Abstract
Social learning theories of drug abuse propose that individuals imitate drug use behaviors modeled by social peers, and that these behaviors are selectively reinforced and/or punished depending on group norms. Historically, animal models of social influence have focused on distal factors (i.e., those factors outside the drug-taking context) in drug self-administration studies. Recently, several investigators have developed novel models, or significantly modified existing models, to examine the role of proximal factors (i.e., those factors that are immediately present at the time of drug taking) on measures of drug self-administration. Studies using these newer models have revealed several important conclusions regarding the effects of social learning on drug abuse: 1) the presence of a social partner influences drug self-administration, 2) the behavior of a social partner determines whether social contact will increase or decrease drug intake, and 3) social partners can model and imitate specific patterns of drug self-administration. These findings are congruent with those obtained in the human laboratory, providing support for the cross-species generality and validity of these preclinical models. This mini-review describes in detail some of the preclinical animal models used to study social contact and drug self-administration to guide future research on social learning and drug abuse.
Keywords: animal, drug abuse, drug use, model, social influence, social contact, social learning
1. Introduction
The social environment plays a critical role in the etiology and persistence of drug use disorders. Many social factors are distal to the drug-taking context and are not immediately present at the time in which drugs are used. These factors include those found in the individual’s home, school, and community environments, and these factors may serve to increase or decrease the probability of drug use. Preclinical animal studies have successfully modeled many of these factors and have consistently revealed their role in drug self-administration. For example, studies using rodents and nonhuman primates have revealed that social stress and social isolation increase, whereas social enrichment and social dominance decrease, drug self-administration across a wide variety of drug classes and schedules of reinforcement (see reviews by Burke and Miczek, 2014; Nader et al., 2012; Stairs and Bardo, 2009). That drug use in humans is similarly affected by distal social factors (e.g., Gordon, 2002) supports the validity of these models for predicting human drug use and for evaluating putative interventions for drug use disorders.
Unfortunately, preclinical studies examining proximal social factors (i.e., those factors that are immediately present during the drug-taking event) have not advanced at the same pace. Historically, preclinical investigators have been hampered by a relative dearth of models that adequately assess and allow manipulation of proximal social variables. Only recently has the design and validation of models that allow extensive and systematic evaluation of these factors increased. For example, several recent studies have examined the effects of proximal social contact on conditioned drug reward using the conditioned place preference (CPP) procedure. In the CPP procedure, a Pavlovian association is formed between a stimulus (e.g., a social peer, an interoceptive drug cue) and an environmental context, and a preference for the stimulus-paired environment serves as a measure of the rewarding effects of the stimulus. These studies have consistently revealed enhanced rewarding effects when drug and social stimuli are conditioned together (Thiel et al., 2008; Watanabe, 2011). Similarly, when conditioned as mutually exclusive conditions, social contact can compete with the rewarding effects of drug stimuli (Fritz et al., 2011a, 2011b).
Recent research has also begun examining the effects of social contact on drug self-administration, and several recent reviews have described the effects of social contact on various measures of drug intake. These reviews have examined the neurobiological mechanisms at the nexus of social contact and drug self-administration (Bardo et al., 2013), the behavioral mechanisms mediating the effects of social contact on drug self-administration (Strickland and Smith, 2014), the intersection of emotional valance and social context in drug self-administration (Neisewander et al., 2012), and the effects of social contact on conditioned drug reward (Zernig et al., 2013). The primary objective of this mini-review is to provide an evaluation of several preclinical models of proximal social contact and their application to the study of drug self-administration (see Figure 1 for schematic overview of the described models). To this end, we have identified three key conclusions from this literature and will discuss the models within this context. The collective findings from these studies indicate that: 1) the presence of a social peer influences drug self-administration, 2) the effects of social contact are moderated by the behavior of that peer, and 3) animals can model and imitate specific patterns of drug self-administration.
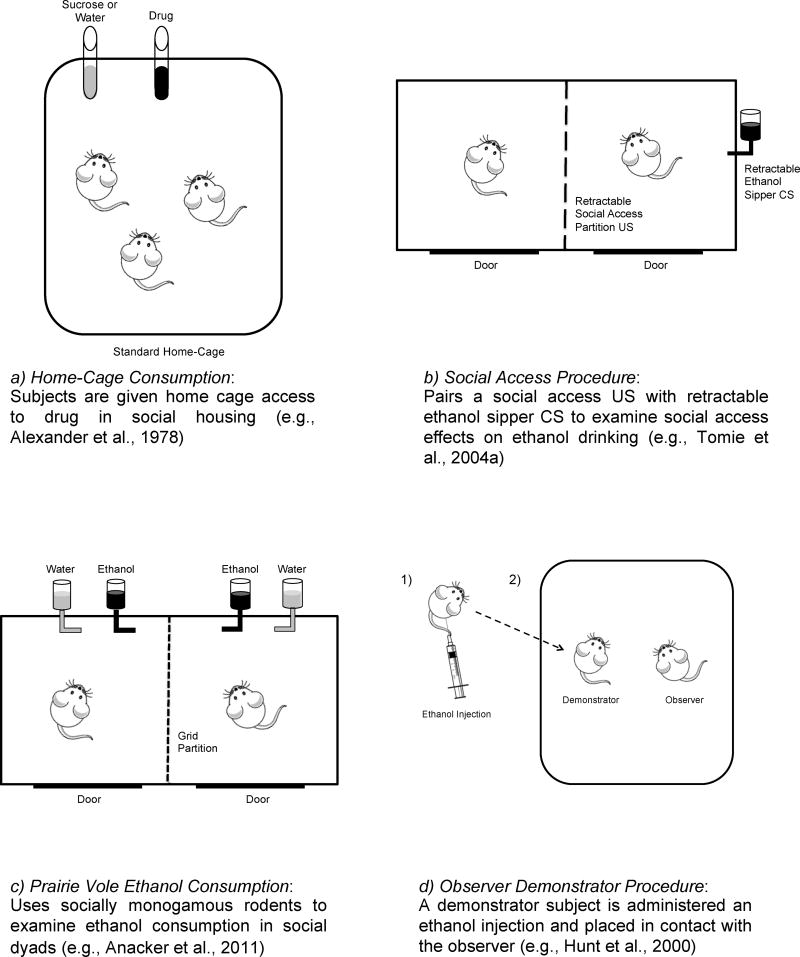
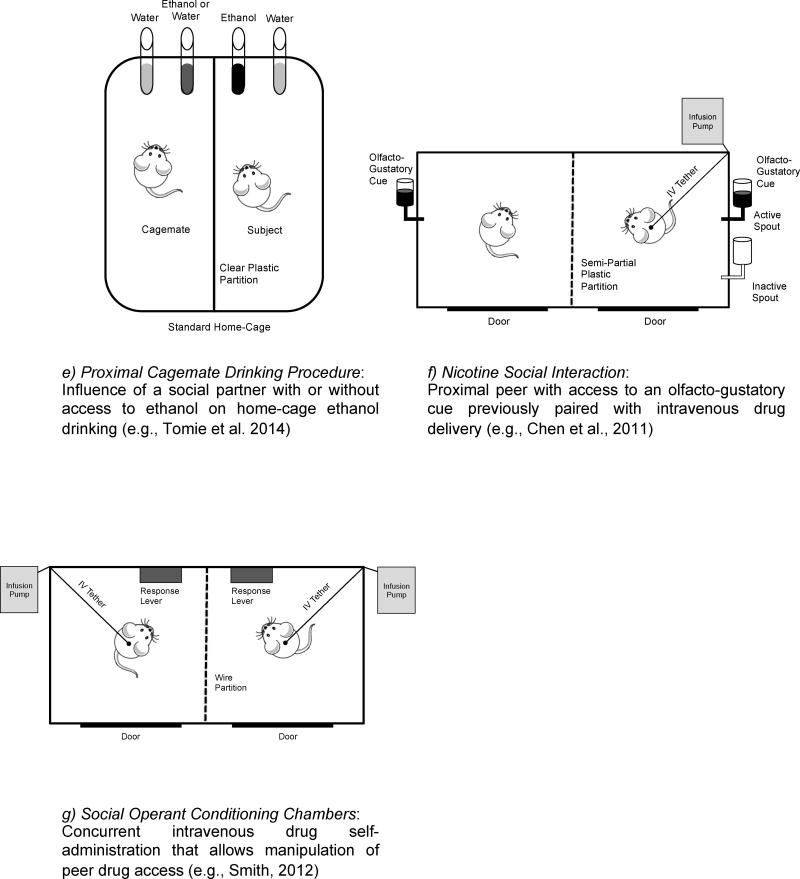
Figure 1. Schematic overview of models of proximal social contact.
Schematic diagrams of each model are provided with a short description of procedure and manipulation.
2. Social Learning Theories of Social Contact
One of the strongest prognosticators of adolescent and young adult drug use is the drug-use behavior of peers (Bahr et al., 2005; Walden et al., 2004). A number of epidemiological studies have explored the link between social factors and drug self-administration, and have provided cogent support for the influence of peers (Bot et al., 2005; Kelly et al., 2013; Salvy et al., 2014). The results of this research indicate that peer influence occurs at both a distal (e.g., incorporation of group norms) and proximal (e.g., spending time with others who use drugs) level. Equally important to note is recent evidence suggesting that distal factors become less salient over time while proximal factors become more influential (Salvy et al., 2014). Thus, at the broadest level, peers play a critical role in the expression and continuation of drug use, with proximal influences having a progressively greater impact over time.
Although several theories exist to explain peer influence and the high concordance of drug use among members of peer groups, social learning theories (i.e., group socialization theories) have garnered a great deal of attention. Social learning theories posit that attitudes, beliefs, and behaviors held by a social group are transmitted to individual members, and that social interactions determine future member behavior (Akers, 1977; Kandel, 1986). Further, peers may selectively reinforce desired behaviors (e.g., drug use) and punish undesired behaviors (e.g., drug abstinence) of other group members. The emergence of novel preclinical models of social contact offers a platform by which hypotheses derived from social learning theories can be tested. This is important because social learning theories propose a number of mechanisms by which the social environment can be manipulated to prevent and treat drug use and abuse (see Strickland and Smith, 2014). Thus, the development of preclinical models of social contact has the potential to contribute to the translational design and implementation of interventions for drug use disorders, as well as a broader understanding of the social mechanisms of drug self-administration.
3. Effects of the Presence of a Social Peer
At the broadest level, studies of social contact and drug self-administration have consistently revealed that the presence of a social peer influences drug intake. In the early models of social contact (Figure 1a; “Home-Cage Consumption”), a social manipulation was used in which subjects were given home-cage access to an oral morphine solution under isolated or social housing conditions (Alexander et al., 1978, 1981; Hadaway et al., 1979). In the first of these studies, socially housed rats drank more of a morphine solution than isolated rats during periods of forced consumption; however, isolated rats drank more of this solution in a two-bottle choice procedure (i.e., water versus morphine; Alexander et al., 1978). Females in that study were more sensitive to the housing manipulation, with a greater difference in consumption between isolated and social conditions in females than males. A follow-up study demonstrated the importance of social housing during development, in that rats reared in isolation but tested in social conditions showed greater levels of morphine consumption than rats reared and tested in social conditions (Alexander et al., 1981). Later studies extended these findings across a range of morphine concentrations and found that a period of social interaction prior to testing reduced consumption in the isolated subjects (Hadaway et al., 1979; Raz and Berger, 2010b). Further, a recent study indicated that daily treatment with fluoxetine attenuated, whereas daily treatment with parachlorophenylalanine (a serotonin synthesis blocker) enhanced, the increased morphine consumption in isolated rats (Raz and Berger, 2010a). Given the demonstrated role of serotonergic signaling in anxiety (see review by Gordon and Hen, 2004), these findings suggest that isolation-induced anxiety and its alleviation by social interaction may contribute to the observed effects of social contact.
A number of preclinical studies have used a similar home-cage consumption model to examine oral ethanol consumption. These studies have typically shown decreased ethanol consumption in socially housed rats relative to isolated rats (Deatherage, 1972; Ehlers et al., 2007; Wolffgramm and Heyne, 1991; but see Doremus et al., 2005; Weisinger et al., 1989). Some of these studies have revealed a “dose-dependent” relationship between the level of social interaction and changes in ethanol intake (Wolffgramm, 1990; Wolffgramm and Heyne, 1991). For example, rats housed in partial-contact caging (i.e., wire bars between cages) showed reduced ethanol consumption relative to isolated subjects, but greater consumption than subjects given full social access (Wolffgramm, 1990). These studies also suggest that social hierarchy may moderate the relationship between social housing and ethanol consumption. Studies conducted in rodents (Blanchard et al., 1987; Pohorecky, 2006, 2008) and non-human primates (McKenzie-Quirk and Miczek, 2008) indicate that home-cage ethanol consumption is linked to dominance rank, with the highest rates of consumption in subordinate subjects. Such findings are consistent with the notion that home-cage, free-consumption models may capture the effects of stress induced by social isolation and social hierarchies (for more on the role of social dominance in drug abuse, see review by Nader et al., 2012).
A series of studies conducted by Tomie and colleagues examined the effects of intermittent and continuous social interaction on rodent ethanol intake (Figure 1b; “Social Access Procedure”). In this procedure, rats are given intermittent access to a social partner separated by a wire partition via a retractable door. The use of a retractable ethanol sipper tube also allows the experimenter control over ethanol access, as well as the temporal relationship between drug and social presentation. In the first of these studies, rats presented with intermittent social access showed increased ethanol consumption in both paired and unpaired conditions (Tomie et al., 2004b). These results suggest that social interaction enhances ethanol intake, and that this effect is independent of a Pavlovian association between access to ethanol and social interaction. Importantly, increased fluid consumption was not observed when social access was paired with a water-containing sipper or when the ethanol sipper was presented without social access (Tomie et al., 2004b). A follow-up study replicated these findings and extended them across a range of ethanol concentrations (Tomie et al., 2004a). Parametric studies revealed that enhanced ethanol consumption was greatest when social access and the ethanol sipper were each presented intermittently, rather than when they were continuously available (Tomie et al., 2005, 2007). These effects were specific to the social manipulation because mechanical operation of an access door without social presentation failed to increase ethanol intake (Tomie et al., 2005). Taken together, these results suggest that access to intermittent social interaction enhances the reinforcing effects of ethanol, and that these effects are specific to drug consumption rather than a general increase in consummatory behavior.
More recently, several studies have examined social contact and ethanol intake using a prairie vole model (“Prairie Vole Ethanol Consumption”; Figure 1c). In contrast to traditional laboratory rodents, prairie voles form strong and socially monogamous male-female and sibling pair bonds. Thus, prairie voles may provide unique insight into the effects of social interaction on drug self-administration. In the first of these studies, same-sex, adult prairie vole siblings were housed in either social pairs (separated by wire mesh) or isolation, and then tested for ethanol consumption using a two-bottle, home-cage choice test (ethanol versus saccharin; Anacker et al., 2011a). Relative to siblings housed in isolation, pair-housed siblings showed a greater preference for ethanol and a greater similarity in drinking patterns. A later study found that these effects were dependent on the sex composition of the dyad, with no differences in ethanol consumption or preference observed for opposite-sex dyads in social versus isolated housing (Hostetler et al., 2012). A subsequent study indicated that the presence of a social partner reduces measures of relapse following abstinence (Hostetler and Rynabinin, 2014). Specifically, access to a social partner with or without access to ethanol after a period of abstinence attenuated relapse-like elevations in ethanol intake typically observed in isolated animals. These findings indicate that the influence of a social peer may depend on the stage of drug use examined; however, further studies are needed to clarify these effects.
Additional studies have revealed similar effects of social contact with other drugs and other routes of administration. For example, monkeys trained to respond in an oral PCP versus water choice procedure showed increased PCP consumption when tested in the presence of another monkey with access to PCP (separated by a grid partition) relative to when tested in isolation (Newman et al., 2007). Similarly, rats trained on a progressive-ratio (PR) schedule exhibited greater amphetamine-maintained breakpoints when tested in the presence of a same-sex conspecific without access to amphetamine (separated by a clear partition) than when tested in isolation (Gipson et al., 2011). In that study, the presence of a social peer without drug access increased responding maintained by a high dose of amphetamine (0.1 mg/kg); however, this effect was limited to the first social pairing and was not observed at a lower dose (0.01 mg/kg). Collectively, these studies demonstrate that the presence of a peer influences drug self-administration and other measures of drug-seeking behavior.
4. Effects of Manipulating a Peer’s Access to Drugs
Recent advances in animal models have made possible the direct manipulation of access to drugs among peers in order to examine the role of peer behavior in drug self-administration. This is important because it allows the testing of predictions made by social learning theories of drug abuse, namely that drug-use behaviors are learned and transmitted within peer groups. Collectively, the results of these studies provide cogent evidence that the drug use of peers critically influences the effects of social contact on drug self-administration.
Some of the first studies investigating the effects of peer drug use examined the social transmission of ethanol intake using the observer-demonstrator procedure (“Observer-Demonstrator Procedure”; Figure 1d). Broadly, these studies allow an observer to interact with a social demonstrator that is either acutely intoxicated by ethanol or injected with a control substance. Ethanol consumption is then measured in the observer to determine if the interaction(s) with an intoxicated demonstrator influenced later drug intake. Both preweanling and adolescent rats that interacted with an intoxicated conspecific showed increased ethanol intake in later tests (Hunt et al., 2000, 2001); however, increases in consumption of ethanol and a control solution (i.e., decaffeinated coffee) were not seen in observers interacting with a demonstrator administered the control solution (Hunt et al., 2001). This finding indicates that the observed effects were specific to ethanol-related outcomes and not a general transfer to all novel liquid consumption. Several studies have replicated these effects and suggested that social transmission may depend on the age, sex, and novelty of the social partner (Hallmark et al., 2004; Honey et al., 2004; Maldonado et al., 2008). Importantly, shifts in ethanol preference were not observed after interactions with an ethanol stimulus alone (i.e., ethanol-soaked cotton ball) or with a sedated partner administered ethanol (Fernandez-Vidal et al., 2004). Although it is important to note that these studies do not specifically examine the influence of a peer at the time of drug consumption, they do indicate an individual’s behavior under an acutely intoxicated state later influences the ethanol intake of a social partner.
More recently, Tomie and colleagues developed a Proximal Cagemate Drinking (PCD) procedure that allows manipulation of peer ethanol access and examination of its effects on cagemate ethanol consumption (“Proximal Cagemate Drinking Procedure”; Figure 1e). In the PCD procedure, mice are given continuous, home-cage access to a two-bottle choice of ethanol versus water, and housed in the presence or absence of a cagemate. A transparent plastic barrier separates social partners, affording the manipulation of ethanol access and measurement of consumption for each social partner. The first of these studies found that having a cagemate with access to ethanol increased ethanol intake in male mice living in male-female dyads, but not male-male dyads (Tomie et al., 2014). In a follow up study, having a cagemate with access to ethanol increased ethanol intake in female mice regardless of whether the cagemate was male or female (Tomie et al., 2015). The presence of two cagemates with access to ethanol further enhanced ethanol intake relative to when a single cagemate was present, suggesting that a dose-dependent relationship may exist between the number of social peers and ethanol intake. Notably, when two cagemates were housed on the same side of the plastic partition, lower rates of ethanol intake were observed compared to when subjects were separated by the partition (Tomie et al., 2015). Taken together, these data indicate that ethanol consumption of one individual can influence ethanol consumption of other individuals within a peer group.
A series of studies conducted by Chen and colleagues have examined the interaction between social contact, drug-associated cues, and intravenous nicotine self-administration (“Nicotine Social Interaction”; Figure 1f). This model uses a translucent partition with nose holes to divide a modified operant chamber into two sides, thereby allowing partial contact between rats. A test subject is given access to intravenous nicotine along with the simultaneous delivery of an olfactogustatory (OG) stimulus, a manipulation that in isolation attenuates acquisition rates by means of a conditioned taste aversion. The interaction between this drug-associated cue and a social partner is examined by presenting a partner that has access to the OG stimulus but not nicotine. In the first of these studies, the presence of the partner with the OG stimulus was required for stable acquisition of nicotine self-administration by test subjects (Chen et al., 2011). Importantly, these effects were dependent on the partner having access to the OG stimulus. This finding suggests that the interaction between the social partner and OG stimulus produced a different effect on drug self-administration than either stimulus alone. Although continued social access was not required for the maintenance of self-administration after acquisition, the availability of a social partner and OG stimulus enhanced nicotine intake in subsequent sessions. Interestingly, this social transmission of nicotine intake has been mechanistically linked to carbon disulfide (Wang and Chen, 2014), a volatile chemical in the expired breath of rodents that also mediates social learning of food preferences (Galef et al., 1988). Furthermore, rats that underwent context-induced extinction later reinstated nicotine-seeking behavior when presented in the original context with the OG stimulus and a social partner (Wang et al., 2014). A partner with access to the OG stimulus produced a greater increase in context-induced reinstatement than a partner without access, demonstrating the importance of combining the OG stimulus with social contact (Wang and Chen, 2014). Notably, measures of social interaction (e.g., an open field social interaction test) significantly predicted the degree of socially facilitated nicotine intake and reinstatement in these studies (Wang et al., 2014).
Several recent studies have used modified operant conditioning chambers to examine intravenous drug self-administration in multiple rodents at the same time and in the same chamber. Historically, intravenous self-administration studies of social contact have been limited by practical concerns, such as the small size of commercially available operant chambers, the need to prevent a subject from pressing the response lever of another subject, and the need to prevent infusion lines from becoming entangled. We recently developed custom-built operant conditioning chambers that mitigate these concerns (“Social Operant Conditioning Chambers”; Figure 1g). Specifically, chambers are separated by a wire partition that allows multiple animals to be tested concurrently with complete visual, olfactory, auditory, and limited tactile contact with one another. Importantly, these chambers also allow drug access to be manipulated on an individual basis. In the first of these studies, intravenous cocaine self-administration on both a fixed-ratio (FR) and PR schedule of reinforcement was examined for rats housed in isolation or housed with a social partner that either did or did not have access to cocaine (Smith, 2012). When both subjects had access to cocaine, cocaine self-administration was facilitated on both FR and PR schedules. In contrast, when only one subject had drug access, cocaine self-administration was inhibited in the partner that had access to cocaine. A later econometric analysis revealed that although elasticity of demand (i.e., sensitivity to the price of cocaine) did not vary among these groups, cocaine consumption was decreased when a subject was paired with a partner without access to cocaine (Peitz et al., 2013). Furthermore, in acquisition studies examining the percentage of rats acquiring drug self-administration and the rate at which they acquired drug self-administration, rats tested with a cocaine-experienced partner were more likely to acquire the behavior and had faster acquisition rates than those tested with a cocaine-naïve partner (Smith et al., 2014).
Another benefit of this model is that the operant conditioning chambers can be reconfigured to allow a theoretically unlimited number of subjects to be tested simultaneously. For example, a recent study revealed that when rats were tested in triads (i.e., three to a chamber), rats with access to cocaine emitted more responses on a lever in close proximity to a partner with access to cocaine than to a partner without access to cocaine (Smith and Pitts, 2014). These data suggest that rats “choose” or “prefer” to be in the presence of a partner that is also self-administering cocaine relative to a partner that is not self-administering cocaine. These findings were supported in a follow-up study reporting that rats prefer to spend time in the presence of other rats with a similar history of drug exposure as determined by a partner preference test (Smith et al., 2015). Taken in combination with results from the ethanol and nicotine literature, these findings provide cogent evidence that the behavior of a peer (i.e., whether or not that peer is also self-administering a drug) determines whether drug intake will be facilitated or inhibited by social contact.
5. Evidence of Modeling and Imitation in Drug Self-Administration
Most of the evidence for modeling and imitation in drug self-administration is indirect. Obtaining direct evidence is difficult because separating imitation from other social learning phenomena (e.g., social facilitation, local enhancement) requires the use of numerous control groups not traditionally included in drug self-administration studies (Zentall, 2012). Furthermore, it is likely that the social transmission of learned drug behaviors involves a combination of behavioral mechanisms that are not mutually exclusive (see review by Strickland and Smith, 2014). Despite these caveats, several findings support the possibility that individuals imitate the behavior of social partners, which influences both total drug intake and specific patterns of use.
Some of the evidence of modeling and imitation in drug self-administration has been obtained from the prairie vole model of ethanol consumption. In one such study, baseline ethanol drinking levels were determined in isolation and prairie voles were matched into same-sex dyads based on drinking levels (Anacker et al., 2011b). When a same-sex, high-intake drinker was paired with a low-intake drinker, increasing correspondence in ethanol consumption was observed, which was attributed to decreased consumption by the high-intake subject. In contrast, no change in ethanol intake was seen in same-sex social partners with similar levels of ethanol intake at pairing. Importantly, these effects did not extend to changes in consumption of an alternative reinforcer (i.e., saccharin), providing evidence for the specificity of this change to ethanol self-administration. A follow-up study indicated that these effects might depend on the sex of the social partner because similar outcomes were not observed in male-female dyads (Hostetler et al., 2012). Although correlations derived from cumulative lick records in a later study indicated that drinking bouts of dyads occurred in close temporal proximity to one another, similarly high correlations were observed for drinking bouts during a baseline isolation period (Anacker and Ryabinin, 2013), which may indicate a ceiling effect under baseline conditions. Taken together, these findings suggest that modeling and imitation may be responsible for the similar patterns of ethanol consumption often observed among members of peer groups.
Studies conducted using social operant conditioning chambers provide additional evidence of modeling and imitation by social peers. In male dyads, for instance, rats without access to cocaine responded on an inactive lever in a pattern that mimicked the responding of a social partner self-administering cocaine (Smith, 2012). A later study directly tested the hypothesis that social partners would show increased correspondence in drug-maintained responding over time (Lacy et al., 2014). In that study, subjects were first trained to respond on a fixed interval (FI) schedule of reinforcement and then tested daily with a social partner. Quarterlife values (i.e., the time at which the 25th percentile response occurs within an interval) became increasingly similar within social dyads over time, and reached statistical significance by the third day of testing. Although additional studies are needed to further test predictions about modeling and imitation, these data are consistent with the possibility that social learning mechanisms are responsible for the development of similar patterns of drug self-administration among members of peer groups.
6. Influence of Peer Presence, Peer Behavior, and Peer Imitation in the Human Laboratory
A full review of the human literature on social contact and drug self-administration is beyond the scope of this mini-review; however, representative studies will serve to demonstrate the cross-species generality of the effects of social contact on drug taking and provide tentative support for the predictive validity of these animal models. Our discussion of social contact in the human laboratory will focus on alcohol consumption, given that the vast majority of human studies examining social factors have used this drug. Importantly, the collective findings from these studies correspond with conclusions from the animal laboratory in that the presence of a peer influences drug intake, that this influence depends on the behavior of the peer, and that individuals imitate the behavior of their peers.
Early studies of social contact and drug intake in humans revealed that the presence of a peer could influence alcohol self-administration. For example, Doty and De Wit (1995) found that a greater number of subjects chose to self-administer alcohol over placebo when given the opportunity to do so in a social versus isolated condition. Notably, subjects in the social condition also reported greater positive subject-rated effects (e.g., euphoria) after alcohol administration, whereas subjects in the isolated condition reported greater negative subject-rated effects (e.g., dysphoria). Similarly, subjects in another study reported higher ratings of intoxication when tested with a social partner who also drank alcohol than when tested with a social partner who did not drink alcohol (Kirkpatrick and de Wit, 2013). Consistent with increased alcohol consumption and subject-rated effects in social contexts, Griffiths and colleagues (1974) reported that placing time-outs from social interaction contingent on alcohol choice was sufficient to suppress the choice to drink alcohol. Taken together, these findings reveal that the presence of a peer and his or her drinking behavior critically influence the subjective and reinforcing effects of alcohol.
Some of the first studies manipulating peer behavior to determine the effects on human alcohol consumption used a wine-tasting paradigm. In this task, subjects are told to compare wines by sampling multiple glasses. While sampling, a confederate acts as a social stimulus, wherein he or she drinks heavily (e.g., 700 ml of wine), lightly (e.g., 100 ml of wine), or not at all throughout the tasting. Numerous studies have demonstrated that subjects mimic the drinking behavior of a confederate, with subjects exposed to a heavy-drinking partner consuming more alcohol throughout the session (Caudill and Marlatt, 1975; Caudill and Kong, 2001; Lied and Marlatt, 1979). Recent studies extended these findings by using a model with greater ecological validity, namely a naturalistic bar setting with ad lib drinking availability. In these studies, subjects are given unlimited access to alcoholic (e.g., beer, wine) and non-alcoholic (e.g., soda) beverages over a set period of time (e.g., 30 minutes). Similar to the wine-tasting paradigm, confederates model heavy, light, or non-drinking during this period, and the subject’s drinking behavior is recorded. In this procedure, subjects exposed to a same-sex, heavy-drinking confederate drank more alcoholic beverages than those exposed to a light or non-drinking partner (Larsen et al., 2009). Later studies revealed that these effects are consistent across a variety of social manipulations and environments (Larsen et al., 2010, 2012, 2013). Notably, the effects of social contact on alcohol use are not dependent on explicit awareness of social influence. For example, one study indicated that a majority of subjects (i.e., 80%) reported being unaware of a partner’s influence and disagreed that the partner affected their drinking, even when alcohol consumption increased in the presence of that partner (Dallas et al., 2014). Collectively, these findings provide convincing evidence that the alcohol use of a peer can influence drinking within a peer group.
Although more limited in number, recent studies also provide tentative evidence that humans imitate patterns of alcohol use in addition to imitating total levels of consumption. Koordeman and colleagues (2011) reported that subjects given the opportunity to drink an alcoholic beverage while watching a contemporary movie with scenes containing alcohol use were more likely to take a sip following an actors’ sipping. Men in that study were more likely than women to imitate sipping behavior, and this effect was independent of the actors’ sex. Similarly, Larsen and colleagues (2010) found that social dyads showed correspondence in sip initiation in a naturalistic bar setting. In that study, subjects were more likely to take a sip of a beverage following a confederate’s sip, and this probability was greatest when both subjects were drinking alcohol. Together, these findings are concordant with the animal literature demonstrating that a social peer can influence both total drug intake and individual patterns of drug self-administration.
7. Future Directions
The emergence of novel animal models of social contact is an encouraging development in the study of drug use and abuse, and these models provide a fertile environment for additional research on the role of social contact in drug self-administration. Future research will need to examine how age and sex influence the effects of social contact on measures of drug self-administration. Despite the prevalence of opposite sex dyads in human social interactions, only a few studies have included such manipulations on measures of drug self-administration (e.g., Hostetler et al., 2012, Tomie et al., 2014, 2015). Similarly, although some studies have revealed age-dependent effects of social contact (e.g., Doremus, 2005), far fewer studies have examined if age differences within a social group may alter the effects of social contact on drug self-administration.
More complex models of social contact will also need to be developed. Social contact is in itself reinforcing, and social learning theories of drug abuse predict that drugs and social contact should mutually enhance the positive reinforcing effects of one another. For example, in humans, alcohol pretreatment increased the number of choice responses for social interaction versus money (Griffiths et al., 1975). Modifications to current animal models could allow manipulation of social contact in reinforcement or punishment contingencies. For instance, drug use could be reinforced by social contact by making access to a partner contingent on drug self-administration. Such findings would be consistent with those obtained in CPP studies (e.g., Thiel et al., 2008; Watanabe, 2011) and would significantly advance current models of social contact. Alternatively, drug use could be punished by removing access to a partner via a response-cost contingency, a manipulation that also decreases alcohol self-administration in the human laboratory (Griffiths et al., 1974).
Most broadly, cross-laboratory collaborations between animal and human laboratories must be encouraged throughout model development. Traditionally, animal and human models of drug self-administration have developed concurrently but independently of one another. The building of models through explicit communication between animal and human laboratory researchers will help strengthen the cross-species sensitivity of derived models and better link the animal and human laboratory outcomes. This animal-to-human approach to model building will improve the reliability and predictive validity of future models, as well as enhance the translational appeal and clinical utility of existing models.
8. Conclusions
The emergence of novel animal models of social contact with enhanced predictive validity and translational appeal is an exciting development for the study of drug self-administration. The comparative utility of many of these models rests on the research question and resources at hand. For example, the Home-Cage Consumption model (Figure 1a) and Observer-Demonstrator Procedure (Figure 1d) provide rapid and easy-to-implement designs that require only traditional laboratory equipment. Alternatively, the Social Access Procedure (Figure 1b) provides the opportunity to examine operant contingencies related to social interaction and access. Researchers concerned about monogamous and sibling pair bonds will likely find value in the Prairie Vole Ethanol Consumption model (Figure 1c), as will researchers interested in dyadic responding. The Nicotine Social Interaction model (Figure 1f) provides a method to examine the relationship between social stimuli and other drug-related cues and could be adapted for use with other substances. Finally, the Proximal Cagemate Drinking Procedure (Figure 1e) and Social Operant Conditioning Chambers (Figure 1g) are ideally suited for testing hypotheses about the influence of peer drug use at the time of drug taking. Modifications to these models that recognize their individual strengths will further the study of social contact and drug self-administration.
Taken together, a review of animal models of social contact and drug self-administration reveals three key conclusions: 1) a peer that is immediately present at the time of drug use influences drug consumption, 2) the behavior of that peer (i.e., whether that peer is also using drugs) determines whether social contact will increase or decrease drug self-administration, and 3) peers model and imitate specific patterns of drug self-administration. These findings are congruent with those obtained in the human laboratory, suggesting that these models have both face and predictive validity for the human condition. Further advances in these models will increase our understanding of the role of social contact in drug self-administration and will identify the behavioral mechanisms that mediate this relationship.
Highlights.
We reviewed models of social contact at the time of drug self-administration
The presence of a social partner influences drug self-administration
The behavior of a social partner determines the effect of social contact
Social partners can model and imitate specific patterns of drug self-administration
Acknowledgments
This work was supported by the National Institutes of Health (NIDA Grants R01DA031725 and R01DA027485 to MAS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akers RL. Deviant behavior: a social learning approach. Belmont, CA: Wadsworth; 1977. [Google Scholar]
- Alexander BK, Beyerstein BL, Hadaway PF, Coambs RB. Effect of early and later colony housing on oral ingestion of morphine in rats. Pharmacol Biochem Behav. 1981;15:571–6. doi: 10.1016/0091-3057(81)90211-2. [DOI] [PubMed] [Google Scholar]
- Alexander BK, Coambs RB, Hadaway PF. The effect of housing and gender on morphine self-administration in rats. Psychopharmacology (Berl) 1978;58:175–9. doi: 10.1007/BF00426903. [DOI] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol. 2011a;16:92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Ryabinin AE. Alcohol intake in prairie voles is influenced by the drinking level of a peer. Alcohol Clin Exp Res. 2011b;35:1884–90. doi: 10.1111/j.1530-0277.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AM, Ryabinin AE. Identification of subpopulations of prairie voles differentially susceptible to peer influence to decrease high alcohol intake. Front Pharmacol. 2013;4:84. doi: 10.3389/fphar.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr SJ, Hoffmann JP, Yang X. Parental and peer influences on the risk of adolescent drug use. J Prim Prev. 2005;26:529–51. doi: 10.1007/s10935-005-0014-8. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–90. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Hori K, Tom P, Blanchard DC. Social structure and ethanol consumption in the laboratory rat. Pharmacol Biochem Behav. 1987;28:437–42. doi: 10.1016/0091-3057(87)90502-8. [DOI] [PubMed] [Google Scholar]
- Bot SM, Engels RC, Knibbe RA, Meeus WH. Friend’s drinking behaviour and adolescent alcohol consumption: the moderating role of friendship characteristics. Addict Behav. 2005;30:929–47. doi: 10.1016/j.addbeh.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl) 2014;231:1557–80. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill BD, Kong FH. Social approval and facilitation in predicting modeling effects in alcohol consumption. J Subst Abuse. 2001;13:425–41. doi: 10.1016/s0899-3289(01)00099-2. [DOI] [PubMed] [Google Scholar]
- Caudill BD, Marlatt GA. Modeling influences in social drinking: an experimental analogue. J Consult Clin Psych. 1975;43:405–15. doi: 10.1037/h0076689. [DOI] [PubMed] [Google Scholar]
- Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacol. 2011;36:2629–38. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas R, Field M, Jones A, Christiansen P, Rose A, Robinson E. Influenced but unaware: social influence on alcohol drinking among social acquaintances. Alcohol Clin Exp Res. 2014;38:1448–53. doi: 10.1111/acer.12375. [DOI] [PubMed] [Google Scholar]
- Deatherage G. Effects of housing density on alcohol intake in the rat. Physiol Behav. 1972;9:55–7. doi: 10.1016/0031-9384(72)90264-8. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doty P, de Wit H. Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacology (Berl) 1995;118:19–27. doi: 10.1007/BF02245245. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–9. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vidal JM, Molina JC. Socially mediated alcohol preferences in adolescent rats following interactions with an intoxicated peer. Pharmacol Biochem Behav. 2004;79:229–41. doi: 10.1016/j.pbb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, et al. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol. 2011a;16:273–84. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Fritz M, Klement S, El Rawas R, Saria A, Zernig G. Sigma1 receptor antagonist BD1047 enhances reversal of conditioned place preference from cocaine to social interaction. Pharmacol. 2011b;87:45–8. doi: 10.1159/000322534. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Mason JR, Preti G, Bean NJ. Carbon disulfide: a semiochemical mediating socially-induced diet choice in rats. Physiol Behav. 1988;42:119–24. doi: 10.1016/0031-9384(88)90285-5. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Yates JR, Beckmann JS, Marusich JA, Zentall TR, Bardo MT. Social facilitation of d-amphetamine self-administration in rats. Exp Clin Psychopharmacol. 2011;19:409–19. doi: 10.1037/a0024682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HW. Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocrinology. 2002;27:115–26. doi: 10.1016/s0306-4530(01)00039-7. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Hen R. The serotonergic system and anxiety. NeuroMolecular Med. 2004;5:27–40. doi: 10.1385/NMM:5:1:027. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Bigelow G, Liebson I. Suppression of ethanol self-administration in alcoholics by contingent time-out from social interactions. Behav Res Ther. 1974;12:327–34. doi: 10.1016/0005-7967(74)90007-2. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Bigelow G, Liebson I. Effect of ethanol self-administration on choice behavior: money vs. socializing. Pharmacol Biochem Behav. 1975;3:443–6. doi: 10.1016/0091-3057(75)90054-4. [DOI] [PubMed] [Google Scholar]
- Hadaway PF, Alexander BK, Coambs RB, Beyerstein B. The effect of housing and gender on preference for morphine-sucrose solutions in rats. Psychopharmacology (Berl) 1979;66:87–91. doi: 10.1007/BF00431995. [DOI] [PubMed] [Google Scholar]
- Hallmark RA, Hunt PS. Social learning about ethanol in preweanling rats: role of endogenous opioids. Dev Psychobiol. 2004;44:132–9. doi: 10.1002/dev.10163. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Venturelli PJ, Fleckenstein AE. Drugs and society. 11. Burlington, MA: Jones and Bartlett; 2011. [Google Scholar]
- Honey PL, Varley KR, Galef BG., Jr Effects of ethanol consumption by adult female rats on subsequent consumption by adolescents. Appetite. 2004;42:299–306. doi: 10.1016/j.appet.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Anacker AM, Loftis JM, Ryabinin AE. Social housing and alcohol drinking in male-female pairs of prairie voles (Microtus ochrogaster) Psychopharmacology (Berl) 2012;224:121–32. doi: 10.1007/s00213-012-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE. Social partners prevent alcohol relapse behavior in prairie voles. Psychoneuroendocrinology. 2014;39:152–7. doi: 10.1016/j.psyneuen.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Holloway JL, Scordalakes EM. Social interaction with an intoxicated sibling can result in increased intake of ethanol by periadolescent rats. Dev Psychobiol. 2001;38:101–9. doi: 10.1002/1098-2302(200103)38:2<101::aid-dev1002>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Lant GM, Carroll CA. Enhanced intake of ethanol in preweanling rats following interactions with intoxicated siblings. Dev Psychobiol. 2000;37:90–9. [PubMed] [Google Scholar]
- Kandel DB. Processes of peer influences in adolescence. In: Silbereisen RK, Eyeferth K, Rudinger G, editors. Development as action in context: problem behavior and normal youth development. New York, NY: Springer; pp. 203–228. [Google Scholar]
- Kelly BC, Wells BE, Leclair A, Tracy D, Parsons JT, Golub SA. Prevalence and correlates of prescription drug misuse among socially active young adults. Int J Drug Policy. 2013;24:297–303. doi: 10.1016/j.drugpo.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, de Wit H. In the company of others: social factors alter acute alcohol effects. Psychopharmacology (Berl) 2013;230:215–26. doi: 10.1007/s00213-013-3147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koordeman R, Kuntsche E, Anschutz DJ, van Baaren RB, Engels RC. Do we act upon what we see? Direct effects of alcohol cues in movies on young adults’ alcohol drinking. Alcohol Alcohol. 2011;46:393–8. doi: 10.1093/alcalc/agr028. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Smith MA. Cocaine self-administration in social dyads using custom-built operant conditioning chambers. J Neurosci Meth. 2014;236:11–8. doi: 10.1016/j.jneumeth.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H, Engels RC, Granic I, Huizink AC. Does stress increase imitation of drinking behavior? An experimental study in a (semi-)naturalistic context. Alcohol Clin Exp Res. 2013;37:477–83. doi: 10.1111/j.1530-0277.2012.01942.x. [DOI] [PubMed] [Google Scholar]
- Larsen H, Engels RC, Granic I, Overbeek G. An experimental study on imitation of alcohol consumption in same-sex dyads. Alcohol Alcohol. 2009;44:250–5. doi: 10.1093/alcalc/agp002. [DOI] [PubMed] [Google Scholar]
- Larsen H, Engels RC, Souren PM, Granic I, Overbeek G. Peer influence in a micro-perspective: imitation of alcoholic and non-alcoholic beverages. Addict Behav. 2010;35:49–52. doi: 10.1016/j.addbeh.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Larsen H, Overbeek G, Granic I, Engels RC. The strong effect of other people’s drinking: two experimental observational studies in a real bar. Am J Addiction. 2012;21:168–75. doi: 10.1111/j.1521-0391.2011.00200.x. [DOI] [PubMed] [Google Scholar]
- Lied ER, Marlatt GA. Modeling as a determinant of alcohol consumption: effect of subject sex and prior drinking history. Addict Behav. 1979;4:47–54. doi: 10.1016/0306-4603(79)90020-0. [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Finkbeiner LM, Kirstein CL. Social interaction and partner familiarity differentially alter voluntary ethanol intake in adolescent male and female rats. Alcohol. 2008;42:641–8. doi: 10.1016/j.alcohol.2008.08.003. [DOI] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 2008;201:137–45. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Nader SH, Morgan D. Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology (Berl) 2012;224:57–67. doi: 10.1007/s00213-012-2843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology (Berl) 2012;224:33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JL, Perry JL, Carroll ME. Social stimuli enhance phencyclidine (PCP) self-administration in rhesus monkeys. Pharmacol Biochem Behav. 2007;87:280–8. doi: 10.1016/j.pbb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitz GW, Strickland JC, Pitts EG, Foley M, Tonidandel S, Smith MA. Peer influences on drug self-administration: an econometric analysis in socially housed rats. Behav Pharmacol. 2013;24:114–23. doi: 10.1097/FBP.0b013e32835f1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA. Housing and rank status of male Long-Evans rats modify ethanol’s effect on open-field behaviors. Psychopharmacology (Berl) 2006;185:289–97. doi: 10.1007/s00213-005-0257-3. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Psychosocial stress and chronic ethanol ingestion in male rats: effects on elevated plus maze behavior and ultrasonic vocalizations. Physiol Behav. 2008;94:432–47. doi: 10.1016/j.physbeh.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Raz S, Berger BD. Effects of fluoxetine and PCPA on isolation-induced morphine self-administration and startle reactivity. Pharmacol Biochem Behav. 2010a;96:59–66. doi: 10.1016/j.pbb.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Raz S, Berger BD. Social isolation increases morphine intake: behavioral and psychopharmacological aspects. Behav Pharmacol. 2010b;21:39–46. doi: 10.1097/FBP.0b013e32833470bd. [DOI] [PubMed] [Google Scholar]
- Salvy SJ, Pedersen ER, Miles JN, Tucker JS, D’Amico EJ. Proximal and distal social influence on alcohol consumption and marijuana use among middle school adolescents. Drug Alcohol Depend. 2014;144:93–101. doi: 10.1016/j.drugalcdep.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology (Berl) 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lacy RT, Strickland JC. The effects of social learning on the acquisition of cocaine self-administration. Drug Alcohol Depend. 2014;141:1–8. doi: 10.1016/j.drugalcdep.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Social preference and drug self-administration: A preclinical model of social choice within peer groups. Drug Alcohol Depend. 2014;135:140–5. doi: 10.1016/j.drugalcdep.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Strickland JC, Bills SE, Lacy RT. The effects of a shared history of drug exposure on social choice. Behav Pharmacol. 2015 doi: 10.1097/FBP.0000000000000139. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacol Biochem Behav. 2009;92:377–82. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Smith MA. The effects of social contact on drug use: behavioral mechanisms controlling drug intake. Exp Clin Psychopharmacol. 2014;22:23–34. doi: 10.1037/a0034669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–12. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Burger KM, Di Poce J, Pohorecky LA. Social opportunity and ethanol drinking in rats. Prog Neuro-Psychoph. 2004a;28:1089–97. doi: 10.1016/j.pnpbp.2004.05.044. [DOI] [PubMed] [Google Scholar]
- Tomie A, DeFuria AA, Jones HA, Edwards SD, Yu L. Effects of Cagemate Gender and the Cagemate’s access to ethanol on ethanol and water intake of the proximal male or the proximal female CD-1 mouse. Alcohol. 2014;48:73–82. doi: 10.1016/j.alcohol.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Tomie A, Gittleman J, Dranoff E, Pohorecky LA. Social interaction opportunity and intermittent presentations of ethanol sipper tube induce ethanol drinking in rats. Alcohol. 2005;35:43–55. doi: 10.1016/j.alcohol.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Tomie A, Lewis K, Curiotto J, Pohorecky LA. Intermittent exposure to a social stimulus enhances ethanol drinking in rats. Pharmacol Biochem Behav. 2007;87:341–8. doi: 10.1016/j.pbb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A, Samuel AG, Sprung DM, Malul Y, Yu L. Effects of number of cagemates on home cage ethanol drinking during proximal cagemate drinking (PCD) procedures in male and female CD-1 mice. Prog Neuro-Psychoph. 2015;57:1–10. doi: 10.1016/j.pnpbp.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Tomie A, Uveges JM, Burger KM, Patterson-Buckendahl P, Pohorecky LA. Effects of ethanol sipper and social opportunity on ethanol drinking in rats. Alcohol Alcohol. 2004b;39:197–202. doi: 10.1093/alcalc/agh055. [DOI] [PubMed] [Google Scholar]
- Walden B, McGue M, Lacono WG, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: the respective roles of peers and parents. J Abnorm Psychol. 2004;113:440–50. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- Wang T, Chen H. Carbon disulfide mediates socially-acquired nicotine self-administration. PloS One. 2014;9:e115222. doi: 10.1371/journal.pone.0115222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Han W, Wang B, Jiang Q, Solberg-Woods LC, Palmer AA, et al. Propensity for social interaction predicts nicotine-reinforced behaviors in outbred rats. Genes Brain Behav. 2014;13:202–12. doi: 10.1111/gbb.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S. Drug-social interactions in the reinforcing property of methamphetamine in mice. Behav Pharmacol. 2011;22:203–6. doi: 10.1097/FBP.0b013e328345c815. [DOI] [PubMed] [Google Scholar]
- Weisinger RS, Denton DA, Osborne PG. Voluntary ethanol intake of individually- or pair-housed rats: effect of ACTH or dexamethasone treatment. Pharmacol Biochem Behav. 1989;33:335–41. doi: 10.1016/0091-3057(89)90510-8. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–9. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol Biochem Behav. 1991;38:389–99. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- Zentall TR. Perspectives on observational learning in animals. J Comp Psychol. 2012;126:114–28. doi: 10.1037/a0025381. [DOI] [PubMed] [Google Scholar]
- Zernig G, Kummer KK, Prast JM. Dyadic social interaction as an alternative reward to cocaine. Front Psychiatry. 2013;4:100. doi: 10.3389/fpsyt.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]