Abstract
Background
Long-term psychosocial outcomes of cystic fibrosis (CF) patients diagnosed through newborn screening remains unknown.
Methods
This cross-sectional study compared three groups of youths (16 to 22 years): CF patients diagnosed through NBS (CF-NBS, n=13), CF patients diagnosed through standard practice (CF-SP, n=26) and healthy peers (H, n=42), plus 72 of their parents. We hypothesized that adolescent psychological functioning would be mediated by parent depression and quality of parent-child communication and cohesiveness.
Results
A path analysis showed significantly more depression among CF-NBS group parents (p=.006–.008). Parent-child cohesiveness was related to communication (p<.001). Cohesiveness and communication were associated with youth internalizing problems (p=.037, p=.009), emotional symptoms (p=0.018, p=0.022), and personal adjustment (communication only, p=0.009). Parent depression was related to youth personal adjustment (p=0.022).
Conclusions
CF patients report psychosocial function similar to healthy peers. Parents of children diagnosed with CF through NBS may be at risk for depressive symptoms when their children reach adolescence.
Keywords: adolescence, cohesiveness, communication, cystic fibrosis, newborn screening, psychosocial function
INTRODUCTION
Early diagnosis of cystic fibrosis (CF) resulting from newborn screening and innovative treatments have led to increased longevity for people with CF. Affected families also face new challenges as well as opportunities as a consequence of such advances. Mothers of newborns with CF tend to choose bottle feeding over breast feeding [1]. Parents of young children with CF reportedly perceived their children as being more vulnerable to illness than parents whose infants either have no health problems or have another health conditions identified through newborn screening, e.g. congenital hypothyroidism [2]. Evidence also shows a CF diagnosis made during early infancy can be associated with parental depressive symptoms [3, 4]. Maternal depression can adversely affect mothers’ capacities to provide their infants with sensitive and responsive caregiving [5], which provides the foundation for infants to form secure attachments with their parents and close interpersonal relationships later in life [6]. Toddlers diagnosed with CF in early infancy (due to the presence of CF symptoms) were observed to have higher rates of insecure infant-mother attachments than children diagnosed with CF later in infancy [7]. These findings raise questions about the long-term impact of a neonatal CF diagnosis resulting from NBS on the quality of parent-child relationships and youth psychosocial functioning later in life.
Psychological Functioning of Adolescents with CF
As children with CF reach adolescence they often experience disease progression that forces them to rely more heavily on their parents for health care during a developmental phase that typically involves striving for individuation from parents [8, 9]. Concurrently, they should be shifting their attention to strengthening peer relationships. However, school absence due to illness and hospitalization limit access to friends [10], while medical protocols, designed to prevent cross-infection, preclude CF patients from socializing with one another. Female teens, who tend to have more severe lung disease than males [11], may be particularly susceptible to stressors associated with self-care and social isolation [12]. Not surprising, children and adolescents with CF often report higher rates of depression and anxiety than the general population [13] which is also associated with non-adherence to airway clearance and insecure attachment to parents [14]. One study reported 32% of adolescents and young adults with CF had clinical levels of anxiety and 3% had clinical levels of depression [15]. Another study showed anxiety and depression in adolescents and young adults with CF to be lower than healthy controls [16]. One report showed 10% of CF patients ages 5–18 years met the diagnostic criteria for attention-deficit hyperactivity, 60% of whom were non-adherent to prescribed CF treatment [17]. This rate of ADHD in CF patients was higher than the 6–7% reported in the general population [18]. Studies of youths with ADHD not associated with CF suggested the quality of parent-child relationships can affect the symptom manifestation and social functioning of individuals with ADHD [19]. While adolescents with CF may be at risk for psychosocial problems, the mechanism for developing such symptoms remains unclear.
Parent Psychological Functioning and Family Relationships
Parents of children with CF have reported more overall stress and more illness-related stress than parents of healthy children [20, 21]. Mothers report anxiety and depression; fathers report depression and other internalizing problems [21, 22, 23]. It has been suggested that parental anxiety and/or depression can lead to similar symptoms in their children [24].
Family cohesion, expressiveness, and organization, as reported by children with CF, have been negatively correlated with child self-reported anxiety and depression [16]. Family cohesiveness, flexibility, and positive interactions have been associated with adherence to treatment as reported by children with CF and their parents [25, 26]. The quality of parent-child relationships represents a potential mediating factor in the psychological well-being of youths with CF.
The quality of parent-adolescent relationships in normative samples has been found to affect various aspects of teens’ psychosocial development [27, 28]. It is well documented that secure attachments (particularly to mothers) allow adolescents to move towards cognitive and emotional autonomy [27, 29]. Adolescent attachment security has been correlated with the capacity to effectively communicate emotion [30]. Insecure parent-child attachment has been linked to adolescent internalizing symptoms [28] and discrepancies between adolescent self-report and parent report of adolescent psychological symptoms [31]. Thus, research with normative samples and patients with CF points to the quality of parent-child relationships as a critical factor influencing adolescent psychosocial functioning.
This study drew from the Circumplex conceptual model of family systems [32] to examine the relationship between a neonatal CF diagnosis resulting from NBS and youth psychosocial functioning in late adolescence and early adulthood. Two central concepts in this model are communication and cohesiveness. Communication includes sharing thoughts and feelings, respectful listening, and empathy for other’s feelings and experiences. Cohesiveness is the affective bond or emotional attachment between family members. Effective communication facilitates cohesiveness. We posit that a neonatal diagnosis (from NBS) is likely to produce parental distress and perceptions of child vulnerability that could adversely affect the quality of parent-child cohesiveness and patterns of communication early in the child’s life and continue well into the child’s adolescence. Given the progressive nature of CF, parents are likely to experience either on-going or recurrent emotional distress. Observing their children’s repeated pulmonary infections and/or emergence of new CF complications is likely to reinforce parents’ perceptions of their children’s vulnerability. Consequently, patterns of interaction between parent and their children that transpire from a neonatal diagnosis are likely to become entrenched in ways that could affect children’s long-term psychosocial development. We hypothesized that adolescent psychosocial functioning would be mediated by parent depressive symptoms, parent-child communication, and parent-child cohesiveness. We also controlled for child age and gender, and parent education based on previous research.
METHODS
This cross-sectional study was conducted within the Wisconsin NBS Project, a longitudinal evaluation of benefits and risks of NBS for CF [33]. The original study, conducted from 1985–1994, involved random assignment of newborns into two groups: infants diagnosed through NBS and infants diagnosed through methods that were standard practice at the time, e.g., symptom development and/or family history. Median age at diagnosis was 6.9 weeks (range=3 days to 5.4 years, including 5 false-negatives) for the NBS group and 24 weeks (range=4 days to 15.7 years) for the standard practice group. Parents of 145 children with CF, confirmed by sweat chloride levels of ≥60 mmol/L, consented to enroll their children into the original randomized controlled trial (RCT) [34].
The current study assessed long-term psychosocial functioning of adolescents and young adults, hereafter referred to as youths, who participated in the original study and compared their functioning to a group of healthy peers. Both studies were approved by participating Institutional Review Boards (IRBs).
Sample
The current sample included youths, ages 16–22 years (born between 1985–1994), diagnosed through NBS (CF-NBS group, n=13), or through standard practice (CF-SP group, n=26), and healthy peers (H group, n=42). Eighty-one youths and 72 parents (64 mothers, 8 fathers) participated.
Recruitment, Consent, Incentives
CF patients were recruited from two Wisconsin CF Centers. Invitational recruitment letters explained the study purpose and assessment procedures. Based on respective IRB recommendations, an opt-out approach was used at one site and opt-in procedure at the other site. The opt-out approach involved contacting all families who did not respond to recruitment letters indicating they did not wish to participate. For the opt-in approach, we only contacted families who returned a document indicating interest in the study. Clinical staff also recruited participants during regularly scheduled clinic visits. We recruited H group participants by sending recruitment letters to parents of patients who participated in the study to invite healthy siblings or cousins to participate. We also distributed flyers in primary care clinics, posted ads on the Department of Pediatrics website, and published ads in local newspapers. Written informed consent was obtained from patients ≥18 years, parents and written assent was obtained from patients 16–17 years old. Youths received $100 after completing all assessments; families were reimbursed up to $50 for travel.
Assessments
Assessments were performed at CF centers or community sites, e.g., libraries that were convenient for participants. Data collectors had advanced degrees in school psychology and extensive clinical experience.
To measure youth psychological functioning, we used the Adolescent self-report form (for 16–21 years) of the Behavioral Assessment System for Children–Second Edition (BASC-2) [35]. Youths completed this 176-item, self-report inventory that produces five composite scores generated from 16 subscales. The Personal Adjustment composite (33 items, four subscales) measures relations with parents and peers, self-esteem, and self-reliance. The Emotional Symptoms Index (58 items, six subscales) is a global indicator of emotional disturbance. The Internalizing Problems (70 items, seven subscales) measures inwardly-directed distress, e.g., anxiety and depression. The Inattention/Hyperactivity composite (16 items, two subscales) measures inattentiveness and excessive activity. The School Problems composite (25 items, three subscales) measures adaptation to high school or college. Items emphasize how adolescents think about themselves and their behavior, as well as their perceptions of other people’s opinions about them. Responses range from 0=never to 3=almost always. Summed scores can be converted to standardized T scores based on gender. T scores ≥70 suggest clinical levels of maladjustment on the following subscales: School Problems, Internalizing Problems, Inattention/Hyperactivity, and Emotional Symptoms Index. T scores ≤30 suggest clinically significant results on the Personal Adjustment subscale. Internal consistency coefficients are reportedly in the mid-0.90s for the Internalizing Problems and Emotional Symptoms Index composites are in the mid to upper-0.80s for the School Problems, Inattention/Hyperactivity, and Personal Adjustment composites [31]. The median test-retest reliability coefficient ranges from 0.74 for Personal Adjustment to 0.84 for School Problems. Intercorrelations of subscales for normative and clinical samples range from 0.52 to 0.93 [31]. We used BASC-2 ASSIST computer program to enter BASC-2 responses and calculate scale and composite scores.
To measure parent-child communication and cohesiveness, we asked parents to complete the Communication and Attachment scales of the Parenting Relationship Questionnaire (PRQ) [36] for children ages of 6–18 years. The Communication scale (9 items) measures parent perceptions about the quality of verbal exchanges between the parent and child, particularly parents’ listening capacities. Items emphasize the quality and frequency of thoughts, behaviors, and actions associated with parent-child interactions (ranging from 0=never to 3=almost always). The Attachment scale (11 items) measures parents’ perceptions about their emotional closeness to the child, empathy for the child’s feelings, and ability to comfort the child when distressed. Summed scores can be converted to T scores and percentiles. Normative scores are based on the child’s age (16–18 years for our study) and parent’s gender. T scores ≤30 on the Attachment or the Communication scales suggest clinically significant relationship problems for which additional assessment and possible intervention may be warranted. Alpha coefficients assessing internal consistency for the Attachment scale reportedly are 0.85 for female raters and 0.86 for male raters in youths ages 16–18 years; coefficients for the Communication are 0.85 female and 0.87 male raters in youths ages 16–18 years [36]. Test-retest reliability coefficients are 0.76 for Attachment and 0.84 for Communication. Assessments of construct validity show correlations of −0.34 between the Attachment scale and the comparable subscale of the Parenting Stress Index and 0.53 for Communication scale and comparable subscale of the Parent-Child Relationship Inventory [36].
Parents completed the Center for Epidemiological Studies – Depression (CES-D) [37]. This 20-item, self-report scale screens for depressive symptoms in the general population. Items emphasize the frequency of particular symptoms during the past week (ranging from 0=rarely or not at all to 3=most of the time). Sum scores range from 0 to 60. Scores ≥ 16 suggest clinical levels of depressive symptoms. Internal consistency coefficients have been 0.85 in non-clinical samples and 0.90 in clinical samples with a test-retest reliability coefficient of 0.54.
Analysis
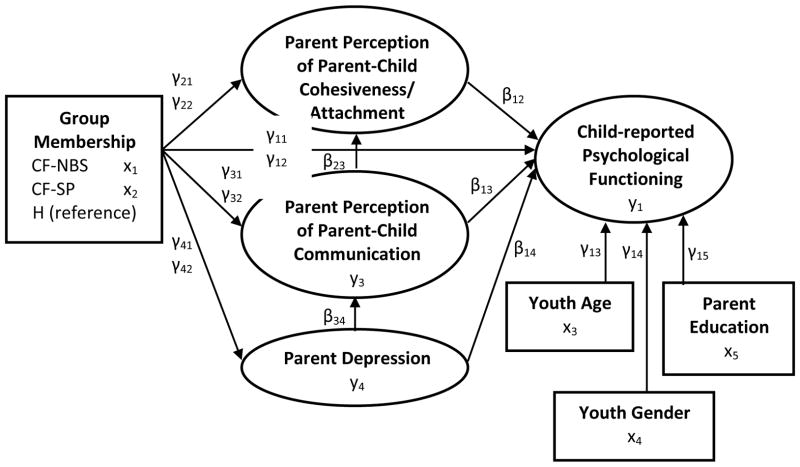
Descriptive analyses were conducted on the demographic variables by groups. We used maximum likelihood estimates of summed subscale scores in path analyses (Figure 1) to estimate parameters in five structural models (School Problems, Internalizing Problems, Inattention/Hyperactivity, Emotional Symptom Index, and Personal Adjustment). Mediational analysis was conducted to assess possible indirect effects of parent depression on Attachment and Communication. Sobel tests [38] assessed statistically significant mediation in models. We used Mplus version 6.12 [39, 40] to construct all models. To determine how representative this sample was of the original sample, we used an exact proportional difference test [41] to compare patient gender, race, genotype, and pancreatic status in each CF group. Genotype and pancreatic status were included because both have been associated with objective and subjective measures of patient health in this population [42].
Figure 1. Heuristic Model of Factors Associated with Youth Psychosocial Functioning*.
*Child Psychological Functioning includes Personal Adjustment, Internalizing Problems, Emotional Symptoms, Inattention-Hyperactivity, and School Problems.
CF-NBS= Cystic Fibrosis Diagnosis made through Newborn Screening
CF-SP= Cystic Fibrosis Diagnosis made through Standard Practice at time
H= Healthy Comparison Group without Cystic Fibrosis or other Chronic conditions
Evaluation of Missing Data
The BASC-2 program uses floating denominators to adjust for missing items. If there were more than two missing items in a scale, that scale score and the composite score to which it belonged were not generated. Following the PRQ manual’s instruction, unrated items for Attachment or Communication scales were assigned a score of two. If any items were missing on the CES-D, the sum score was not generated. An initial appraisal of missing data for each measure indicated <5% total items missing. All items missed met the conditions of missing completely at random (MCAR) based on Little’s test [43].
RESULTS
Demographics and Sample Comparisons
Thirty-nine youths with CF (NBS group, n=13, standard practice, n=26) from the original RCT study, and 42 healthy controls (2 were siblings of CF patients), ages 16–22 years, participated in this study. In all but nine cases, one parent of each youth also participated in the study. Participants were predominantly white; most parents were female and married (Table 1). Youth gender was fairly evenly divided. Parents in the healthy group tended to be more highly educated than those in either CF group. The original RCT sample and the subsample in this study did not differ significantly in demographics, CF genotype or pancreatic status (Table 2).
Table 1.
Sample Demographics by Group
| Healthy | CF-NBS | CF-SP | |
|---|---|---|---|
| PARENT INFROMATION | |||
| Gender | n=39* | n=11** | n=22*** |
| Mothers (%) | 33 (84.6) | 11 (100.0) | 20 (90.0) |
| Fathers (%) | 6 (15.4) | 0 (0.0) | 2 (10.0) |
| Mean Age in Years (SD) | 50.1 (4.9) | 48.1 (4.7) | 49.2 (7.9) |
| Marital Status | n=38 | n=11 | n=20 |
| Married (%) | 30 (76.9) | 7 (63.6) | 18 (90.0) |
| Single/Divorced (%) | 7 (18.0) | 4 (36.4) | 2 (10.0) |
| Widowed (%) | 1 (2.6) | 0 (0) | 0 (0) |
| Other (%) | 1 (2.6) | 0 (0) | 0 (0) |
| Race | |||
| African American/Black (%) | 2 (5.1) | 0 (0) | 0 (0) |
| American Indian (%) | 0 (0) | 0 (0.0%) | 0 (0.0) |
| European American/White (%) | 31 (79.5) | 11 (100.0) | 19 (95.0) |
| Asian American (%) | 1 (2.6) | 0 (0) | 0 (0) |
| Hispanic (%) | 2 (5.1) | 0 (0) | 1 (5.0) |
| Other (%) | 3 (7.7) | 0 (0.0) | 0 (0.0) |
| Education | |||
| High School/GED (%) | 5 (12.8) | 5 (45.5) | 7 (35.0) |
| Community College/Trade School (%) | 5 (12.8) | 3 (27.3) | 6 (30.0) |
| Baccalaureate College Degree (%) | 14 (35.9) | 2 (18.2) | 6 (30.0) |
| Graduate Degree/Professional Degree (%) | 15 (38.5) | 1 (9.1) | 1 (5.0) |
| Income | |||
| Less than $20,000 (%) | 1 (2.6) | 2 (18.2) | 0 (0) |
| $20,000–$40,000 (%) | 5 (13.2) | 2 (18.2) | 2 (10.0) |
| $41,000–$60,000 (%) | 4 (10.5) | 3 (27.3) | 5 (25.0) |
| $61,000–$80,000 (%) | 1 (2.6) | 0 (0) | 4 (20.0) |
| $81,000–$100,000 (%) | 5 (13.2) | 2 (18.2) | 5 (25.0) |
| Over $100,000 (%) | 22 (57.9) | 2 (18.2) | 4 (20.0) |
|
| |||
| YOUTH INFORMATION | |||
| Gender | n=42 | n=13 | n=26 |
| Female (%) | 21 (50.0%) | 6 (46.2%) | 14 (53.9%) |
| Male (%) | 21 (50.0%) | 7 (53.8%) | 12 (46.2%) |
| Mean Age in Years (SD) | 17.9 (1.7) | 18.15 (1.8) | 18.26 (1.8) |
CF-NBS, cystic fibrosis-newborn screening group; CF-SP, cystic fibrosis-standard practice group; SD, standard deviation;
3 H group parents did not report demographic information
2 CF-NBS group parents did not report demographic information
4 CF-SP group parents did not report demographic information, and 1 did not report age
Table 2.
Comparison of Original RCT Sample with Current Sample
| Current CF-NBS N=13 |
Original CF-NBS N=77 |
Z-value | p-valuea | Current CF-SP N=26 |
Original CF-SP N=81 |
Z-value | p-valuea | |
|---|---|---|---|---|---|---|---|---|
| Female | 46.2% | 40.0% | 0.399 | 0.717 | 53.8% | 46.0% | 0.725 | 0.450 |
| White | 100% | 95.0% | 0.773 | 0.716 | 86.4% | 89.0% | −0.327 | 0.999 |
| Genotype: | ||||||||
| Homozygous F508del | 61.5% | 53.0% | −0.551 | 0.826 | 34.6% | 43.0% | 0.774 | 0.626 |
| Heterozygous F508del | 38.5% | 43.0% | 0.296 | 0.999 | 46.2% | 38.0% | −0.713 | 0.511 |
| Combine other and unknown | 0.0% | 4.0% | 0.723 | 0.770 | 19.2% | 18.5% | −0.081 | 0.954 |
| Pancreatic Status: | ||||||||
| Insufficiency | 92.3% | 79.0% | −1.11 | 0.296 | 65.4% | 58.0% | −0.666 | 0.526 |
| Sufficiency | 7.7% | 8.0% | 0.012 | 0.999 | 23.1% | 21.0% | −0.225 | 0.855 |
| Unknown | 0.0% | 13.0% | 1.37 | 0.185 | 11.5% | 21.0% | 1.07 | 0.325 |
RCT, randomized controlled trial; CF-NBS, cystic fibrosis-newborn screening group; CF-SP, cystic fibrosis-standard practice group
Group Differences
All five models used the H group as the reference and controlled for parent education because H group parents tended to have higher education levels than parents in both CF groups. Based on the literature regarding the potential influence of youth gender and age on outcomes, we controlled for these variables in each model. Results (Tables 3 and 4) showed parents in the CF-NBS group reported significantly more depressive symptoms than H group parents (p=0.006–0.008). There was no significant difference between parents in the CF-SP group and H group on depression scores. There were no significant differences between either CF study groups and H group on parent-reported Communication, Attachment, or youth-reported measures of psychological functioning on any models.
Table 3.
Effect Paths for Youth Personal Adjustment, Youth Internalizing Problems and Youth Emotional Symptoms Index
| Youth Personal Adjustment |
Youth Internalizing Problems |
Youth Emotional Symptoms Index |
|||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Effect Path | Model Parameter |
Non- Standardized Parameter (SE) |
P [95% CI] |
Non- Standardized Parameter (SE) |
P [95% CI] |
Non- Standardized Parameter (SE) |
P [95% CI] |
| CF-NBS → Attachment | γ21 | 1.204 (1.369) | 0.379 [−1.480, 3.888] | 1.148 (1.373) | 0.403 [−1.543, 3.839] | 1.102 (1.370) | 0.421 [−1.582, 3.789] |
| CF-SP → Attachment | γ22 | 0.805 (1.080) | 0.456 [−1.313, 2.922] | 1.026 (1.082) | 0.343 [−1.094, 3.145] | 0.963 (1.077) | 0.372 [−1.149, 3.075] |
| CF-NBS → Communication | γ31 | −2.211 (1.983) | 0.265 [−6.097, 1.675] | −2.195 (1.985) | 0.269 [−6.086, 1.695] | −2.281 (1.989) | 0.252 [−6.180, 1.618] |
| CF-SP → Communication | γ32 | 2.040 (1.529) | 0.182 [−0.957, 5.037] | 1.753 (1.535) | 0.253 [−1.255, 4.761] | 1.871 (1.536) | 0.223 [−1.139, 4.881] |
| CF-NBS → Parent Depression | γ41 | 10.424 (3.760) | 0.006 [3.055, 17.793] | 10.181 (3.761) | 0.007 [2.809, 17.553] | 10.243 (3.757) | 0.006 [2.879, 17.606] |
| CF-SP → Parent Depression | γ42 | 1.045 (3.024) | 0.730 [−4.883, 6.972] | 0.802 (3.027) | 0.791 [−5.131, 6.735] | 0.864 (3.023) | 0.775 [−5.061, 6.788] |
| CF-NBS → Youth Ad | γ11 | 3.231 (8.051) | 0.688 [−12.549, 19.01] | −0.187 (15.815) | 0.991 [−31.185, 30.811] | −5.711 (13.418) | 0.670 [−32.011, 20.589] |
| CF-SP → Youth Ad | γ12 | −2.352 (5.897) | 0.690 [−13.911, 19.206] | 8.470 (11.664) | 0.468 [−14.391, 31.332] | 5.349 (9.861) | 0.588 [−13.979, 24.677] |
| Parent Depression → Communication | β34 | −0.018 (0.066) | 0.782 [−0.147, 0.111] | −0.018 (0.066) | 0.781 [0.148, 0.111] | −0.023 (0.066) | 0.728 [−0.153, 0.107] |
| Communication → Attachment | β23 | 0.751 (0.094) | <0.001 [0.568, 0.935] | 0.749 (0.094) | <0.001 [0.565, 0.933] | 0.750 (0.094) | <0.001 [0.566, 0.934] |
| Attachment → Youth Ad | β12 | −1.397 (0.725) | 0.054 [−2.818, 0.024] | 3.061 (1.471) | 0.037 [0.178, 5.943] | 2.932 (1.236) | 0.018 [0.509, 5.354] |
| Communication → Youth Ad | β13 | 2.025 (0.778) | 0.009 [0.501, 3.550] | −4.102 (1.578) | 0.009 [−7.196, −1.009] | −3.048 (1.327) | 0.022 [−5.648, −0.448] |
| Parent Depression → Youth Ad | β14 | −0.565 (0.247) | 0.022 [−1.049, −0.081] | 0.543 (0.493) | 0.271 [−0.423, 1.510] | 0.695 (0.415) | 0.094 [−0.119, 1.509] |
| Youth Age → Youth Ad | γ13 | 3.588 (1.486) | 0.016 [0.676, 6.499] | −0.817 (2.939) | 0.781 [−6.577, 4.944] | −1.536 (2.488) | 0.537 [−6.413, 3.340] |
| Youth Gender → Youth Ad | γ14 | 6.931 (4.978) | 0.164 [−2.827, 16.688] | 9.258 (9.903) | 0.350 [−10.152, 28.668] | 1.052 (8.355) | 0.900 [−15.324, 17.428] |
| Parent Education → Youth Ad | γ15 | 1.799 (2.553) | 0.481 [−3.205, 6.803] | 4.462 (5.075) | 0.379 [−5.484, 14.408] | 5.274 (4.296) | 0.220 [−3.146, 13.693] |
CF-NBS, cystic fibrosis-newborn screening group; CF-SP, cystic fibrosis-standard practice group; CI, confidence interval; Ad, Adjustment; IP, Internalizing Problems; ESI, Emotional Symptoms Index; Bold results indicate statistical significance at p<0.05.
Table 4.
Effect Paths for Youth Inattention-Hyperactivity and Youth School Problems
| Youth Inattention-Hyperactivity | Youth School Problems | ||||
|---|---|---|---|---|---|
|
| |||||
| Effect Path | Model Parameter | Non-Standardized Parameter (SE) | P [95% CI] | Non-Standardized Parameter (SE) | P [95% CI] |
| CF-NBS → Attachment | γ21 | 1.339 (1.374) | 0.330 [−1.354, 4.032] | 1.350 (1.374) | 0.326 [−1.342, 4.043] |
| CF-SP → Attachment | γ22 | 0.995 (1.091) | 0.362 [−1.143, 3.133] | 0.987 (1.092) | 0.366 [−1.154, 3.129] |
| CF-NBS → Communication | γ31 | −2.315 (1.979) | 0.242 [−6.194, 1.565] | −2.318 (1.977) | 0.241 [−6.192, 1.556] |
| CF-SP → Communication | γ32 | 1.653 (1.543) | 0.284 [−1.370, 4.677] | 1.618 (1.545) | 0.295 [−1.410, 4.647] |
| CF-NBS → Parent Depression | γ41 | 10.044 (3.759) | 0.008 [2.677, 17.412] | 9.993 (3.761) | 0.008 [2.622, 17.364] |
| CF-SP → Parent Depression | γ42 | 0.665 (3.024) | 0.826 [−5.263, 6.593] | 0.614 (3.027) | 0.839 [−5.318, 6.546] |
| CF-NBS → Youth IH | γ11 | −2.727 (6.553) | 0.677 [−15.571, 10.117] | −1.980 (8.116) | 0.807 [−17.887, 13.927] |
| CF-SP → Youth IH | γ12 | −3.002 (4.900) | 0.540 [−12.606, 6.602] | 3.468 (6.054) | 0.567 [−8.397, 15.334] |
| Parent Depression → Communication | β34 | −0.029 (0.066) | 0.663 [−0.157, 0.100] | −0.030 (0.066) | 0.651 [−0.158, 0.099] |
| Communication → Attachment | β23 | 0.749 (0.094) | <0.001 [0.565, 0.933] | 0.749 (0.094) | <0.001 [0.566, 0.933] |
| Attachment → Youth IH | β12 | 0.534 (0.623) | 0.391 [−0.687, 1.754] | 0.532 (0.751) | 0.479 [−0.940, 2.004] |
| Communication → Youth IH | β13 | −1.064 (0.665) | 0.109 [−2.367, 0.239] | −1.194 (0.810) | 0.141 [−2.782, 0.394] |
| Parent Depression → Youth IH | β14 | 0.217 (0.202) | 0.282 [−0.178, 0.613] | −0.177 (0.249) | 0.477 [−0.665, 0.311] |
| Child Age → Youth IH | γ13 | −0.098 (1.199) | 0.935 [−2.449, 2.253] | 0.595 (1.478) | 0.687 [−2.302, 3.492] |
| Child Gender → Youth IH | γ14 | −3.871 (4.151) | 0.351 [−12.007, 4.264] | −6.891 (5.164) | 0.182 [−17.013, 3.231] |
| Parent Education → Youth IH | γ15 | 0.968 (2.139) | 0.651 [−3.244, 5.161] | −1.185 (2.659) | 0.656 [−6.396, 4.026] |
CF-NBS, cystic fibrosis-newborn screening group; CF-SP, cystic fibrosis-standard practice group; CI, confidence interval; IH, Inattention-Hyperactivity;
Sch P, School Problems;
Bold results indicate statistical significance at p<0.05.
Youth Psychological Function
All models showed a significant positive relationship between Attachment and Communication (p<0.001). Parent-reported Communication effectiveness was associated with parent perceptions of close relationships with their children. Significant relationships were found between parent-reported Attachment relative to youth-reported outcomes of Internalizing Problems (p=0.037) and Emotional Symptoms Index (p=0.018). Significant relationships were found between parent-reported Communication relative to youth-reported Internalizing Problems (p=0.009) and Emotional Symptoms Index (p=0.022), as well as between Communication and Personal Adjustment (p=0.009). Thus, parent-child relationships characterized by close relationships and effective communication were associated with youth-reported favorable psychological functioning. Parent-reported Attachment and Communication were not significantly associated with youth-reported School Problems or Inattention/Hyperactivity. Although parent depression was not associated with Communication, a significant negative association was found between parent depression and youth Personal Adjustment (p=0.022). Youths whose parents reported more depressive symptoms were more likely to experience difficulties with Personal Adjustment than children of parents who reported fewer depressive symptoms. Older youths tended to report better Personal Adjustment than younger adolescents. Parent age and child gender were not significant factors associated with any youth outcomes. Mediating factors (depression on Communication, Communication on Attachment) were not significant. We also assessed Attachment and Communication as mediators and no relationships were significant. A proportional difference test examined the percent of participants within each group whose scores fell within a clinical range. Results were only statistically significant for the CES-D measure of parent depression; the CF-NBS group had the highest percentage within the clinical range (CF-NBS=58%, CF-SP=27%, H=22%). Most scores in all groups on all measures fell within the normal range.
DISCUSSION
This report describes the first investigation of long-term psychosocial outcomes for patients who were diagnosed with CF through NBS. The only group difference was that parents of youths diagnosed through NBS, but not those diagnosed through standard practice, reported more depressive symptoms than parents of children with no chronic health conditions. This finding supports one aspect of our proposed mechanistic model. Receipt of news that one’s child has a potentially life-shortening condition so early in the child’s life could set the stage for parental distress that continues throughout the child’s life. However, an alternative explanation is that such depressive symptoms were due to well documented evidence that children in the CF-NBS group tended to have genotypes and related clinical profiles associated with more severe pulmonary disease than those diagnosed through standard practices [42, 44]. Having a child with serious complications of CF certainly could give rise to depressive symptoms. The lack of group differences on other measures suggests that even in the presence of parental depressive symptoms, the quality of parent-child relationship and adolescent psychological function appear to be fairly similar to the healthy comparison group. Our findings are consistent with the Circumplex model [32] and previous research with normative samples [27] that show effective communication and close parent-youth relationships, even in the presence of chronic health problems, are associated with favorable adolescent psychosocial functioning. It is noteworthy that depressive symptoms among parents, regardless of whether or not the child had CF, were linked to less favorable adolescent psychosocial development. These findings of depressive symptoms among care-givers concur with previous research [21].
NBS for CF and other genetic conditions clearly benefits affected infants, their families, and society by preventing mortality, morbidity and “diagnostic odysseys” [45, 46]. Early diagnosis of rare conditions can also enhance our understanding of and capacity to treat individuals with these conditions. However, the psychosocial consequences of an unsolicited diagnosis so early in life is not inconsequential [47]. The mother of a child with CF most eloquently stated, “From the moment you hear that diagnosis, your relationship with that child, for better or worse, is forever changed.” Screening programs for CF and other genetic conditions are likely to expand as new genetic/genomic technologies become ubiquitous in health care. Thus, increasing numbers of infants are likely to be diagnosed prenatally and after birth. Our findings point to the need for early intervention programs for families with newly diagnosed infants designed to prevent long-term psychosocial complications. These programs could be made easily accessible to parents through on-line offerings that help parents build coping capacities, establish effective communication patterns, and optimize cohesive family relationships. Ideally, such programs should be created as parent-professional partnerships using strength-based models that promote family resilience. It is also imperative that depression screening and assessment of family dynamics be part of routine CF care. Mental health referrals for families struggling to cope may be particularly valuable during times of transition, such as late adolescence.
Limitations
We acknowledge that our sample size was small relative to the complexity of the path analysis used to construct the models. However, we were able to obtain convergence on these models and parameter estimates were stable. The small sample raises questions about the sensitivity of the standard errors. Still, we were able to identify several statistically significant relationships. Although there was a lack of statistically significant difference between this study sample and the original RCT sample, given the low rate of enrollment (only 39 of the 145 in the original study), we exercise caution in concluding that findings definitively reflect the entire original Wisconsin NBS cohort. The different recruitment procedures used at each site and inclusion of siblings (n=2) in the control group could have introduced some bias—again cause for caution in generalizing the findings. Given the lack of a child self-report version of the Attachment and Communication assessment, data were based solely on parent reports. Future studies should include adolescent perspectives about their relationships with their parents and objective observations of parent-child interactions. Some youths exceeded the age limit for which some instruments were standardized. We chose to do so because instruments to measure the constructs in this study were not available for older individuals and using different versions of instruments posed analytic problems.
CONCLUSION
Psychosocial functioning of youths with CF, irrespective of diagnostic method, is similar to healthy peers. Parents of CF patients diagnosed through NBS may be at risk for depressive symptoms when their children are adolescents, though this finding may be related to the child’s health status. Parental depression can adversely affect children’s personal adjustment, thus routine mental health screening is optional. Future research is needed to identify effective psychosocial interventions to prevent or ameliorate the adverse impact of a neonatal diagnosis.
Acknowledgments
We thank the families who generously volunteered their time and efforts to make this project possible. We gratefully acknowledge Dr. Rebecca Koscik for her early contributions and Dr. Caroline Racine for her assistance with data collection. Finally, we remain grateful to the entire Wisconsin Neonatal CF Screening Project team in Madison and Milwaukee. This work was supported by Cystic Fibrosis Foundation A001-5-01.
Abbreviations
- CF
cystic fibrosis
- NBS
newborn screening
- ADHD
attention deficit hyperactivity
- RCT
randomized control trial
- IRB
Institutional Review Board
- BASC-2
Behavioral Assessment System for Children-Second Edition
- PRQ
Parenting Relationship Questionnaire
- MCAR
Missing completely at random
Footnotes
Conflict of Interest: None of the authors has a potential conflicts of interest or financial relationship to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tluczek A, Clark R, McKechnie AC, Orland KM, Brown RL. Task-oriented and bottle feeding adversely affect the quality of mother-infant interactions after abnormal newborn screens. J Dev Behav Pediatr. 2010;31:414–26. doi: 10.1097/DBP.0b013e3181dd5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tluczek A, McKechnie AC, Brown RL. Factors associated with parental perception of child vulnerability 12 months after abnormal newborn screening results. Res Nurs Health. 2011;34:389–400. doi: 10.1002/nur.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glasscoe C, Lancaster GA, Smyth RL, Hill J. Parental depression following the early diagnosis of cystic fibrosis: a matched, prospective study. J Pediatr. 2007;150:185–191. doi: 10.1016/j.jpeds.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Tluczek A, Clark R, Kosick RL, Farrell PM. Mother-infant relationship in the context of neonatal CF diagnosis: preliminary findings. Pediatr Pulmonol. 2005;28:179–180. [Google Scholar]
- 5.Goodman SH. Depression in mothers. Annu Rev Clin Psychol. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- 6.DeKlyen M, Greenberg MT. Attachment and psychopathology in childhood. In: Cassidy J, Shaver PR, editors. Handbook of Attachment: Theory, Research, and Clinical Applications. 2. Ch 27. New York, NY: The Guilford Press; 2008. pp. 637–665. [Google Scholar]
- 7.Simmons R, Goldberg S, Washington J, Fischer-Fay A, Maclusky I. Infant-mother attachment and nutrition in children with CF. J Dev Behav Pediatr. 1995;16:183–186. [PubMed] [Google Scholar]
- 8.Cystic Fibrosis Foundation. Patient Registry 2010 Annual Data Report. 2010. [Google Scholar]
- 9.Badlan K. Young people living with cystic fibrosis: An insight into their subjective experience. Health Soc Care Community. 2006;14:264–270. doi: 10.1111/j.1365-2524.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- 10.Ernst MM, Johnson MC, Stark LJ. Developmental and psychosocial issues in CF. Pediatr Clin N Am. 2010;54:865–85. doi: 10.1016/j.pcl.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Konstan MW, Wagener JS, Vandevanter DR, et al. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros. 2012;11:405–11. doi: 10.1016/j.jcf.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrop M. Psychosocial impact of cystic fibrosis in adolescence. Pediatr Nurs. 2007;19:41–45. doi: 10.7748/paed2007.12.19.10.41.c6432. [DOI] [PubMed] [Google Scholar]
- 13.Cruz IC, Marciel KK, Quittner AL, et al. Anxiety and depression in cystic fibrosis. Semin Respir Crit Care Med. 2009;30:569–578. doi: 10.1055/s-0029-1238915. [DOI] [PubMed] [Google Scholar]
- 14.Smith BA, Modi AC, Quittner AL, et al. Depressive symptoms in children with cystic fibrosis and parents and its effects on adherence to airway clearance. Pediatr Pulmonol. 2010;45:756–763. doi: 10.1002/ppul.21238. [DOI] [PubMed] [Google Scholar]
- 15.Modi AC, Driscoll KA, Montag-Leifling K, et al. Screening for symptoms of depression and anxiety in adolescents and young adults with cystic fibrosis. Pediatr Pulmonol. 2011;46:153–159. doi: 10.1002/ppul.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szyndler JE, Towns SJ, van Asperen PP, et al. Psychological and family functioning and quality of life in adolescents with cystic fibrosis. J Cyst Fibros. 2005;4:135–144. doi: 10.1016/j.jcf.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Georgiopoulos AM, Hua LL. The diagnosis and treatment of attention deficit-hyperactivity disorder in children and adolescents with cystic fibrosis: a retrospective study. Psychosomatics. 2011;52(2):160–6. doi: 10.1016/j.psym.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–9. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deault LC. A systematic review of parenting in relation to the development of comorbidities and functional impairments in children with attention-deficit/hyperactivity disorder (ADHD) Child Psychiatry Hum Dev. 2010;41(2):168–92. doi: 10.1007/s10578-009-0159-4. [DOI] [PubMed] [Google Scholar]
- 20.Quittner AL, Opipari LC, Espelage DL, Carter B, Eid N, Eigen H. Role strain in couples with and without a child with a chronic illness: associations with marital satisfaction, intimacy, and daily mood. Health Psychol. 1998;17:112–24. doi: 10.1037//0278-6133.17.2.112. [DOI] [PubMed] [Google Scholar]
- 21.Driscoll KA, Montag-Leifling K, Acton JD, et al. Relations between depressive and anxious symptoms and quality of life in caregivers of children with cystic fibrosis. Pediatr Pulmonol. 2009;44:784–792. doi: 10.1002/ppul.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besier T, Goldbeck L. Anxiety and depression in adolescents with CF and their caregivers. J Cyst Fibros. 2011;10:435–442. doi: 10.1016/j.jcf.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Smith BA, Modi AC, Quittner AL, Wood BL. Depressive symptoms in children with cystic fibrosis and parents and its effects on adherence to airway clearance. Pediatr Pulmonol. 2010;45:756–63. doi: 10.1002/ppul.21238. [DOI] [PubMed] [Google Scholar]
- 24.Besier T, Born A, Henrich G, et al. Anxiety, depression, and life satisfaction in parents caring for children with cystic fibrosis. Pediatr Pulmonol. 2011;46:672–682. doi: 10.1002/ppul.21423. [DOI] [PubMed] [Google Scholar]
- 25.White T, Miller J, Smith G, et al. Adherence and psychopathology in children and adolescents with cystic fibrosis. Eur Child Adolesc Psychiatry. 2009;18:96–104. doi: 10.1007/s00787-008-0709-5. [DOI] [PubMed] [Google Scholar]
- 26.DeLambo KE, Levers-Landis CE, Drotar D, et al. Association of observed family relationship quality and problem-solving skills with treatment adherence in older children and adolescents with cystic fibrosis. J Pediatr Psychol. 2004;29:343–353. doi: 10.1093/jpepsy/jsh038. [DOI] [PubMed] [Google Scholar]
- 27.Allen JP, Manning N. From safety to affect regulation: Attachment from the vantage point of adolescence. New Dir Child Adolesc Dev. 2007;2007:23–39. doi: 10.1002/cd.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen JP, Moore C, Kuperminc G, et al. Attachment and adolescent psychosocial functioning. Child Dev. 1998;69:1406–1419. [PMC free article] [PubMed] [Google Scholar]
- 29.Allen JP, McElhaney KB, Land DJ, et al. A Secure Base in Adolescence: Markers of attachment security in the Mother-Adolescent Relationship. Child Dev. 2003;74:292–307. doi: 10.1111/1467-8624.t01-1-00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker-Stoll F, Delius A, Scheitenberger S. Adolescents’ nonverbal emotional expressions during negotiation of a disagreement with their mothers: An attachment approach. Int J Behav Dev. 2001;25:344–353. [Google Scholar]
- 31.Berger LE, Jodl KM, Allen JP, et al. When adolescents disagree with others about their symptoms: Differences in attachment organizations as an explanation of discrepancies between adolescent-, parent-, and peer-reports of behavior problems. Dev Psychopathol. 2005;17:489–507. doi: 10.1017/s0954579405050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson DH, Gorall DM. Circumplex model of marital and family systems. In: Walsh F, editor. Normal family processes: Growing diversity and complexity. 3. New York: Guilford Press; 2003. pp. 514–548. [Google Scholar]
- 33.Farrell PM, Kosorok MR, Rock MJ, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Pediatrics. 2001;107:1–13. doi: 10.1542/peds.107.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Farrell PM Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Improving the health of patients with cystic fibrosis through newborn screening. Adv Pediatr. 2000;47:79–115. [PubMed] [Google Scholar]
- 35.Reynolds CR, Kamphaus RW. BASC-2: Behavior Assessment System for Children. 2. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- 36.Kamphaus RW, Reynolds CR. Parenting Relationship Questionnaire. Minneapolis, MN: NCS Pearson; 2006. [Google Scholar]
- 37.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 38.Sobel ME. Some new results on indirect effects and their standard errors in covariance structure models. In: Tuma N, editor. Sociological Methodology. Washington, DC: American Sociological Association; 1986. pp. 159–186. [Google Scholar]
- 39.Muthén LK, Muthén BO. Mplus User’s Guide. Los Angeles: Muthén and Muthén; 1998. [Google Scholar]
- 40.Muthén LK, Muthén BO. Mplus VERSION 2.12: Addendum to the Mplus User’s Guide. Los Angeles: Muthén and Muthén; 2010. [Google Scholar]
- 41.Mehta CR, Patel NR. StatXact 8 PROCs for SAS. Cambrige, MA: Cytel Inc. Pfeffer PE; 2007. [Google Scholar]
- 42.Tluczek A, Becker T, Grieve A, et al. Relationships among health-related quality of life, pulmonary health, and newborn screening for cystic fibrosis. Chest. 2011;140:170–177. doi: 10.1378/chest.10-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198–1202. [Google Scholar]
- 44.Farrell PM, Li Z, Kosorok MR, et al. Bronchopulmonary disease in children with cystic fibrosis after early or delayed diagnosis. Am J Respir Crit Care Med. 2003;168:1100–1108. doi: 10.1164/rccm.200303-434OC. [DOI] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. CDC Grand Rounds: Newborn screening and improved outcomes. CDC Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report (MMWR) 61:390–393. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6121a2.htm. [PubMed] [Google Scholar]
- 46.Kharrazi M, Kharrazi LD. Delayed diagnosis of cystic fibrosis and the family perspective. Journal of Pediatrics. 2005;147:S21–S25. doi: 10.1016/j.jpeds.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Grob R. Testing Baby: The transformation of newborn screening, parenting, and policymaking. Piscataway, New Jersey: Rutgers University Press; 2011. [Google Scholar]