Abstract
The role of mitochondrial manganese-superoxide dismutase (Mn-SOD) in the maintenance of vascular function has not yet been studied. Thus we examined flow- and agonist-induced dilations in isolated mesenteric arteries (~90 µm in diameter) of Mn-SOD heterozygous (Mn-SOD+/−) and wild-type (WT) mice. Increases in flow elicited dilations in all vessels, but the magnitude of the dilation was significantly less in vessels of Mn-SOD+/− mice than in those of WT mice (64 vs. 74% of passive diameter). Nω-nitro-l-arginine methyl ester inhibited the dilation in vessels of WT mice but had no effect on vessels of Mn-SOD+/− mice. Tempol or tiron (superoxide scavengers) increased flow-induced dilation in vessels of Mn-SOD+/− mice. Acetylcholine- and sodium nitroprusside-induced, but not adenosine-induced, dilations were also decreased in arteries of Mn-SOD+/− mice. Superoxide levels in the arteries of Mn-SOD+/− mice were significantly increased. Western blot analysis confirmed a 50% reduction of Mn-SOD protein in the vessels of Mn-SOD+/− mice. A 41% reduction in endothelial nitric oxide synthase (eNOS) protein and a 37% reduction in eNOS activity were also found in the vessels of Mn-SOD+/− mice. Whereas there was no difference in eNOS protein in kidney homogenates of WT and Mn-SOD+/− mice, a significant reduction of nitric oxide synthase activity was found in Mn-SOD+/− mice, which could be restored by the administration of tiron. We conclude that an increased concentration of superoxide due to reduced activity of Mn-SOD, which inactivates nitric oxide and inhibits eNOS activity, contributes to the impaired vasodilator function of isolated mesenteric arteries of Mn-SOD+/− mice. These results suggest that Mn-SOD contributes significantly to the regulation of vascular function.
Keywords: shear stress, endothelial nitric oxide, superoxide distmutase-2
Ample evidence suggests that endothelium-dependent, nitric oxide (NO)-mediated arteriolar dilation is impaired in many pathological conditions because of either decreased production or increased inactivation of NO or because of the synergistic effects of these two factors. Superoxide dismutases (SOD), the major antioxidant enzymes, importantly contribute to the regulation of the steady-state levels of superoxide and other reactive oxygen species. There are three isoforms of SOD: cytosolic SOD or copper zinc SOD (CuZn-SOD or SOD-1), mitochondrial SOD or manganese SOD (Mn-SOD or SOD-2), and extracellular CuZn-SOD (EC-SOD or SOD-3). The functional importance of each individual isoform in vascular protection against oxidative stress is still not resolved.
Previous studies using dietary restriction of copper and nonselective pharmacological inhibition of copper-containing SODs (6, 21, 29), as well as more recent studies using genetic models of deficiency of CuZn-SOD (7) and EC-SOD (10), demonstrated that the endogenous activity of copper-containing SODs is essential for endothelial dilator functions. Clinical studies found that reduced EC-SOD activity is closely associated with increased vascular oxidative stress and is responsible for the impaired endothelium-dependent vasodilatation in patients with coronary artery disease (14) and chronic heart failure (15). However, few studies have examined the specific role of Mn-SOD in the regulation of vascular endothelial function, especially in microvessels.
The physiological importance of Mn-SOD has been confirmed because SOD-2 gene knockout mice die of a cardiomyopathy within 2 wk after birth (9, 17, 24). Interestingly, overexpression of CuZn-SOD does not prevent neonatal death in mice that lack Mn-SOD (5), indicating an unique function of Mn-SOD in vivo. Mn-SOD heterozygous (Mn-SOD+/−) mice have been used previously to examine the effects of Mn-SOD deficiency on cardiac and cerebral responses to ischemia-reperfusion injury and mitochondrial function (2, 11, 13, 18, 28, 33). The importance of Mn-SOD in protecting against ischemia-reperfusion injury of the heart has been demonstrated by the fact that Mn-SOD+/− mice had a significant deficit in postischemic myocardial function compared with wild-type (WT) mice (2). This altered postischemic functional recovery, however, was not observed in hearts of CuZn-SOD heterozygous mice (2). In the cerebral circulation of Mn-SOD+/− mice, an increase in mitochondrial exacerbates cerebral infarction after ischemia-reperfusion (11, 28). Increased mitochondrial in Mn-SOD+/− mice has also been shown to decrease NO bioactivity, increase myocardial O2 consumption (18), and decrease exercise capacity (12). Also, the decreased activity of Mn-SOD is associated with increased oxidative stress-induced apoptosis in multiple organs (13, 33). Conversely, a recent study found that in the aortas of Mn-SOD+/− mice, levels were not different from those of WT mice (1). As a result, NO-mediated relaxation to ACh of the aortic rings of WT and Mn-SOD+/− mice was also not different. Collectively, the aforementioned studies reveal a close relationship between mitochondria-derived and endothelial NO-mediated responses. However, the role of Mn-SOD in the regulation of arterial function, especially the endothelial mechanisms responsible for shear stress-induced dilation, is not fully understood. Thus this study was designed to compare endothelium-dependent NO-mediated vasodilator responses to shear stress and vasoactive agents in isolated arteries of Mn-SOD+/− and WT mice and to clarify the specific effect of Mn-SOD deficiency in the regulation of microvascular function.
METHODS
Animals
Male Mn-SOD heterozygous (n = 24) and WT (n = 26) mice, 20–23 wk old and ~30 g in weight, were used. Mice were interbred and genotyped at our facility (18). All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conformed to the current guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals.
Flow-induced dilation
Flow-induced dilation (FID) was assessed in second-order mesenteric arteries [~160 µm in maximal diameter (passive diameter, PD) at 80 mmHg of intravascular pressure] of Mn-SOD+/− and WT mice. Vessels, ~1 mm in length, were isolated and cannulated in a water-jacketed (37°C) perfusion device, which consisted of two parallel perfusion chambers (1 ml in volume). Two vessels, one from each group, were perfused (1 ml/min) with 3-(N-morpholino) propanesulfonic acid (MOPS)-buffered physiological salt solution containing (in mM) 142.0 NaCl, 5.0 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 10.0 glucose, 2.0 pyruvate, 0.02 EDTA, and 3.0 MOPS at pH 7.4. Intravascular pressure was maintained at 80 mmHg by a pressure-servocontroller (Living Systems, Burlington, VT) connected to the outflow side of the chamber. Intraluminal flow was established by a syringe pump (model 100, KD Scientific), coupled with an inflow pressure transducer, which was connected at the inflow side of the chamber. During intraluminal flow, intravascular pressure was maintained by lowering outflow pressure to an amount equal to the increase in inflow pressure. Perfusate flow was increased from 0 to 15 µl/min, in 5 µl/min steps. FID was measured in control and after the administration of Nω-nitro-l-arginine methyl ester (l-NAME, 3 × 10−4 M), Tempol (10−3 M), or Tempol plus l-NAME.
In separate experiments, dilations to ACh (10−8 to 10−6 M), sodium nitroprusside (SNP, 10−8 to 10−6 M), and adenosine (ADO, 10−7 to 10−5 M) were assessed in the arteries of Mn-SOD+/− and WT mice.
Because of the insufficient amount of protein that can be isolated from second-order mesenteric arteries of each mouse, in the following experimental protocols, isolated first-order or homogenates of pooled first- and second-order mesenteric arteries were used.
Chemiluminescent detection of concentration
Rate of release of in isolated first-order mesenteric arteries from mice of both genotypes was assayed by the lucigenin chemiluminescence method (26). Vessels were equilibrated in PSS at 37°C for 30 min and then transferred into 2 ml of Krebs-HEPES buffer containing lucigenin (5 µM). Chemiluminescence was measured continuously with a scintillation counter (LS 700, Beckman Instruments) for 10 min after dark adaptation was allowed. After that, every vessel was completely digested with 50 µl of 1 N NaOH and total protein was assayed. level was normalized by protein content of each vessel and expressed as counts per minute per microgram of protein.
Protein expression with Western blotting
First- and second-order mesenteric arteries of Mn-SOD+/− and WT mice were isolated and pooled. Homogenates containing 10 µg of protein were separated on SDS-PAGE gels (10% acrylamide), transferred to a polyvinyl difluroide membrane, and probed with primary antibodies of endothelial NO synthase (NOS) (eNOS), Mn-SOD (BD Transduction Laboratories), and CuZn-SOD (Calbiochem). Secondary antibodies were conjugated to horseradish peroxidase according to the Amersham ECL-Plus protocol. The exposed film was developed in a Kodak X-Omat developer. Image acquisition and density of specific bands on the film were obtained by an image system (Alpha Innotech).
NOS activity
NOS activity of pooled first- and second-order mesenteric arteries (10 µg/sample) and the kidney (100 µg/sample) of Mn-SOD+/− and WT mice was assayed by measuring the rate of conversion of radiolabeled l-[3H]citrulline from l-[3H]arginine (Amersham) by using an assay kit (Cat. 482700, Calbiochem) and a scintillation counter (Beckman LS6500). The reaction was carried out at 37°C for 60 min. The values of samples treated with l-NAME (3 × 10−4 M) were used as background controls, and the average of background readings from each batch of assays was subtracted from the value of each sample to reflect actual NOS activity. Final production of l-[3H]citrulline was expressed as picomoles per hour per milligram protein. In kidney samples, NOS activities were assayed in control and in the presence of pyrogallol (0.2–0.8 mM) or 0.4 mM of pyrogallol plus 10 mM tiron.
SOD activity
SOD activity of mesenteric arteries was assessed by measuring the inhibition of pyrogallol autoxidation (22). First- and second-order mesenteric arteries were isolated. Forty micrograms of protein (pooled from 2 to 3 mice) were used in each measurement, and the reaction was monitored spectrophotometrically at room temperature for 3 min. The activity was calculated against a standard curve of SOD (0–1.6 U/ml; S-2515, Sigma). To assess Mn-SOD activity, KCN was added to vessel homogenates at a final concentration of 0.4 mM to inhibit copper-containing SOD activity.
Statistical analysis
Data are expressed as means ± SE; n refers the number of mice in each group. Statistical analysis was performed using repeated-measures of ANOVA followed by the Tukey-Kramer post hoc test and Student’s t-test. Statistical significance was accepted at a level of P < 0.05.
RESULTS
Flow-induced dilation
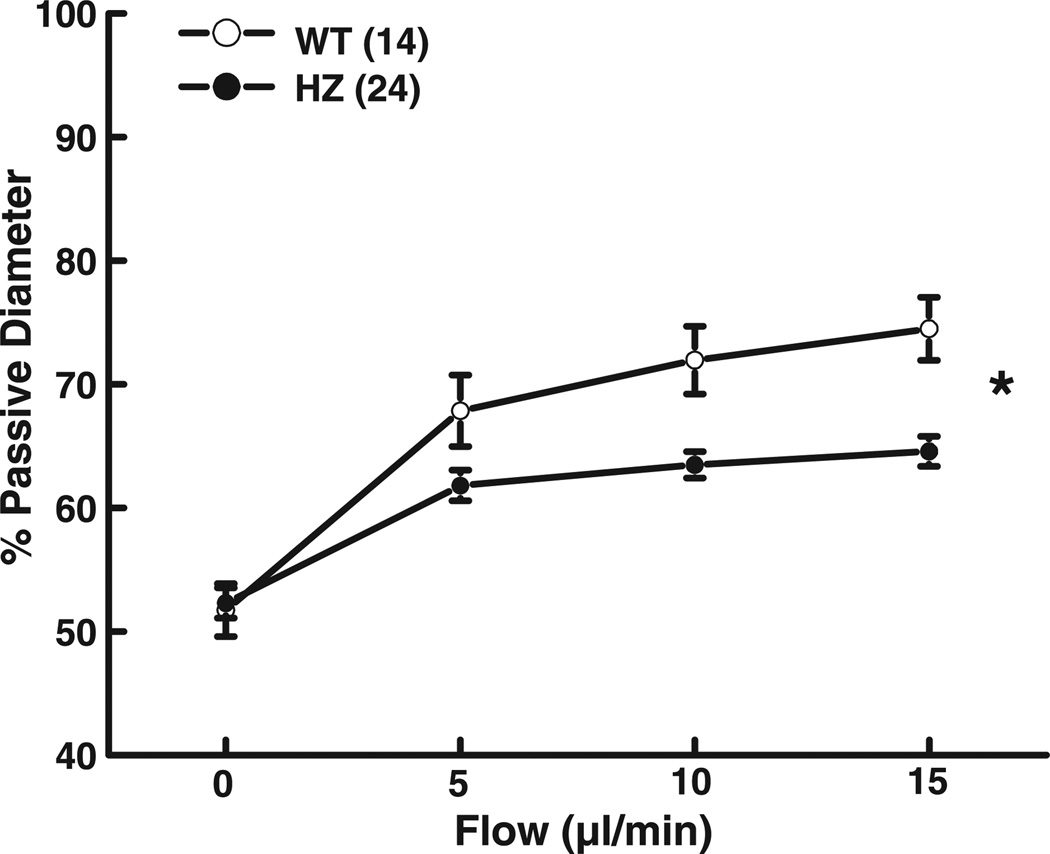
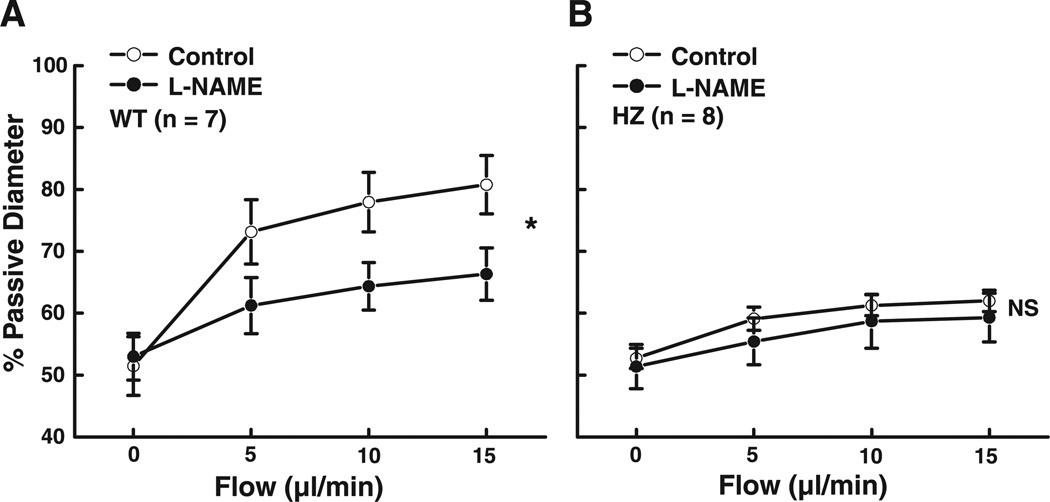
Isolated mesenteric arteries of Mn-SOD WT and Mn-SOD+/− mice developed active tone (51.7 ± 2.1 and 52.3 ± 1.1% PD, respectively) during a 1-h equilibration at 80 mmHg of intravascular pressure. In the presence of constant intravascular pressure, increases in perfusate flow from 0 to 15 µl/min elicited significant increases in arterial diameter in both groups of mice (Fig. 1). However, FID in the arteries of Mn-SOD+/− mice was significantly reduced compared with that in WT mice. For example, in response to 15 µl/min of flow, the diameter of vessels of Mn-SOD+/− mice was 64.6 ± 2.3% PD compared with 74.5 ± 2.6% PD (P < 0.05) in vessels of WT mice. Inhibition of NO synthesis with l-NAME significantly decreased FID in vessels of WT mice (Fig. 2A) but had no effect on FID in vessels of Mn-SOD+/− mice (Fig. 2B). These results indicate that FID in the mesenteric arteries of Mn-SOD-deficient mice is impaired, and the mechanism involved is related to an impaired NO-mediated dilation.
Fig. 1.
Flow-induced dilation (total average of different experimental protocols) in mesenteric arteries of wild-type (WT, n = 14) and Mn-SOD+/− (HZ, n = 24) mice. *P < 0.05 between WT and HZ mice (ANOVA).
Fig. 2.
Flow-induced dilation in mesenteric arteries of WT (A, n = 7) and HZ (B, n = 8) mice in control and after inhibition of nitric oxide (NO) synthesis with Nω-nitro-l-arginine methyl ester (l-NAME, 3 × 10−4 M). *P < 0.05 between control and l-NAME (ANOVA). NS, no significance.
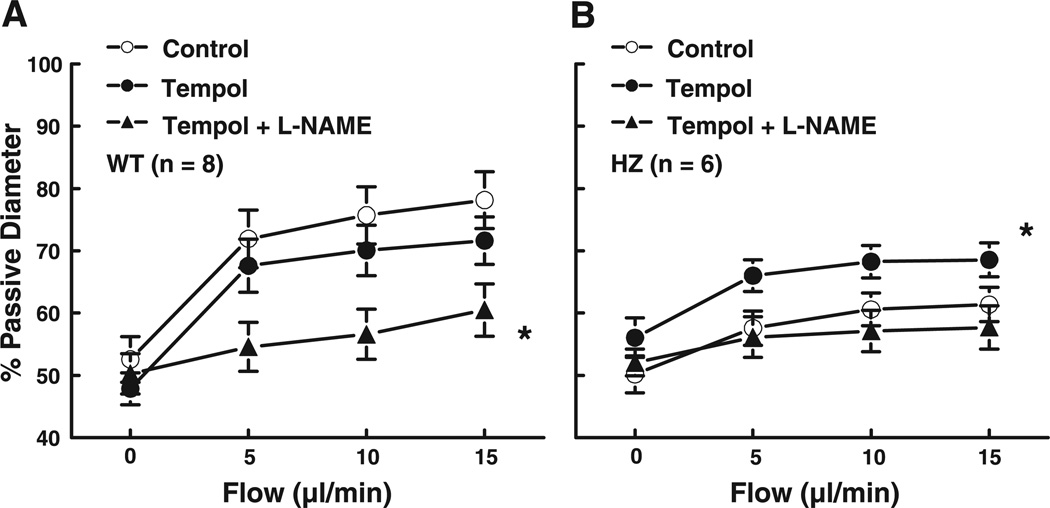
To investigate whether inactivation of NO by radicals is responsible for the decreased FID in vessels of Mn-SOD+/− mice, FID was assessed in control and after incubation of vessels, intra- and extraluminally, with the scavenger Tempol (10−3 M) for 30 min. Tempol did not affect the basal diameter of arteries in either group of mice nor FID in vessels of WT mice but significantly increased FID in the vessels of Mn-SOD+/− mice (Fig. 3) to a level comparable to that in the vessels of WT mice. Tiron (an intracellular scavenger, 3 × 10−4 M) also significantly increased FID in the vessels of Mn-SOD+/− mice (data not shown). In the presence of Tempol, l-NAME inhibited FID not only in WT mice but also in the arteries of Mn-SOD+/− mice.
Fig. 3.
Flow-induced dilation in mesenteric arteries of WT (A, n = 8) and HZ (B, n = 6) mice in control, after scavenging with Tempol (10−3 M) and after inhibition of NO synthesis with l-NAME (3 × 10−4 M). *P < 0.05 between control and Tempol + l-NAME in WT and between Tempol and Tempol + l-NAME in HZ (ANOVA).
Agonist-induced dilation
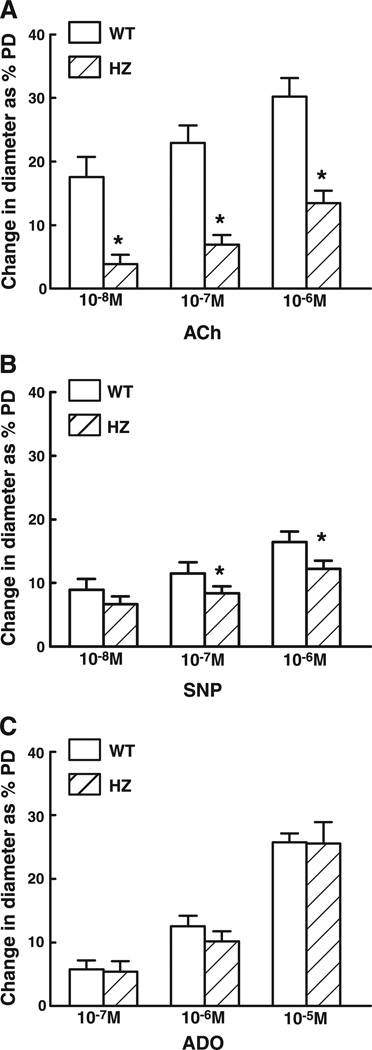
ACh (10−8, 10−7, and 10−6 M)-, SNP (10−8, 10−7, and 10−6 M)-, and ADO (10−7, 10−6, and 10−5 M)-induced dilations were compared in the mesenteric arteries of Mn-SOD+/− and WT mice (Fig. 4). Dilation to ACh was greatly reduced in the vessels of Mn-SOD+/− mice. For example, dilation to 10−7 M of ACh decreased by 56% compared with that of vessels of WT mice. Dilation to SNP was also reduced in the vessels of Mn-SOD+/− mice, but the magnitude of reduction was less. For example, dilation to 10−7 M SNP was decreased by 26% compared with that in vessels of WT mice. In contrast, dilations to ADO were comparable in vessels of the two groups of mice.
Fig. 4.
Changes in diameter as percentage of passive diameter (PD) of mesenteric arteries of WT and HZ mice in response to acetylcholine (A, ACh, n = 19 in WT and n = 18 in HZ), sodium nitroprusside (B, SNP, n = 19 in WT and n = 14 in HZ), and adenosine (C, ADO, n = 15 in WT and n = 15 in HZ). *P < 0.05 vs. WT.
level and SOD activity
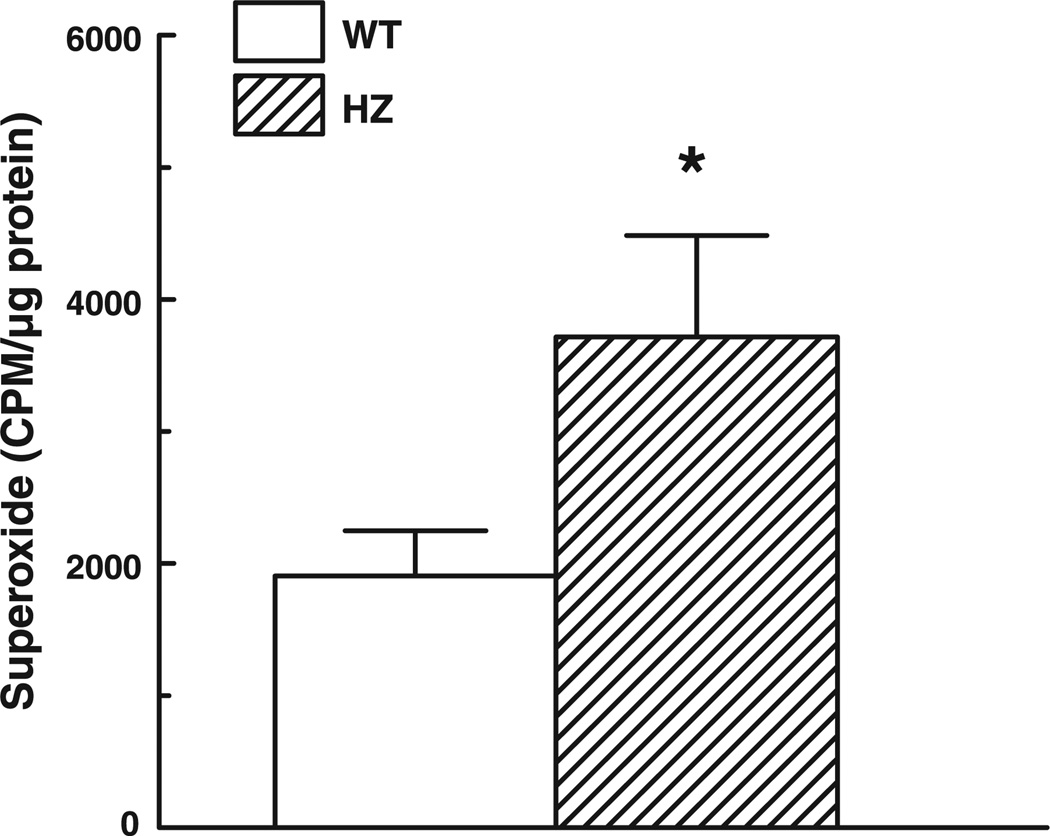
Basal release of in the mesenteric arteries of Mn-SOD+/− and WT mice was assessed by lucigenin-derived chemiluminescence. Figure 5 shows that the level of in the vessels of Mn-SOD+/− mice was significantly higher than that in vessels of WT mice.
Fig. 5.
Basal level of formation in mesenteric arteries of WT (n = 16) and HZ (n = 15) mice determined by lucigenin (5 µM) chemiluminescence. *P < 0.05 vs. WT.
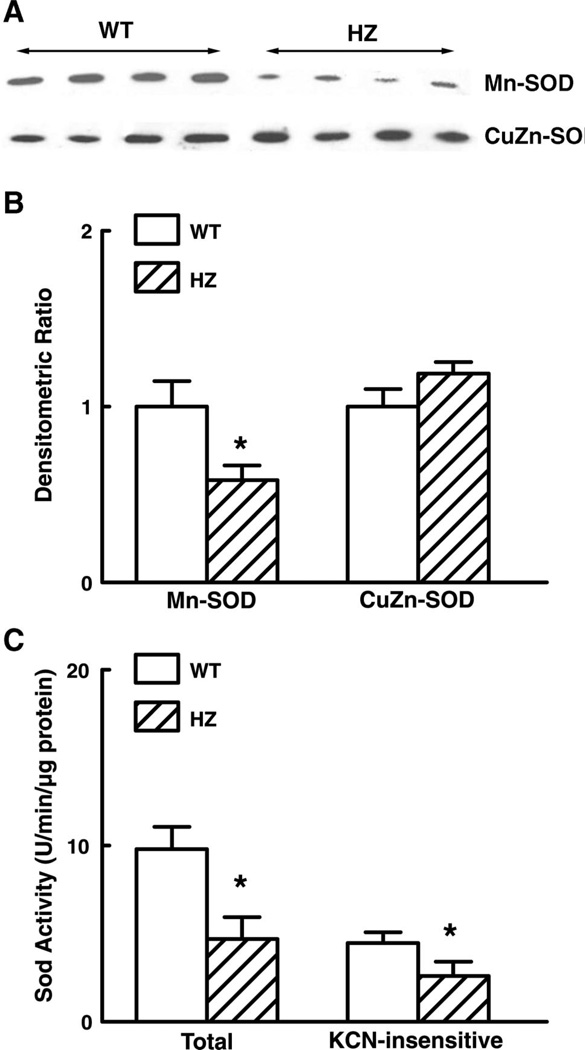
Protein levels of CuZn-SOD and Mn-SOD in mesenteric arteries of Mn-SOD+/− and WT mice were assessed by Western analysis. A decreased density of Mn-SOD bands was seen in the vessels of Mn-SOD+/− mice (Fig. 6A). The summary data of densitometric ratios, shown in Fig. 6B, further indicate that expression of Mn-SOD was significantly decreased (by 42%) in Mn-SOD+/− mice compared with that of WT mice. Expression of CuZn-SOD was not different between the two groups. In correspondence with the decreased Mn-SOD expression, total SOD activity in the vessels of Mn-SOD+/− mice decreased by 44% (Fig. 6C). To determine Mn-SOD activity, KCN (0.4 mM) was added to inhibit the activity of copper-containing SODs. In the presence of KCN, SOD activity was 47% lower in vessels of Mn-SOD+/− than in vessels of WT mice (Fig. 6C).
Fig. 6.
A: representative Western blot analysis of Mn-SOD and CuZn-SOD proteins in mesenteric arteries of WT and HZ mice. B: summary data of three Western analysis blots. Data are normalized by means of densitometry of Mn-SOD and CuZn-SOD from vessels of WT mice. C: total and KCN (2 mM)-insensitive SOD activity as determined by measuring the inhibition of pyrogallol autoxidation in 40 µg of protein of pooled mesenteric arteries of WT (n = 8) and HZ (n = 6) mice. *P < 0.05 vs. WT.
eNOS activity
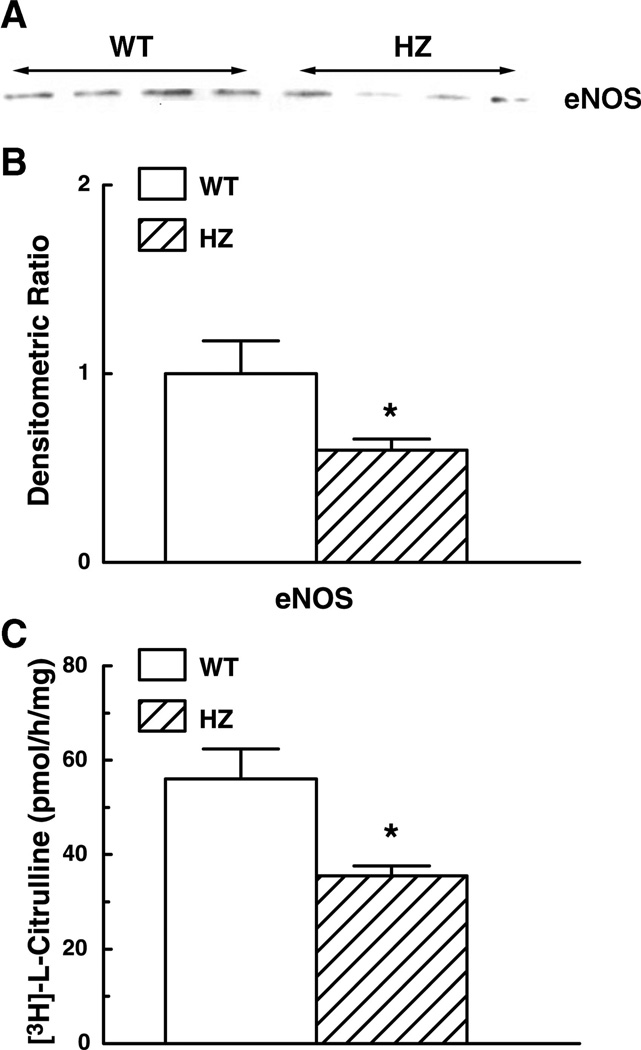
To determine whether the impairment of eNOS function was an additional cause of the decreased FID in the vessels of Mn-SOD+/− mice, we assessed eNOS expression and NOS activity in the mesenteric arteries of Mn-SOD+/− and WT mice (Fig. 7). Contrary to our expectation, expression of eNOS protein was reduced in vessels of Mn-SOD+/− mice compared with those of WT mice (Fig. 7A). Figure 7B shows that the densitometric ratio of eNOS in the vessels of Mn-SOD+/− mice significantly decreased (by 41%). Basal production of l-[3H]citrulline in the homogenates of pooled mesenteric arteries of Mn-SOD+/− mice was also significantly decreased (by 37%) (Fig. 7C).
Fig. 7.
A: representative Western analysis blot of endothelial NO synthase (eNOS) protein in mesenteric arteries of WT (n = 15) and HZ mice. B: summary data of three Western analysis blots. Data are normalized by means of densitometry of eNOS from vessels of WT mice. C: basal activity of NOS as determined by measuring the rate of conversion of l-[3H]citrulline from l-[3H]arginine in 10 µg of protein of homogenate of mesenteric arteries of WT (n = 8) and HZ (n = 8) mice. *P < 0.05 vs. WT.
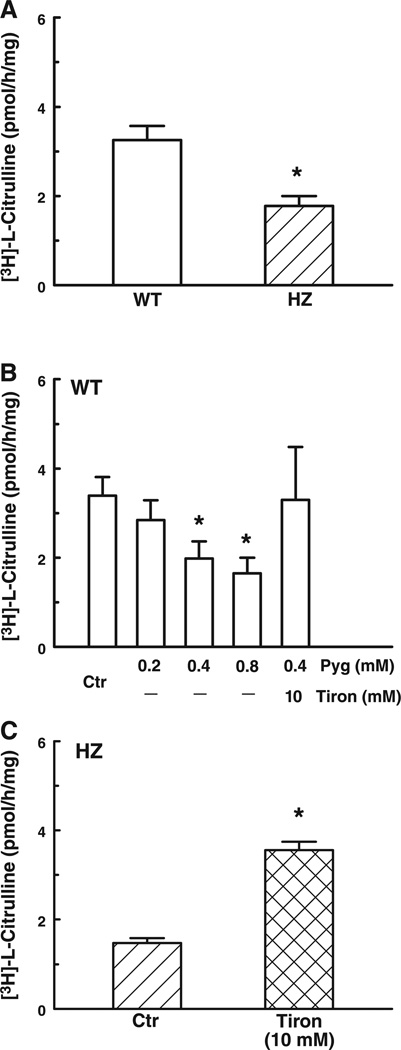
To further investigate the influence of on NOS activity, we assessed eNOS expression and NOS activity from kidney homogenates of both groups of mice. Protein expression of eNOS was not different in the two groups (data not shown). However, l-[3H]citrulline production was significantly decreased (by 46%) in the Mn-SOD+/− mice compared with that of the WT mice (Fig. 8A). In WT mice, pyrogallol, a donor, dose dependently (0.2–0.8 mM) decreased NOS activity. In the presence of pyrogallol (0.4 mM), tiron (10 mM) fully restored NOS activity (Fig. 8B). Furthermore, in kidney homogenates of Mn-SOD+/− mice, tiron (10 mM) significantly increased NOS activity that became comparable to that of WT mice (Fig. 8C). These data indicate that an increased level of in Mn-SOD+/− mice inhibits the activity of NOS.
Fig. 8.
A: basal activity of NOS in 100 µg of protein of kidney homogenate of WT and HZ (n = 21) mice. *P < 0.05 vs. WT. B: effect of pyrogallol (Pyg) and tiron on basal activity of NOS in kidney homogenates of WT (n = 7) mice. *P < 0.05 vs. control (Ctr). C: effect of Tiron on basal activity of NOS in kidney homogenate of HZ (n = 8) mice. *P < 0.05 vs. control (Ctr).
DISCUSSION
The present study is the first to provide evidence for the role of Mn-SOD in the maintenance of endothelium-dependent, flow- or shear stress-induced dilation by use of Mn-SOD+/− mice and demonstrates that the impaired NO-mediated FID in the arteries of Mn-SOD+/− mice is most likely due to an increased level and a decreased activity of eNOS.
Impaired NO-mediated dilator responses in arteries of Mn-SOD+/− mice
FID is an important physiological phenomenon and often used to determine endothelial function in small arteries and arterioles. It has been demonstrated by many studies in a variety of pathological conditions that increased concentration of or decreased activity of SOD impairs FID. Clinical studies also show that in patients with coronary artery disease and heart failure, a decreased FID is closely associated with a reduced activity of EC-SOD (14, 15). In the present study, we further demonstrate that deficiency of mitochondrial SOD also impairs FID (Fig. 1). Because we found that inhibition of NO synthesis had no effect on FID in Mn-SOD+/− mice and because, in the presence of l-NAME, FID was comparable between vessels of Mn-SOD+/− and WT mice (Figs. 2 and 3), we conclude that the impaired FID in Mn-SOD+/− mice is due to an impaired NO-mediated dilation.
It has been reported that in small mesenteric arteries of mice, the ACh-induced relaxation, in the absence of eNOS activity, is mainly mediated by H2O2-induced hyperpolarization of vascular smooth muscle cells (23). The mechanism involved in the ACh-induced synthesis of H2O2 is dependent on the function of CuZn-SOD (27). In the present study, ACh-induced dilation in arteries of Mn-SOD+/− mice is reduced (Fig. 4). Whether the reduced response is due to inactivation of NO by increased level of or to decreased H2O2-mediated dilation (eNOS activity and SOD activity are both reduced in these vessels) is not clear, and more extensive studies would be needed to address these questions. Nevertheless, the results showing that SNP-induced dilations are reduced in the mesenteric arteries of Mn-SOD+/− mice (Fig. 4) (indicating an increased interaction between NO and within the vascular wall) and that dilation to ADO, which serves as a control for smooth muscle-dependent dilation, as well as the basal tone of arteries are not different in Mn-SOD+/− and WT mice, suggest that increased mitochondria-derived impairs endothelium-dependent agonist-induced dilations and that impaired NO-mediated responses are associated with Mn-SOD deficiency.
Several mechanisms may contribute to the impaired NO-mediated FID in the arteries of Mn-SOD+/− mice. One of them could be that oxidative stress significantly contributes to endothelial dysfunction via inactivation of NO by an increased formation of (3, 19), as has been shown in variety of pathological states (4). In the present study, we found that basal release of in the mesenteric arteries of Mn-SOD+/− mice was increased twofold over control (Fig. 5). Although, it is not clear whether application of shear stress would elicit an additional increase in the release of in the vessels of Mn-SOD+/− mice, the results showing that administration of Tempol by scavenging restores FID, reveal an imbalance among the level of , the activity of SOD, and the bioavailability of NO in response to shear stress in vessels of Mn-SOD+/− mice (Fig. 3). It can be debated whether as a charged molecule can cross mitochondrial membranes. Recent studies suggest that is released from the mitochondria into the cytosol via voltage-dependent anion channels located on the outer membrane of mitochondria (8) and demonstrate that the rate of release from the mitochondria is increased severalfold when mitochondrial production is increased. From these reports, and as a consequence of the heterozygous deletion of the Mn-SOD gene, the decreased Mn-SOD protein and activity in mesenteric arteries of Mn-SOD+/− mice (Fig. 6) could cause an increased level of mitochondrial , which when released into the cytosol would inactivate NO, resulting in reduced endothelium-dependent and NO-mediated dilations. In addition, produced in smooth muscle and perhaps other cells in the vascular wall could also inactivate NO generated in endothelial as well as smooth muscle cells. This concept is supported by the observed -dependent reduction in dilation of vessels of Mn-SOD+/− mice to SNP.
H2O2 plays an important role in many vascular beds as an endogenous vasodilator. In human coronary resistance arteries, FID is mediated by H2O2 (25), and the mitochondria are believed to be the source of H2O2 generated in response to shear stress (20). With the application of these mechanisms to our findings, the reduced FID in vessels of Mn-SOD+/− mice may also result from impaired H2O2-mediated dilation, because insufficiency of Mn-SOD could potentially result in a reduced ability to convert in mitochondria to H2O2. However, our results showed that the magnitude of the remaining FID after inhibition of NO synthesis with l-NAME in the vessels of WT and Mn-SOD+/− mice were comparable, suggesting that the impaired FID in arteries of Mn-SOD+/− mice is mainly due to reduced NO bioavailability caused by an increased level of mitochondrial .
With regard to the decreased eNOS protein expression in the arteries of Mn-SOD+/− mice (Fig. 7), we have no ready mechanistic explanation. The reduction seems to be tissue specific because eNOS protein was not found to be reduced in the kidney or myocardium (12). Nevertheless, the reduced basal production of NO could contribute to the impaired NO-mediated FID in the vessels of these mice. In addition, the increased concentration in Mn-SOD+/− mice may inhibit eNOS activity via several mechanisms to reduce FID (see below).
Increased level of and reduced activity of eNOS in Mn-SOD+/− mice
In the present study, we found that an acute increase in level by administering an donor pyrogallol decreased eNOS activity in the kidney homogenates of Mn-SOD WT mice (Fig. 8B). Conversely, in Mn-SOD+/− mice, an acute decrease in the level by administering the scavenger tiron increased eNOS activity (Fig. 8C). These results suggest that an increased level of inhibits eNOS activity. In contrast, in the mesenteric vessels of Mn-SOD+/− mice, it seems likely that the reduction (41%) in eNOS protein is responsible for the reduced basal production of NO (Fig. 7, B and C). However, we cannot rule out the possibility that under a stimulation of shear stress, the inhibitory role of on the activation of eNOS may provide for additional mechanisms that could contribute to the reduced FID in vessels of Mn-SOD+/− mice. It has been demonstrated by many studies that the formation of peroxynitrite, as a result of the reaction of with NO, inhibits in variety of vascular functions that are dependent on the activation of eNOS. One of the underlying mechanisms is the uncoupling of eNOS via the oxidation of BH4 (16). On the other hand, it has also been demonstrated that peroxynitrite may attack the zinc-thiolate center of eNOS directly to cause enzymatic uncoupling (34). In either case, eNOS uncoupling decreases NO synthesis and increases the level.
Recently, the role of oxidative stress-induced formation of asymmetric dimethylarginine (ADMA), an endogenous eNOS inhibitor, in contributing to cardiovascular dysfunction has attracted considerable attention. It has been demonstrated that oxidative stress increases ADMA concentrations via upregulation of ADMA synthase or inhibition of the ADMA degrading enzyme dimethylaminohydrolase. In the presence of high levels of ADMA, eNOS can become a source of vascular production that further enhances vascular oxidative stress burden (31, 32). In patients with peripheral arterial disease, FID is impaired, accompanied by an enhanced plasma concentration of ADMA (30). Thus a variety of -related mechanisms may contribute to the decreased eNOS activity in mesenteric vessels and kidney homogenates of Mn-SOD+/− mice.
In summary, this study elucidates the specific role of Mn-SOD in the regulation of shear stress-dependent mechanisms in microvessels. The results demonstrate that deletion of the gene for Mn-SOD blunts eNOS activity and causes impaired flow- and agonist-induced dilation, caused by increased levels.
Acknowledgments
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-31069, HL-50412, HL-68813, HL-70653, and PO-1-HL-43023.
REFERENCES
- 1.Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. Am J Physiol Heart Circ Physiol. 2004;287:H1141–H1148. doi: 10.1152/ajpheart.01215.2003. [DOI] [PubMed] [Google Scholar]
- 2.Asimakis GK, Lick S, Patterson C. Postischemic recovery of contractile function is impaired in SOD2(+/−) but not SOD1(+/−) mouse hearts. Circulation. 2002;105:981–986. doi: 10.1161/hc0802.104502. [DOI] [PubMed] [Google Scholar]
- 3.Buerk DG, Lamkin-Kennard K, Jaron D. Modeling the influence of superoxide dismutase on superoxide and nitric oxide interactions, including reversible inhibition of oxygen consumption. Free Radic Biol Med. 2003;34:1488–1503. doi: 10.1016/s0891-5849(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 4.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 5.Copin JC, Gasche Y, Chan PH. Overexpression of copper/zinc superoxide dismutase does not prevent neonatal lethality in mutant mice that lack manganese superoxide dismutase. Free Radic Biol Med. 2000;28:1571–1576. doi: 10.1016/s0891-5849(00)00280-x. [DOI] [PubMed] [Google Scholar]
- 6.Didion SP, Hathaway CA, Faraci FM. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am J Physiol Heart Circ Physiol. 2001;281:H1697–H1703. doi: 10.1152/ajpheart.2001.281.4.H1697. [DOI] [PubMed] [Google Scholar]
- 7.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 8.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 9.Huang TT, Carlson EJ, Kozy HM, Mantha S, Goodman SI, Ursell PC, Epstein CJ. Genetic modification of prenatal lethality and dilated cardiomyopathy in Mn superoxide dismutase mutant mice. Free Radic Biol Med. 2001;31:1101–1110. doi: 10.1016/s0891-5849(01)00694-3. [DOI] [PubMed] [Google Scholar]
- 10.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 11.Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- 12.Kinugawa S, Wand Z, Kaminski PM, Wolin MS, Edwards J, Kaley G, Hintze TH. Limited exercise capacity in heterozygous manganese superoxide dismutase gene knockout mice. Roles of superoxide anion and nitric oxide. Circ Res. doi: 10.1161/01.CIR.0000159261.11520.63. First published January 6, 2005, 10.1161/01.RES.0000155331.09458.A7. [DOI] [PubMed] [Google Scholar]
- 13.Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci USA. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, Hornig B. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–2270. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 15.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 16.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 17.Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Jue T, Edwards J, Wang X, Hintze TH. Changes in NO bioavailability regulate cardiac O2 consumption: control by intramitochondrial SOD2 and intracellular myoglobin. Am J Physiol Heart Circ Physiol. 2004;286:H47–H54. doi: 10.1152/ajpheart.00730.2003. [DOI] [PubMed] [Google Scholar]
- 19.Liochev SI, Fridovich I. Superoxide and nitric oxide: consequences of varying rates of production and consumption: a theoretical treatment. Free Radic Biol Med. 2002;33:137–141. doi: 10.1016/s0891-5849(02)00864-x. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 21.Lynch SM, Frei B, Morrow JD, Roberts LJ, Xu A, Jackson T, Reyna R, Klevay LM, Vita JA, Keaney JF., Jr Vascular superoxide dismutase deficiency impairs endothelial vasodilator function through direct inactivation of nitric oxide and increased lipid peroxidation. Arterioscler Thromb Vasc Biol. 1997;17:2975–2981. doi: 10.1161/01.atv.17.11.2975. [DOI] [PubMed] [Google Scholar]
- 22.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 23.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- 25.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 26.Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol Heart Circ Physiol. 1994;266:H2568–H2572. doi: 10.1152/ajpheart.1994.266.6.H2568. [DOI] [PubMed] [Google Scholar]
- 27.Morikawa K, Shimokawa H, Matoba T, Kubota H, Akaike T, Talukder MA, Hatanaka M, Fujiki T, Maeda H, Takahashi S, Takeshita A. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest. 2003;112:1871–1879. doi: 10.1172/JCI19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuschke DA, Saari JT, Miller FN. A role for dietary copper in nitric oxide-mediated vasodilation. Microcirculation. 1995;2:371–376. doi: 10.3109/10739689509148281. [DOI] [PubMed] [Google Scholar]
- 30.Stuhlinger MC, Oka RK, Graf EE, Schmolzer I, Upson BM, Kapoor O, Szuba A, Malinow MR, Wascher TC, Pachinger O, Cooke JP. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation. 2003;108:933–938. doi: 10.1161/01.CIR.0000085067.55901.89. [DOI] [PubMed] [Google Scholar]
- 31.Sydow K, Munzel T. ADMA and oxidative stress. Atheroscler Suppl. 2003;4:41–51. doi: 10.1016/s1567-5688(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 32.Sydow K, Schwedhelm E, Arakawa N, Bode-Boger SM, Tsikas D, Hornig B, Frolich JC, Boger RH. ADMA and oxidative stress are responsible for endothelial dysfunction in hyperhomocyst(e)inemia: effects of l-arginine and B vitamins. Cardiovasc Res. 2003;57:244–252. doi: 10.1016/s0008-6363(02)00617-x. [DOI] [PubMed] [Google Scholar]
- 33.Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, Carlson EJ, Epstein CJ, Huang TT, Richardson A. Knockout mice heterozygous for SOD2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Heart Circ Physiol. 2001;281:H1422–H1432. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- 34.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]