.npep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Carrillo M, Ricci LA, Coppersmith GA, Melloni RH., Jr The effect of increased serotonergic neurotransmission on aggression: a critical meta-analytical review of preclinical studies. Psychopharmacology (Berl.) 2009;205:349–368. doi: 10.1007/s00213-009-1543-2. [DOI] [PubMed] [Google Scholar]
- Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, Meddle SL. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J. Neuroendocrinol. 2011;23:1113–1124. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- Cox ET, Jarrett TM, McMurray MS, Greenhill K, Hofler VE, Williams SK, Joyner PW, Middleton CL, Walker CH, Johns JM. Combined norepinephrine/serotonergic reuptake inhibition: effects on maternal behavior, aggression, and oxytocin in the rat. Front Psychiatry. 2011;2:34. doi: 10.3389/fpsyt.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchfield KL, Levy KN, Clarkin JF, Kernberg OF. The relational context of aggression in borderline personality disorder: using adult attachment style to predict forms of hostility. J. Clin. Psychol. 2008;64:67–82. doi: 10.1002/jclp.20434. [DOI] [PubMed] [Google Scholar]
- da Veiga CP, Miczek KA, Lucion AB, de Almeida RM. Social instigation and aggression in postpartum female rats: role of 5-Ht1A and 5-Ht1B receptors in the dorsal raphe nucleus and prefrontal cortex. Psychopharmacology (Berl.) 2011;213:475–487. doi: 10.1007/s00213-010-2083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida RM, Lucion AB. Effects of intracerebroventricular administration of 5-HT receptor agonists on the maternal aggression of rats. Eur. J. Pharmacol. 1994;264:445–448. doi: 10.1016/0014-2999(94)00548-6. [DOI] [PubMed] [Google Scholar]
- Dhakar MB, Rich ME, Reno EL, Lee HJ, Caldwell HK. Heightened aggressive behavior in mice with lifelong versus postweaning knockout of the oxytocin receptor. Horm. Behav. 2012;62:86–92. doi: 10.1016/j.yhbeh.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JL, Roache L, Nguyen KN, Cushing BS, Troyer E, Papademetriou E, Raghanti MA. Organizational effects of oxytocin on serotonin innervation. Dev. Psychobiol. 2012;54:92–97. doi: 10.1002/dev.20566. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, van Erp AM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Europ J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: effects of ibotenic acid lesion and oxytocin antisense. Physiol. Behav. 1998;63:351–359. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock E, Veenstra-VanderWeele J, Yan Z, Kerr TM, Morris M, Anderson GM, Carter CS, Cook EH, Jacob S. Examining autism spectrum disorders by biomarkers: example from the oxytocin and serotonin systems. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:712–721. doi: 10.1016/j.jaac.2012.04.010. e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi F, Tekes K, Laufer R, Szegi P, Tothfalusi L, Csaba G. Effect of a single neonatal oxytocin treatment (hormonal imprinting) on the biogenic amine level of the adult rat brain: could oxytocin-induced labor cause pervasive developmental diseases? Reprod. Sci. 2013;20:1255–1263. doi: 10.1177/1933719113483010. [DOI] [PubMed] [Google Scholar]
- Heiming RS, Monning A, Jansen F, Kloke V, Lesch KP, Sachser N. To attack, or not to attack? The role of serotonin transporter genotype in the display of maternal aggression. Behav. Brain Res. 2013;242:135–141. doi: 10.1016/j.bbr.2012.12.045. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Fyodorov D, Wegman L, Lelutiu N, Pehek E, Yamamoto B, Silver J, Weeber E, Sweatt J, Deneris E. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hogg S, Hof M, Wurbel H, Steimer T, de Ruiter A, Koolhaas J, Sluyter F. Behavioral profiles of genetically selected aggressive and nonaggressive male wild house mice in two anxiety tests. Behav. Genet. 2000;30:439–446. doi: 10.1023/a:1010246717180. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol. Psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Ieni JR, Thurmond JB. Maternal aggression in mice: effects of treatments with PCPA, 5-HTP and 5-HT receptor antagonists. Eur. J. Pharmacol. 1985;111:211–220. doi: 10.1016/0014-2999(85)90758-7. [DOI] [PubMed] [Google Scholar]
- Jia R, Tai FD, An SC, Broders H, Ding XL, Kong Q, Zhao L, Zhang H. Effects of neonatal oxytocin treatment on aggression and neural activities in mandarin voles. Physiol. Behav. 2008 doi: 10.1016/j.physbeh.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Johns JM, Joyner PW, McMurray MS, Elliott DL, Hofler VE, Middleton CL, Knupp K, Greenhill KW, Lomas LM, Walker CH. The effects of dopaminergic/serotonergic reuptake inhibition on maternal behavior, maternal aggression, and oxytocin in the rat. Pharmacol. Biochem. Behav. 2005;81:769–785. doi: 10.1016/j.pbb.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler MS, Bosch OJ, Bunck M, Landgraf R, Neumann ID. Maternal care differs in mice bred for high vs. low trait anxiety: impact of brain vasopressin and cross-fostering. Soc. Neurosci. 2011;6:156–168. doi: 10.1080/17470919.2010.495567. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Progr Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav. Neurosci. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Stepp JE, Lee HJ, Young WS, 3rd, Caldwell HK. Normal maternal behavior, but increased pup mortality, in conditional oxytocin receptor knockout females. Behav. Neurosci. 2010;124:677–685. doi: 10.1037/a0020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsee MA. Reactive aggression and posttraumatic stress in adolescents affected by Hurricane Katrina. J. Clin. Child Adolesc. Psychol. 2008;37:519–529. doi: 10.1080/15374410802148152. [DOI] [PubMed] [Google Scholar]
- Martin CB, Ramond F, Farrington DT, Aguiar AS, Jr, Chevarin C, Berthiau AS, Caussanel S, Lanfumey L, Herrick-Davis K, Hamon M, Madjar JJ, Mongeau R. RNA splicing and editing modulation of 5-HT(2C) receptor function: relevance to anxiety and aggression in VGV mice. Mol. Psychiatry. 2013;18:656–665. doi: 10.1038/mp.2012.171. [DOI] [PubMed] [Google Scholar]
- Maskey M, Warnell F, Parr JR, Le Couteur A, McConachie H. Emotional and behavioural problems in children with autism spectrum disorder. J. Autism Dev. Disord. 2013;43:851–859. doi: 10.1007/s10803-012-1622-9. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, De Bold JF, De Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl.) 2002;163:434–458. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl Psychiatry. 2012;2:e122. doi: 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato T. Dual modes of extracellular serotonin changes in the rat ventral striatum modulate adaptation to a social stress environment, studied with wireless voltammetry. Exp. Brain Res. 2013;230:583–596. doi: 10.1007/s00221-012-3168-7. [DOI] [PubMed] [Google Scholar]
- Nehrenberg DL, Rodriguiz RM, Cyr M, Zhang X, Lauder JM, Gariepy JL, Wetsel WC. An anxiety-like phenotype in mice selectively bred for aggression. Behav. Brain Res. 2009;201:179–191. doi: 10.1016/j.bbr.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci. Biobehav. Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Front. Behav. Neurosci. 2010;4:12. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg JM, Vekovischeva O, Sandnabba NK. Anxiety profiles of mice selectively bred for intermale aggression. Behav. Genet. 2003;33:503–511. doi: 10.1023/a:1025718531997. [DOI] [PubMed] [Google Scholar]
- Pagani JH, Lee HJ, Young WS., 3rd Postweaning, forebrain-specific perturbation of the oxytocin system impairs fear conditioning. Genes Brain Behav. 2011;10:710–719. doi: 10.1111/j.1601-183X.2011.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani JH, Zhao M, Cui Z, Williams Avram SK, Caruana DA, Dudek SM, Young WS. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatry. 2014 doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova NK, Skrinskaya YA, Amstislavskaya TG, Vishnivetskaya GB, Seif I, de Meier E. Behavioral characteristics of mice with genetic knockout of monoamine oxidase type A. Neurosci. Behav. Physiol. 2001;31:597–602. doi: 10.1023/a:1012364910091. [DOI] [PubMed] [Google Scholar]
- Pugliese CE, White BA, White SW, Ollendick TH. Social anxiety predicts aggression in children with ASD: clinical comparisons with socially anxious and oppositional youth. J. Autism Dev. Disord. 2013;43:1205–1213. doi: 10.1007/s10803-012-1666-x. [DOI] [PubMed] [Google Scholar]
- Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, Muglia LJ, Pfaff DW. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Rutherford HJ, Williams SK, Moy S, Mayes LC, Johns JM. Disruption of maternal parenting circuitry by addictive process: rewiring of reward and stress systems. Front Psychiatry. 2011;2:37. doi: 10.3389/fpsyt.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Vorhees CV, Williams MT. Mouse plasmacytoma-expressed transcript 1 knock out induced 5-HT disruption results in a lack of cognitive deficits and an anxiety phenotype complicated by hypoactivity and defensiveness. Neuroscience. 2009;164:1431–1443. doi: 10.1016/j.neuroscience.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft CT, Weatherill RP, Woodward HE, Pinto LA, Watkins LE, Miller MW, Dekel R. Intimate partner and general aggression perpetration among combat veterans presenting to a posttraumatic stress disorder clinic. Am J Orthopsychiat. 2009;79:461–468. doi: 10.1037/a0016657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., 3rd Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Williams SK, Lauder JM, Johns JM. Prenatal Cocaine Disrupts Serotonin Signaling-Dependent Behaviors: Implications for Sex Differences, Early Stress and Prenatal SSRI Exposure. Curr. Neuropharmacol. 2011;9:478–511. doi: 10.2174/157015911796557957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm. Behav. 2000;37:145–155. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Kinney JW, Marriott LK, Holmes A, Harris AP, Saavedra MC, Starosta G, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Wenk GL, Crawley JN. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Europ J Neurosci. 2004;19:1384–1396. doi: 10.1111/j.1460-9568.2004.03214.x. [DOI] [PubMed] [Google Scholar]
- Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Adv. Genet. 2011;75:151–169. doi: 10.1016/B978-0-12-380858-5.00005-8. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J. Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;21:52S–60S. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.