Abstract
The purpose of this study was to investigate the mechanisms that regulate superoxide production as a function of an acute elevation of intravascular pressure and age. Mesenteric arteries isolated from young (6 mo) and aged (24 mo) male Fischer 344 rats were used. production in vessels in response to 80 (normal pressure, NP) and 180 (high pressure, HP) mmHg was determined by the superoxide dismutase-inhibitable nitroblue tetrazolium (NBT) reduction assay. In vessels exposed to NP, production was significantly higher in aged than in young vessels (32.7 ± 7.0 vs. 15.4 ± 2.4 nmol·mg−1·30 min−1). HP enhanced production in vessels of both groups, but the enhancement was significantly greater in aged than in young vessels (63.4 ± 6.7 vs. 32.7 ± 4.3 nmol·mg−1·30 min−1). Apocynin (100 µmol/l) attenuated HP-induced increases in production in both groups, whereas allopurinol (100 µmol/l) and Nω-nitro-l-arginine methyl ester (100 µmol/l) inhibited the response only in aged vessels. Confocal microscopy showed increases in in response to HP in endothelial and smooth muscle layers of both groups, with much greater fluorescent staining in aged than in young rats and in the endothelium than in smooth muscle cells. No significant changes in NAD(P)H oxidase gene and protein expressions were observed in vessels of the two groups. Upregulation of protein expression of xanthine oxidase was detected in aged vessels. We conclude that NAD(P)H oxidase contributes importantly to HP-induced enhanced production in vessels of both young and aged rats, whereas xanthine oxidase and nitric oxide synthase-dependent production also contribute to the enhancement in mesenteric arteries of aged rats.
Keywords: endothelium, reactive oxygen species, NAD(P)H oxidase, xanthine oxidase, endothelial nitric oxide synthase uncoupling
Diminished nitric oxide (NO) bioavailability by increased superoxide production is one of the major mechanisms responsible for the impaired endothelium-dependent vasodilator responses in hypertension and aging (13, 18, 29). In experimental and human essential hypertension, activation of NADPH oxidase and xanthine oxidase are the two major sources responsible for the increased production (31). Vascular can be also produced by endothelial NO synthase (eNOS) uncoupling, which further decreases NO synthesis and increases oxidative stress in hypertension (9, 19). However, the mechanism of increased production in aged vessels is not well understood, and this may be complicated by accompanying pathological conditions.
Similar to the endothelial dysfunction found in hypertension, a brief increase in intravascular pressure in isolated arterioles of young and normotensive rats reduced NO-mediated, flow-induced dilation through an increased production (16). It was also demonstrated that high pressure or stretch enhances production via a protein kinase C-dependent activation of NAD(P)H oxidase (26, 32). It is known that hypertension accelerates aging-related structural and functional changes in the vascular wall and reduces life expectancy (10); on the other hand, arterial aging further increases blood pressure in hypertension (2, 11, 23). Thus it is interesting to examine whether arteries from aged animals are more susceptible to the damage caused by high pressure and whether the mechanisms involved in production are different from those in young animals. Furthermore, it has been demonstrated that hypertension, aging, or acute high pressure cause selective endothelial dysfunction (5, 16, 18, 29, 32).
In the present study, we investigated the mechanisms that are responsible for production in response to acute increases in intravascular pressure in isolated arteries of young and aged rats, and we also aimed to identify the difference in production between the endothelial and smooth muscle cells.
MATERIALS AND METHODS
Isolated arteries
First-order mesenteric arteries (~350 µm in diameter and 10–15 mm in length) were isolated from 6- and 24-mo-old male Fischer 344 rats (29). Eight to ten arteries were isolated from each rat. Each vessel was used for only one experimental protocol. The vessels were cannulated in a perfusion chamber (4 ml) and perfused with MOPS-buffered (pH 7.4) physiological salt solution (PSS). All side branches along the vessels were carefully ligated to ensure no leakages. The intravascular pressure was then increased to 80 mmHg, and the vessels were equilibrated at 37°C for 60 min. All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conformed to the current guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals.
Nitroblue tetrazolium reduction assay
Vessels were incubated with nitroblue tetrazolium (NBT, 10 µM) in different experimental conditions for 30 min to allow generated from vessels to reduce NBT to formazan, which was then dissolved in DMSO and determined spectrophotometrically at 630 nm (27).
In the first protocol, we compared production in response to intravascular pressures of 80 and 180 mmHg in the mesenteric arteries of 6- and 24-mo-old rats. After the equilibration, NBT was administered intra- and extraluminally with intravascular pressures at 80 or 180 mmHg for 30 min. In a group of experiments, vessels were cannulated and pressurized at zero intravascular pressure for an hour and then NBT was administered. The reaction was stopped by the addition of 1 ml of 1 N HCl into the vessel chamber. The unreduced NBT dye was completely removed by repeated washing of the vessels intra- and extraluminally with cold (4°C) PSS. The vessels were then removed from the cannula and snap frozen in liquid nitrogen for further assays. Negative controls were established by the addition of SOD (100 U/ml) with NBT to the vessels for the same period of time.
In the second protocol, production in response to 180 mmHg of pressure was characterized by using different pharmacological inhibitors. Apocynin (Apo, 100 µM), allopurinol (Allop, 100 µM), and Nω-nitro-l-arginine methyl ester (l-NAME; 100 µM) were used as specific inhibitors of NAD(P)H oxidase, xanthine oxidase, and NOS, respectively. Vessels were equilibrated at 80 mmHg for 60 min, followed by intra- and extraluminal administration of the inhibitors for 30 min. NBT was then administered with the inhibitors to the vessels, and the intravascular pressure was increased to 180 mmHg for 30 min. In one group of vessels, the effect of Apo on production was assessed at 80 mmHg.
Collected vessels were completely digested in 2 M KOH, and the protein content was then determined. The samples were further mixed with DMSO in a ratio of 1:1.2, and the absorbance at 630 nm (OD630) was determined against a mixture of KOH and DMSO as a blank. Standard curves of NBT (0–10 µM) were constructed by using the mixture as a vehicle. The SOD-inhibitable NBT reduction was calculated by subtracting the average of the negative controls from all other samples. Final production was expressed as nmoles of NBT per milligram protein per 30 min of incubation time.
Western blot analysis
Isolated mesenteric arteries were homogenized in Laemmli buffer with a Pellet Pestle Mixer for 30 s followed by 2-min sonication and 5-min boiling. Samples were separated on a 10% SDS-PAGE gel and transferred on a polyvinyldifluoride membrane. The membrane was incubated with antibodies of Nox-1, gp91phox, or Nox-4 (Santa Cruz Biotechnology), xanthine oxidase (Novus Biologicals), or inducible NOS (iNOS, BD Transduction Laboratories) overnight at 4°C. The secondary antibody was conjugated to horseradish peroxidase according to the Amersham ECL-Plus protocol. β-Actin was used to normalize for loading variations.
RT-PCR
In this series of experiments, the impact of increased intraluminal pressure on vascular NAD(P)H gene expression was assessed by comparison of gp91phox and Nox-4 mRNA at 80 and 180 mmHg pressure in rats of both ages. To match the pressure profile used in determination, vessels were incubated at 80 mmHg for 60 min, and then the intravascular pressure was maintained at 80 mmHg for another 60 min or increased to 180 mmHg for 10, 30, and 120 min, respectively. Vessels were then taken off the cannulas and immersed immediately into liquid nitrogen for determination of RNA. Freshly isolated vessels were used as controls. Total RNA of single vessels was purified using a mini RNA isolation kit (Zymo Research, Orange, CA). Reverse transcription was performed using 0.5 µg RNA and Superscript II (Invitrogen) as per manufacturer’s instructions. Done in duplicate, 10% of the RT product was used for PCR amplification in the presence of SYBR green, and increased fluorescence was determined in real time using a Stratagene Mx3000P (Stratagene, La Jolla, CA). Expression of gp91phox and Nox-4 was normalized using β-actin.
Confocal microscopy
formation in the endothelium and smooth muscle cells of mesenteric arteries were determined by dihydroethidium staining and confocal microscopy. Dihydroethidium (DHE, 1 µM) was administered intra- and extraluminally at 80- and 180-mmHg pressures for 30 min. After extra DHE was washed out with cold (4°C) PSS, the vessels were cut longitudinally, removed from the cannula, and fixed in 4% paraformaldehyde for 20 min. After a brief wash in PBS, the vessel segment was adhered to glass slides with the endothelium facing up and coverslipped with antifading solution for fluorescent confocal microscopy (Bio-Rad MRC 1024ES/Olympus 1×70). With the use of a double-blind design, three images of the endothelial and smooth muscle layers were consistently obtained per vessel segment. All images were taken with an UPlanFI×40 objective and identical program settings. A histogram of full-sized fluorescent images was then created to measure the total number and average intensity of red pixels. The product of these two values gives a total fluorescent intensity of the image measured, which corresponds to the level of . The final data were normalized by subtracting the fluorescence intensity in vessels treated with DHE at zero intravascular pressure for the same period of time.
Statistical analysis
Data are expressed as means ± SE. Statistical significance was calculated by Student’s t-test and by repeated-measures ANOVA, followed by the Tukey-Kramer multiple-comparison test. n refers to the number of rats from which mesenteric arteries were isolated. If more than one vessel of a rat was used in the same protocol, the average was obtained and counted as n = 1. Significance level was taken at P < 0.05.
RESULTS
A total of 25 6-mo-old and 16 24-mo-old male Fischer 344 rats were used in this study. The average body weight for young and aged rats was 360 ± 6 and 451 ± 6 g (P < 0.05), respectively. The systolic blood pressure and heart rate in young and aged rats were 135 ± 6 and 130 ± 5 mmHg and 472 ± 13 and 452 ± 11 beats/min (n = 6 rats in each group), respectively, measured with the tail-cuff method (IITC, Life Science). The average diameter of the first-order mesenteric arteries, at 80 mmHg of pressure, in young and aged rats was 356 ± 10 and 373 ± 9 µm, respectively. No significant difference was found.
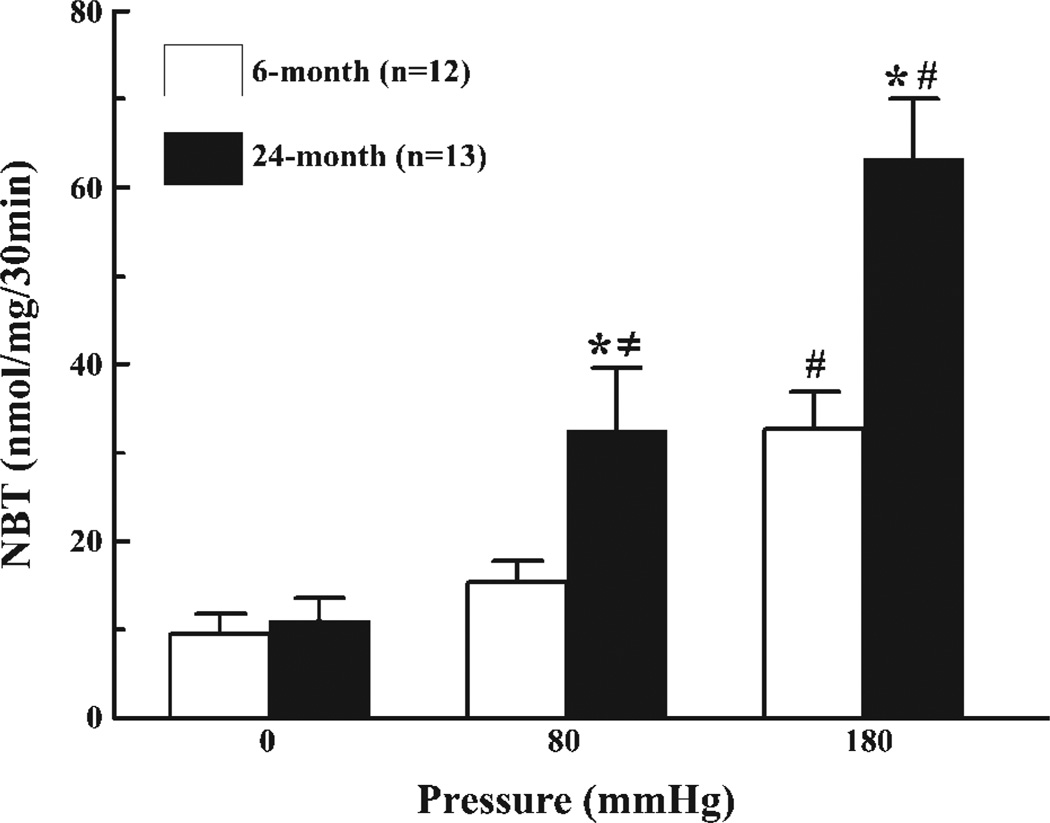
production as a function of aging and intravascular pressure in isolated mesenteric arteries is depicted in Fig. 1, showing first that at zero intravascular pressure, production was not different between the two groups. At 80 mmHg, production in vessels of aged rats increased significantly more than in vessels of young rats, and the level of was significantly higher than at zero pressure only in vessels of aged rats. At 180 mmHg, production in both groups about doubled from the levels at 80 mmHg; however, the increase was significantly greater in vessels of aged than in young rats (~16 vs. 29 nmol·mg−1·30 min−1 in young and aged rats, respectively). Overall, when the intravascular pressure increased from 0 to 180 mmHg, production increased threefold in vessels of young rats and sixfold in vessels of aged rats. These data clearly indicate that an increase in pressure enhances production in vessels and that vascular aging potentiates pressure-activated production.
Fig. 1.
Nitroblue tetrazolium (NBT) reduction assay detecting production in mesenteric arteries of 6- and 24-mo-old male Fischer rats in response to 0, 80, and 180 mmHg of intravascular pressure. *Significant difference vs. 6-mo-old rats. ≠ and #Significant differences vs. 0 and 80 mmHg, respectively.
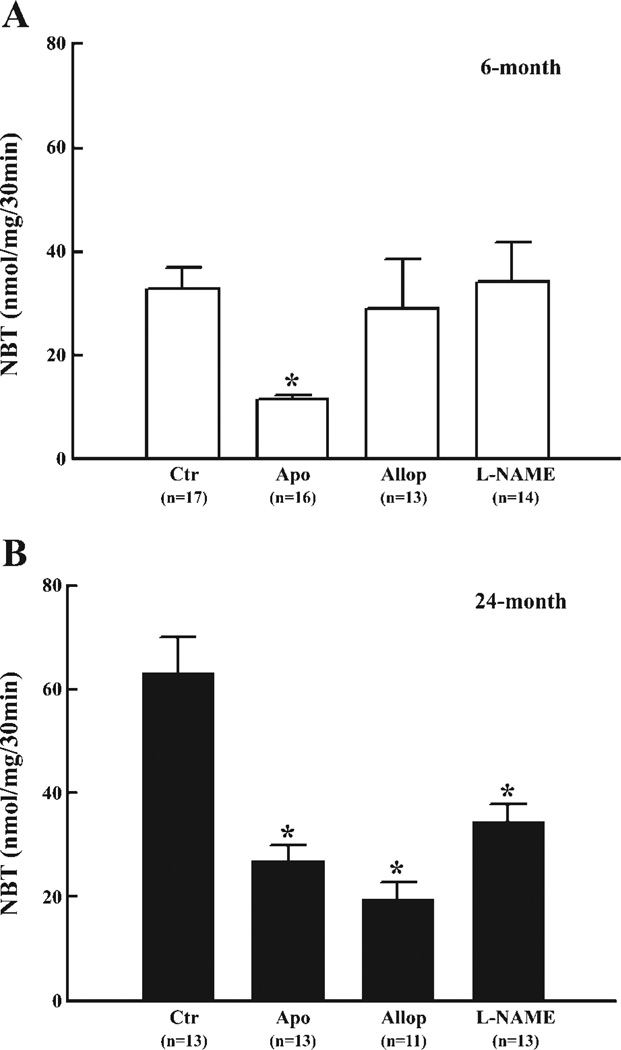
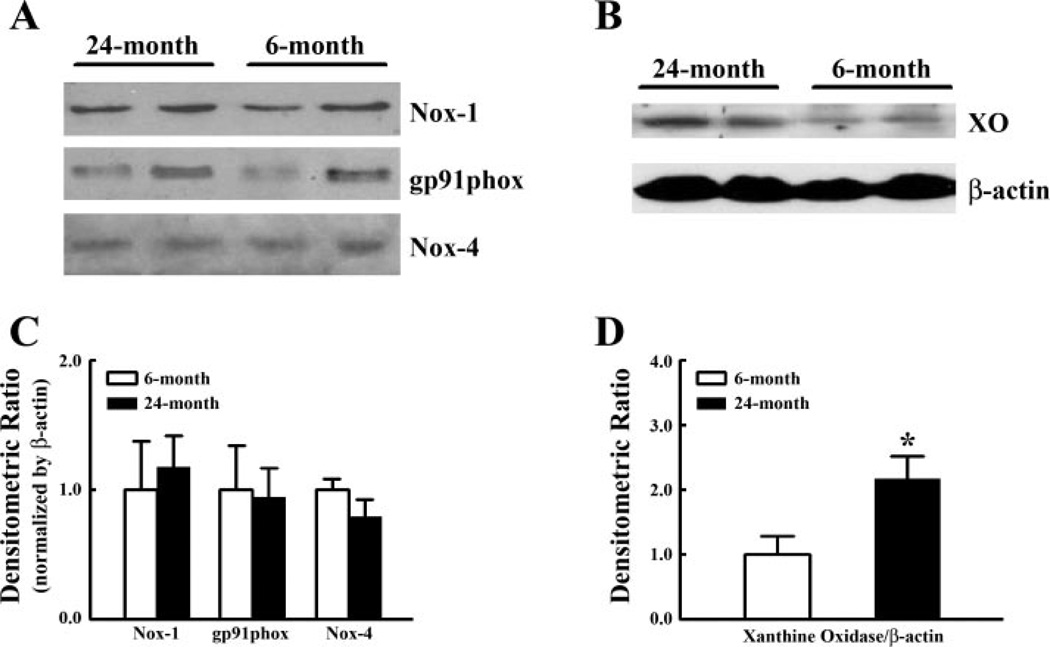
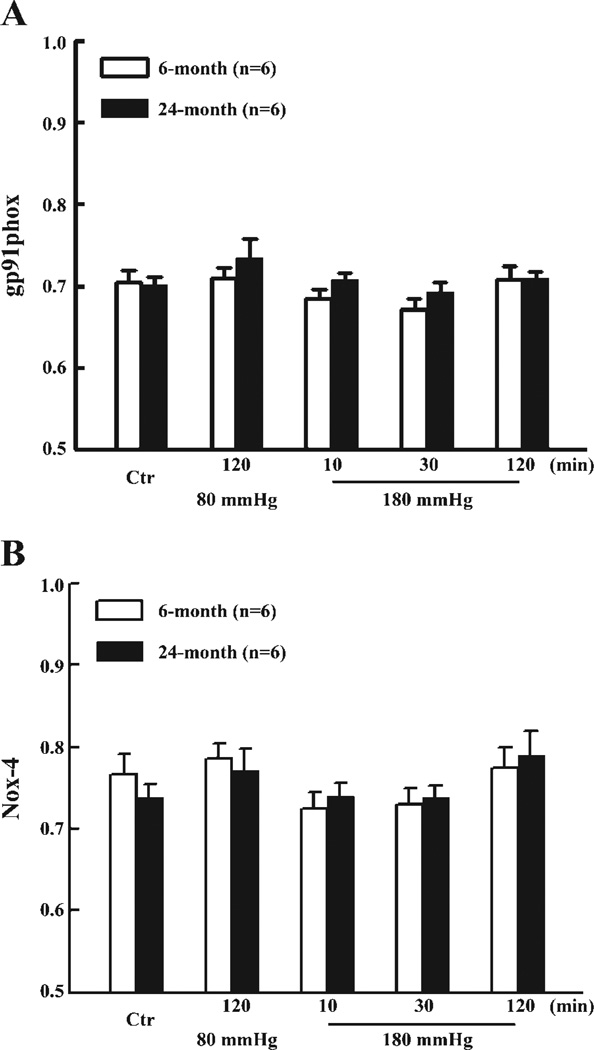
To identify the specific sources responsible for the enhanced production of , as a consequence of increases in intravascular pressure at both ages, vessels were treated with inhibitors of NAD(P)H oxidase, xanthine oxidase, or NOS, respectively. At 80 mmHg, Apo had no significant effect on production in young vessels (15.4 ± 2.4 vs. 12.1 ± 3.4 nmol NBT·mg−1·30 min−1) but significantly decreased production in aged vessels (32.7 ± 7.0 vs. 17.4 ± 3.1 nmol NBT·mg−1·30 min−1). At 180 mmHg of intravascular pressure, as shown in Fig. 2, the increased production of in vessels of young rats (Fig. 2A) was inhibited by Apo but was not significantly affected by Allop and l-NAME, indicating that activation of NAD(P)H oxidase is responsible for the pressure-induced increase in . However, in vessels of aged rats (Fig. 2B), not only Apo, but also Allop and l-NAME, significantly inhibited production, indicating that NAD(P)H oxidase, xanthine oxidase, and NOS are all contributing to pressure-activated production. Western blot analysis did not reveal significant differences in protein expression of Nox-1, gp91phox, and Nox-4 in vessels from rats of both ages, but protein expression of xanthine oxidase was significantly increased in vessels of aged rats (Fig. 3). iNOS protein was undetectable in vessels of either group of rats (data not shown). Using similar pressure protocols, we found no significant changes in vascular expression of gp91phox and Nox-4 mRNA at different pressure steps or between young and old rats (Fig. 4).
Fig. 2.
production in response to 180 mmHg of intravascular pressure in control (Ctr) and after inhibition of NADPH oxidase with apocynin (Apo, 10−4 M), xanthine oxidase with allopurinol (Allop, 10−4 M), and nitric oxide synthase with Nω-nitro-l-arginine methyl ester (l-NAME, 10−4 M), respectively, in mesenteric arteries of 6- (A) and 24-mo-old rats (B). *Significant difference vs. control. n, Number of rats.
Fig. 3.
A and B: representative Western blot analyses of NADPH oxidase subunits Nox-1, gp91phox, and Nox-4, and xanthine oxidase (XO), respectively, in mesenteric arteries of 6- and 24-mo-old rats. β-Actin was used to normalize for loading variations. C and D: summarized data of three Western blot analyses (12 rats in each group). Data are normalized by means of densitometric ratios of proteins of interest and β-actin from vessels of 6-mo-old rats. *Significant difference vs. 6-mo-old rats.
Fig. 4.
Expression of gp91phox (A) and Nox-4 (B) mRNA in mesenteric vessels exposed to 80 and 180 mmHg for 10, 30, and 120 min, of 6- and 24-mo-old rats. Controls were freshly isolated vessels without pressure treatment. Quantitative RT-PCR was used to determine expression of gp91-phox and Nox-4 relative to β-actin expression as described in MATERIALS AND METHODS.
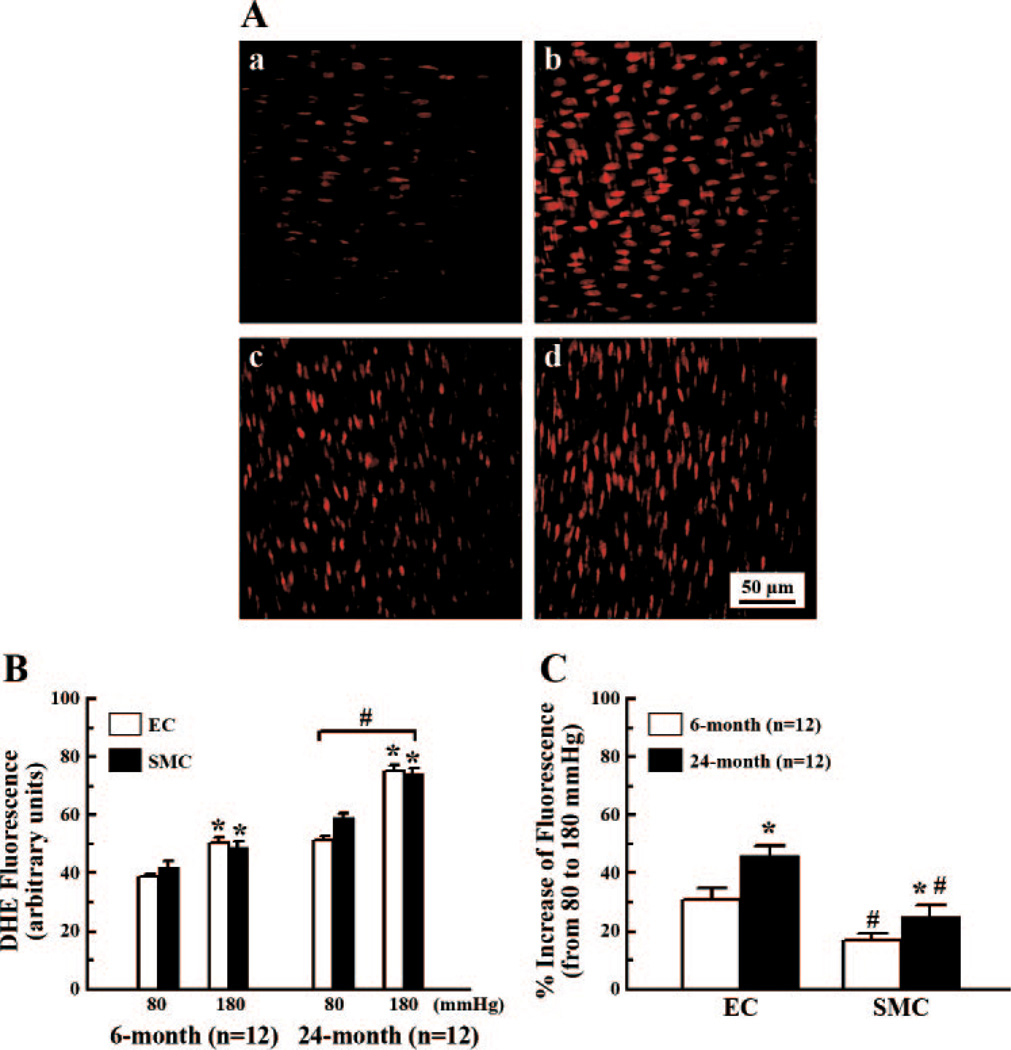
Confocal microscopy of DHE staining was used to determine the production in endothelial and smooth muscle layers of vessels. The endothelial and smooth muscle cells were identified and fluorescence intensity of a single scanning layer was obtained (Fig. 5A). Fluorescence intensity between the endothelial and smooth muscle layers within each age group was not significantly different. Fluorescence intensity increased significantly at 180 mmHg, compared with 80 mmHg, in the endothelial and smooth muscle layers of both 6- and 24-mo-old rats. Similar to the data shown in Figs. 1 and 2, fluorescence intensity in the endothelial and smooth muscle layers was significantly higher in aged than in young rats (Fig. 5B). Figure 5C further reveals that the percent increase in fluorescence intensity in the endothelial layers, in vessels of both groups, was significantly greater than in the smooth muscle layers and was also significantly greater in aged than in young vessels, implying that the endothelium plays a dominant role, particularly in aged vessels, in pressure-induced production.
Fig. 5.
A: sample images of confocal microscopy comparing fluorescence intensity of dihydroxyethidium (DHE) (red color) in response to 80 and 180 mmHg of intravascular pressure in the endothelial and smooth muscle layers of mesenteric arteries of 6-mo-old rats. a and b are the endothelial layers; c and d are smooth muscle layers; a and c, and b and d are vessels treated with 80 and 180 mmHg of pressure, respectively. B: summarized histogram data detecting fluorescence intensity of DHE in the endothelial and smooth muscle layers of 6- and 24-mo-old rats. *Significant difference compared with 80 mmHg. #Significant difference compared with 6-mo-old rats. C: percent increase in fluorescence intensity from 80 to 180 mmHg of intravascular pressure. *Significant difference compared with 6-mo-old rats. #Significant difference compared with EC.
DISCUSSION
In the present study, we provided evidence for pressure-activated and age-dependent increases in vascular . It is known that rapidly reacts with NO, thereby decreasing NO bioavailability (12). Excessive production is, therefore, one of the major causes contributing to the development of endothelial dysfunction in cardiovascular disease states (14). We found previously that a 30-min elevation of intravascular pressure reduces NO-mediated, flow- and agonist-induced dilations in arterioles of normal rats and that administration of SOD restored the responses (16, 32), suggesting that elevation of pressure, even for a short period of time, can induce endothelial dysfunction. In the present study, we further compared arteries isolated from young and aged rats in response to acute high pressure and measured production directly from these vessels. We found that arteries of aged rats released significantly more than those of young rats. At 80 mmHg of intravascular pressure, which is the normal pressure of first-order mesenteric arteries in rats, released from arteries of young rats was not different from that released at zero pressure. In aged vessels, however, release was significantly increased and reached a level similar to that in young vessels at 180 mmHg. These data suggest that the sensitivity of aged arteries to intravascular pressure is increased and is released in these vessels, even at a normal pressure, to produce endothelial dysfunction. Indeed, we have previously demonstrated that at 80 mmHg of pressure, shear stress-induced dilation and release of NO in mesenteric arteries were reduced in aged rats (29). Others have also shown increased and impaired endothelium-dependent vasodilator responses in aging humans (30) and rats (1, 5). Furthermore, in the present study we found that in response to a brief period of high intravascular pressure, produced in aged arteries was twice as great than that produced in young arteries. Undoubtedly, this excessive will have a further deleterious impact on what is already an existing endothelial dysfunction in aged vessels.
The mechanisms responsible for pressure-activated release in the vasculature have not been well documented. The body weight in aged rats was greater than that in young rats, but the diameter of vessels used in the experiments was not different between the two groups. Therefore, it is unlikely that the production in response to pressure was due to the influence of body weight. In isolated first-order mesenteric arteries, there was only a minimal pressure-induced myogenic constriction and the arterial diameter reached a plateau after the intravascular pressure was increased to 80 mmHg (28). production, however, was increased in response to high pressure in arteries of young and aged rats, whereas at a normal pressure (80 mmHg) it was increased only in aged arteries. In this context, pressure-induced production is not related to the changes in vascular diameter. On the other hand, lines of evidence show that cyclic or sustained stretch (26), and turbulent flow or oscillatory shear stress increase oxidative stress (17). Together, these data suggest that multiple signaling pathways are involved in mechanical force-induced formation. NAD(P)H oxidases are the major source of in the vascular wall. Increased evidence indicates that NAD(P)H oxidases have distinct subcellular localization and are associated with mechanotransduction components, such as the cytoskeleton, focal adhesion sites, and caveolae (15, 22). However, whether these cellular components are involved in pressure-induced activation of NAD(P)H oxidases is not known. Recent studies indicate that the signaling cascade of high pressure-induced NAD(P)H oxidase-dependent production involves an increase in intracellular Ca2+ and protein kinase C phosphorylation (21, 32). This finding is congruent with a previous report showing that mechanosensitive production of reactive oxygen species is linked to calcium-dependent myogenic constriction in arterioles (25). On the other hand, cytosolic Ca2+ level can in turn, be increased by per se through the mobilization of intracellular Ca2+ stores and/or through the influx of extracellular Ca2+, a positive feedback that further accelerates oxidative stress of the vessels (6). Nevertheless, we found that the sources of in arteries of young and aged rats were distinct. In vessels of young rats, NAD(P)H oxidase contributes to the high pressure-induced production. In vessels of aged rats, however, NAD(P)H oxidase, xanthine oxidase, and eNOS all contribute to the augmented production of , since not only Apo, but also Allo and l-NAME, inhibit the responses.
A role for NAD(P)H oxidase in the age-dependent potentiation of oxidative stress and endothelial dysfunction has been well documented (13). Inhibition of NAD(P)H oxidase decreases production and improves endothelial function. However, increased NAD(P)H oxidase-dependent oxidative stress in aged vessels is not necessarily coupled with an increased protein expression of the enzyme. Csiszar et al. (5) demonstrated previously that NAD(P)H oxidase subunits p47phox, p67phox, Nox-1, and p22phox were not increased in coronary arterioles of aged compared with young rats. Similarly, in the present study, protein and gene expressions of NAD(P)H oxidase were not different in mesenteric arteries of the two groups of rats. Thus pressure-induced increases in the activity of NADPH oxidase in aged mesenteric arteries is suggested. Indeed, at 80 mmHg, Apo inhibited production in only aged vessels. At high pressure, Apo decreased production significantly more in vessels of aged than that of young rats (Fig. 2). This is different from hypertension-induced activation of NADPH oxidase, where both protein expression and activity of the enzyme are increased (13). Acute high pressure-induced activation of NAD(P)H oxidase in aged vessels is more likely due to posttranslational modulation of the enzyme via altered mechanotransduction in the vascular wall. It is known that activation of NAD(P)H oxidase requires assembly of the enzyme subunits on the plasma membrane or intracellular vesicles (15). It has also been demonstrated that high pressure increases the phosphorylation of p47phox that facilitates association of the membrane-bound and cytoplasmic subunits of NAD(P)H oxidase and the activity of the enzyme (32). However, whether structural changes in the wall of aged arteries, such as arterial stiffness, will facilitate pressure-induced mechanotransduction, thereby enhancing the activation of NAD(P)H oxidase, needs to be further explored.
In addition to the activity of NAD(P)H oxidase, the increased in response to high pressure was significantly contributed to by xanthine oxidase and NOS in aged arteries, as evidenced by the inhibitory role of Allo and l-NAME (Fig. 2) and a more than twofold increase in xanthine oxidase expression (Fig. 3). Xanthine oxidase has been implicated to be a major source of oxidative stress in certain disease conditions, such as heart failure (20). In vascular aging, the role of xanthine oxidase-dependent oxidative stress is unclear. A recent human study reported that impaired flow-induced dilation in brachial arteries was not restored by administration of Allo, suggesting that endothelial dysfunction in older individuals is not xanthine oxidase related (7). In the aorta and kidney of aged rats, however, xanthine oxidase activity and expression were increased (4, 24). In line with these findings, our data indicate that vascular oxidative stress in aging, at least in rats, is dependent, at least in part, on xanthine oxidase. In our previous study, we found that in mesenteric arteries of aged compared with young rats eNOS activity was reduced, whereas eNOS expression was not (29). Previous studies have documented the existence of iNOS mRNA and protein expression in aorta and coronary arteries of aged rats (3, 5). To clarify whether iNOS is involved in the inhibitory role of l-NAME in superoxide production, we assessed iNOS protein in the arteries. Concomitantly, we found iNOS was not detectable in mesenteric arteries of rats. Together, the data suggest that eNOS, the predominant NOS isoform in the vasculature, when uncoupled, is the most probable source of . This is further suggested by the fact that l-NAME reduced the pressure-induced production. Evidence for eNOS uncoupling has been obtained in cultured endothelial cells and different animal models (9). Landmesser et al. (19) reported that increased production in DOCA-salt hypertension was inhibitable by l-NAME and that the fundamental mechanism for eNOS uncoupling was the oxidation of tetrahydrobiopterin, a critical cofactor for NO synthesis. Therefore, NAD(P)H and xanthine oxidase-dependent oxidative stress in aged vessels may enhance oxidation of tetrahydrobiopterin, which may well lead to eNOS-dependent production in response to high pressure. Other studies have also demonstrated that oxidative stress increases the oxidation of eNOS and decreases the dimer-to-monomer ratio of the enzyme, resulting in eNOS uncoupling (35).
Using confocal microscopy and en face preparation of mesenteric arteries, we provided evidence (Fig. 5) indicating that both vascular endothelial and smooth muscle cells are sources of high pressure- and age-related release of . These studies also revealed that, at a given confocal scanning layer, the endothelium is more sensitive to high pressure-induced production, which was demonstrated by a greater percent increase in production in the endothelium than in smooth muscle. It is therefore reasonable to suggest that an excessive production, particularly in the endothelium, will cause a severely diminished NO bioavailability and impaired endothelium-dependent, NO-mediated vasodilator responses in high pressure-treated arterioles (16, 32). We also speculate that the increased susceptibility to high pressure of the endothelium of aged arteries, as evidenced by the greater production, may also occur in other pathological conditions, such as hypertension, resulting in endothelial dysfunction.
This study elucidates the specific interaction between a brief increase in intravascular pressure and age in the regulation of vascular production. The results demonstrate that aging and high pressure act synergistically to cause a greater increase in production, particularly in the endothelium. Thus, in response to high pressure and aging, excessive production in the endothelium could diminish NO bioavailability and cause endothelial dysfunction. Accordingly, the use of antioxidants to reduce oxidative stress in various pathological conditions and aging would be a reasonable therapeutic strategy. However, clinical trials of vitamin E supplementation have shown not to have significant effects on cardiovascular outcomes (34). A recent study further revealed that chronic antioxidant supplementation impairs endothelial function by increasing eNOS uncoupling (33). Apparently, antioxidant supplementation alone is not sufficient enough to decrease vascular oxidative stress. However, based on our results, multiple mechanisms, including NAD(P)H oxidase, xanthine oxidase and eNOS-dependent production, are involved in endothelial dysfunction in aged vessels. Will a therapeutic strategy that combines antioxidants with other agents that enhance eNOS function, such as supplementation of BH4 to prevent eNOS uncoupling, improve clinical outcomes? Based on our study, the increased susceptibility of aged vessels to high pressure, by producing more , suggests that the prevention of acute increases in blood pressure, which could be caused by a variety of factors, will reduce the possibility of additional damage to endothelial function in the elderly.
ACKNOWLEDGMENTS
The authors thank Dr. Alan D. Springer for suggestions in the analysis of DHE fluorescent images.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-43023, HL-68813, and HL-070653.
REFERENCES
- 1.Bauersachs J, Bouloumie A, Mulsch A, Wiemer G, Fleming I, Busse R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovasc Res. 1998;37:772–779. doi: 10.1016/s0008-6363(97)00250-2. [DOI] [PubMed] [Google Scholar]
- 2.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, Thomas F, Pannier B, Asmar R, Zureik M, Safar M, Guize L. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 3.Cernadas MR, de Miguel LS, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez-Farre A. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 4.Chung H, Song S, Kim H, Ikeno Y, Yu B. Modulation of renal xanthine oxidoreductase in aging: gene expression and reactive oxygen species generation. J Nutrition Health Aging. 1999;3:19–23. [PubMed] [Google Scholar]
- 5.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 6.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 7.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forstermann U. Endothelial NO synthase as a source of NO and superoxide. Eur J Clin Pharmacol. 2006;62(Suppl 13):5–12. [Google Scholar]
- 10.Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005;46:280–286. doi: 10.1161/01.HYP.0000173433.67426.9b. [DOI] [PubMed] [Google Scholar]
- 11.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 12.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 14.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 16.Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress- and agonist-induced dilations. Circ Res. 1998;83:960–965. doi: 10.1161/01.res.83.9.960. [DOI] [PubMed] [Google Scholar]
- 17.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koller A, Huang A. Impaired nitric oxide-mediated flow-induced dilation in arterioles of spontaneously hypertensive rats. Circ Res. 1994;74:416–421. doi: 10.1161/01.res.74.3.416. [DOI] [PubMed] [Google Scholar]
- 19.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, Harrison DG, Hornig B, Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 21.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 22.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 23.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 24.Newaz MA, Yousefipour Z, Oyekan A. Oxidative stress-associated vascular aging is xanthine oxidase-dependent but not NAD(P)H oxidase-dependent. J Cardiovasc Pharmacol. 2006;48:88–94. doi: 10.1097/01.fjc.0000245402.62864.0a. [DOI] [PubMed] [Google Scholar]
- 25.Nowicki PT, Flavahan S, Hassanain H, Mitra S, Holland S, Goldschmidt-Clermont PJ, Flavahan NA. Redox signaling of the arteriolar myogenic response. Circ Res. 2001;89:114–116. doi: 10.1161/hh1401.094367. [DOI] [PubMed] [Google Scholar]
- 26.Oeckler RA, Kaminski PM, Wolin MS. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res. 2003;92:23–31. doi: 10.1161/01.res.0000051860.84509.ce. [DOI] [PubMed] [Google Scholar]
- 27.Rook GA, Steele J, Umar S, Dockrell HM. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by gamma-interferon. J Immunol Methods. 1985;82:161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]
- 28.Sun D, Messina EJ, Kaley G, Koller A. Characteristics and origin of myogenic response in isolated mesenteric arterioles. Am J Physiol Heart Circ Physiol. 1992;263:H1486–H1491. doi: 10.1152/ajpheart.1992.263.5.H1486. [DOI] [PubMed] [Google Scholar]
- 29.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 31.Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- 32.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- 33.Versari D, Daghini E, Rodriguez-Porcel M, Sattler K, Galili O, Pilarczyk K, Napoli C, Lerman LO, Lerman A. Chronic antioxidant supplementation impairs coronary endothelial function and myocardial perfusion in normal pigs. Hypertension. 2006;47:475–481. doi: 10.1161/01.HYP.0000201445.77125.26. [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 35.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]