Abstract
A single nucleotide polymorphism (SNP) in the human µ-opioid receptor gene (OPRM1 A118G) has been widely studied for its association in a variety of drug addiction and pain sensitivity phenotypes; however, the extent of these adaptations and the mechanisms underlying these associations remain elusive. To clarify the functional mechanisms linking the OPRM1 A118G SNP to altered phenotypes, we used a mouse model possessing the equivalent nucleotide/amino acid substitution in the Oprm1 gene. In order to investigate the impact of this SNP on circuit function, we used voltage-sensitive dye imaging in hippocampal slices and in vivo electroencephalogram recordings of the hippocampus following MOPR activation. As the hippocampus contains excitatory pyramidal cells whose activity is highly regulated by a dense network of inhibitory neurons, it serves as an ideal structure to evaluate how putative receptor function abnormalities may influence circuit activity. We found that MOPR activation increased excitatory responses in wild-type animals, an effect that was significantly reduced in animals possessing the Oprm1 SNP. Furthermore, in order to assess the in vivo effects of this SNP during MOPR activation, EEG recordings of hippocampal activity following morphine administration corroborated a loss-of-function phenotype. In conclusion, as these mice have been shown to have similar MOPR expression in the hippocampus between genotypes, these data suggest that the MOPR A118G SNP results in a loss of receptor function.
Keywords: Mu-Opioid Receptor, Single Nucleotide Polymorphism, OPRM1, A112G, A118G, N40D, mice, sex, genotype, hippocampus
INTRODUCTION
Mu-opioid receptors (MOPR) modulate several pathways including pain and pleasure. The A118G single nucleotide polymorphism (SNP) in the µ-opioid receptor gene (OPRM1) has been associated with an altered vulnerability to opioid addiction (Drakenberg et al., 2006; Ray and Hutchison, 2004; van den Wildenberg et al., 2007), a decreased response to opioid-induced analgesia (Chou et al., 2006a; Sia et al., 2008), and an enhanced response to therapies for alcohol (Anton et al., 2008; Ray and Hutchison, 2007) and nicotine addiction (Lerman et al., 2004). Mice possessing the equivalent SNP (A112G) have decreased MOPR expression and morphine-evoked behaviors, altered GTPγS binding to the MOPR and downstream intracellular signaling cascades, and sex-specific deficits in morphine reward (Knapman et al., 2014; Mague et al., 2009; Wang et al., 2014; Wang et al., 2012). Additionally, recent studies in humans possessing the A118G SNP (Troisi et al., 2012; Way et al., 2010; Way et al., 2009) and in this mouse model of the A118G SNP (Briand et al., 2015) have shown in parallel that this SNP may have important ramifications for more complex behaviors, such as stress resiliency, which would also impact relapse behaviors in addiction. However, it has not been determined whether these effects result from decreased receptor availability or altered receptor function. For instance, male and female mice homozygous for the G112 allele (G/G) show equivalent MOPR expression decreases in reward-related brain regions; however, only the females show decreased morphine reward ((Mague et al., 2009; Wang et al., 2014; Wang et al., 2012).
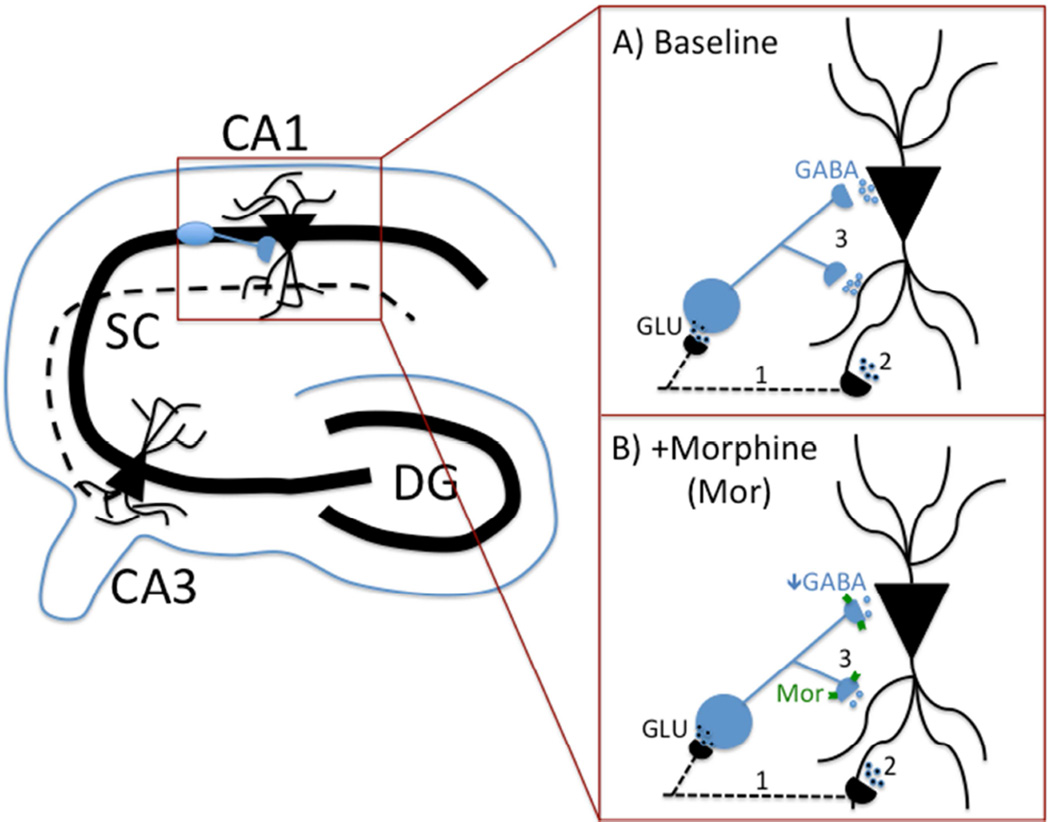
In order to address if alterations in receptor function were responsible for these changes, we evaluated circuit function in the hippocampus, a region displaying similar MOPR expression between genotypes and sexes (Mague et al., 2009; Wang et al., 2014; Wang et al., 2012). In CA1, MOPRs are predominantly found on somatodendritic and axonal aspects of fast-spiking, parvalbumin (PV)-containing GABAergic basket cells (Drake and Milner, 1999; Drake and Milner, 2002). Activation of MOPRs hyperpolarizes these cells and decreases GABAergic neurotransmission, thereby disinhibiting glutamatergic neurons and providing net excitatory activity (Glickfeld et al., 2008; Neumaier et al., 1988); for visual, see Figure 1. As perisomatic GABAergic inhibition can induce fast changes in neuronal polarity and gate cell firing at high frequencies (Csicsvari et al., 2003; Uhlhaas and Singer, 2010), regulation of excitatory output by PV neurons may underlie network synchrony and gamma-band oscillatory activity (Whittington and Traub, 2003) and influence memory storage/retrieval (Montgomery et al., 2009; Montgomery and Buzsaki, 2007). Indeed, loss of GABAergic modulation induced by MOPR activation has been shown to reduce high-frequency oscillations in the hippocampus (Whittington et al., 1998) and cortex (Sun et al., 2006; Zuo et al., 2007) and mediate conditioned effects of morphine (Rezayof et al., 2007).
Figure 1. Schematic highlighting MOPR role in hippocampal circuit.
(A, B) Shaffer Collateral projections (represented by dotted line) from pyramidal cells in CA3 synapse with dendrites of CA1 pyramidal cells as well as local inhibitory interneurons (shown in blue). A) Thus, stimulating CA3 axons (1) will produce direct activation of CA1 dendrites (2) as well as indirect (i.e., feed-forward) inhibition of these dendrites/cell b odies through GABAergic interneuron activation (3). B) MOPR activation (e.g., with DAMGO or morphine) during CA3 axonal firing (1) will decrease GABA release from the interneuron (3), resulting in a net increase in excitatory influences on CA1 pyramidal cells (2).
In order to better understand the synaptic and circuit-level alterations conferred by the A112G SNP, we employed voltage-sensitive dye imaging (VSDi) techniques in hippocampal slice preparations to evaluate opioid-stimulated responses. CA1 pyramidal cells supply a clear view of inhibition because they do not generate recurrent excitation; accordingly, excitatory postsynaptic potentials (EPSPs) induced by afferents are followed almost exclusively by locally-induced inhibitory postsynaptic potentials (IPSPs). As a result, CA1 IPSPs form a temporally distinct and measurable VSDi component (Ang et al., 2005; Carlson and Coulter, 2008). In these studies, we found that while baseline net circuit activity elicited by a single excitatory stimulus was similar between wild-type A/A and G/G mice, DAMGO-mediated increases in circuit activity were significantly attenuated in G/G mice, suggesting a loss-of-function of the MOPR. Furthermore, in vivo hippocampal EEG recordings showed a MOPR loss-of-function in G/G mice following a systemic injection of morphine. These data, which support clinical findings of decreased responses to opioidergic modulation, demonstrate both ex vivo and in vivo functional receptor deficits resulting from this SNP.
MATERIALS AND METHODS
Animals
All experiments utilized adult male and female mice (3–5 months of age; 20–35 g). Estrous cycle for female mice was not determined. Mice used in these experiments were either homozygous for the A112 (wild-type) or G112 (knock-in) allele [for detailed description of generation of Oprm1tm1Jabl mice, see (Mague et al., 2009)]. Briefly, an equivalent N-linked glycosylation site to the A118G SNP found in humans was eliminated using site-directed mutagenesis in a bacterial artificial chromosome containing the C57BL/6 mouse oprm1. This was accomplished by replacing the adenine at nucleotide position 112 with a guanine, resulting in an aspartic acid substitution of asparagine at amino acid position 38. These mice were maintained on a C57BL/6 background and were bred, group housed, and maintained on a 12 h light/dark cycle with food and water available ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee.
Voltage-sensitive dye imaging (VSDi)
VSDi experiments were performed according to previous studies [(Ang et al., 2005; Ang et al., 2006); for detailed methodology, see (Carlson and Coulter, 2008)]. Briefly, mice were decapitated following isoflurane anesthesia. The brain was removed and horizontal hippocampal slices (350 µm) were cut using an Integraslice 7550 PSDS vibrating microtome (Campden Instruments, Lafayette, IN) in ice-cold sucrose artificial cerebrospinal fluid (ACSF), in which NaCl was replaced with an equiosmolar concentration of sucrose. ACSF consisted of 130 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO3, 10 mM glucose, 1 mM MgCl2, 2 mM CaCl2 (pH 7.2–7.4 when saturated with 95% O2/5% CO2). Slices were then transferred to a static interface chamber (34°C) for 30 min and kept at 22–25°C thereafter. The osmolarity of all solutions was 305–315 mOsm.
Slices were stained for 20 min with 0.125 mg/ml (in ACSF) of the voltage-sensitive dye di-3-ANEPPDHQ (D36801, Invitogen), and imaged in an oxygenated interface chamber using an 80 × 80 CCD camera recording at a 1 kHz frame rate (NeuroCCD: RedShirtImaging, Decatur, GA). Epi-illumination was provided by a custom LED illuminator. Compared to the more commonly used photodiode array, the CCD chip well size (215,000 electrons) requires use of relatively low light-intensities, thereby minimizing photodynamic damage. The structures and regions of the slice were identified thusly: SO – stratum oriens, SR – stratum radiatum, SLM – stratum lacunosum moleculare, CTX – cortex, DG – dentate gyrus. Schaffer collateral stimulation using a single 20-mA, 200-ms pulse was administered with the electrode placed in SR near the CA3/CA1 border (Figure 2a,b). This stimulation protocol was utilized to highlight influences of PV interneurons, as these cells have been shown to respond with high reliability to initial, but not repeated, afferent input (Pouille and Scanziani, 2004; Spruston, 2008). A field-recording electrode was also placed in SR to monitor population responses following stimulation; these data, however, were not analyzed or included in this manuscript. After initial electrode-placement and evaluation of population responses, the slice was allowed to recover for at least 5 min prior to testing. Baseline responses elicited by 12 single-stimulus trials, each separated by 20s, were recorded during bath application of ACSF. Following these recordings, the control ACSF was replaced by ACSF containing the selective MOPR agonist [d-Ala(2),N-Me-Phe(4),Gly(5)-ol]-enkephalin [DAMGO; 1 mM (Sigma-Aldrich)], which bathed the slice for at least 10 min prior to the presentation of 12 single-stimulus trials of 20-mA, 200-ms pulses.
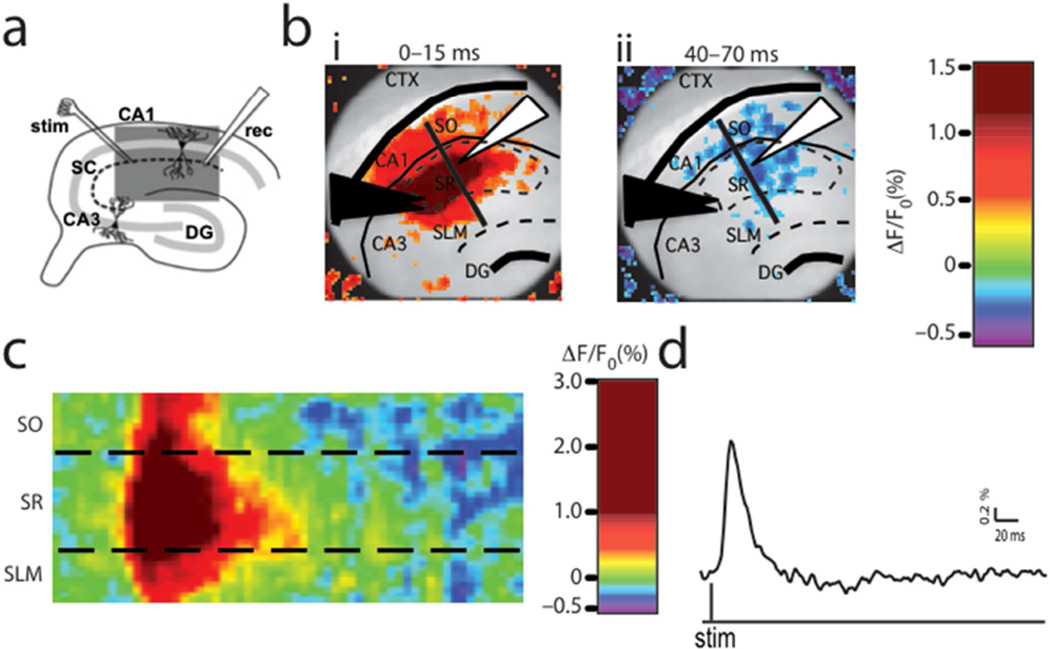
Figure 2. VSDi procedure, quantification, and analysis.
(a) A diagram of hippocampus circuitry illustrates the stimulus protocol utilized in this study. A stimulating electrode was placed in the Shaffer collateral axons from CA3 pyramidal cells and a recording electrode was placed in the distal end of SR in CA1. The light gray line represents the pyramidal cell layer and the dotted black line delineates the path of the Shaffer collateral axons. The dark gray box depicts the area visualized in b(b) Horizontal slices containing the hippocampus were visualized under a 10× lens. The black triangles show the stimulating electrode placement and the white triangle shows the placement of the recording electrode. The structures and regions are labeled thusly: SO – stratum oriens, SR – stratum radiatum, SLM – stratum lacunosum moleculare, CTX – cortex, DG – dentate gyrus. The average normalized pixel-changes for the duration indicated following stimulation demonstrates the peak excitatory (bi) and inhibitory (bii) responses for a representative wild-type animal. Changes in membrane voltage are illustrated in red (excitation) or blue (inhibition). The black line corresponds to the raster plot shown in c(c) Raster plots corresponding to the pixels along the black line drawn in figure 2b show the average pixel-changes over time for the SO, SR, and SLM during baseline. Changes in membrane voltage are illustrated in red (excitation) or blue (inhibition). (d) A 2D trace of the SR region from c.
VSDi data analyses
VSD data was analyzed in IGOR (Wavemetrics, Lake Oswego, OR) on 12-trial-averages as previously described (Ang et al., 2005; Ang et al., 2006). Briefly, fluorescence-changes were calculated as the percent change in fluorescence divided by the resting fluorescence (%DF/F0). Fitted double exponentials were subtracted from the normalized fluorescence to compensate for photobleaching. Raster Plot Quantification: Raster plots were generated by plotting the fluorescence signals across an imaginary line drawn through the peak of the response from SO to the SLM over the slice image and plotting the fluorescence signal from those pixels that fall under the line for all sampling points in time (Figure 2c). To determine net excitatory changes resulting from DAMGO administration, we employed a raster plot subtraction method that compared pixel-changes before and after DAMGO administration. Specifically, we subtracted the DAMGO raster plot (Figure 4aii) from the basal raster plot (Figure 4ai), resulting in a representation of the alteration in inhibitory regulation as a result of MOPR stimulation (Figure 4aiii). From 2D traces corresponding to average subtracted pixel-changes for SO, SR, and SLM, we determined 1) the peak amplitude of disinhibition, determined by the greatest change in fluorescence (%ΔF/F0), 2) the duration of disinhibition, measured as time (ms) that the loss of inhibition remained elevated, and 3) the area under the curve (AUC), which summed the subtracted changes in fluorescence for a 50-ms window following the stimulation (Figure 4biii). Statistical analyses were performed with GraphPad Prism 5.0 software package (GraphPad Software, San Diego, CA). Differences between groups (genotype and sex) wereassessed using two-way ANOVAs.
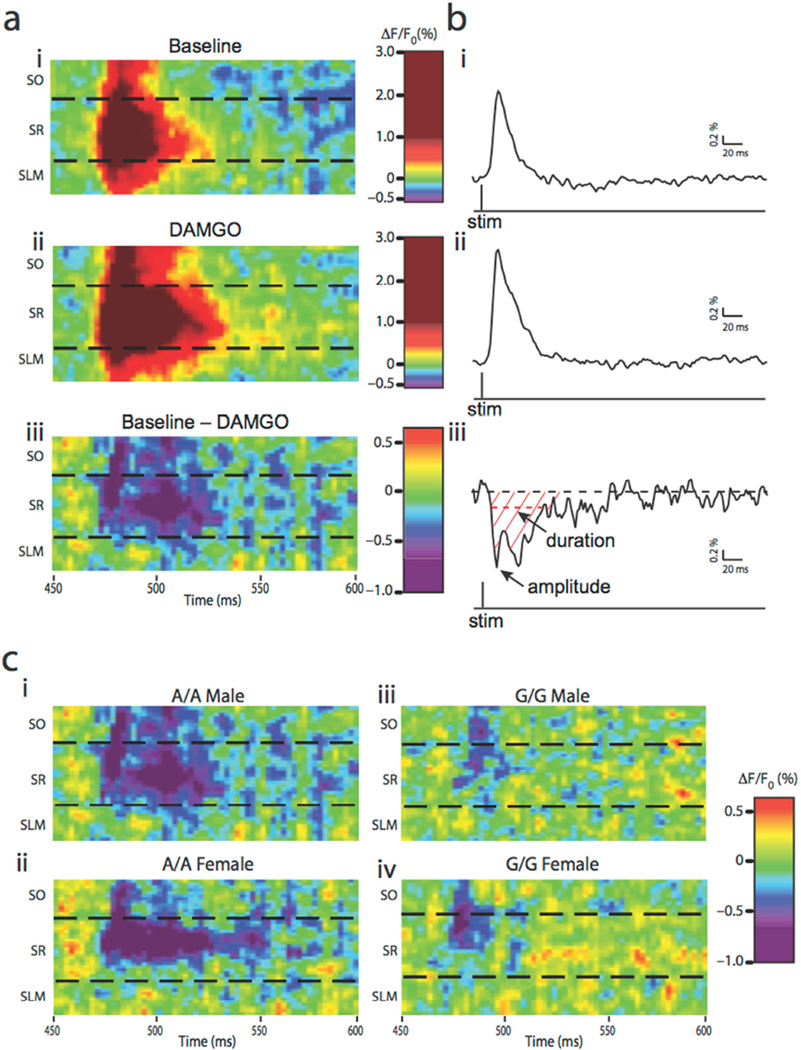
Figure 4. Raster plot subtraction analyses.
(a) Raster plots corresponding to the pixels along the black line drawn in figure 2b show the average pixel-changes over time for the SO, SR, and SLM during baseline (ai) or DAMGO application (aii). Subtraction of the baseline plots from the DAMGO plots shows the net disinhibition resulting from MOPR activation (ciii). Changes in membrane voltage are illustrated in red (excitation) or blue (inhibition). (b) A 2D trace of the SR region shows the quantification of subtracted raster plots. The amplitude was determined by the peak disinhibitory response. The duration, shown as the horizontal dashed red line, measured the time (ms) during which disinhibition was elevated above noise. The area under the curve (AUC; diagonal red lines) was calculated for a 50-ms window following stimulation. For all 2D plots, the scales of response amplitudes correspond to the numerical axis of the color scales drawn to the left of each trace. (c) Representative subtracted raster plots for A/A male (ci), A/A female (cii), G/G male (ciii), and G/G female (civ) show the loss of inhibition resulting from DAMGO administration.
Surgery for electroencephalogram (EEG) recordings
Male and female A112G mice underwent stereotaxic implantation of bipolar, twisted, stainless steel electrodes into region CA1 of the hippocampus (200 µm diameter, Plastics One, Roanoke VA). All surgeries used aseptic techniques and were performed with sterile gloves and full gown. The surgical field, table and stereotaxic frame were disinfected prior to surgery. Animals were anaesthetized with isoflurane and then placed in a stereotaxic frame for hippocampal depth electrode placement with continuous isoflurane inhalation by facemask. The scalp was cleaned with betadine and a midline incision was made, exposing the bregma and lambdoid sutures. Hippocampal depth electrodes (stainless steel) were placed using stereotaxic coordinates (AP −1.82 mm, ML 1.10 mm, DV −1.9 mm). The electrodes were cemented in place and the animals were kept under a warming light and carefully observed until fully awake and mobile.
Recording of EEG activity
Recording of EEG activity was performed between 10AM and 4PM, after a minimum of two weeks recovery from surgery. The mice were tested in their home cages, which were fitted with special tops to accommodate speakers and electrode cables, and placed inside a Faraday cage. The mice were acclimatized to the testing apparatus for 30 min before the first stimulus onset. Following a baseline trial, animals were injected with increasing doses (0, 1, 2, 7, and 20 mg/kg; i.p.) of morphine sulfate (NIDA) dissolved in saline. Subsequently, in order to demonstrate that the effects of morphine were mediated by MOPRs, naloxone (1 mg/kg; i.p.) was co-administered with a second 20 mg/kg injection of morphine. The head stage was connected to a three-channel electrode cable, which was connected to a high-impedance differential AC amplifier (A-M Systems, Carlsborg, Washington, USA). Auditory stimuli were generated by Micro1401 hardware and Spike 6 software (Cambridge Electronic Design) and delivered through speakers attached to the cage top. All experiments were done in the presence of background white noise of 55 dB. For gating experiments, 150 white-noise clicks pairs (S1, S2) were presented with a 500 msec inter-stimulus interval and a 9 second inter-trial interval. EEG signal was bandpass filtered online between 1 and 500 Hz, and grand average waveforms were created from −500 ms to 1000 ms relative to the auditory stimulus. To remove movement artifacts, trials containing activity over 2 SD of the mean were rejected. Initial peak analysis was performed in Microsoft Excel (Redmond, WA) or Igor (Wavemetrics, OR) on the remaining averaged trials. The baseline was corrected at stimulus onset of S1 and S2 independently.
EEG data analysis
Spectral decomposition of auditory-evoked response waveforms were performed using the EEGLab toolbox in Matlab (Delorme and Makeig, 2004), as published previously (Gandal et al., 2010). Single-trial epochs between −0.1 and 0.2 seconds relative to the first stimulus (S1) were extracted from the continuous EEG data sampled at 1667 Hz. For each epoch, total power (i.e., event-related spectral perturbation, ERSP) was calculated using Morlet wavelets in 100 linearly spaced frequency bins between 2.0 and 100 Hz, with wavelet cycles increasing from 3 (at low frequencies) to 6 (at high frequencies). Total power was calculated in decibels (dB) relative to baseline power (−100 to 0 ms) in each frequency band. Data from specific time and frequency windows were extracted as the average power for each window. For comparisons of group level data, only saline, 20mg/kg morphine, and 20mg/kg morphine + Naloxone were considered. Since the differential effect of genotype on morphine’s ability to modulate responses was of primary interest and total power at baseline (saline condition) was not different between A/A and G/G mice, results for the 20mg/kg morphine condition and 20mg/kg morphine + Naloxone conditions were normalized to the saline condition. A repeated measures mixed-model (genotype × condition) ANOVA assessed significance of power changes (IBM SPSS Statistics, Version 22, IBM Corp, Armonk, NY); pairwise comparisons were deduced using least squares differences.
RESULTS
Quantification of baseline responses
To evaluate differences in circuit responses to afferent activity, compound population responses in CA1 were induced with a single 20-µA, 200-µs pulse delivered to Schaffer collateral axons passing through SR of CA1. VSDi of area CA1 recorded an evoked fast depolarization followed by a rapid repolarization (Figure 2), reflecting responses at the single-cell level (Ang et al., 2005; Ang et al., 2006; Carlson and Coulter, 2008). As previously validated, these alterations in fluorescence depict net functional changes in neuronal activity, which have been shown to be comparable to AMPA/NMDA-mediated EPSPs and GABA-mediated IPSPs measured by intracellular electrophysiological techniques (Ang et al., 2005; Ang et al., 2006; Carlson and Coulter, 2008). The initial depolarization, which directly activated CA1 dendrites and local interneurons, propagated to distal regions of SR and outwards towards SLM and SO and was followed by a longer hyperpolarization. This can be visualized spatially in snapshots of the averaged peak excitatory (Figure 2bi) or inhibitory (Figure 2bii) responses and temporally as 2D traces of fluorescence changes over time (Figure 2d) or raster plots of activity, which show changes in fluorescence across space and time (Figure 2c).
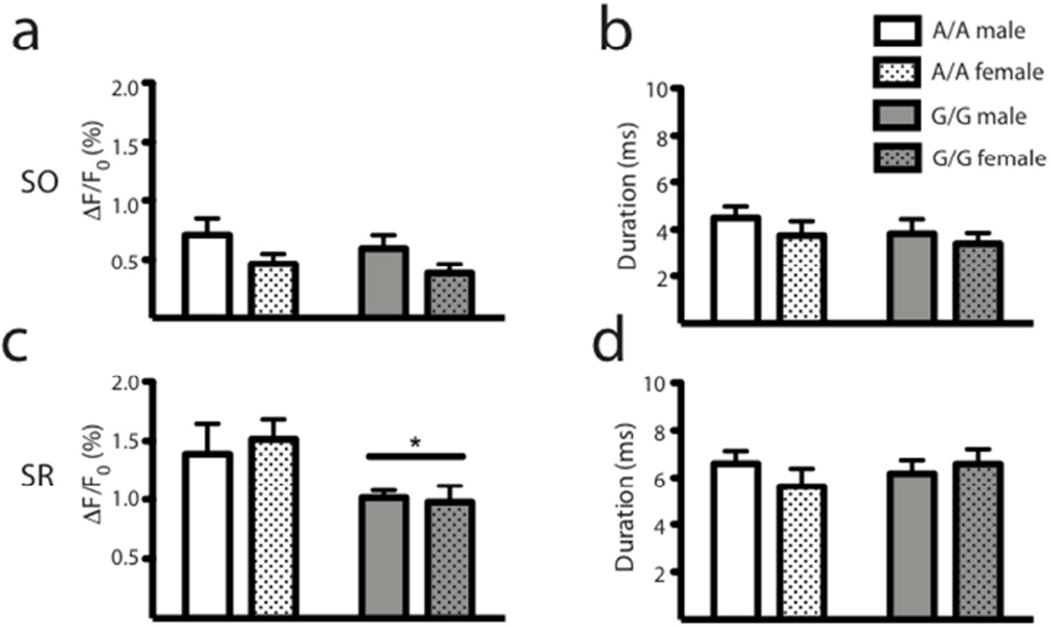
A112 and G112 animals, both male and female, displayed similar VSDi responses to Schaffer collateral stimulation under basal conditions (Figure 3). However, in SR, there was a significant reduction in the peak amplitude of the response in G112 animals regardless of sex (main effect of genotype, F1,19 = 5.26, p < 0.05; Figure 3c). While a trend was observed in the SO peak amplitude in response to Schaffer collateral stimulation in females (Figure 3a), this effect was not significant.
Figure 3. Baseline responses.
There were no differences between genotypes or sexes in the SO for amplitude (a) or tau (b). In the SR, there was a significant reduction in the peak excitation for G/G mice (c) but not for the tau (d). All data are presented as mean ± SEM, n = 5; * p < 0.05 compared to A/A.
Analysis of DAMGO-mediated response-changes
To determine if the A112G SNP alters hippocampal circuit activity during MOPR stimulation, we first examined responses following application of the highly specific MOPR agonist DAMGO (1 mM) and normalized these to each animal’s basal response. In accordance with previous studies (McQuiston, 2007; McQuiston and Saggau, 2003) and expectations for agents that inhibit GABAergic release, we found increases in neuronal activation following DAMGO administration in wild-type mice (Figure 4a).
In order to compare baseline and DAMGO-mediated responses, we subtracted the raster plot pixel-changes following DAMGO application from the raster plot responses observed under basal conditions in order to highlight the loss of inhibition due to MOPR-stimulated GABA inactivation. Since MOPR stimulation with DAMGO effectively decreases GABA transmission, any increase in excitatory events must have occurred due to this reduction in inhibitory modulation; the subtracted raster plots illustrate this MOPR-mediated loss of inhibition. An advantage of this approach is that it allowed us to more reliably compare drug treatment effects across genotypes and sexes. By subtracting the actual pixel responses between sessions, we eliminated the requirement for excessive numerical transformations and were able to analyze 2D traces quantified directly from the subtracted plot.
Comparing the subtracted raster plots between genotypes and sexes showed that while all groups showed an initial decrease in inhibitory modulation following DAMGO application, the wild-type animals had an elevated and prolonged response compared to the G/G mice (Figure 4c). Indeed, these observations were supported by quantification of raster plots for each of the regions within CA1. In order to identify differences between groups, we used a 2D trace of the subtracted pixel-changes over time for each region of CA1 and measured the peak amplitude and duration of the response in addition to the area under the curve (AUC) (Figure 5). In the SO, there were significant reductions in the ability of DAMGO to disinhibit excitatory responses both in G/G animals and in females, without an interaction between these effects. The G/G genotype and the females showed reduced disinhibition compared to their respective counterparts for the peak amplitude (main effects of genotype, F1,19 = 7.45, p < 0.05 and sex, F1,19 = 6.30, p < 0.05; Figure 5a), duration (main effects of genotype, F1,19 = 22.58, p < 0.001 and sex, F1,19 = 6.05, p < 0.05; Figure 5b), and the AUC (main effects of genotype, F1,19 = 20.68, p < 0.001 and sex, F1,19 = 9.94, p < 0.01; Figure 5c).
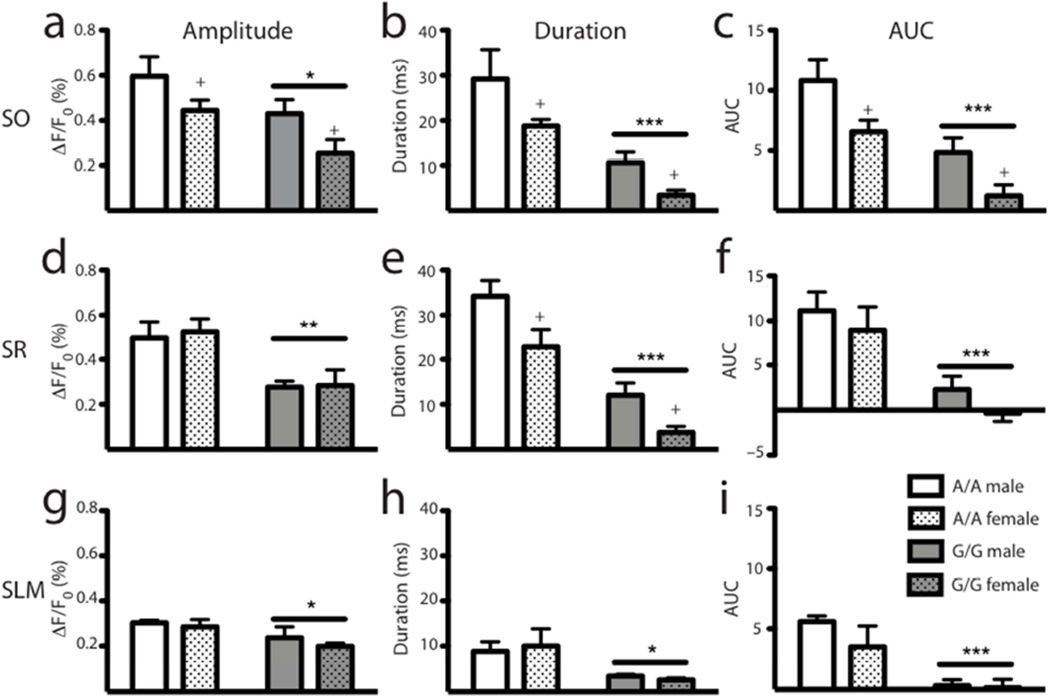
Figure 5. DAMGO-mediated inhibition is reduced in G/G animals.
(a) Analysis of 2D traces from each strata of CA1 reveals alterations in genotype or sex responses to DAMGO administration. In the SO, both the G/G animals and females, each compared to their respective counterparts, showed decreases in the amplitude (a), duration (b) and AUC (c). In the SR, G/G animals showed reductions in amplitude (d), duration (e), and AUC (f); additionally, there was a significant reduction in females compared to males for duration only (e). In the SLM, levels of disinhibition were lower compared to the other CA1 regions; however, G/G animals still showed a decreased response to DAMGO administration measured by the amplitude (g), duration (h), and AUC (i). All data are presented as mean ± SEM, n = 5; * p < 0.05, ** p < 0.01, *** p < 0.001, † p < 0.0001 compared to A/A; + p < 0.05 compared to males.
We found a similar pattern in the SR, in which there was a significant reduction in the ability of DAMGO to disinhibit excitatory responses in G/G animals, as demonstrated by decreases in the peak amplitude (main effect of genotype, F1,19 = 13.76, p < 0.01; Figure 5d), duration (main effect of genotype, F1,19 = 47.12, p < 0.0001; Figure 5e), and the AUC (main effect of genotype, F1,19 = 22.37, p < 0.001; Figure 5f). There was also a main effect of sex for the duration of response, in which the females of both genotypes showed reduced disinhibition compared to their male counterparts (main effect of sex, F1,19 = 10.67, p < 0.01; Figure 5e); there was not, however, an interaction between genotype and sex main effects. Another advantage of this analysis was that it allowed us to evaluate differences in SLM, a region that, due to its lower basal responses, we could not otherwise have analyzed. Though the responses for all groups were lower in this region compared to the SR and SO, there was still a significantly reduced disinhibition in the SLM for the G/G animals for the peak (main effect of genotype, F1,19 = 6.30, p < 0.05; Figure 5g), duration (main effect of genotype, F1,19 = 8.42, p < 0.05; Figure 5h), and AUC (main effect of genotype, F1,19 = 18.76, p < 0.001; Figure 5i).
EEG
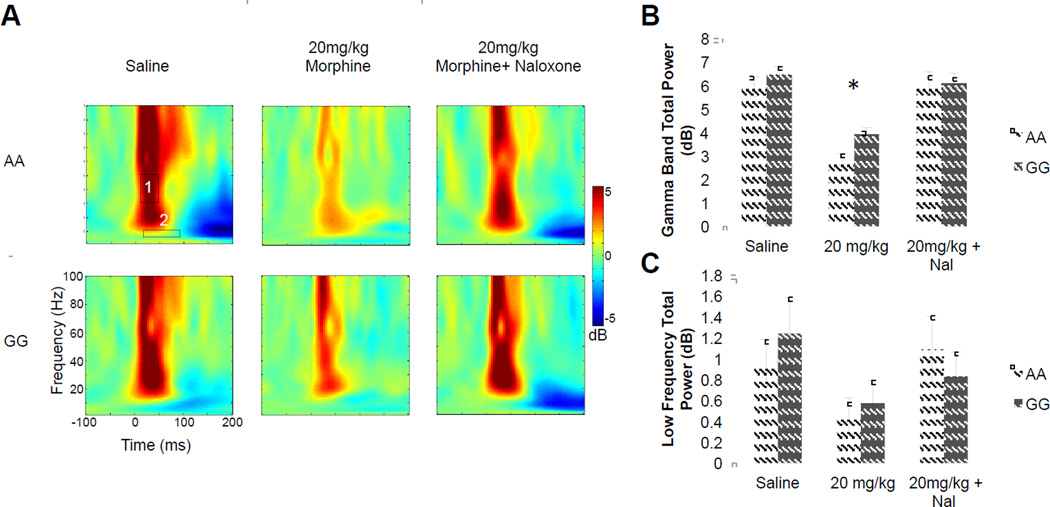
MOPRs located on terminals of PV interneurons can strongly modulate oscillatory activity of the hippocampus (Gulyas et al., 2010). Indeed, recent work has demonstrated that PV cells are critical for the generation of gamma oscillations in the hippocampus and neocortex (Fuchs et al., 2007; Lodge et al., 2009). In order to test if CA1-specific A118G differences in MOPR modulation of inhibition examined in vitro are reflected in gamma-band brain activity recorded in vivo, mice were implanted with low impedance hippocampal depth electrodes to measure EEG activity. Using an auditory stimulus protocol, tone-evoked gamma-band power was evaluated before and after an acute injection of morphine. At baseline (saline condition), total gamma power did not differ between genotypes (A/A vs G/G; T36.31=0.9552, p = 0.34). In order to investigate the change in responses following MOPR activation, EEG power following morphine injection and morphine + Naloxone injection were normalized to the baseline data. Similar to prior work in rats (Zuo et al., 2007), we found that morphine (20 mg/kg, i.p.) reduced auditory-evoked gamma activity in the range of 31 to 51 Hz in all mice. However, this response was significantly reduced in G/G animals (genotype × condition interaction: F1,38 = 4.49, p < 0.05; Figure 6a,b). Co-administration of naloxone (1 mg/kg; i.p.) reversed the effect of morphine similarly in all animals (Figure 6c). As such, EEG recordings demonstrate a differential reduction in Gamma activity in after MOPR activation dependent on A112G substitution. This specific reduction in gamma activity after MOPR activation is consistent with the cell-type specific localization of these receptors (Drake and Milner, 1999; Drake and Milner, 2002), and previous in vivo findings (Zuo et al., 2007). The reduced effect of morphine in G/G mice is consistent with the loss-of-function phenotype identified in the hippocampal slice preparation.
Figure 6. G/G mice exhibit reduced gamma-related responses to MORP activation.
A) Total power response for representative A/A (top) and G/G (bottom) mice, for Saline (left), 20 mg/kg Morphine (center), and 20 mg/kg Morphine + 1mg/kg Naloxone (Nal) (right). Note the decrease in high frequency (gamma) activity (box 1). This did not occur at lower frequencies (box 2). B) Group average results for gamma activity (31–51 Hz), * = p < 0.05 compared to A/A C) Group Average results for low frequency 6– 11 Hz, demonstrate no significant group differences. B and C are presented as mean ± SEM, n = 8–11.
DISCUSSION
MOPR stimulation in the hippocampus increases net excitatory activity by decreasing GABAergic inhibition from local interneurons, resulting in the disruption of pyramidal cell firing synchrony and an alteration in hippocampal function (Faulkner et al., 1998). A common SNP in the gene encoding the MOPR has been shown to alter a variety of behaviors and drug responses in clinical populations [for review, (Mague and Blendy, 2010)] and in animal models (Barr and Goldman, 2006; Mague et al., 2009; Ramchandani et al., 2010; Zhang et al., 2014). Neither the extent of these changes nor the mechanisms mediating the effects are completely understood. We used VSDi techniques to investigate circuit changes in the hippocampus in order to determine if functional alterations resulting from this SNP could better inform results from previous clinical and preclinical studies. Additionally, we utilized in vivo EEG recordings to evaluate changes in oscillatory activity in the hippocampus in awake animals that were acutely administered opiates. Overall, we found that the augmentation of excitatory responses elicited by opiate administration in wild-type animals was reduced in animals homozygous for the G112 allele. This reduction was particularly striking in raster plot subtraction analyses in which DAMGO-mediated responses of individual pixels were subtracted from basal responses, revealing the loss of inhibition caused by the MOPR activation. Similar results were found when evaluating hippocampal circuitry in vivo: reductions in gamma band activity caused by MOPR activation were decreased in G/G mice. Furthermore, our data suggests that the A112G SNP results in the reduced functionality of the receptor, as it has been shown that MOPR expression levels in the hippocampus are similar between genotypes and sexes in A112G mice (Mague et al., 2009; Wang et al., 2014; Wang et al., 2012; Zhang et al., 2014).
Ex Vivo VSDi Recordings Demonstrate that the MOPR A112G SNP Results in a Loss of MOPR Functionality and Altered Hippocampal Circuitry Evoked Responses
While baseline responses were similar between genotypes and sexes, there was a significantly lower peak response in the SR in the G/G animals. This could result from enhanced tonic GABAergic activity, possibly suggesting either a reduction in efficacy of endogenous MOPR modulation of GABA activity or, alternatively, a reduction in endogenous opioidergic tone in G/G animals. However, this effect was not seen for other measures of responses in the SR or SO, suggesting only a subtle consequence of these potential baseline alterations. Similarly, previous behavioral work with these mice did not uncover robust baseline differences, but only reductions in morphine-mediated behaviors (Mague et al., 2009).
Despite similar basal responses, there was a pronounced difference between genotypes following application of DAMGO. Raster plot subtraction analysis revealed robust MOPR deficits in the G/G animals in all CA1 regions tested. Though both genotypes showed an initial peak disinhibitory effect of DAMGO, this response was more intense and prolonged in A/A animals. Since the extent and duration of responses seem to be most affected, these data suggest that there could potentially be alterations in the desensitization or trafficking of the receptor.
In these experiments, we also found significant reductions in the female responses to MOPR activation compared to males, regardless of genotype. This was not surprising given the frequency of reported sex-differences in response to opioid administration (Craft, 2008). Opioids have been shown to be more efficacious in males compared to females in both rodent (Kepler et al., 1989) and human (Cepeda and Carr, 2003) studies investigating sex-differences in the analgesic properties of opioids. In contrast, female rats respond more robustly to the rewarding properties of opioids (Cicero et al., 2003) and women are more likely to abuse prescription opioid analgesics (Roe et al., 2002). Specifically in the hippocampus, ovarian steroid hormones have been shown to influence levels of opioid peptides (Roman et al., 2006; Williams et al., 2011) and the availability of MOPRs on the surface of PV cells (Torres-Reveron et al., 2009). In contrast to our previous studies, however, we did not demonstrate interactions between genotype and sex. This could suggest that differences in CA1 responses to MOPR activation may not directly underlie the sex-specific reduction in morphine-conditioned place-preference studies (Mague et al., 2009).
Since VSDi responses show net activity of entire circuits, we were unable to isolate responses of specific subpopulations of interneurons and, thus, cannot unequivocally ascribe our findings to MOPR modulation of PV basket cells. However, previous studies have shown that MOPRs are found predominantly on these interneurons (Drake and Milner, 1999; Drake and Milner, 2002) and that stimulation of these cells disinhibits glutamatergic dendrites (Glickfeld et al., 2008). This is supported by the findings provided by the stimulation protocol utilized in these studies, in which a single 200-ms pulse was administered, as the PV interneurons have been shown to respond with high reliability to initial, but not repeated, afferent input (Pouille and Scanziani, 2004; Spruston, 2008). Also, GABAA receptors located opposite PV cell terminals produce IPSPs that rise and decay very rapidly (Klausberger et al., 2002; Lavoie et al., 1997). Indeed, other studies evaluating MOPR-mediated elevations of CA1 responses to Shaffer collateral stimulation found that paired current pulses, similar to the single pulses utilized in the present studies, were mediated by GABAA receptors (McQuiston and Saggau, 2003), while DAMGO-induced augmentations of CA1 responses following prolonged stimulation were mediated by GABAB receptors (McQuiston, 2007). These features of PV-containing, fast-spiking interneurons enable them to induce a reliable and brief, yet intense, somatic shunting of postsynaptic conductance (Bartos et al., 2007; Vida et al., 2006). Thus, reduced PV interneuron inhibition is a plausible explanation for the augmentation of CA1 responses following DAMGO administration in these current experiments. The reduced disinhibition demonstrated by the G/G animals following DAMGO administration suggests a disruption in MOPR modulation of these PV interneurons resulting from the A112G SNP.
In Vivo EEG Electrophysiological Recordings Also Demonstrate that the MOPR A112G SNP Results in Altered Hippocampal Responses
A consequence of the increase in excitatory responses demonstrated in wild-type mice could be a reduction in both neuronal synchrony and the formation of high-frequency oscillatory activity. Indeed, PV interneurons have been shown to be important in generating gamma-band oscillations in the hippocampus (Bartos et al., 2007; Fuchs et al., 2007). Given the reduced MOPR-mediated augmentation of responses in the G/G animals, we would predict that reductions in gamma-activity resulting from MOPR activation (Sun et al., 2006; Zuo et al., 2007) would be decreased in these animals. Our findings support this prediction. Gamma-band power was decreased in all mice after administration of morphine, though blunted in G/G mice. Naloxone blocked all gamma frequency-related effects of morphine for both genotypes, suggesting that the reductions in gamma were mediated by actions at the MOPR. It should be noted, however, that as we did not measure the effects of naloxone alone, we cannot conclude unequivocally that the reversal in gamma responses results from the blockade of morphine action and not some other mechanism, such as inhibition of endogenous opioids. However, while one study did find significant effects of naloxone alone in monkeys on EEG measures (Ehlers, 1989), similar studies have found no effect of naloxone alone in vitro (Lynch, Jensen, McGaugh, Davila, & Oliver, 1981), in conscious rats (Coltro Campi & Clarke, 1995; Tortella, Moreton, & Khazan, 1978), or in children (Nalin, Petraglia, Genazzani, Frigieri, & Facchinetti, 1988), suggesting that naloxone’s effects in the EEG studies were most likely due to blockade of the exogenously administered morphine.
Clinical studies, as well as those in rodents and non-human primates, have shown that reductions in evoked gamma power are an intermediate phenotype with broad relevance to neuropsychiatric disorders, pain sensitivity and, in the hippocampus, cognition (Carlson et al., 2011; Uhlhaas and Singer, 2011). Thus, these EEG data provide initial evidence those differences in sensitivity shown in vitro may act via PV-cells to mediate some of the behavioral phenotypes associated with the A118G allele. Additionally, because similar EEG studies can be performed in humans, the EEG phenotype provides a framework for translating neurophysiological and behavioral correlates from the A118G mouse model to identifying functional neural substrates underlying the A118G genotype and behavior interaction identified humans.
Conclusions
We observe an alteration in hippocampal function as a result of the Mu Opioid Receptor SNP, A112G, and the reduced effect of DAMGO and morphine in G/G animals further supports a loss-of-function of the MOPR as a consequence of this SNP. Previous work with this mouse line has provided evidence for reduced MOPR expression and decreased behavioral responses to acute morphine administration; likewise, clinical findings have demonstrated a reduced response to the analgesic properties of opioids (Chou et al., 2006b; Sia et al., 2008). In support of these findings, authors often cite in vitro studies showing decreases in MOPR expression (Befort et al., 2001; Zhang et al., 2005). However, the present data were derived from evaluation of MOPR function in the hippocampus, which possesses equivalent MOPR expression between genotypes and sexes as demonstrated using RT-PCR (Mague et al., 2009) and quantitative in vitro autoradiography (Wang et al., 2014; Wang et al., 2012). From our data, it appears that the strong disinhibition controlled by MOPRs on interneurons of the hippocampus is absent, therefore leading us to conclude that this polymorphism represents a “loss of function” phenotype in the context of hippocampal microcircuitry (Figure 1). While others have observed that MOPRs in cultured cells perhaps follow a gain of function phenotype (Bond et al., 1998; Margas et al., 2007), these models do not possess well-organized circuits and therefore do not accurately reflect complex brain structures. In contrast, our ex vivo slices maintain microcircuitry and are representative of a “loss of function” phenotype at the circuitry level, which is closely paralleled by our in vivo EEG hippocampal findings following systemic administration of opiates. We posit that the loss of function in our microcircuitry model occurs due to the reduced inhibitory drive onto pyramidal cells (Figure 1). However, the mechanism underlying changes in receptor function remains unknown. Attempts to identify changes in agonist binding, protein coupling, downstream signaling, and receptor trafficking have not produced conclusive evidence for the specific deficit [for review, see (Knapman and Connor, 2014)]. For instance, in whole-cell patch-clamp studies using a mouse line possessing humanized A/A or G/G alleles, Mahmoud and colleagues found a functional reduction of voltage-gated Ca2+ activation following morphine administration in isolated sensory neurons from G/G animals (Mahmoud et al., 2011), consistent with a loss of function of the receptor. In separate experiments using this A112G mouse line, a reduction in GTPγS binding was detected in several brain regions (Wang et al., 2014). In addition, human positron emission topography studies have demonstrated a decreased binding potential of [11C]carfentanil, indicating a reduced MOPR availability, in those with the G allele (Ray et al., 2011; Weerts et al., 2013). Together, these results suggest deficits in the early stages of binding and signal transduction and would be consistent with the immediate changes seen after acute MOPR agonist administration. However, these data do not rule out other changes to protein binding, downstream signaling, internalization, or dimerization. Future studies specifically aimed at these characteristics will help elucidate the mechanisms whereby this polymorphism disrupts MOPR function. In summary, the modeling of this SNP in mice identified reduced function in the absence of reduced expression and suggests that human SNPs may have more complex consequences on phenotypes than previously appreciated.
MOPR activation increased excitatory responses in wild-type animals
This effect was significantly reduced in animals possessing the Oprm1 SNP
In vivo EEG recordings during morphine administration corroborated a loss of function phenotype
These data suggest that the MOPR A118G SNP results in a loss of function at the circuit level
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants R00-DA-032681 (to J.R.T.) and T32-DA-007241–15 (to S.D.M.). We would like to thank Dr. Julie A. Blendy for generously providing the A112G MOPR mice used in these experiments and Ms. Mary McMullen for assistance with statistical analysis. We would like to thank Dr. Luyi Zhou and Ms. Miranda Fisher for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ang CW, Carlson GC, Coulter DA. Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J Neurosci. 2005;25(42):9567–9580. doi: 10.1523/JNEUROSCI.2992-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26(46):11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65(2):135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Goldman D. Non-human primate models of inheritance vulnerability to alcohol use disorders. Addict Biol. 2006;11(3–4):374–385. doi: 10.1111/j.1369-1600.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Befort K, Filliol D, Decaillot FM, Gaveriaux-Ruff C, Hoehe MR, Kieffer BL. A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling. J Biol Chem. 2001;276(5):3130–3137. doi: 10.1074/jbc.M006352200. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Hilario M, Dow HC, Brodkin ES, Blendy JA, Berton O. Mouse Model of OPRM1 (A118G) Polymorphism Increases Sociability and Dominance and Confers Resilience to Social Defeat. J Neurosci. 2015;35(8):3582–3590. doi: 10.1523/JNEUROSCI.4685-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Coulter DA. In vitro functional imaging in brain slices using fast voltage-sensitive dye imaging combined with whole-cell patch recording. Nat Protoc. 2008;3(2):249–255. doi: 10.1038/nprot.2007.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Talbot K, Halene TB, Gandal MJ, Kazi HA, Schlosser L, Phung QH, Gur RE, Arnold SE, Siegel SJ. Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci U S A. 2011;108(43):E962–E970. doi: 10.1073/pnas.1109625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97(5):1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- Chou WY, Wang CH, Liu PH, Liu CC, Tseng CC, Jawan B. Human opioid receptor A118G polymorphism affects intravenous patient-controlled analgesia morphine consumption after total abdominal hysterectomy. Anesthesiology. 2006a;105(2):334–337. doi: 10.1097/00000542-200608000-00016. [DOI] [PubMed] [Google Scholar]
- Chou WY, Yang LC, Lu HF, Ko JY, Wang CH, Lin SH, Lee TH, Concejero A, Hsu CJ. Association of mu-opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 2006b;50(7):787–792. doi: 10.1111/j.1399-6576.2006.01058.x. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Aylward SC, Meyer ER. Gender differences in the intravenous self-administration of mu opiate agonists. Pharmacol Biochem Behav. 2003;74(3):541–549. doi: 10.1016/s0091-3057(02)01039-0. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol. 2008;16(5):376–385. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37(2):311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res. 1999;849(1–2):203–215. doi: 10.1016/s0006-8993(99)01910-1. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12(2):119–136. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- Drakenberg K, Nikoshkov A, Horvath MC, Fagergren P, Gharibyan A, Saarelainen K, Rahman S, Nylander I, Bakalkin G, Rajs J, et al. Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci U S A. 2006;103(20):7883–7888. doi: 10.1073/pnas.0600871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner HJ, Traub RD, Whittington MA. Disruption of synchronous gamma oscillations in the rat hippocampal slice: a common mechanism of anaesthetic drug action. Br J Pharmacol. 1998;125(3):483–492. doi: 10.1038/sj.bjp.0702113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53(4):591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ. Validating gamma oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiatry. 2010;68(12):1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Atallah BV, Scanziani M. Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci. 2008;28(8):1824–1832. doi: 10.1523/JNEUROSCI.4700-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30(45):15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ. Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacol Biochem Behav. 1989;34(1):119–127. doi: 10.1016/0091-3057(89)90363-8. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci. 2002;22(7):2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapman A, Connor M. Cellular signalling of non-synonymous single-nucleotide polymorphisms of the human mu-opioid receptor (OPRM1) Br J Pharmacol. 2014 doi: 10.1111/bph.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapman A, Santiago M, Connor M. Buprenorphine signaling is compromised at the N40D polymorphism of the human mu-opioid receptor in vitro. Br J Pharmacol. 2014 doi: 10.1111/bph.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABA(A) receptor channels are dependent on alpha-subunit isoform. Biophys J. 1997;73(5):2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, Shields PG, Kaufmann V, Redden D, Benowitz N, et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J. 2004;4(3):184–192. doi: 10.1038/sj.tpj.6500238. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29(8):2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Blendy JA. OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend. 2010;108(3):172–182. doi: 10.1016/j.drugalcdep.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci U S A. 2009;106(26):10847–10852. doi: 10.1073/pnas.0901800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud S, Thorsell A, Sommer WH, Heilig M, Holgate JK, Bartlett SE, Ruiz-Velasco V. Pharmacological consequence of the A118G mu opioid receptor polymorphism on morphine- and fentanyl-mediated modulation of Ca(2)(+) channels in humanized mouse sensory neurons. Anesthesiology. 2011;115(5):1054–1062. doi: 10.1097/ALN.0b013e318231fc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margas W, Zubkoff I, Schuler HG, Janicki PK, Ruiz-Velasco V. Modulation of Ca2+ channels by heterologously expressed wild-type and mutant human micro-opioid receptors (hMORs) containing the A118G single-nucleotide polymorphism. J Neurophysiol. 2007;97(2):1058–1067. doi: 10.1152/jn.01007.2006. [DOI] [PubMed] [Google Scholar]
- McQuiston AR. Effects of mu-opioid receptor modulation on GABAB receptor synaptic function in hippocampal CA1. J Neurophysiol. 2007;97(3):2301–2311. doi: 10.1152/jn.01179.2006. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Saggau P. Mu-opioid receptors facilitate the propagation of excitatory activity in rat hippocampal area CA1 by disinhibition of all anatomical layers. J Neurophysiol. 2003;90(3):1936–1948. doi: 10.1152/jn.01150.2002. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Betancur MI, Buzsaki G. Behavior-dependent coordination of multiple theta dipoles in the hippocampus. J Neurosci. 2009;29(5):1381–1394. doi: 10.1523/JNEUROSCI.4339-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci U S A. 2007;104(36):14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, Mailheau S, Chavkin C. Opioid receptor-mediated responses in the dentate gyrus and CA1 region of the rat hippocampus. J Pharmacol Exp Ther. 1988;244(2):564–570. [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429(6993):717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28(12):1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64(9):1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray R, Ruparel K, Newberg A, Wileyto EP, Loughead JW, Divgi C, Blendy JA, Logan J, Zubieta JK, Lerman C. Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc Natl Acad Sci U S A. 2011;108(22):9268–9273. doi: 10.1073/pnas.1018699108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezayof A, Razavi S, Haeri-Rohani A, Rassouli Y, Zarrindast MR. GABA(A) receptors of hippocampal CA1 regions are involved in the acquisition and expression of morphine-induced place preference. Eur Neuropsychopharmacol. 2007;17(1):24–31. doi: 10.1016/j.euroneuro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Roe CM, McNamara AM, Motheral BR. Gender- and age-related prescription drug use patterns. Ann Pharmacother. 2002;36(1):30–39. doi: 10.1345/aph.1A113. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Gustafsson L, Meyerson BJ, Nylander I. Variations in opioid peptide levels during the estrous cycle in Sprague-Dawley rats. Neuropeptides. 2006;40(3):195–206. doi: 10.1016/j.npep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Sia AT, Lim Y, Lim EC, Goh RW, Law HY, Landau R, Teo YY, Tan EC. A118G single nucleotide polymorphism of human mu-opioid receptor gene influences pain perception and patient-controlled intravenous morphine consumption after intrathecal morphine for postcesarean analgesia. Anesthesiology. 2008;109(3):520–526. doi: 10.1097/ALN.0b013e318182af21. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9(3):206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Sun N, Li Y, Tian S, Lei Y, Zheng J, Yang J, Sui N, Xu L, Pei G, Wilson FA, et al. Dynamic changes in orbitofrontal neuronal activity in rats during opiate administration and withdrawal. Neuroscience. 2006;138(1):77–82. doi: 10.1016/j.neuroscience.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Williams TJ, Chapleau JD, Waters EM, McEwen BS, Drake CT, Milner TA. Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Exp Neurol. 2009;219(1):319–327. doi: 10.1016/j.expneurol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, Siracusano A, Gross C. Variation in the mu-opioid receptor gene (OPRM1) moderates the influence of early maternal care on fearful attachment. Soc Cogn Affect Neurosci. 2012;7(5):542–547. doi: 10.1093/scan/nsr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37(3):514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg E, Wiers RW, Dessers J, Janssen RG, Lambrichs EH, Smeets HJ, van Breukelen GJ. A functional polymorphism of the mu-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol Clin Exp Res. 2007;31(1):1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Vida I, Bartos M, Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49(1):107–117. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Huang P, Blendy JA, Liu-Chen LY. Brain region- and sex-specific alterations in DAMGO-stimulated [(35) S]GTPgammaS binding in mice with Oprm1 A112G. Addict Biol. 2014;19(3):354–361. doi: 10.1111/j.1369-1600.2012.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Huang P, Ung A, Blendy JA, Liu-Chen LY. Reduced expression of the mu opioid receptor in some, but not all, brain regions in mice with OPRM1 A112G. Neuroscience. 2012;205:178–184. doi: 10.1016/j.neuroscience.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Creswell JD, Eisenberger NI, Lieberman MD. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10(1):12–24. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci U S A. 2009;106(35):15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, McCaul ME, Kuwabara H, Yang X, Xu X, Dannals RF, Frost JJ, Wong DF, Wand GS. Influence of OPRM1 Asn40Asp variant (A118G) on [11C]carfentanil binding potential: preliminary findings in human subjects. Int J Neuropsychopharmacol. 2013;16(1):47–53. doi: 10.1017/S146114571200017X. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26(12):676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Faulkner HJ, Jefferys JG, Chettiar K. Morphine disrupts long-range synchrony of gamma oscillations in hippocampal slices. Proc Natl Acad Sci U S A. 1998;95(10):5807–5811. doi: 10.1073/pnas.95.10.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Mitterling KL, Thompson LI, Torres-Reveron A, Waters EM, McEwen BS, Gore AC, Milner TA. Age- and hormone-regulation of opioid peptides and synaptic proteins in the rat dorsal hippocampal formation. Brain Res. 2011 doi: 10.1016/j.brainres.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Ho A, Blendy JA, Kreek MJ. Mouse Model of the OPRM1 (A118G) Polymorphism: Differential Heroin Self-Administration Behavior Compared to Wild Type Mice. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280(38):32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- Zuo YF, Wang JY, Chen JH, Qiao ZM, Han JS, Cui CL, Luo F. A comparison between spontaneous electroencephalographic activities induced by morphine and morphine-related environment in rats. Brain Res. 2007;1136(1):88–101. doi: 10.1016/j.brainres.2006.11.099. [DOI] [PubMed] [Google Scholar]