Abstract
We hypothesized that shear stress stimulates the release of epoxyeicosatrienoic acids (EETs) from arteriolar endothelium, which directly hyperpolarize smooth muscle. To test this hypothesis, a perfusion system, consisting of two separate, serially connected chambers (A and B), was used. A donor vessel, isolated from gracilis muscle of female NO-deficient mice and rats, was cannulated in chamber A. In chamber B, an endothelium-denuded detector vessel isolated from mesentery of these animals was cannulated. In the presence of indomethacin, 5, 10, and 20 dyne/cm2 shear stress elicited dilation of donor vessels, followed by dilation of detector vessels. Changes in membrane potential of the detector vessel smooth muscle cells in response to the perfusate from 5 and 10 dyne/cm2 shear stress–stimulated donor vessels was also recorded (by ≈ −12 to −15 and −20 to −30 mV, respectively). Exposing detector vessels to 30 mmol/L KCl or pretreating them with iberiotoxin abolished their hyperpolarization and dilation to the flow of perfusate. Pretreatment of donor vessels with PPOH, an inhibitor of cytochrome P-450/epoxygenase, eliminated dilator responses in both donor and detector vessels, as well as the hyperpolarization of detector vessels. GC-MS analysis showed increasing release of EETs into the perfusate collected from 1, 5, and 10 dyne/cm2 shear stress–stimulated arterioles, which was abolished by PPOH. Thus, EETs, released from endothelial cells of donor vessels stimulated with shear stress, hyperpolarize smooth muscle of downstream detector vessels, confirming their identity as endothelium-derived hyperpolarizing factors and suggesting that gap junctional communication may not be necessary for shear stress–stimulated EDHF-mediated vasodilation.
Keywords: NO deficiency, shear stress, EET, hyperpolarization, arterioles
Although the chemical identity of endothelium-derived hyperpolarizing factor (EDHF) remains controversial, epoxyeicosatrienoic acids (EETs), metabolites of arachidonic acids by cytochrome P450 (CYP)/epoxygenase, have been identified as a potential EDHF in a variety of vascular beds, including coronary, cerebral, renal, skeletal muscle vasculature, and human forearm and subcutaneous microvessels.1 However, some studies that question the existence of EDHF suggest that electrical coupling of endothelial and smooth muscle cells through gap junctions mediates the activity. The idea that EDHF/EETs activate potassium channels in endothelial cells, leading to either the release of K+,2–4 or spread of current from endothelial cells,5–9 to hyperpolarize smooth muscle, has attracted attention, because histological evidence of the existence of gap junctions between endothelial and smooth muscle cells and between endothelial cells was provided.10,11 It was also reported that hyperpolarization of endothelial cells by injection of current or administration of ACh was conducted downstream to cause dilation of arterioles through endothelial gap junctions,12,13 a response that was inhibited by removal of the endothelium, or attenuated in arteries of mice deficient in connexin 40, a protein component of endothelial gap junctions.13,14 On the other hand, by using patch clamp techniques, some studies contradicted this conclusion and suggested that EET(s) hyperpolarize smooth muscle cells directly via activation of potassium channels, leading to vasodilation.15,16
Our previous studies have demonstrated that in NO deficiency, EDHF/EET is responsible for the mediation of endothelium-dependent, flow/shear stress–induced dilation of arterioles from female, but not that of arterioles from male, NO-deficient mice and rats, a mediation that is sensitive to potassium channel inhibitors.17–20 In the present study, using an EDHF bioassay and an electrophysiological technique, we aimed to test our hypothesis that EDHF/EETs are released in response to shear stress and that they dilate arterioles via direct hyperpolarization of smooth muscle. The major advantage of this technique is our ability to measure synchronously smooth muscle membrane potential and diameter of endothelium-denuded detector vessels in response to EETs that are released from endothelial cells of shear stress–stimulated donor vessels.
Materials and Methods
Animals
Twelve- to 14-week-old male and female eNOS-KO and wild-type (WT) mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and 8-week-old female rats from Charles River Laboratories (Wilmington, Mass). Rats were treated with NG-nitro-l-arginine methyl ester (l-NAME, 50 mg/dL) in the drinking water for 3 to 4 weeks, as described previously.17,19,20 All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conform to the guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals.
Arterioles
Donor vessels were isolated from gracilis muscle of female and male (as control) eNOS-KO and WT mice, and l-NAME–treated female rats. Detector vessels were isolated from mesentery of the corresponding animals, and then their endothelium was removed by injection of air into the vessel lumen. Mesenteric arterioles used for the measurement of shear stress–stimulated EET production were isolated from l-NAME–treated female rats.
Experimental Design
A perfusion system consisting of two separate, serially connected chambers (Chambers A and B) was used (Figure 1). A donor vessel was cannulated in chamber A, and an endothelium-denuded detector vessel was cannulated in chamber B and was connected serially to the outflow site of chamber A. One syringe pump was connected to the inflow site of chamber A. Instant changes in the rate of flow of the intraluminal perfusate were achieved precisely by a pump (1 mL BD syringe, minimum flow 0.02 mL/h). A micropipette (≈2 µL volume) connected chambers A and B. Thus, when shear stress was initiated by perfusion of the donor vessel, the perfusate flowed through the detector vessel. The outflow site of chamber B was connected to a pressure-servo controller. Intraluminal pressure of the two vessels was maintained at 80 mm Hg by both the in- and outflow pressure transducers. A known level of initial shear stress was applied to the donor vessel and changes in diameter of both donor and detector vessels were recorded in turn (30 seconds apart) by moving the microscope stage onto a video tape along with time signals generated from a digital counter (VC-450, Thalner Electronic Laboratories). Corresponding flow rates to achieve the designed shear stress provided by the inflow pump were calculated based on the basal diameter of donor vessels and the viscosity of the perfusate (0.0069 poise in MOPS-PSS), according to the equation of shear stress (τ)=4ηQ/πr3. The perfusion system was also used to record changes in smooth muscle membrane potential and diameter of the detector vessels (in chamber B) simultaneously, in response to the perfusate that has flown through shear stress-stimulated donor vessels or to exogenous vasoactive agents.
Figure 1.
Schema of a perfusion system designed for EDHF bioassay and electrophysiology in isolated arterioles. T indicates transducer; PSC, pressure-servo control.
Assessment of Smooth Muscle Membrane Potential of Detector Vessels
The methods for the measurement of membrane potential were similar to those published earlier.21 Briefly, the perfusion system was mounted on a fixed stage of an inverted microscope (IMT-2, Olympus) on a vibration isolation table shielded by a Faraday cage. Membrane potential was recorded with an electrometer (IE-210, Warner) using microelectrodes pulled (P-97, Sutter Instrument Co) from glass capillary tubing (BF 100 to 50–10, Sutter). Electrode tips were filled with 0.1% propidium iodide (P-1304, Molecular Probes) in 3 mol/L KCl and backfilled with 3 mol/L KCl; tip resistance is ≈50 MΩ. An Ag/AgCl reference electrode was connected to the effluent of chamber B via an agar bridge (3% agar/3 mol/L KCl). The electrode was positioned via a micromanipulator (Narishige) and further advanced into the vessels with an oil hydrostatic micromanipulator (TrentWells). The output of the electrometer and the video calipers were connected to a data acquisition system (DI-700, Dataq Instruments).
Criteria for successful intracellular recording are as follows: (1) a sharp negative drop of potential on entering the cell; (2) a stable potential recording for at least 6 to 8 minutes (1 to 2 minutes for initial stabilization, 2 to 3 minutes during flow stimulation, 2 minutes of positive current injection, 0.5 nA, for propidium staining, and 1 minute to reconfirm a stable intracellular potential); (3) a sharp return to reference potential when withdrawing from the cell; and (4) a clear positive propidium labeling of the nucleus of a single smooth muscle cell (see Figure 4). The fluorescent image of propidium iodide staining was visualized after each recording with a 150W mercury lamp through a HQ Cy-3 filter set (41007a, HQ545/30-HQ610/75-Q570LP, Chroma) using an ultra-long working distance objective (ULWD M/Splan 20/0.4, Olympus). Cell labeling was also imaged with a cooled CCD camera (CoolSNAP Color, Roper Scientific).
Figure 4.
a, Original tracing of changes in smooth muscle membrane potential (mV) and diameter (µm) of a detector vessel in response to the perfusate from a donor vessel isolated from female eNOS-KO mice, subjected to IND and stimulated by 10 dyne/cm2 shear stress. Insert shows the nucleus of the smooth muscle cell stained with propidium iodide. b and c, Summarized data showing changes in smooth muscle membrane potential (b) and diameter (c) of detector vessels in response to the perfusate flowing through donor vessels isolated from female eNOS-KO mice, subjected to IND, and stimulated by 5 and 10 dyne/cm2 shear stress, with and without presence of IBTX in detector vessels (V2) or PPOH in donor vessels (V1), respectively (n=9). *Significant difference from that in presence of inhibitors. #Significant difference from that stimulated by 5 dyne/cm2 shear stress.
Experimental Protocols
Changes in arteriolar diameter of donor and detector vessels isolated from eNOS-KO mice in response to shear stress applied to donor vessels were assessed. To have sufficient release of EDHF when stimulated with shear stress, to elicit responses of detector vessels, a long segment of a donor vessel (≈3 to 5 mm in average length) with all branches ligated (≈2 to 3 branches) was isolated. Indomethacin (IND, 5×10−5 mol/L), an inhibitor of cyclooxygenase, was present in the superfusion of donor vessels during the entire period of the experiments.
In the first series of the experiments, initial shear stress of 5, 10, and 20 dyne/cm2, calculated based on the basal diameter, was applied to donor vessels, and then changes in diameter of both donor and detector vessels were, in turn (30 seconds apart), recorded for 6 minutes. After these control experiments, 30 mmol/L KCl or iberiotoxin (IBTX, 10−7 mol/L), an inhibitor of large-conductance, calcium-activated K+ channels (BKCa), was administered to detector vessels; or PPOH (5×10−5 mol/L), an inhibitor of CYP/epoxygenase, was administered to donor vessels, for 45 minutes. Then changes in diameter of both donor and detector vessels in response to 20 dyne/cm2 shear stress were once more recorded.
In the second series of studies, changes in smooth muscle membrane potential and diameter of detector vessels, in response to the perfusate that flowed from donor vessels stimulated by shear stress (5 and 10 dyne/cm2), were simultaneously recorded. This series of studies was performed with vessels of three different groups of animals: female eNOS-KO and WT mice, and female l-NAME–treated rats. During the entire experiment, donor vessels were subjected to adenosine (ADO, 10−4 mol/L) in addition to IND and l-NAME (5×10−4 mol/L) (the latter was only given to donor vessels isolated from WT mice), to maintain a stable, maximum (passive) diameter of the vessels during ≈5 to 6 minutes of perfusion. These experiments were repeated in the presence of IBTX in detector vessels, or PPOH in donor or detector vessels, respectively. In separate experiments, changes in smooth muscle membrane potential and diameter of detector vessels in response to perfusion of exogenous 11,12-EET (5×10−11 mol/L), and a bolus administration of phenylephrine (10−7 mol/L) were also assessed.
Quantitation of EETs in Perfusate From Shear Stress–Stimulated Vessels
Preparation of Perfusate Samples From Isolated Arterioles
Mesenteric arterioles of l-NAME–treated female rats were used for the measurement of perfusate EETs because of their adequate length that is necessary for collecting sufficient volume of samples. Isolated arterioles (≈13 mm in average length) with all (≈6–8) branches ligated were cannulated in a vessel chamber (14 mL in volume) filled with MOPS-PSS (37°C) at 80 mm Hg of intraluminal pressure. Adenosine (10−4 mol/L) was added to both perfusion and suffusion solutions to fully dilate the vessel. The average diameter of the vessel was obtained by measuring the diameter along the entire length of the vessel at 100 µm distances. After a one-hour equilibration in a no-flow condition, a known level of shear stress (1, 5, or 10 dyne/cm2) was administered to the vessel for 5 minutes, and then perfusate was collected in the outflow tubing. Each sample, collected at different flow rates (2 to 5 mL), was normalized by adding MOPS-PSS to a final volume of 6 mL. In separate experiments, 10 dyne/cm2 was applied to the vessels that had been treated with PPOH (5×10−5 mol/L) to inhibit CYP/epoxygenase.
Purification of EETs
Before extraction, 4.5 ng of a mixture of D8-EETs was added to each sample as internal standards. After extraction, the samples were reconstituted in 20 µL methanol and injected into reverse phase HPLC to obtain EET fractions.
Derivatization and Quantitation With GC-MS Analysis
After derivatization, the samples were reconstituted in 50 µL isooctane, and a 10-µL aliquot was injected into a GCMS (HP-5890/5989A, Hewlett-Packard). Endogenous EETs were identified (ion mass-to-charge ratio=319) by comparison of GC retention times with authentic D8-EETs (mass-to-charge ratio=327) standards, quantified by calculating the ratio of abundance and further normalized by the time of perfusion and the area (mm2) of the vascular endothelium.
Chemicals and Statistics
All chemicals were obtained from Sigma. PPOH was synthesized by J.R. Falck (University of Texas Southwestern Medical center, Dallas, Texas). Data are expressed as mean±SEM. n refers to the number of animals in each group. Statistical analysis was performed using repeated-measures of ANOVA followed by the Tukey-Kramer post hoc test and Student t test. Statistical significance was accepted at a level of P<0.05.
Results
A perfusion system designed for the bioassay of EDHF and measurement of smooth muscle membrane potential in response to shear stress is illustrated in Figure 1. Functional denudation of the endothelium in detector vessels was confirmed by the abolishment of acetylcholine (ACh, 10−6 mol/L)-induced, but maintenance of sodium nitroprusside-induced, dilations (data not shown).
Changes in Diameter of Donor and Detector Vessels of eNOS-KO Mice in Response to Shear Stress Applied to Donor Vessels Treated With IND
Figure 2 shows that 5, 10, and 20 dyne/cm2 initial shear stress, calculated based on basal diameter of the donor vessel (61.3±3.7 µm), elicited significant dose-dependent dilations of donor vessels, followed by a dilation of similar magnitude of detector vessels, indicating that shear stress-stimulated endothelial mediator(s), other than those derived from eNOS or cyclooxygenase, account for the dilator responses of both donor and detector vessels. Identical experiments performed on male donor vessels of eNOS-KO mice, as control, showed that neither donor nor detector vessels dilated in response to 20 dyne/cm2 shear stress when donor vessels were pretreated with IND (online Figure I, available in the online data supplement at http://circres.ahajournals.org), confirming our previous findings showing that in vessels of male mice and rats, in the absence of NO, prostaglandins are responsible for flow-induced dilation.20,22 However, after exposure of detector vessels of female eNOS-KO mice to 30 mmol/L KCl (Figure 3, top panel) or treatment of vessels with IBTX (Figure 3, middle panel), dilation of detector vessels was completely eliminated without affecting the dilation of donor vessels stimulated with 20 dyne/cm2 shear stress. The results indicate that dilation of detector vessels caused by the perfusate that flowed through donor vessels is dependent on the activation of BKCa channel of smooth muscle, a response that fits the criteria of EDHF-mediated vasodilator responses. Data in Figure 3 (bottom panel), showing that neither donor nor detector vessels dilated when 20 dyne/cm2 shear stress was applied to donor vessels that had been pretreated with PPOH, suggest that the characteristics of the mediator(s) responsible for the dilation of donor and detector vessels conform to those of EETs.
Figure 2.
Changes in diameter of gracilis arterioles (donor vessels, treated with indomethacin, IND 5×10−5 mol/L) of female eNOS-KO mice, in response to an initial shear stress of 5, 10, and 20 dyne/cm2, and of endothelium-denuded (−EC) mesenteric arterioles (detector vessels), in response to the perfusate first flowing through donor vessels, as a function of time (n=11 to 14).
Figure 3.
Changes in diameter of donor vessels isolated from female eNOS-KO mice, in response to an initial shear stress of 20 dyne/cm2, and of detector vessels treated with either 30 mmol/L KCl (top panel, n=4) or iberiotoxin (IBTX, 10−7 mol/L, middle panel, n=7), or treatment of donor vessels with PPOH (5×10−5 mol/L, bottom panel, n=7), in response to the perfusate first flowing through donor vessels, as a function of time. *Significant difference between two curves.
Changes in Diameter and Membrane Potential of Detector Vessels in Response to the Perfusate Flowing Through IND-Treated Donor Vessels Stimulated by Shear Stress
We also examined whether the EETs that are released from female NO-deficient donor vessels would not only dilate, but also hyperpolarize detector vessels. To this end, a shear stress equal to 5 and 10 dyne/cm2 was applied to fully dilated (by adenosine) donor vessels. The Table provides general information of detector vessels used. Figure 4 shows that in the presence of 5 dyne/cm2 shear stress, the perfusate passing through donor vessels isolated from female eNOS-KO mice elicited smooth muscle hyperpolarization of detector vessels by −12.9±1.5 mV, associated with an increase in diameter by 13.1±1.5 µm. When shear stress was increased to 10 dyne/cm2, a further hyperpolarization (−27.3±2.2 mV) and greater dilation (20.7±1.7 µm) were observed. In the presence of IBTX in detector vessels, or PPOH in donor vessels, hyperpolarization and dilation of detector vessels in response to 10 dyne/cm2 shear stress administered to donor vessels, were eliminated, indicating that it is EET(s) that are released into the perfusate from the endothelium of donor vessels stimulated by shear stress to hyperpolarize smooth muscle of detector vessels via activation of BKCa channels.
Characteristics of Detector Vessels of Female eNOS-KO (n=9) and WT (n=5) Mice and Rats (n=8)
| eNOS-KO | WT With l-NAME–Treated Donor Vessels |
l-NAME– Treated Rats |
|
|---|---|---|---|
| Resting membrane potential, mV | −23.8±1.9 | −23.6±1.0 | −22.0±0.7 |
| Basal diameter, µm | 74.9±4.7 | 86.6±4.8 | 88.2±5.0 |
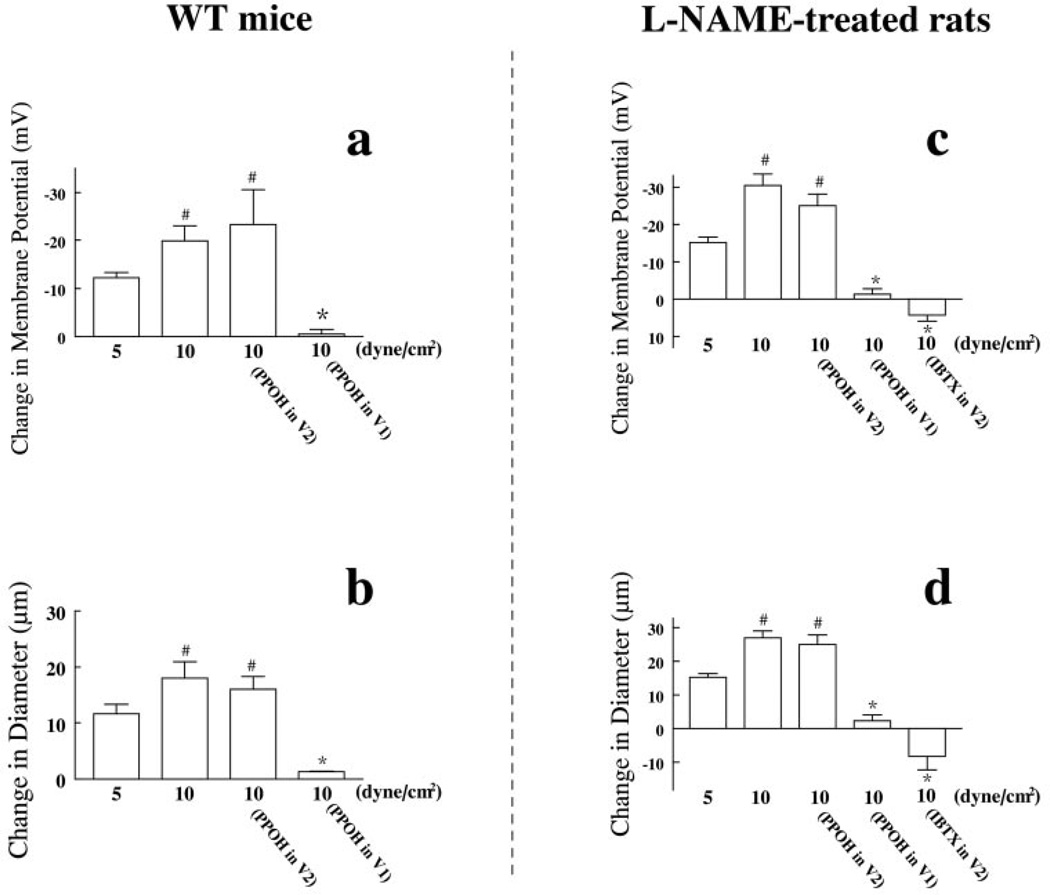
Consistent results were also obtained when similar experiments were done in vessels isolated from female WT mice after acute (30 to 45 minutes) exposure of l-NAME in the superfusion of donor vessels and those isolated from female rats after chronic (3 to 4 weeks) treatment with l-NAME in the drinking water. In WT mice, 5 and 10 dyne/cm2 shear stress passing through donor vessels elicited smooth muscle hyperpolarization of detector vessels by −12.2±1.1 and −19.0±3.1mV, associated with vasodilations of 11.6±1.7 and 18.8±2.9 µm, respectively (Figure 5a and 5b). In l-NAME–treated rats, the same shear stresses elicited smooth muscle hyperpolarization of detector vessels by −15.5±1.4 and −30.4±3.1mV, associated with vasodilation of 15.3±1.1 and 26.4±2.0 µm, respectively (Figure 5c and 5d). On the other hand, PPOH, when administered to detector vessels, had no effect on their dilator and hyperpolarizing responses, but it essentially eliminated the responses when administered to donor vessels (Figure 5), confirming further that the source of EETs is the endothelial cells of donor vessels. Perfusion of 11,12-EET (5×10−11 mol/L) for 6 minutes elicited smooth muscle hyperpolarization (by −21±2.4 mV) and vasodilation (by 19.7±1.5 µm) (online Figure II), whereas phenylephrine (10−7 mol/L) caused depolarization and vasoconstriction of detector vessels (data not shown).
Figure 5.
a and b, Changes in smooth muscle membrane potential (a) and diameter (b) of detector vessels in response to the perfusate first flowing through donor vessels isolated from female WT mice, subjected to l-NAME (5×10−4 mol/L)+IND and stimulated by 5 and 10 dyne/cm2 shear stress, with presence of PPOH in detector vessels (V2, n=4) or in donor vessels (V1, n=7), respectively. c and d, Changes in smooth muscle membrane potential (c) and diameter (d) of detector vessels in response to the perfusate first flowing through donor vessels of l-NAME–treated female rats, subjected to IND and stimulated by 5 and 10 dyne/cm2 shear stress, with presence of PPOH (5×10−5 mol/L) in detector vessels (V2, n=4) or in donor vessels (V1, n=6); or with IBTX (10−7 mol/L) in detector vessels (V2, n=4), respectively. *Significant difference from those stimulated by 5 or 10 dyne/cm2 shear stress and those with PPOH in detector vessels (V2). #Significant difference from that stimulated by 5 dyne/cm2 shear stress.
Quantitation of EETs in Perfusate From Shear Stress–Stimulated Vessels
EETs were identified in the perfusate collected from shear stress–stimulated arterioles (Figure 6), suggesting that shear stress dose-dependently stimulates the release of EETs from vascular endothelium, a response that is prevented by PPOH.
Figure 6.
Quantitation of EETs by GC-MS analysis in the perfusate collected from isolated mesenteric arterioles of l-NAME–treated female rats, stimulated by 1, 5, and 10 dyne/cm2 shear stress in control conditions and in the presence of PPOH (n=6; a total of 13 vessels in each group). *Significant difference from that stimulated by 1dyne/cm2 shear stress. #Significant difference from that stimulated by 5 dyne/cm2 shear stress and that by 10 dyne/cm2 shear stress in the presence of PPOH, respectively.
Discussion
The present study is the first to provide direct evidence for shear stress–stimulated release of EETs in microvessels. Moreover, the released EETs, in addition to be responsible for the mediation of flow-induced dilation of donor vessels, traffic downstream to endothelium-denuded detector vessels to cause hyperpolarization and dilation. These findings do not necessarily challenge the evidence for other possible mechanisms by which, as reported by others,8,9,23 EDHF/EETs mediate hyperpolarizing responses, or other mediators that might contribute to the mediation of the responses, but rather to confirm that EETs can be directly released from endothelial cells to hyperpolarize and dilate vessels via a mechanism that is independent of gap junctional communications.
Mechanical factors like shear stress cause vasodilation that is linked to factors released from the endothelium, such as NO and prostaglandins (PGs). Our previous studies have demonstrated that although in physiological conditions, NO and PGs are the main contributors of shear stress–dependent vasodilation, EDHF/EETs serve as a back-up mechanism in NO deficiency. We observed previously that in the absence of NO, the mechanism responsible for shear stress–dependent regulation of arteriolar tone is purely EDHF/EET-dependent, a response which, moreover, is especially prominent in the presence of estrogen.17–20 Because the characteristics of gender-specific regulation of EDHF/EETs have already been reported previously, the present study focused on the issue as to whether EETs, similar to NO and PGs, are releasable and transferable factors that can hyperpolarize smooth muscle via directly activating K+ channels.
We found that increases in shear stress dilated donor vessels of female eNOS-KO mice, followed by dilation of detector vessels (Figure 2). Because the entire experiment was performed in the presence of indomethacin, the dilator responses observed could be characterized as non-NO, non–PG-dependent responses. When control experiments were performed on vessels isolated from male eNOS-KO mice treated with indomethacin, neither donor nor detector vessels dilated in response to shear stress, whereas in the absence of indomethacin, shear stress did elicit dilation of both vessels (online Figure I), indicating, as demonstrated previously,22 a PG-mediated response. Additionally, after the onset of intraluminal flow, an increase in perfusion pressure was observed. As measured in preliminary studies, application of 20 dyne/cm2 of initial shear stress to arterioles elicited approximately 10 to 12 mm Hg increases in perfusion pressure, which however, could not be adjusted by lowering outflow pressure, as is the case when flow-induced dilation is performed in a single vessel. The reasons for this are as follows: first, donor and detector vessels are serially connected, and thus the method of maintaining a constant intraluminal pressure by increasing and decreasing inflow and outflow pressures equally is not feasible; and second, because lowering outflow pressure may elicit a myogenic dilation in the detector vessel, which could be misleading when trying to understand the reasons for the dilation of these vessels. Thus, as shown in our data, detector vessels dilated even in the presence of minimal increases in perfusion pressure and myogenic tone, indicating further that the bioassay is sensitive enough to detect vasodilator substances released from donor vessels.
The involvement of smooth muscle KCa channels in the shear stress–induced non–NO-, non–PG-mediated dilations of vessels was confirmed by the results shown in Figure 3 (top and middle panels). Results indicate that although donor vessels dilated in response to 20 dyne/cm2 shear stress, no dilator responses were observed in detector vessels that had been subjected to depolarizing concentrations of K+ or treated with IBTX. On the other hand, the characterization of the mediators responsible for the dilator responses of donor and detector vessels was made possible by the evidence that PPOH administered to donor vessels abolished the responses of both types of vessel (Figure 3, bottom panel), indicating that it is EETs that dilate both donor and detector vessels, although other mediator(s) released as a function of activation of CYP could also play a role in the mediation of the responses. Because EETs could be released from both endothelial and smooth muscle cells,24 PPOH was also administered to detector vessels. We found that unlike administration of PPOH to donor vessels, pretreatment of detector vessels with PPOH did not affect their dilation and hyperpolarization in response to the perfusate from shear stress–stimulated donor vessels (Figure 5). Moreover, previous studies have confirmed that the shear stress–dependent dilation is purely endothelium-dependent.17,18,21,22 Thus, the hyperpolarization and dilation of detector vessels were elicited by EETs that were released from endothelial cells of donor vessels, rather than smooth muscle cells of detector vessels. Our results are unlike the studies published previously on ACh-stimulated EDHF/EET-mediated conducted vasodilation, in which EETs activated KCa channels only at the site of vessel stimulation but not at distant sites, as indicated by the fact that application of IBTX at the remote site did not alter dilation, but blockade of KCa channels or CYP/epoxygenase at the ACh-stimulated site significantly attenuated both local and conducted responses. The authors claimed therefore, that a local release of EETs accounts for the hyperpolarization of the vessels, which then evokes a conducted dilation independent of K+ channels.8 Although, in the present study, the possibility of a contribution of myoendothelial gap junctional communication to the mediation of EET-induced dilation of donor vessels is not excluded, the fact that IBTX-treatment or depolarization of detector vessels completely eliminated their dilator responses, while dilation of donor vessels was unaffected, strongly suggests that EETs dilate detector vessels via direct activation of K+ channels on smooth muscle, a finding that corresponds to that observed in single smooth muscle cells subjected to exogenous EETs.15,16,25
Synchronous recording of changes in detector vessel membrane potential and diameter in response to the perfusate that first flowed through shear stress–stimulated donor vessels provides solid evidence of an EDHF/EET-mediated hyperpolarization and vasodilation. A ≈22- to 24-mV resting membrane potential was recorded in vessels in which a ≈50% basal myogenic tone developed spontaneously. In the presence of 5 and 10 dyne/cm2 shear stress, increasing hyperpolarization and dilation of detector vessels were elicited that could be prevented by blocking K+ channels in detector vessels or by inhibiting EET synthesis in donor vessels (Figures 4 and 5). Because EDHF-mediated responses can be discerned in most instances only when NO synthesis is impaired, and also because a role for estrogen in the upregulation of EET synthesis has already been demonstrated,17–20 we repeated our studies in vessels of two additional NO-deficient female animal models caused by acute (donor vessels of WT mice subjected to l-NAME for 30 to 45 minutes) and chronic (l-NAME–treated rats for 3 to 4 weeks) inhibition of NO synthesis. The result showed EET-mediated responses to shear stress (Figure 5) that were identical to those obtained in vessels of female eNOS-KO mice (Figures 2 to 4). These results illustrate the general nature of compensatory activity of EETs, as an EDHF in the maintenance of endothelial function when the bioavailability of NO is impaired. It is of note that perfusion of a low concentration of 11,12-EET (5×10−11 mol/L) directly elicited hyperpolarization and dilation of detector vessels (online Figure II) comparable in magnitude to that caused by 10 dyne/cm2 shear stress applied to donor vessels in bioassay studies (Figures 4 and 5), confirming further our conclusions.
Our previous study demonstrated a genomic upregulation of EET production in NO-deficient microvessels, which was responsible for the switch from shear stress–dependent dilation mediated by prostaglandins to that mediated by EDHF in male phenotypic vessels treated with estrogen.19 In the present study, we quantified EETs in the perfusate collected from shear stress–stimulated isolated microvessels of female NO deficient rats, providing for the first time direct biochemical evidence to link the release of EETs from endothelial cells to stimulation of the vessels by shear stress (Figure 6). Apart from activation of K+ channels and hyperpolarization, other intracellular EET targets have been identified, ie, tyrosine kinases and phosphatases, mitogen-activated protein kinase (ERKs, P44/42 and P38 MAPK, and stress-activated protein kinase), PI3K/Akt, and IκB kinase.24 Also, roles for EETs, for instance, 11,12- and 14,15-EET, in the mediation of antimigratory effects of smooth muscle cells via a cAMP/PKA-dependent mechanism,26 a signaling pathway that could be activated by binding of EETs to their specific receptor,27 have been reported. Although the specific intracellular signaling cascades responsible for detecting and then converting this physical stimulus into chemical signals, as manifested by the release of EETs via the metabolism of arachidonic acid, is as yet unknown, the present study could serve as the basis for further investigation of the signal transduction cascades involved and the relationship between shear stress and EETs.
In summary, shear stress stimulates the release of EETs from the endothelium of arterioles isolated from female NO-deficient mice and rats. The released EETs, in addition to be responsible for flow-induced dilation of donor vessels, can be transferred to downstream detector vessels to hyperpolarize and dilate them, via the activation of smooth muscle BKCa channels. Our data demonstrate that EETs are released to mediate shear stress–induced hyperpolarization/dilation of arterioles, a response that is independent of the presence of gap junctional communications. Moreover, further studies by using these newly developed experimental devices and biochemical approaches could yield deeper insight into the signal transduction pathways responsible for the release of EETs by shear stress and their role in the regulation of microvascular function.
Supplementary Material
Acknowledgments
This study was supported by NIH grants HL 070653, HL 68813, and HL 43023.
References
- 1.Campbell WB, Gauthier KM. What is new in endothelium-derived hyperpolarizing factors? Curr Opin Nephrol Hypertens. 2002;11:177–183. doi: 10.1097/00041552-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Dora KA, Garland CJ. Properties of smooth muscle hyperpolarization and relaxation to K+ in the rat isolated mesenteric artery. Am J Physiol Heart Circ Physiol. 2001;280:H2424–H2429. doi: 10.1152/ajpheart.2001.280.6.H2424. [DOI] [PubMed] [Google Scholar]
- 3.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 4.Coleman HA, Tare M, Parkington HC. K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels. J Physiol. 2001;531:359–373. doi: 10.1111/j.1469-7793.2001.0359i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman HA, Tare M, Parkington HC. EDHF is not K+ but may be due to spread of current from the endothelium in guinea pig arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2478–H2483. doi: 10.1152/ajpheart.2001.280.6.H2478. [DOI] [PubMed] [Google Scholar]
- 6.Coleman HA, Tare M, Parkington HC. Myoendothelial electrical coupling in arteries and arterioles and its implications for endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol. 2002;29:630–637. doi: 10.1046/j.1440-1681.1999.03701.x. [DOI] [PubMed] [Google Scholar]
- 7.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 8.Hoepfl B, Rodenwaldt B, Pohl U, De Wit C. EDHF, but not NO or prostaglandins, is critical to evoke a conducted dilation upon ACh in hamster arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H996–H1004. doi: 10.1152/ajpheart.01082.2001. [DOI] [PubMed] [Google Scholar]
- 9.Welsh DG, Segal SS. Role of EDHF in conduction of vasodilation along hamster cheek pouch arterioles in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1832–H1839. doi: 10.1152/ajpheart.2000.278.6.H1832. [DOI] [PubMed] [Google Scholar]
- 10.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 11.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 12.Emerson GG, Segal SS. Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol. 2001;280:H160–H167. doi: 10.1152/ajpheart.2001.280.1.H160. [DOI] [PubMed] [Google Scholar]
- 13.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 14.De Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier KM, Falck JR, Reddy LM, Campbell WB. 14,15-EET analogs: characterization of structural requirements for agonist and antagonist activity in bovine coronary arteries. Pharmacol Res. 2004;49:515–524. doi: 10.1016/j.phrs.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am J Physiol Heart Circ Physiol. 2001;280:H2430–H2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]
- 17.Huang A, Wu Y, Sun D, Koller A, Kaley G. Effect of estrogen on flow-induced dilation in NO deficiency: roles of prostaglandins and EDHF. J Appl Physiol. 2001;91:2561–2566. doi: 10.1152/jappl.2001.91.6.2561. [DOI] [PubMed] [Google Scholar]
- 18.Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol. 2001;280:H2462–H2469. doi: 10.1152/ajpheart.2001.280.6.H2462. [DOI] [PubMed] [Google Scholar]
- 19.Huang A, Sun D, Wu Z, Yan C, Carroll MA, Jiang H, Falck JR, Kaley G. Estrogen elicits cytochrome P450–mediated flow-induced dilation of arterioles in NO deficiency: role of PI3K-Akt phosphorylation in genomic regulation. Circ Res. 2004;94:245–252. doi: 10.1161/01.RES.0000111525.96232.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Huang A, Sun D, Falck JR, Koller A, Kaley G. Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation. Am J Physiol Heart Circ Physiol. 2001;280:H2456–H2461. doi: 10.1152/ajpheart.2001.280.6.H2456. [DOI] [PubMed] [Google Scholar]
- 21.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 22.Sun D, Huang A, Smith CJ, Stackpole CJ, Connetta JA, Shesely EG, Koller A, Kaley G. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ Res. 1999;85:288–293. doi: 10.1161/01.res.85.3.288. [DOI] [PubMed] [Google Scholar]
- 23.Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium- dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci U S A. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming I. To move or not to move? Cytochrome P450 products and cell migration. Circ Res. 2002;90:936–938. doi: 10.1161/01.res.0000019742.48706.f0. [DOI] [PubMed] [Google Scholar]
- 25.Pratt PF, Li P, Hillard CJ, Kurian J, Campbell WB. Endothelium-independent, ouabain-sensitive relaxation of bovine coronary arteries by EETs. Am J Physiol Heart Circ Physiol. 2001;280:H1113–H1121. doi: 10.1152/ajpheart.2001.280.3.H1113. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Sui X, Bradbury JA, Zeldin DC, Conte MS, Liao JK. Inhibition of vascular smooth muscle cell migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ Res. 2002;90:1020–1027. doi: 10.1161/01.res.0000017727.35930.33. [DOI] [PubMed] [Google Scholar]
- 27.Wong PY, Lai PS, Falck JR. Mechanism and signal transduction of 14 (R), 15 (S)-epoxyeicosatrienoic acid (14,15-EET) binding in guinea pig monocytes. Prostaglandins Other Lipid Mediat. 2000;62:321–333. doi: 10.1016/s0090-6980(00)00079-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.