Abstract
Glucose is the primary energy source for eukaryotic cells and the predominant substrate for the brain. GLUT3 is essential for trans-placental glucose transport and highly expressed in the mammalian brain. To further elucidate the role of GLUT3 in embryonic development, we utilized the vertebrate whole animal model system of Danio rerio as a tractable system for defining the cellular and molecular mechanisms altered by impaired glucose transport and metabolism related to perturbed expression of GLUT3. The comparable orthologue of human GLUT3 was identified and the expression of this gene abrogated during early embryonic development. In a dose-dependent manner embryonic brain development was disrupted resulting in a phenotype of aberrant brain organogenesis, associated with embryonic growth restriction and increased cellular apoptosis. Rescue of the morphant phenotype was achieved by providing exogenous GLUT3 mRNA. We conclude that GLUT3 is critically important for brain organogenesis and embryonic growth. Disruption of GLUT3 is responsible for the phenotypic spectrum of embryonic growth restriction to demise and neural apoptosis with microcephaly.
Keywords: Embryonic growth restriction, microcephaly, apoptosis
Introduction
Facilitative glucose transporter isoform GLUT3 is primarily expressed in mammalian neurons and trophoblasts. In both locations, GLUT3 mediates glucose transport and availability for fueling oxidative metabolism (16). Previous investigations employing a mouse model reveal that homozygous loss of GLUT3 caused early embryonic demise related to the absence of GLUT3 in the trophectoderm (5). In contrast, mice heterozygous for the null mutation are viable, exhibiting phenotypic changes characterized by mild fetal growth retardation that resolves soon after birth (5). Additionally, aberrant neurobehavior and seizures are observed but only by electroencephalogram (26). Thus, heterozygous expression of GLUT3 (50% reduction from that of wild type) did not result in major phenotypic abnormalities related to structural development of either the feto-placental unit or the brain (5). Therefore, we hypothesized that the embryonic effects of impaired GLUT3 expression may occur independent of placental effects and may not be phenotypically evident until greater inhibition than heterozygosity is achieved. To test this hypothesis, we employed zebrafish (Danio rerio), a unique vertebrate animal model that lacks a placenta - which allowed us to selectively knock-down GLUT3 and explore the impact of a dose-dependent decrease in GLUT3 expression on embryonic development. These studies revealed that dose-dependent impaired expression of this glucose transporter resulted in a spectrum of phenotypes ranging from relatively mild embryonic growth restriction to complete embryonic demise. Additionally, structural changes in brain development were evident related to increased apoptosis and microcephaly. Interestingly, this morphant phenotype could be rescued by the introduction of either zebrafish or rat GLUT3 mRNA but not zebrafish GLUT1 mRNA, thus supporting and assigning specificity of GLUT3 expression in the causation of these observed phenotypic effects.
Experimental Procedures
Zebrafish maintenance and general procedures
Zebrafish were maintained and staged as described previously (10,22). Wild-type zebrafish stocks were used throughout this study. Fish were maintained in a photoperiod of 14L:10D in egg water at a constant temperature of 28.5°C and fed three times daily. For all experiments, embryos were obtained by in vitro fertilization according to standard procedures (22). Embryos were dechorionated with watchmaker forceps. To inhibit pigmentation, egg water was supplemented with 0.003% (wt/vol) 2-phenylthiourea (PTU). All chemicals were from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Microscopy and image analysis
Adult zebrafish were maintained under standard conditions (22), and embryos were staged by standard methods (10). To image zebrafish, embryos were positioned in a drop of 3% methylcellulose on microscope slides. Differential interference contrast images (DIC) were obtained using an Olympus IX71 microscope fitted with a Nomarski objective and images were acquired with a TH4-100 camera (Olympus,Melville, NY) and Olympus microsuite software. The high resolution images of in-situ hybridization studies (Figure 1) were taken under a stereo-microscope (Stemi 2000-C; Zeiss, Germany) using an AxioCam MRc5 CCD sensor and AxioVision AC software (Zeiss, Germany). Fluorescently labeled embryos were visualized utilizing a laser-scanning confocal microscope (BX61WI FV500; Olympus, Melville, NY) utilizing the 10× objective. Representative fish were imaged using identical confocal settings and serial Z stacks were acquired using a pinhole aperture of 150 µM. Images were collected with Fluoview software (Olympus, Melville, NY).
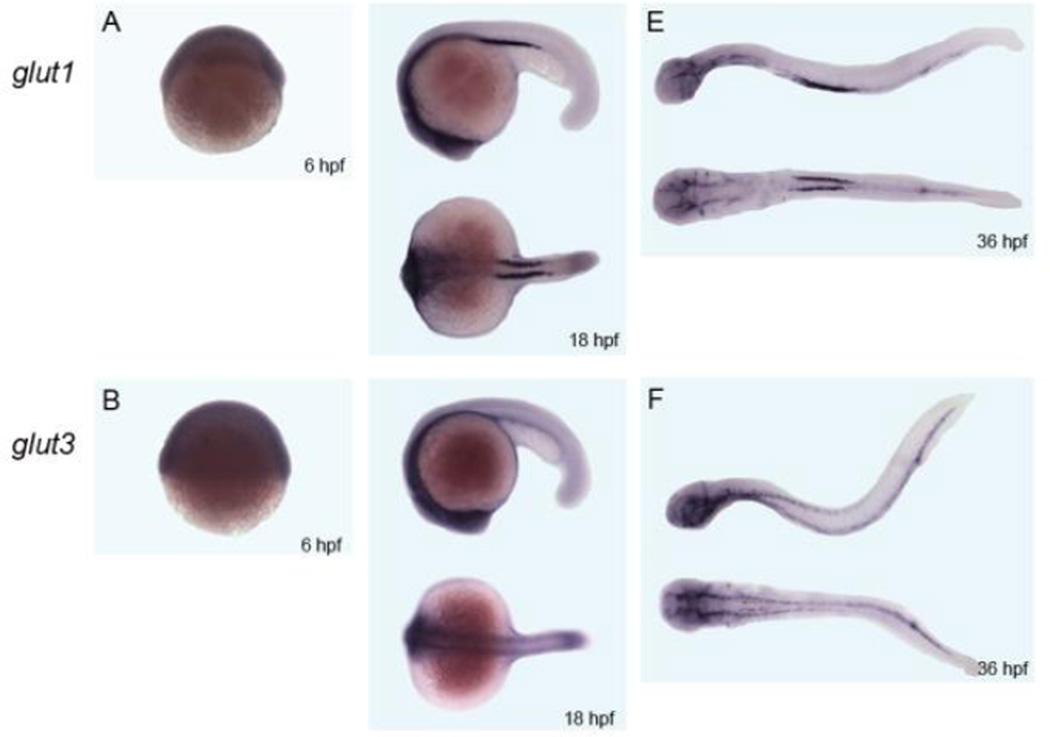
Figure 1. Whole mount in situ hybridization of glut1 and glut3 in zebrafish embryos.
RNA whole mount in situ hybridization (ISH) was carried out to determine glut1 and glut3 expression in zebrafish embryos at 6, 18 and 36 hours post fertilization (hpf). A and B, at 6 hpf, glut1 and glut3 are expressed ubiquitously with a similar general expression pattern. C and D, at 18 hpf, both glut1 and glut3 are expressed throughout the developing embryo, as previously reported. In addition, glut1 has restricted expression in part of pronephros whereas glut3 shows more expression in the brain region. E and F, at 36 hpf, glut1 continues to express strongly in pronephros and some parts of anterior gut, whereas glut3 has restricted expression in neurons as well as some weaker expression in spinal neurons and gut.
In Situ Hybridization and Immunohistochemistry
To generate in situ probes for glut1 and glut3, PCR products were amplified from zebrafish cDNA (Gene Bank #NM_001002643). cDNA was prepared from total RNA extracted from 24-h post-fertilization (hpf) embryos and reverse-transcribed to single-stranded cDNA using Superscript III (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. D. rerio glut1 and glut3 specific primers are listed in Table 1. PCR products from corresponding primers were used as templates to prepare riboprobes using the DIG-labeling kit (Roche Applied Science, Indianapolis, IN). Various stage embryos were collected and fixed in fresh 4% paraformaldehyde overnight at 4°C. Fixed embryos were dechorionated, dehydrated with an ascending methanol series (30, 50 and 100%), and stored in methanol at −20°C overnight. In situ hybridization was performed as previously described (13, 17) with minor modifications (25).
Table 1.
Danio Rerio sequences of primers used in creating ISH probes and rescue mRNAs.
| Primer name | Gene | Species | Primer sequence |
|---|---|---|---|
| G1ProbeF | glut1 | D.Rerio | GGCATCCTCATGGCACAG |
| G1ProbeR | glut1 | D.Rerio | CTGTGATCTCTTCAAACGACCG |
| G3ProbeF | glut3 | D.Rerio | GGGAGAAGAAACAAGTAACATG |
| G3ProbeR | glut3 | D.Rerio | GGAGCGGAAGAGTTCTGGG |
| G1mRNAF | glut1 | D.Rerio | CACCATGGAGTCTAATAAAAAGGAGGTGACT |
| G1mRNAR | glut1 | D.Rerio | TCAGAGTTGTGAATCTGCCC |
| G3mRNA(GA)F | glut3 | D.Rerio | ATGGAaGGGGGAGAAGAAACA* |
| G3mRNA(GA)R | glut3 | D.Rerio | TTACATGGTGGAATTAGATTTCTCC |
A site-directed mutation of “G” to “A” was introduced in the rescue mRNA of Danio Rerio glut3.
F=forward, R=reverse.
Morpholino design and knockdown
To inhibit the expression of glut3, an anti-sense morpholino (MO) targeting the translational start site (5’-CCTCCATCGTGCCGGAGTGGAAAC-3’) of glut3 was synthesized (Gene Tools LLC, Philomath, Oregon). As an injection control, we designed a 5 base pair mismatch (5’-CgTCCATCcTGCCGcAGTGcAAAg-3’) MO. This MO was identical to the glut3 translational start site MO except for a 5-base pair mismatch–indicated by lowercase letters. Additionally, a MO targeting exon 4 and 5 splice sites within the GLUT3 transcript (5’–TTGCGTCTGTGGAAAGAAAAACAGA-3’) was generated. The inhibition of glut1 and glut3 was accomplished using MOs and conditions previously described (9). For knockdown experiments each MO was microinjected into embryos at the 1–2 cell stage in the amounts indicated using pulled glass micro-capillary pipettes attached to a micromanipulator, Model P-97 (Sutter Instrument Co., Novato, CA). Injection was driven by compressed N2 gas using the picopump PLI-100 (Harvard Apparatus, Holliston, MA).
RNA rescue experiments of glut3 morphants
mRNA was generated from constructs containing rat and D. rerio glut3 sequences, and D. rerio glut1 sequences by in vitro transcription using mMESSAGE mMACHINE (Ambion, Inc., Austin, TX) employing the vector’s intrinsic T7 promoter. Primers used to generate the constructs are listed in Table1. Site-directed mutation of “G” to “A” (indicated by lowercase “a” in the primer sequence) was introduced into the glut3 rescue mRNA at the MO targeting site to protect the injected mRNA from being targeted by the injected MO. Resulting mRNA was purified using the RNeasy Mini-kit (Qiagen,Valencia, CA), analyzed by gel electrophoresis and quantified to produce a working concentration of 200 ng/µl. In mRNA rescue experiments, 1 ng/embryo of control, glut1 or glut3 mRNA was co-injected with 7.5 ng/embryo of the glut3 morpholino.
Criteria for morphological assessment of embryos
MO treated embryos were assessed microscopically at the times indicated. Analysis was blinded and performed by two independent evaluators. glut3 morphant embryos are characterized by increased tissue opacity in the head, resulting in the loss of key morphological markers in the CNS. The objective criteria used in scoring embryos was the presence or absence of the midbrain/hindbrain boundary (mhb) and the degree of tissue opacity in the head.
Detection of apoptotic cell death
Apoptosis was detected with the vital dye acridine orange (acridinium chloride hemi-zinc chloride, Sigma, St. Louis, MO). MO treated embryos were dechorionated and incubated with 5 µg/µl acridine orange for 30 minutes at room temperature in the dark. The embryos were then washed three times for 5 minutes with egg water. Embryos were immediately visualized using confocal microscopy.
Statistical analysis
Differences between control values and experimental values were compared by Student's t-test. All data are expressed as means ± SEM. All experiments were performed at least three times. For mRNA rescue experiments, % with normal phenotype was assessed in 25 embryos/group/experiment. Significance was defined as P < 0.005.
Results
Characterization of Zebrafish glut3 expression
The zebrafish glut3 orthologue was recently identified and published (19). This sequence information was utilized to generate glut3 specific probes for in situ hybridization. As illustrated in figure 1, GLUT3 transcripts are expressed ubiquitously at 6 hours post fertilization (hpf) within the developing blastoderm. A similar pattern of expression is seen for GLUT1. By 18 hpf, GLUT3 shows continued expression throughout the developing embryo. Again, GLUT1 has a similar pattern of expression with additional pronounced expression in the developing kidney (pronephric ducts). By 36 hpf, GLUT3 and GLUT1 show expression that is restricted to the brain, with GLUT1 being more caudal and ventral, restricted to endothelial cells as shown previously (27), and GLUT3 being more rostral and dorsal restricted to the neuronal compartment, with GLUT1 continuing to have robust expression in the developing kidney, and GLUT3 expression observed in the dorsal spinal neurons and gastro-intestinal tract.
Knockdown of glut3 results in impaired CNS development, growth restriction and embyronic demise
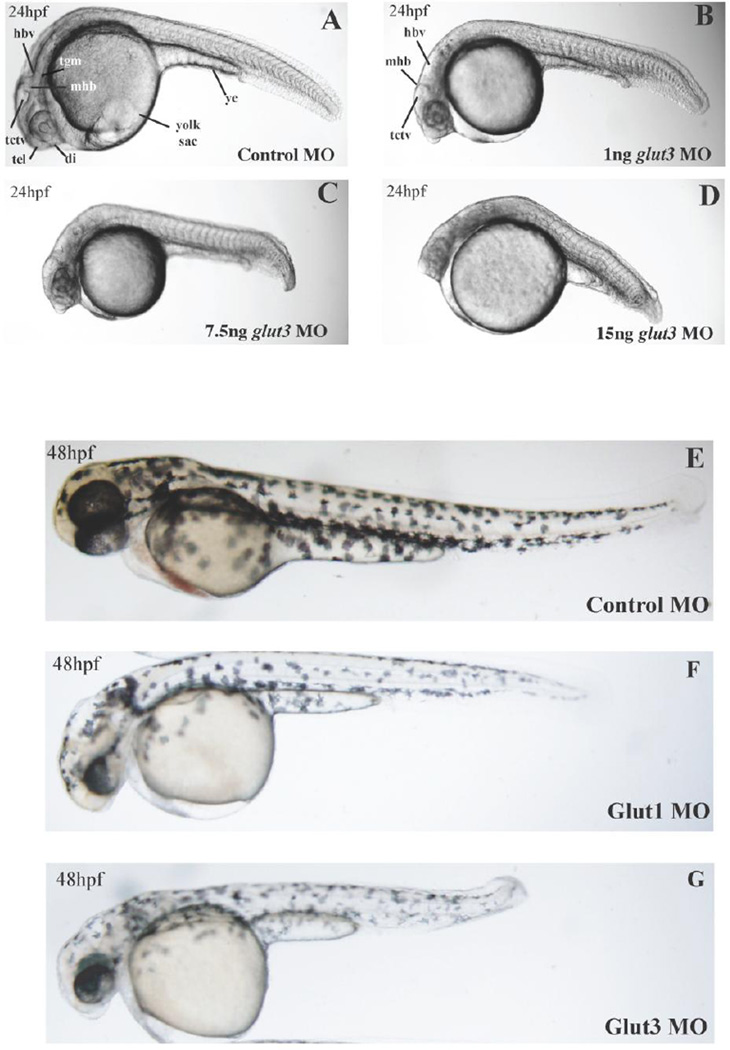
Abrogation of glut3 expression was achieved utilizing MO targeting the start methionine resulting in inhibition of translation of mature glut3 transcripts. As illustrated in Figure 2, inhibiting the expression of glut3 resulted in a severe, dose-dependent phenotype. Embryos were given increasing doses of MO and their phenotype characterized at 24 hpf. At the lowest dose (1 ng) an increase in tissue opacity in the head associated with a lack of distinction of key neuronal markers including the midbrain hindbrain boundary (MHB) was notable. This phenotype was exaggerated at a dose of 7.5 ng and was also associated with a significant decrease in embryo size. Finally, at a dose of 15 ng, all neuronal hallmarks are lost and the embryos are of significantly decreased size and do not survive beyond 48 hpf. Embryos receiving the 7.5 ng dose were also observed at 48 hpf. As illustrated in Figures 2E–G, the glut3 morphant embryo is significantly smaller than both the control and glut1 morphant embryos. The glut3 morphant has apparent microcephaly and is much smaller (growth restricted) overall. Additionally experiments were performed with a MO directed to the splice site between exons 4 and 5 in the glut3 transcript. Injections with this MO resulted in embryonic lethality at all concentrations tested, suggesting that the function of this splice site in the glut3 gene is critical for embryonic survival.
Figure 2. Abrogation of glut3 causes severe morphant phenotype.
A–D, phenotype of embryos injected with a MO targeting the glut3 translational start site (glut3 MO) compared with embryos injected with a control MO (Con MO) illustrates a severe dose dependent phenotype. At 24 hpf (A, C, and E), the following brain structures are distinct in the control embryos and become increasingly difficult to identify with increasing doses of the glut3 MO: diencephalon (di), telencephalon (tel), tectal ventricle (tctv), midbrain/hindbrain boundary (mhb), tegmentum (tgm), and hindbrain ventricle (hbv). Also illustrated is the yolk sac extension (ye). As the dose of glut3 MO increases the neuronal compartment becomes increasingly dense and opaque. E–G, by 48 hpf, the glut3 morphant embryo is significantly smaller than both the control and glut1 morphant embryos. The glut3 morphant has apparent microcephaly and is much smaller overall.
Expression of Glut3, but not Glut1, rescues glut3 morphant phenotype
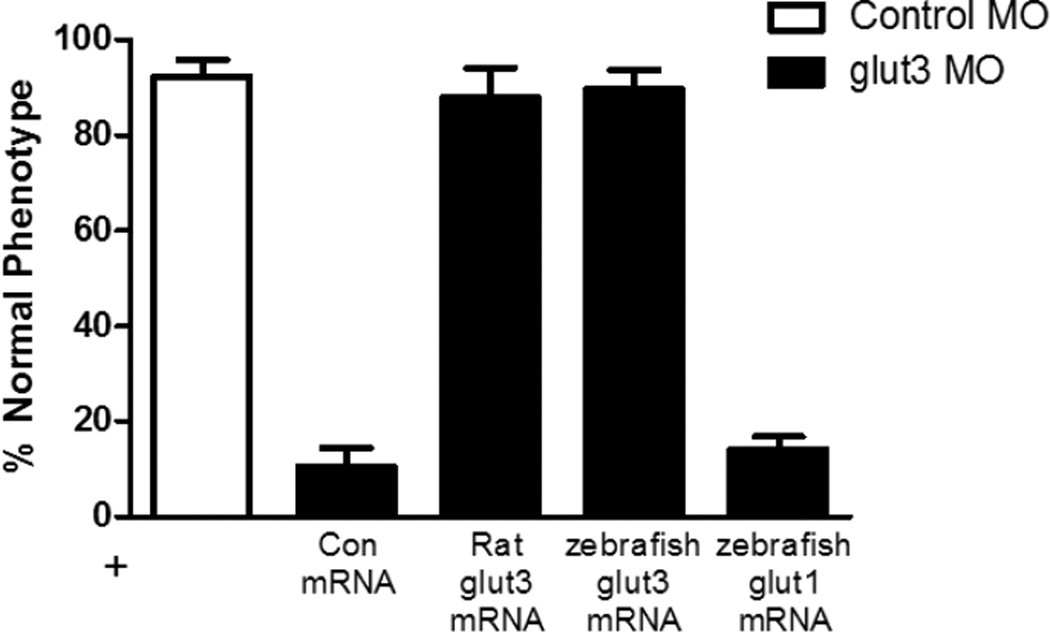
To demonstrate the specificity of the phenotype observed in the glut3 morphants, we performed rescue experiments in which zebrafish glut3 mRNA, rat Glut3 mRNA, zebrafish glut1 mRNA or control mRNA was co-injected into embryos with zebrafish-specific glut3 or control MO. This analysis revealed that both zebrafish glut3 mRNA and rat Glut3 mRNA were able to completely rescue the morphant phenotype, as graphically depicted in Figure 3. The overall normal phenotype in embryos co-injected with control MO and control mRNA was 92.5% ± 6.0% compared to embryos injected with the glut3 MO and control mRNA where only 10.6% ± 6.8% had a normal phenotype. In contrast, embryos injected with the glut3 MO and zebrafish glut3 mRNA expressed a normal phenotype in 80.7% ± 7.1% of embryos. A similar level of rescue was achieved by co-injection of rat glut3 mRNA where 80.7% ± 7.1% of the embryos had a normal phenotype. In contrast, expression of zebrafish glut1 mRNA failed to rescue the glut3 morphant phenotype.
Figure 3. Rescue of glut3 morphant phenotype with GLUT3 mRNA but not GLUT1 mRNA.
The glut3 morphant phenotype is rescued by overexpression of either rat or D. rerio GLUT3 mRNA, but not GLUT1 mRNA. The overall normal phenotype in embryos injected with control MO and control mRNA was 92.5% ± 6.0% compared to embryos co-injected with the glut3 MO + control mRNA where only 10.6% ± 6.8% have a normal phenotype. In embryos injected with the glut3 MO and zebrafish glut3 mRNA, 80.7% ± 7.1% of embryos expressed a normal phenotype. A similar level of rescue was achieved with co-injection of rat Glut3 mRNA where 87.9% ± 10.8% of the embryos had a normal phenotype. In embryos injected with glut3 MO and Glut1 mRNA, only 14.1% ± 4.7% have a normal phenotype.
Abrogation of glut3 results in enhanced apoptotic cell death
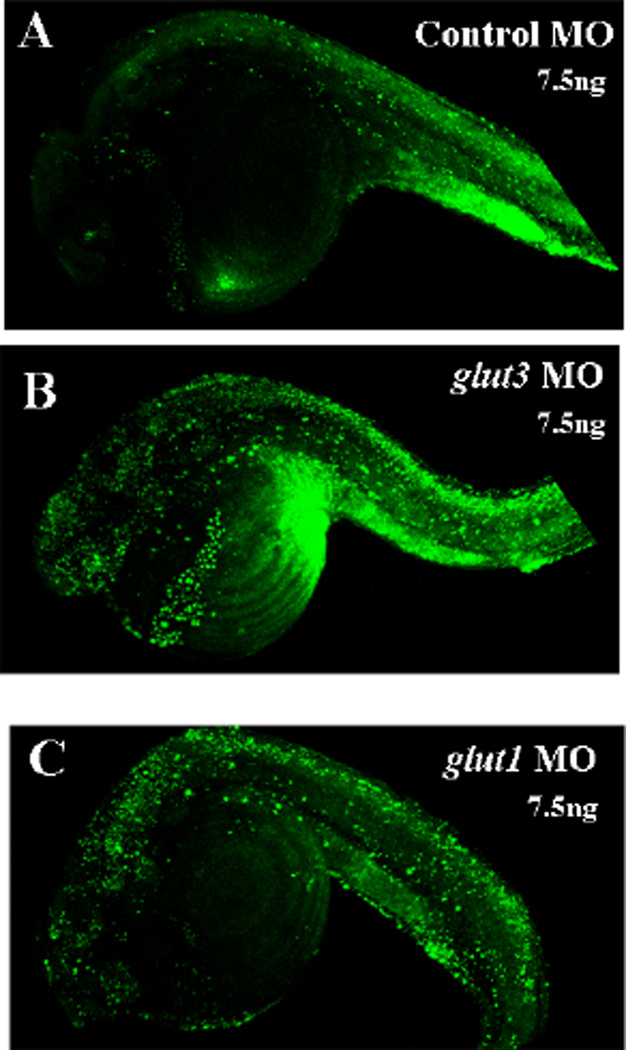
With increased tissue opacity in the head, we hypothesized that inhibiting GLUT3 expression may be affecting cell survival. Therefore, to assess the presence of increased programmed cell death in glut3 morphants, apoptosis was assayed by staining embryos with the vital dye acridine orange. At 24 hpf, an overall increase in apoptosis was observed in morphant (7.5ng dose of MO) embryos compared with controls (Figure 4). glut3 and glut1 morphant embryos display more apoptotic cells than control. Apoptosis appears to be localized primarily in the CNS, with concentrated areas observed in the head and tail regions.
Figure 4. Abrogation of glut3 results in enhanced apoptotic cell death.
To assay for apoptosis, embryos injected with a 7.5ng dose of either control, glut1 or glut3 MO were stained with the vital dye acridine orange, which preferentially stains apoptotic cells. At 24 hpf glut3 and glut1 morphant embryos display more apoptotic cells than control. Apoptosis appears to be localized primarily in the CNS, with concentrated areas observed in the head and tail regions.
Discussion
Facilitative glucose transporters are a family of structurally related membrane-spanning glycoproteins that mediate transport of glucose across lipid bilayers (18). Of the 14 isoforms, GLUT1 and GLUT3 play a significant role in trans-placental glucose transport and embryonic development. Both are expressed in the mammalian trophectoderm and brain with GLUT1 being expressed in the blood brain barrier and GLUT3 in neurons (2,16). GLUT1 and GLUT3 null mice are embryonic lethal due to lack of GLUT3 in the trophectoderm and developing ectoplacental cone, making conclusions about the specific role of these transporters in embryonic development challenging at best (5, 21). Interestingly, assessment of GLUT1 and GLUT3 heterozygotes reveal only mild growth restriction in the glut3(+/−) with no notable phenotype in the GLUT1 heterozygote (5,21). However, the mild fetal growth restriction encountered in the glut3(+/−) mice has been attributed to reduced placental GLUT3 mediated materno-fetal glucose transport (6). To further investigate the role of GLUT3 expression specifically in embryonic development, we have utilized the vertebrate model that lacks a placenta, Danio rerio, and employed morpholino knockdown methods to perturb GLUT3 expression in a dose dependent manner. Previously, similar investigations were performed with GLUT1 and significant changes observed (8). Hence in this study, we used the GLUT1 knockdown for comparison to GLUT3. In the studies presented, impaired embryonic GLUT3 expression is associated with increased apoptosis and an associated decline in growth potential, ultimately leading to embryo demise. In addition, brain development is deranged suggesting that the presence of normal concentrations of GLUT1 expression is not sufficient to save the GLUT3 morphants from developing such phenotypic changes.
Recently, zebrafish have been used to investigate the embryonic contribution of several members of the GLUT family of transporters, specifically, GLUTs 1, 2, and 10. These studies reveal that GLUT10 is localized to the cardiovascular system and the notochord (23) and GLUT2 to the embryonic liver and intestinal bulb. Additionally, GLUT2 is noted to be highly expressed in the brain of adult zebrafish (3). Abrogation of GLUT1 expression results in impaired glucose uptake, increased neural cell apoptosis associated with subsequent ventricular enlargement, trigeminal ganglion cell loss and abnormal hindbrain architecture. Interestingly, this phenotype can be rescued by inhibiting expression of the pro-apoptotic protein Bad independent of glucose uptake (8). Additional studies reveal that impaired GLUT1 expression resulted in a loss of cerebral endothelial cells with concomitant down-regulation of junctional proteins important for intact adherens and tight junctions that impaired cerebral circulation. This leaky blood-brain barrier caused vasogenic brain edema (27). In addition, abrogation of the anti-apoptotic kinase, Akt2, results in a phenotype strikingly similar to the glut1 morphant. Interestingly, overexpression of GLUT1 is able to rescue these morphants, suggesting a role for Akt2 in an integrative pathway directly linking glucose, GLUT1 expression, and activation of apoptosis particularly in the central nervous system (8). Studies have also demonstrated a role for TRPM7, a cation channel, in modulating the cellular response to oxygen-glucose deprivation during ischemia (15). Studies in haplo-insufficient mice when exposed to caloric restriction reveal enhanced placental Glut3 expression aimed at protecting the developing fetus (6). Similarly, in an adult zebrafish model assessing the stress response to decreased temperature, it was discovered that, GLUT1, GLUT3 and HIF-1, protein expression are upregulated in the brain (19), suggesting a shift in metabolism. To our knowledge, the results presented here are the first demonstrating expression of GLUT3 in adult zebrafish brains.
Our present investigation reveals the unique biological importance of GLUT3 expression during embryogenesis and its impact on embryonic growth and structural brain development. While a GLUT3 null mouse exists, embryonic demise observed at e6.5 to e7.5 precludes extensive evaluation during the embryonic period (5). In stark contrast, mice haploinsufficient for GLUT3, develop into structurally normal adults that reproduce effectively (5). While certain aberrations in neurological function were detected in these mice (4,26), there was no observed impact in either fetal or brain structural development (5,26). These mouse investigations prompted our present studies utilizing zebrafish, a well-defined, tractable system for elucidating the cellular and molecular mechanisms perturbed by impaired glucose transport and metabolism. These studies reveal that a dose-dependent decrease in GLUT3 expressed extensively in the developing blastoderm, results subsequently in a visible reduction in embryonic growth and ultimately in embryo demise.
We have previously established zebrafish as a tractable system for defining the cellular and molecular mechanisms affected by perturbed glucose metabolism. Initial studies revealed that interference with GLUT1 expression induced a striking neurodevelopmental syndrome reminiscent of malformations observed with human neural tube defects (8). These results were consistent with observations made in glut1 antisense mice where congenital abnormalities related to aberrant neural tube formation were encountered (7). Additionally, in diabetic mouse models, embryonic development under conditions of maternal diabetes resulted in increased rates of congenital malformation associated with decreased GLUT1 expression (7, 12, 24). Taken together, these data suggest impaired glucose metabolism is associated with developmental abnormalities and the severity of malformations is directly related to the level of glucose transporter expression.
Unlike GLUT1, which is expressed widely in the embryo including the blood-brain barrier and astrocytic cells (2), GLUT3 is limited in its embryonic expression to neuronal progenitor cells and mature neurons (16). Theoretically, neuronal specific knockout of GLUT3 could result in compensatory up-regulation of related GLUT isoforms and subsequent rescue of cells within the neuronal compartment (26). Unlike mouse models utilizing mice haploinsufficient for GLUT3, the current studies describe how a dose-dependent decrease in GLUT3 expression results in impaired embryonic growth and deranged structural brain development. Similar to pre-implantation mouse embryos (5), we also observed increased cellular apoptosis primarily in the cephalad and caudal regions of the developing zebrafish embryos. This process of increased apoptosis was associated with loss of midbrain/hindbrain demarcation and microcephaly in zebrafish. The specificity of the morphant phenotype was confirmed when successful rescue was accomplished by introduction of exogenous rat or zebrafish GLUT3 mRNAs, but not by zebrafish GLUT1. Thus it appears that reduced GLUT3 expression is detrimental to embryonic growth and survival related to its key expression in the blastoderm as early as 6 hpf. In addition, prominent expression of GLUT3 subsequently in the neuronal compartment as illustrated by in situ hybridization (Figure 1) likely led to brain developmental abnormalities apparent in the glut3 morphants and supports the need for further characterization, perhaps by the creation of a conditional neuron-specific GLUT3 knock-out mouse or zebrafish models
The clinical significance of reduced GLUT3 expression is not defined. However, hemizygous expression of GLUT3 in mouse models has been associated with features consistent with the autism spectrum disorders (26). Additionally, GLUT3 expression is increased in glioblastomas and other brain cancers (1) and reduced in Alzheimer’s disease (11). More recently, trans-chromosomal regulation resulting in reduction of GLUT3 expression was associated with dyslexia in human genome-wide single nucleotide polymorphism studies (14). Taken together, these data suggest that GLUT3 is likely critical for normal neurodevelopment. Whether mutations in GLUT3 may contribute to congenital microcephalic syndromes with unknown etiology is intriguing and warrants further investigation.
In summary, our current investigations utilizing a zebrafish model support a role for GLUT3 in fetal growth and survival as well as normal structural brain development. Based on these investigations a stage is set for generating neuron-specific mutations of the GLUT3 gene and examining the impact on brain organogenesis.
Highlights.
-
-
Glucose Transporter Isoform 3 (GLUT3) is critically important for brain organogenesis and embryonic growth.
-
-
Dose-dependent disruption of GLUT3 in zebrafish is responsible for the phenotypic spectrum of embryonic growth restriction to demise and neural apoptosis and microcephaly
Acknowledgments
The authors wish to acknowledge Amit Ganguly, Ph.D. for his assistance. Some of this work was supported by grants from the NIH (to SUD) HD33997 and HD 46979 and (to MOC) HD01459.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boado RJ, Black KL, Pardridge WM. Gene expression of Glut3 and Glut1 glucose transporters in human brain tumors. Brain Res Mol Brain Res. 1994;27(1):51–57. doi: 10.1016/0169-328x(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 2.Carruthers A, DeZutter J, Ganguly A, Devaskar SU. Will the original glucose transporter isoform please stand up! Am J Physiol Endocrinol Metab. 2009;297(4):E836–E848. doi: 10.1152/ajpendo.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castillo J, Crespo D, Capilla E, Diaz M, Chauvigne F, Cerda J, Planas JV. Evolutionary structural and functional conservation of an ortholog of the GLUT2 glucose transporter gene (SLC2A2) in zebrafish. Am J. Physiol. Regul. Integr. Comp. Physiol. 2009;297(5):R1570–R1581. doi: 10.1152/ajpregu.00430.2009. [DOI] [PubMed] [Google Scholar]
- 4.Fung C, Evans E, Shin D, Shin BC, Zhao Y, Sankar R, Chaudhuri G, Devaskar SU. Hypoxic-ischemic brain injury exacerbates neuronal apoptosis and precipitates spontaneous seizures in glucose transporter isoform 3 heterozygous null mice. J Neurosci Res. 2010;88(15):3386–3398. doi: 10.1002/jnr.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganguly A, McKnight RA, Raychaudhuri S, Shin BC, Ma Z, Moley K, Devaskar SU. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1241–E1255. doi: 10.1152/ajpendo.00344.2006. [DOI] [PubMed] [Google Scholar]
- 6.Ganguly A, Collis L, Devaskar SU. Placental glucose and amino acid transport in calorie-restricted wild type and glut3 null heterozygous mice. Endocrinology. 2012;153(8):3995–4007. doi: 10.1210/en.2011-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilig CW, Saunders T, Brosius FC, III, Moley K, Heilig K, Baggs R, Guo L, Conner D. Glucose transporter-1 deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc Natl Acad Sci USA. 2003;100:15613–15618. doi: 10.1073/pnas.2536196100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen PJ, Gitlin JC, Carayannopoulos MO. GLUT1 deficiency links nutrient availability and apoptosis during embryonic development. J. Biol. Chem. 2006;281(19):13382–13387. doi: 10.1074/jbc.M601881200. [DOI] [PubMed] [Google Scholar]
- 9.Jensen PJ, Gunter LB, Carayannopoulos MO. Akt2 modulates glucose availability and downstream apoptotic pathways during development. J. Biol. Chem. 2010;285(23):17673–17680. doi: 10.1074/jbc.M109.079343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582(2):359–364. doi: 10.1016/j.febslet.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moley KH, Chi MM, Knudson CM, Korsmeyer SJ, Mueckler MM. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat Med. 1998;4(12):1421–1424. doi: 10.1038/4013. [DOI] [PubMed] [Google Scholar]
- 13.Nusslein-Volhard CD, Ralf . Zebrafish. New York: Oxford University Press Inc.; 2002. [Google Scholar]
- 14.Roeske D, Ludwig KU, Neuhoff N, Becker J, Bartling J, Bruder J, Brockschmidt FF, Warnke A, Remschmidt H, Hoffman P, Muller-Myhsok B, Nothen MM, Schulte-Korne G. First genome-wide association scan on neurophysiological endophenotypes points to trans-regulation effects on SLC2A3 in dyslexic children. Molecular Psychiatry. 2011;16:97–107. doi: 10.1038/mp.2009.102. [DOI] [PubMed] [Google Scholar]
- 15.Runnels LW. TRPM6 and TRPM7: a Mul-TRP-PLIK-cation of channel functions. Curr. Pharm. Biotechnol. 2011;12(1):42–53. doi: 10.2174/138920111793937880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3:20 years of distinction. Am. J. Physiol. Endocrinol Metab. 2008;295(2):E242–E253. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nature Protocols. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 18.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol. Metab. 2010;298(2):E141–E145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng YC, Chen RD, Lee JR, Liu ST, Lee SJ, Hwang PP. Specific expression and regulation of glucose transporters in zebrafish ionocytes. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R275–R290. doi: 10.1152/ajpregu.00180.2009. [DOI] [PubMed] [Google Scholar]
- 20.Tseng Y-C, Chen R-D, Lucassen M, Schmidt MM, Dringen R, Alele D, Hwang P-P. Exploring uncoupling proteins and antioxidant mechanisms under acute cold exposure in brains of fish. PLoS One. 2011;6(3):e18180. doi: 10.1371/journal.pone.0018180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- 22.Westerfield M. The Zebrafish Book. Eugene, Oregon: University of Oregon Press; 2000. [Google Scholar]
- 23.Willaert A, Khatri S, Callewaert BL, Coucke PJ, Crosby SD, Lee JG, Davis EC, Shiva S, Tsang M, De Paepe A, Urban Z. Glut10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGF-beta signaling. Hum Mol Genet. 2012;21(6):1248–1259. doi: 10.1093/hmg/ddr555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyman A, Pinto AB, Sheridan R, Moley KH. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149(2):466–469. doi: 10.1210/en.2007-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue Y, Kuok C, Xiao A, Zhu Z, Lin S, Zhang B. Identification and expression analysis of mical family genes in zebrafish. J Genet Genomics. 2010;37(10):685–693. doi: 10.1016/S1673-8527(09)60086-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Fung C, Shin D, Shin BC, Thamotharan S, Sankar R, Ehninger D, Silva A, Devaskar SU. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry. 2010;15(3):286–299. doi: 10.1038/mp.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng PP, Romme E, van der Spek PJ, Dirven CM, Willemsen R, Kros JM. Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Ann Neurol. 2010;68(6):835–844. doi: 10.1002/ana.22318. [DOI] [PubMed] [Google Scholar]