Abstract
The discovery and implementation of antibiotics in the early twentieth century transformed human health and wellbeing. Chemical synthesis enabled the development of the first antibacterial substances, organoarsenicals and sulfa drugs, but these were soon outshone by a host of more powerful and vastly more complex antibiotics from nature: penicillin, streptomycin, tetracycline, and erythromycin, among others. These primary defences are now significantly less effective as an unavoidable consequence of rapid evolution of resistance within pathogenic bacteria, made worse by widespread misuse of antibiotics. For decades medicinal chemists replenished the arsenal of antibiotics by semisynthetic and to a lesser degree fully synthetic routes, but economic factors have led to a subsidence of this effort, which places society on the precipice of a disaster. We believe that the strategic application of modern chemical synthesis to antibacterial drug discovery must play a critical role if a crisis of global proportions is to be averted.
Keywords: antibiotics, chemical synthesis, drug discovery, semisynthesis
1. Introduction
The emergence of pathogenic bacteria resistant to many or all current antibiotics is a major public health concern and one of particular importance in clinical settings. The World Economic Forum recently identified antibiotic resistance as one of the greatest threats to human health in its Global Risks 2013 report.[1] The Center for Disease Control and Prevention released a summary of antibiotic resistance threats in the United States in 2013, outlining the “potentially catastrophic consequences of inaction.”[2] Natural selection, assisted by global misuse of existing antibiotics, and the slowing pace of discovery of new antibiotics conspire to place society at or near a crisis point. The innovation deficit is in large measure due to the fact that many major pharmaceutical companies have abandoned antibacterial research and development, a trend which has created or at the very least contributed to the steep decline in the number of new antibacterials launched in the last 30 years (Figure 1).[3] Meanwhile, resistance rates around the world are rising,[4] new resistance mechanisms are emerging,[5] and infections caused by multidrug-resistant Gram-negative bacteria are becoming particularly difficult to treat. The problem is exacerbated by the ease of international travel and increasing global population densities. Our current arsenal of antibiotics is steadily losing its efficacy and there is little sign that it will be adequately replenished in the near future.[3,6] The development of bacterial resistance is an inevitable consequence of evolution, and without continued replenishment of our arsenal of antibacterial agents, humanity runs the risk of returning to a pre-antibiotic era.
Figure 1.
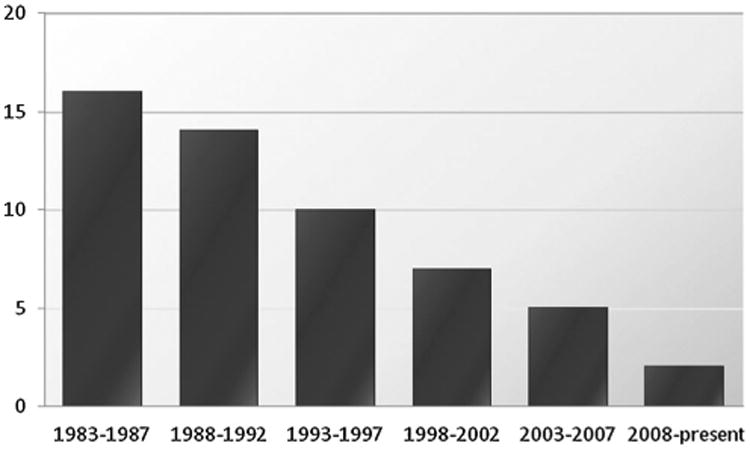
Number of new antibacterials approved by the FDA in 5-year periods from 1983 to present.[3]
In this Review we examine the 100-year history of antibiotics discovery and development from its dawning with the synthesis of the first arsenical agent to those few antibiotic candidates that are currently in late-stage clinical evaluation,[6] highlighting the essential and evolving role of chemical synthesis throughout. Our objectives are to recognize select key contributions of the thousands of scientists who have provided the modern antibacterial pharmacopeia and to make the point that the clearest path forward to discover future generations of life-saving medicines will involve chemical synthesis as its core activity.
More specifically, we suggest that the development of practical, diversifiable, fully synthetic routes to antibiotic natural product scaffolds that are not yet accessible in this way presents the greatest opportunity for rapid discovery and development of new antibiotics in the near term (5–20 years). By this analysis, many of the natural product classes that emerged during and defined the golden era of antibiotics discovery (ca. 1940–1960) represent underutilized resources. As we argue in this Review, the development of practical, fully synthetic routes to antibacterial molecules is a tried-and-tested strategy whose perceived constraints (molecular size and complexity, scalability) need to be reevaluated in light of advances in modern chemical synthesis, both strategic and methodological. We believe that ambitious, translational chemical synthesis must be a core activity of antibiotics research moving forward, as it has been since the inception of the field.
1.1. Scope and Focus of this Review
The vast literature of antibiotics includes several fine review articles,[7] many of them published in this journal.[8] For clear and comprehensive accounts of all aspects of this field—including resistance, mechanisms of action, microbial screening for antibiotic natural products, antibiotic biosynthesis, and drug development—we direct readers to two excellent texts, one authored by Christopher Walsh[9] and the other edited by Thomas Dougherty and Michael Pucci.[10] A detailed understanding of the molecular basis for antibiotic activity and resistance is critical to the success of any drug development program, but these factors are not the focus of this Review. With the exception of an overview of rifampicin, the complex, extraordinarily challenging, and important problem of developing drugs to treat tuberculosis is also not covered here. While others have previously articulated the importance of chemical synthesis in antibiotics drug discovery,[7f, 8c,d] our focal point is the development of platform technologies to access natural product scaffolds (broadly defined) by convergent, component-based, fully synthetic routes. Our intention is to illuminate the evolving role chemical synthesis has played in the discovery and development of new antibacterial agents so as to make clear its potential to contribute to the alleviation of the current innovation deficit. In the final section of this Review, we identify specific opportunities for chemical innovation to fuel future antibiotic drug development. Lastly, we hasten to note that while our expertise and emphasis is chemistry-centered, we recognize that the field of antibiotic discovery would not exist nor could it advance without the essential contributions of individuals from many disciplines: isolation scientists, microbiologists, crystallographers, clinicians, geneticists, toxicologists, and formulations experts, among others.
1.2. Nomenclature of this Review
All antibiotics used in human therapy since the dawn of the antibiotics era in the early 1900s can be divided into three distinct categories according to how they were ultimately manufactured on large scale. These categorizations follow.
Natural products
Compounds manufactured directly by large-scale fermentation of bacteria or fungi.
Semisynthetic antibacterials
Compounds manufactured by chemical synthesis using as starting material a natural product.
Fully synthetic antibacterials
Compounds that are manufactured by fully synthetic routes.
Throughout this article we have attempted to adopt the green, blue, and red color-coding, respectively, in Figures and Schemes to clarify the means of manufacture of the antibiotics presented. While new categories may yet emerge, such as antibiotics produced by metabolic engineering, here we focus on processes that have already yielded clinical agents.
2. Chemical Synthesis Ushers in the Golden Age of Antibiotics Discovery
2.1. Discovery and Development of the First Antibiotics
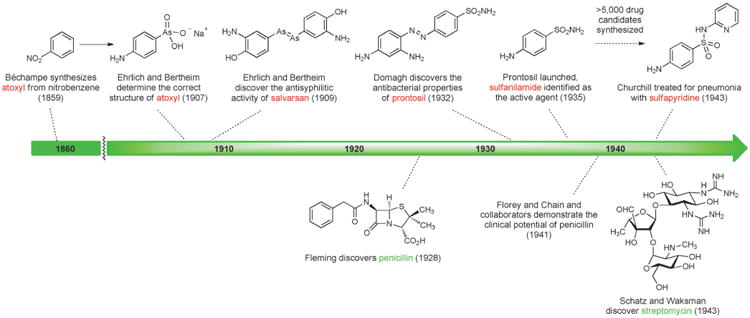
The first effective treatment for a bacterial infection arose from a convergence of disparate advances, including an early chemical synthesis of aniline, Paul Ehrlich's “magic bullet” hypothesis, and the development of the first treatments for African sleeping sickness. In 1854 the French chemist Antoine Béchamp achieved the first economical synthesis of aniline by reduction of nitrobenzene with iron in the presence of hydrochloric acid, a discovery that catalyzed the growth of the synthetic dye industry.[11] Subsequent efforts to prepare aniline derivatives led Béchamp to synthesize a compound known as atoxyl in 1859 by the reaction of aniline with arsenic acid. The chemical structure of atoxyl proposed by Béchamp was later revised (see Scheme 1).
Scheme 1.
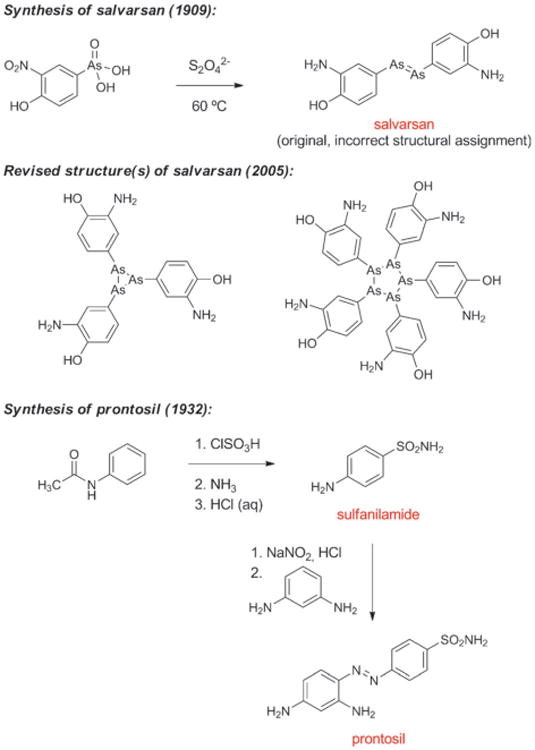
Chemical synthesis of salvarsan and prontosil.
In the latter part of the 19th century, Paul Ehrlich began his prodigious search for a “magic bullet,” a molecule that could combat disease-causing organisms.[12] Ehrlich was broadly interested in fully synthetic dyes, their apparent selective affinities for living tissues, and their therapeutic potential. He hypothesized that the affinity of specific cell types for dye molecules could be harnessed to selectively destroy microorganisms in the body without damaging human cells. An early breakthrough came in 1891 when Ehrlich and Paul Guttmann reported that two patients suffering from malaria had been successfully treated with the fully synthetic thiazine dye methylene blue,[13] possibly the first example of a fully synthetic drug being used in human medicine. Ehrlich was also actively involved in the development of synthetic dye therapeutics for African sleeping sickness, which ravaged equatorial Africa around the turn of the 19th century in an epidemic that claimed between 300 000 and 500 000 lives.[14] His interest was piqued by a paper by H. W. Thomas in 1905 demonstrating that Béchamp's atoxyl exhibited activity against trypanosomes, including the causative organism of sleeping sickness.[15]
Working under Ehrlich's direction, Alfred Bertheim determined in 1907 that the chemical structure of atoxyl had been incorrectly assigned: atoxyl was p-aminophenyl arsenic acid (containing both amine and arsenic acid functional groups, see Figure 2), not an arsenic acid anilide (a less easily derivatized structural isomer containing a nitrogen-arsenic bond), as had been suggested by Béchamp.[16] This was a momentous discovery, as noted later by Bertheim: “Probably for the first time, a biologically effective substance existed whose structure was not only known precisely but also was of a simple composition and extraordinary reactivity, which permitted a wide variety of modifications.” According to Ehrlich, atoxyl “enabled chemotherapy to distance itself from purely empirical trial and error testing and to introduce chemical synthesis.”[17]
Figure 2.
Early history of antibiotics discovery and development.
Bertheim, Ehrlich and co-workers proceeded to synthesize hundreds of structurally related organoarsenic compounds based on atoxyl and test them for activity against trypanosomes. Following a suggestion by Erich Hoffmann, these organoarsenic compounds were also tested against the microorganism found in 1905 by Hoffmann and Schaudinn to be the causal agent of syphilis.[18] This work culminated in the discovery of salvarsan (Figure 2),[19] the first effective treatment for syphilis and the first antibacterial drug. Salvarsan was also known as “Compound 606,” enumerating its place in the sequence of approximately 2000 fully synthetic molecules evaluated during Ehrlich's investigations, and it rapidly became the most widely prescribed drug in the world. By 1920, 2 million doses of salvarsan and neosalvarsan (“Compound 914,” a more water-soluble derivative of salvarsan) were being produced annually in the U.S. alone.[20] Salvarsan was very difficult to administer and had terrible side effects (including deafness), and chemotherapy remained a highly controversial idea.[21]
In the early 1900s Heinrich Hörlein, director of pharmaceutical research at the German chemical conglomerate I.G. Farben, initiated a major effort to find chemotherapeutics for bacterial infections.[22] Hörlein and chemist collaborators had previously discovered that addition of sulfonamide substituents to synthetic dyes often strengthened their binding to wool and silk fibers. They reasoned that the search for a chemotherapeutic agent could build upon this insight from dye chemistry, in that a structural modification that enhanced a molecule's affinity for fibers could also increase its affinity for the protoplasm of bacteria.[23]
Around 1927, I.G. Farben chemists Fritz Mietzsch and Joseph Klarer began to synthesize azo dyes for biological testing. Several factors led them to investigate azo dyes: numerous azo compounds with promising activity against trypanosomes had been discovered during earlier efforts to find therapeutics for sleeping sickness;[14] the azo dye chrysoidine had been found to exhibit in vitro bactericidal effects in 1913;[23] I.G. Farben dominated the global market for synthetic dyes, so the expertise and facilities required to prepare azo dyes were available in-house; and azo compounds of wide structural variability were chemically accessible (a key reason this compound class was appealing).[22] By 1932, Mietzsch and Klarer had synthesized more than 300 azo compounds, including a series containing sulfonamide substituents, and provided these for testing to Gerhard Domagk and others who had developed a suite of in vitro and in vivo biological assays to determine utility against streptococcal infections (among others).[22]
Domagk discovered that the red dye prontosil produced incredible curative effects in mice previously injected with lethal doses of streptococci. In the years that followed prontosil saved the lives of a 10-month-old baby suffering from staphylococcal septicemia and, famously, Domagk's own 6-year-old daughter. In 1935, the same year as the commercial launch of prontosil, it was revealed by researchers at the Pasteur Institute in Paris that the active principle of the first “sulfa drug” was the simpler substance known as sulfanilamide (Figure 2, Scheme 1), a compound very easily prepared in the laboratory even by the relatively primitive methods of the day. This work demonstrated that neither the azo functional group nor the dye character of prontosil were responsible for its therapeutic effect. During the ensuing decade chemists synthesized more than 5000 structural variants of sulfanilamide, and a number of them were launched as drugs.[23] One of these, sulfapyridine (known familiarly as “M&B” after the British manufacturer May & Baker), was used to treat Winston Churchill during a bout of pneumonia in the winter of 1943.[24] Some sulfa drugs such as sulfamethoxazole are still used today, but problematic side effects and the spread of resistance drove many antibacterials from this class out of favor.[25] It is noteworthy that in the history of human medicine the first two antibiotics classes of clinical utility were not natural product-based, but were fully synthetic substances that arose from extensive chemical synthesis and serendipity.
2.2. World War II Catalyzes Production of Penicillin by Fermentation, but not Chemical Synthesis[23]
One of the key scientific breakthroughs of the 20th century occurred when Alexander Fleming discovered in 1928 that a substance produced by the fungus Penicillium chrysogenum (formerly known as Penicillium notatum) exhibited antibacterial activity.[26] Although this finding was made prior to the key achievements of Domagk and collaborators, the fully synthetic sulfa drugs found widespread clinical use many years before penicillin became available for the treatment of bacterial infections. Nearly a decade passed following Fleming's famous discovery before Howard Florey and Ernst Chain received a grant from the Rockefeller Foundation to isolate penicillin and investigate its biological properties. In 1940, the Oxford team member Norman Heatley demonstrated that treatment with crude penicillin significantly extended the lives of mice previously injected with a lethal strain of Streptococcus.[27] The landmark 1940 report in The Lancet begins as follows: “In recent years interest in chemotherapeutic effects has been almost exclusively focused on the sulphonamides and their derivatives. There are, however, other possibilities, notably those connected with naturally occurring substances.” In February 1941, multiple doses of partially-purified penicillin broth were administered to an Oxford policeman suffering from a staphylococcal infection.[28] The policeman's condition improved dramatically following treatment with penicillin, but after five days the limited supply had been exhausted and the policeman succumbed to the resurgent infection. Florey and Chain needed much larger quantities.
By 1941, British industry was engrossed in the war effort and lacked the resources to tackle a large-scale experimental project. Using his Rockefeller connections, Florey crossed the Atlantic and petitioned American pharmaceutical companies to consider mass-production of their therapeutic compound by fermentation. His timing was propitious. In June 1941, President Roosevelt established the Office of Scientific Research and Development (OSRD), a federal agency responsible for coordinating scientific and medical research relating to national defense. The Allies urgently needed to find new treatments for the vast number of troops with disease and wound infections. Sulfa drugs were a hugely important medical breakthrough but also had significant limitations—their spectrum of activity was narrow, and some bacteria acquired resistance rapidly. Furthermore, production of these antibiotics was concentrated in Nazi Germany. The Committee on Medical Research of OSRD initiated a massive project to produce penicillin: one arm of the project aimed to maximize production of penicillin by fermentation, while the other sought to develop a fully synthetic route.
This unprecedented convergence of governments, pharmaceutical companies and academic scientists sparked rapid scientific innovation. Regulatory barriers were knocked down—even impure penicillin had curative effects—and intellectual property concerns were temporarily cast aside.[29] Pfizer scientists James Currie and Jasper Cane achieved a landmark advance by the implementation of deep-tank fermentation techniques for penicillin production, dramatically increasing the production of this life-saving drug.[30] Meanwhile, the synthesis effort involving more than 1000 chemists and 39 major laboratories failed to produce a viable chemical synthesis of penicillin and was terminated in 1945.[23] Disagreement over the true chemical structure of penicillin meant that different groups were trying to synthesize different molecules. Ironically, a team of chemists led by Vincent du Vigneaud did manage to synthesize a minute quantity of penicillin G in spite of the fact that they were targeting a structure that later proved to be incorrect.[31] All efforts to synthesize what turned out to be the correct structure of penicillin, containing a so-called β-lactam or 4-membered cyclic amide function, failed due to the dual challenges of strain and sensitivity posed by the critical β-lactam ring.[32] This “diabolical concatenation of reactive groups”[33] at the core of the penicillin molecule remained essentially inaccessible after perhaps the largest coordinated project (albeit a fairly short-lived one) in the history of organic synthesis.
2.3. A Glimmer of Hope for a Practical, Fully Synthetic Pathway to Penicillins
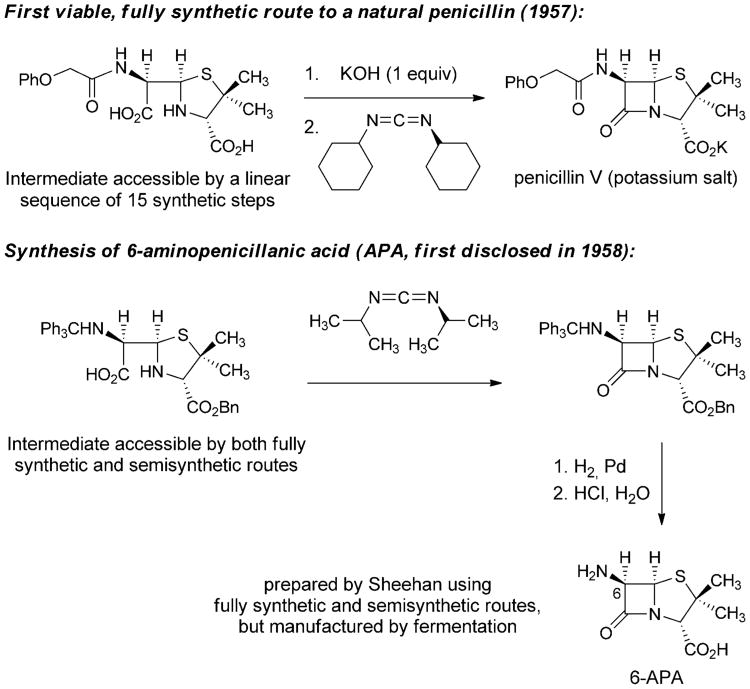
The failure of the penicillin synthesis project during WWII led Ernst Chain to declare in 1946 that the molecule would remain inaccessible by fully synthetic means “unless someone invents an entirely new technique unknown to chemistry.”[34] Shortly thereafter, despite a precipitous drop in research efforts (and research funding) directed toward the development of a fully synthetic route to penicillins, John Sheehan began making progress toward his landmark laboratory synthesis of penicillin V. His research in this area was made possible by the long-term support of the Bristol company. In 1950, Sheehan reported the total synthesis of a penicillin derivative bearing a novel 5-phenyl substituent.[35] Although this non-natural, fully synthetic analog was inactive, the work marked an important step forward. As suggested by Chain, a fully synthetic route to penicillins only became accessible following a transformative chemical innovation. Sheehan brought forward this innovation by inventing an extremely mild method for formation of amide bonds using carbodiimide reagents.[36] This transformation became the key step in the first fully synthetic route to a natural penicillin (penicillin V), published in 1957 by Sheehan and Henery-Logan (Scheme 2).[37]
Scheme 2.
Fully synthetic approaches to penicilin V and 6-aminopenicillanic acid.[23]
In March 1958, Sheehan reported at a symposium that his group had prepared a compound known as 6-aminopenicillanic acid (6-APA) by both fully synthetic and semisynthetic routes, the first public disclosure of a compound that would prove to be critically important to the future discovery of dozens of new β-lactam antibiotics, all with modifications of the C6 sidechain.[38] The following year, scientists at Beecham Research Laboratories in the U.K. reported the isolation of 6-APA from penicillin fermentation broths (having submitted a patent application in 1957),[39] and soon thereafter this intermediate, now produced by fermentation, became the dominant precursor for production of semisynthetic penicillins. Due to the number of steps involved and the low overall yield Sheehan's fully synthetic route to penicillin was not competitive with manufacture by fermentation-semisynthesis, but his pioneering synthetic efforts had led to the discovery of 6-APA and thereby the preparation by semisynthesis of structural analogs that could not have been prepared by other means.
3. Semisynthesis: A Powerful Postwar Engine for Antibacterial Discovery
Bacteria and fungi have continuously evolved over approximately 109 years, producing compounds that confer an evolutionary advantage by killing (other) bacteria, not by their efficacy in treating humans with opportunistic bacterial infections. The evolutionary pressures of human pharmacokinetics, safety, oral bioavailability, and efficacy only came into play in the 1940s when medicinal chemists began to modify fermentation products with the objective of obtaining safer, more efficacious (and proprietary) antibiotics, a process we refer to as “human chemical evolution.” A primary method by which humans have discovered and developed new antibacterial therapies for more than 60 years has been semisynthesis: chemical synthesis using natural products as starting points.
3.1. Origins of Antibacterial Semisynthesis
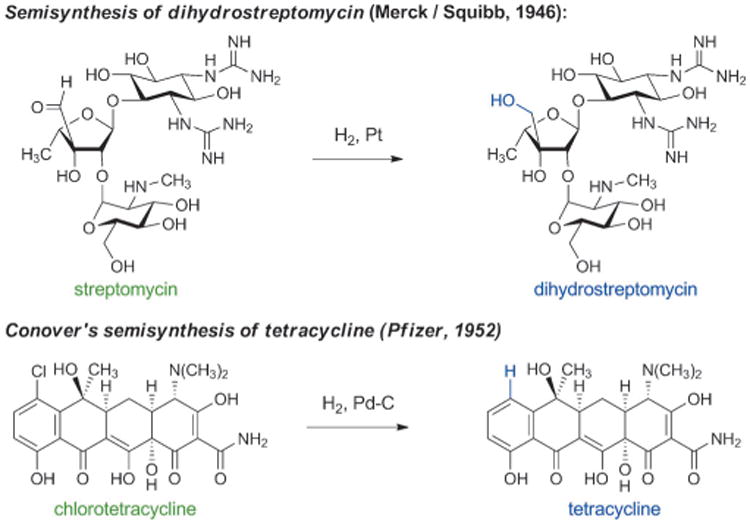
Semisynthesis came to the forefront of antibacterial discovery efforts following innovative chemical alterations of naturally occurring aminoglycosides and tetracyclines. The first aminoglycoside antibiotic was discovered in 1943, when Albert Schatz, a graduate student working with Selman Waksman, isolated streptomycin from the soil microbe Streptomyces griseus. Just as they had done with penicillin a few years previously, pharmaceutical chemists immediately began to probe the structure and properties of streptomycin. In 1946, Robert Peck, Charles Hoffhine, and Karl Folkers at Merck[40] and Quentin Bartz, John Controulis, Harry Crooks, and Mildred Rebstock at Park, Davis & Co.[41] separately discovered that catalytic hydrogenation of streptomycin produced a new compound, dihydrostreptomycin, which exhibited similar antibacterial properties but greater chemical stability (Scheme 3). In 1950, U.S. pharmaceutical firms produced almost 100 tons of streptomycin and dihydrostreptomycin combined, as both antibiotics rapidly found clinical applications.[42] Clinical use of these drugs in humans was later discontinued as a result of their ototoxicity, though they continue to be used in veterinary medicine.
Scheme 3.
The origins of antibacterial semisynthesis.
The first tetracycline antibiotic was discovered in 1948, when Benjamin Duggar of Lederle Laboratories isolated chlorotetracycline (Aureomycin) from the culture broth of Streptomyces aureofaciens,[43] and within two years Pfizer scientists had isolated a second natural tetracycline, oxytetracycline (Terramycin).[44] Chlorotetracycline and oxytetracycline were found to be active against a wide range of Gram-positive and Gram-negative bacteria—together with chloramphenicol (see Section 4.1), they were the first “broad-spectrum” antibiotics. As a brief aside, Gram-positive and Gram-negative bacteria are so called because of their different responses to a common staining protocol developed by Hans Christian Gram. All bacterial cells are bounded by a cytoplasmic membrane, a lipid bilayer that tends to be permeable to uncharged, lipophilic molecules. Gram-negative bacteria (such as Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa) also have an outer membrane that is significantly less permeable to lipophilic molecules. In addition, Gram-negative bacteria often possess multidrug efflux pumps that expel many antibiotics. As a result, the development of antibiotics with activity against Gram-negative bacteria is particularly challenging and currently represents an urgent unmet clinical need.[3, 7i, 45]
Soon after the discovery of chlorotetracycline and oxytetracycline, Lloyd Conover at Pfizer discovered that the carbon-chlorine bond of chlorotetracycline could be cleaved by catalytic hydrogenolysis, producing the first semisynthetic tetracycline antibiotic—tetracycline itself (Scheme 3).[46] The name “tetracycline” is derived from the four linearly fused, six-membered rings that are common to all molecules in this family. Subsequently, tetracycline was found to be a natural product,[47] and by the end of the 1950 s tetracycline was the most prescribed broad-spectrum antibiotic in the U.S.
Although the chemical innovations that enabled the discovery of dihydrostreptomycin and tetracycline may appear trivial today, they had a seismic impact on the strategic mindset of antibiotics discovery and pharmaceutical development more broadly. These innovations demonstrated that natural products could be considered as starting points for the discovery process—extremely useful but not necessarily optimal molecular scaffolds—and henceforth scientists in industry and academia pursued antibiotic research with equal vigor on two fronts: screening of soil samples for new antibacterial natural products, and chemical modification of natural antibiotics to find semisynthetic derivatives with improved therapeutic properties and patentable chemical structures.[29] Semisynthetic innovations have enabled dramatic improvements in antibiotic therapy across all major families of natural antibiotics—here we will discuss the key events and chemical insights that helped overcome the numerous (and constantly evolving) limitations of cephalosporin, tetracycline, and macrolide antibacterials.
3.2. Semisynthesis of β-Lactam Antibiotics
The producing strain of the first cephalosporin antibiotics was discovered in 1948 by Giuseppi Brotzu, Professor of Hygiene at the University of Cagliari. Brotzu observed the propensity of local sewage for self-purification and hypothesized that microorganisms were responsible. He studied the microorganisms present at the outlet of a sewage pipe and discovered that cultures of the mold Cephalosporium acremonium contained one or more substances that were antagonistic to bacteria. Brotzu failed to arouse interest in his discovery in the Italian pharmaceutical industry, and his data and a sample of Cephalosporium acremonium eventually made their way to Edward Abraham at Oxford.[21]
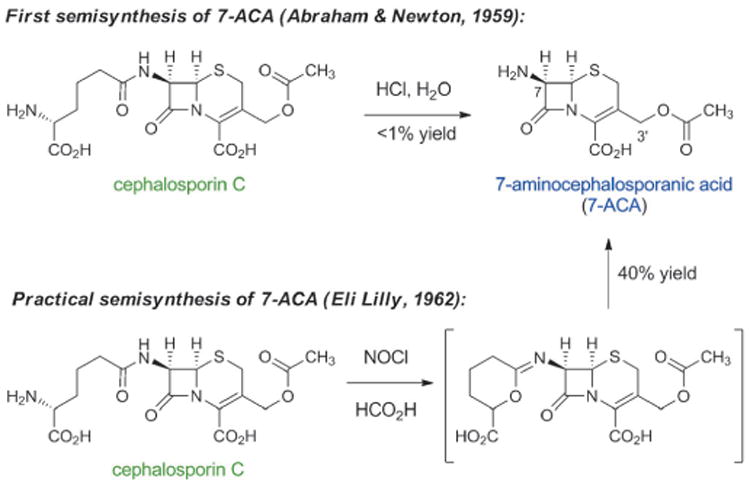
In 1955, Edward Abraham and Guy Newton, two chemists who worked with Florey, succeeded in purifying cephalosporin C from the Cephalosporium culture.[48] Abraham, like others, had observed the subtly or markedly different properties of structurally distinct natural β-lactams and was extremely interested in making chemical modifications to cephalosporins. Regarding cephalosporin C, he later recounted: “There was a great incentive to modify the molecule chemically with a view to increasing its intrinsic activity without affecting its resistance to staphylococcal penicillinase.”[38c] (Penicillinase is a type of β-lactamase with specificity for penicillins; β-lactamases are a collection of bacterial enzymes that hydrolytically open the β-lactam ring, producing inactive molecules). By 1959 Abraham and Newton had synthesized small quantities of 7-aminocephalosporanic acid (7-ACA) by hydrolysis of cephalosporin C under acidic conditions (Scheme 4).[49] Although the yield of this reaction was too low for commercial production, they had discovered a compound that was soon to become (and remains to this day) the key semisynthetic intermediate for production of cephalosporin antibiotics. A few years later, Robert Morin and Bill Jackson at Eli Lilly developed a novel chemical method to remove the side chain of cephalosporin C, providing semisynthetic 7-ACA in a commercially viable yield (40%).[50]
Scheme 4.
Semisynthesis of 7-aminocephalosporanic acid (7-ACA) from cephalosporin C.
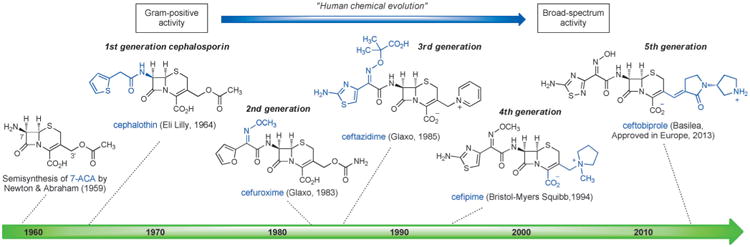
The synthesis of 6-aminopenicillanic acid (6-APA, Sheehan, 1958) and semisynthesis of 7-aminocephalosporanic acid (7-ACA, Abraham & Newton, 1959), and the ensuing development of practical methods for their preparation by fermentation (6-APA, Beecham Research Laboratories, 1959) and by semisynthesis (7-ACA, Eli Lilly, 1962), respectively, opened up the richest treasure trove of antibiotics in human history. More than fifty commercial antibiotics have been discovered and manufactured by chemical modifications of semisynthetic 6-APA and 7-ACA. Here we will limit our discussion to cephalosporins, describing the favorable properties that have been engineered into successive generations through 50 years of human chemical evolution. This evolutionary process began with cephalosporins that exhibited useful activity against Gram-positive bacteria alone and led to the development of compounds that are active against both Gram-positive and Gram-negative organisms (Figure 3). This transition is highly noteworthy and is discussed again later in this Review.
Figure 3.
Human chemical evolution of semisynthetic cephalosporin antibiotics (defining structural features of each generation are highlighted in blue).
First-generation parenteral cephalosporins such as cephalothin (Eli Lilly, approved 1964)[51] exhibited potent activity against Gram-positive organisms but only moderate activity against Gram-negative bacteria. Since the 1960s, chemists have been able to synthesize compounds that possess a broader spectrum of activity, better pharmacological properties, as well as lower susceptibility to resistance mechanisms by introducing innovative side chains at just two modifiable sites of 7-ACA—the amine function at C7, and C3′ (see Figure 3). The expanded-spectrum, second-generation cephalosporins tended to be somewhat less effective against Gram-positive bacteria but significantly more active against Gram-negative bacteria, owing to better cell penetration and resistance to β-lactamases. Importantly, the α-methoxyimino group first introduced in cefuroxime (Glaxo, approved 1983)[52] reduced susceptibility to β-lactamases by sterically blocking cleavage of the β-lactam ring. Gram-negative activity was further improved in third-generation cephalosporins such as ceftazidime (Glaxo, approved 1985).[53] Ceftazidime incorporated an aminothiazole oxime with a charged carboxylate side chain, a combination that enhanced penetration through the porins embedded in the outer membrane of Gram-negative bacteria and helped retain high affinity for the bacterial target (penicillin binding proteins). The emergence of β-lactamases that cleaved third-generation cephalosporins led to the development of fourth-generation molecules such as cefipime (Bristol-Myers Squibb, 1994),[54] which were more active than many third-generation cephalosporins against both Gram-positive and Gram-negative pathogens, including Pseudomonas aeruginosa.[55] The fifth-generation cephalosporin ceftobiprole received approval for use in Europe in 2013 for treatment of hospital-acquired pneumonia.[56]
The human chemical evolution of cephalosporins vividly illustrates the ability of medicinal chemists to continuously tailor the properties of antibacterials to meet specific clinical needs. Widespread clinical use of cephalosporins and other β-lactam antibiotics has selected for bacteria with fierce collections of resistance determinants, but cephalosporins remain critical components of our antibiotic armamentarium. Of the seven drugs currently in advanced clinical development (phase II or III) for the treatment of infections caused by Gram-negative bacilli, three are combinations of a cephalosporin and a β-lactamase inhibitor.[3] Only one of these new combination therapies (ceftolozane/tazobactam, Cubist Pharmaceuticals) incorporates a novel cephalosporin antibiotic, suggesting that the development of new β-lactams is becoming increasingly difficult.
3.3. Semisynthesis of Tetracycline Antibiotics
Beginning with Conover's landmark semisynthesis of tetracycline from chlorotetracycline, the development of semisynthetic tetracyclines has been marked by a series of specific, impactful discoveries. Charles Stephens and collaborators at Pfizer achieved a major enabling advance approximately 10 years after the class had been identified when they demonstrated in 1958 that the C6-hydroxy group of the natural products oxytetracycline, tetracycline and 6-demethyltetracycline could be removed reductively (Scheme 5).[57] The 6-deoxytetracyclines that arose as a consequence were found to be more stable than the parent compounds, yet retained broad-spectrum antibacterial activity. Their enhanced chemical stability enabled further structural modifications that had not been possible with acid- and base-sensitive natural tetracyclines, leading to the discovery of minocycline in 1967 by Michael Martell, Jr. and James Boothe at Lederle laboratories.[57b,58] Minocycline was synthesized from 6-deoxy-6-demethyltetracycline (sancycline) by an electrophilic aromatic substitution reaction at C7, and it exhibited a broader spectrum of activity than prior tetracyclines (including activity against some tetracycline-resistant staphylococci). Like other members of the family, the clinical utility of minocycline declined in the ensuing decades due to increasingly widespread resistance.
Scheme 5.
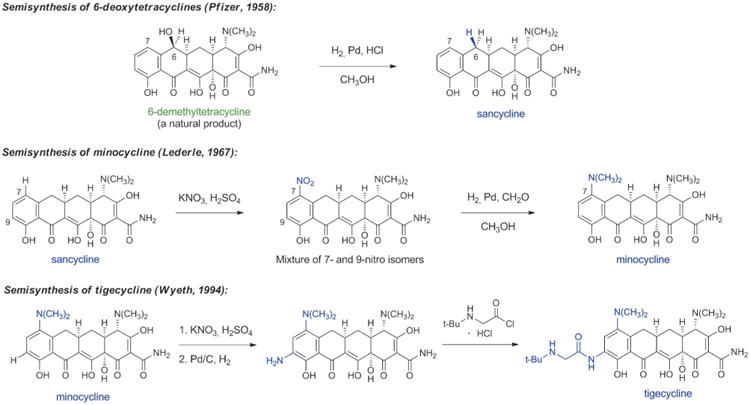
Chemical innovations in tetracycline semisynthesis (important new structural features of each generation are highlighted in blue).
Aiming to overcome tetracycline resistance in the late 1990s, a team of Wyeth scientists led by Frank Tally synthesized 7,9-disubstituted tetracycline derivatives, leading to the discovery of the life-saving antibiotic tigecycline (US approval 2005, Scheme 5).[59] Tigecycline is the defining member of a new class of tetracyclines known as glycylcyclines, which greatly extend the spectrum of tetracyclines, especially toward tetracycline-resistant microorganisms. Tigecycline has become a last line of defense against multidrug-resistant bacteria; for example, it is one of only two approved antibiotics that are active against some carbapenem-resistant bacteria carrying New Delhi metallo-β-lactamase enzymes (NDMs, see Section 4.3 for discussion of carbapenem antibiotics).[5] The other is colistin, which can cause damage to kidneys and nerves. Some of the benefits of tigecycline are attributable to the fact that it binds more strongly than older tetracyclines to the small subunit of the bacterial ribosome (the biological target of all tetracyclines).[60] Its drawbacks include dose-limiting tolerability (nausea, vomiting) and a lack of oral bioavailability. The human chemical evolution of semisynthetic tetracyclines has provided antibacterial therapies that have overcome many limitations of their predecessors, but the slowing pace of discovery in this area is evident (Figure 4).
Figure 4.
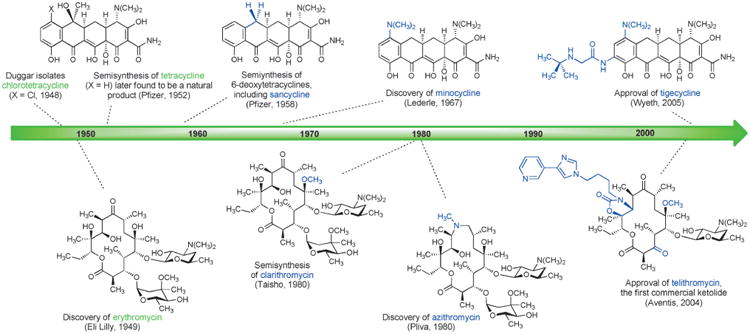
Human chemical evolution of tetracycline and macrolide antibiotics by semisynthesis (important new structural features of each generation are highlighted in blue).
3.4. Semisynthesis of Macrolide Antibiotics
Macrolide antibiotics have undergone serial human chemical evolutionary advances that in many ways parallel those that occurred within tetracycline antibiotics: each semisynthetic advance has built upon prior innovations, and each new (successful) semisynthetic antibiotic has become a starting material for further chemical modification (Figure 4).[61] This strategy is sensible, since it enables favorable characteristics to be carried forward, but it also inevitably leads to a gradual increase in the number of chemical operations required to synthesize new derivatives from the original natural product.
Erythromycin, the first macrolide antibiotic, was discovered in 1949 when scientists at Eli Lilly isolated the natural product from the culture broth of the soil-dwelling fungus Saccharopolyspora erythrea. The term “macrolide” was originally introduced by R. B. Woodward in 1957 to describe metabolic products from Streptomyces that contain a macrolactone ring.[62] Erythromycin was approved for use against a variety of Gram-positive bacterial infections, but upon widespread clinical implementation several limitations were quickly identified. Erythromycin displayed poor oral bioavailability and a short in vivo half-life, and most importantly it was found to be unstable under acidic conditions, giving rise to side effects such as stomach pain. Administration of the antibiotic as an enteric-coated tablet helped sidestep instability to gastric acid; however, innovative chemical solutions were much desired. Studies of chemical instability under acidic conditions revealed that erythromycin decomposes by intramolecular cyclization reactions beginning with addition of the C6 hydroxy group to the C9 ketone, leading to formation of both anhydrohemiketal and spiroketal derivatives (Scheme 6).[63] Knowledge of the chemical basis for instability catalyzed the discovery of semisynthetic macrolides that lacked this significant limitation.
Scheme 6.
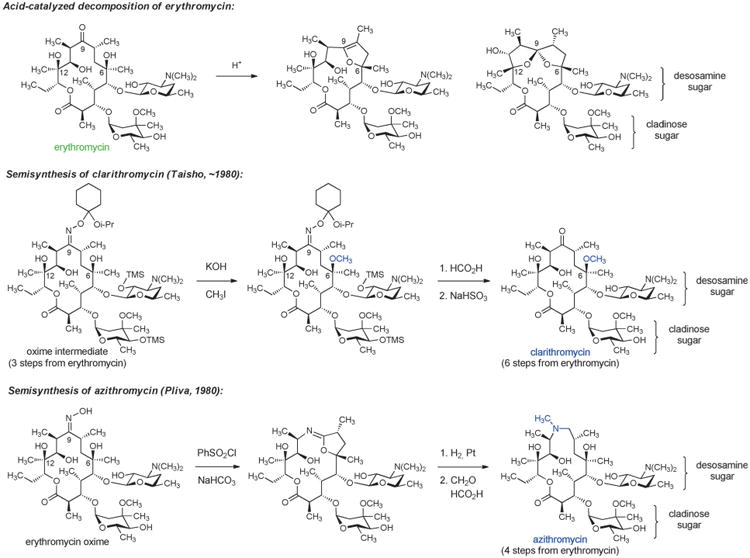
Chemical innovation in macrolide semisynthesis.
One solution was provided by Sadafumi Omura and collaborators at Taisho Pharmaceutical Co. in Japan who developed a 6-step sequence from erythromycin resulting in selective capping of the C6 hydroxy substituent with a methyl group, affording the antibiotic clarithromycin (Scheme 6). Protection of the C9 ketone of erythromycin as an oxime was critical to this work, providing an intermediate whose conformation enabled selective methylation at C6-OH.[64] Clarithromycin displayed a slightly expanded spectrum of activity relative to erythromycin, and it was found to be both acid-stable and orally active.
Another innovative semisynthetic solution to the chemical instability of erythromycin was developed in 1980 by Gorjana Lazarevzki and co-workers at Pliva in Croatia. In this case, the C9 ketone was completely removed from the erythromycin scaffold by a sequence comprising oxime formation, Beckmann rearrangement (ring expansion), and then hydrogenolysis of the resulting iminoether intermediate (Scheme 6).[65] These chemical innovations led to the discovery of an “azalide” structure that became known as azithromycin. Azithromycin was found to have excellent acid stability, oral bioavailability, and an expanded spectrum of activity that included the Gram-negative bacterium Haemophilus influenzae. This macrolide also exhibited a long half-life and achieved very high concentrations in certain tissues. Azithromycin was approved by the FDA in 1991 and rose to be the 7th most prescribed drug (across all therapeutic areas) in the U.S. in 2010 (52.6 million prescriptions). Recent evidence of azithromycin cardiotoxicity, albeit at very low incidence, has raised concerns over such widespread use.[66]
The evolution and widespread distribution of resistance to erythromycin, clarithromycin, and azithromycin has challenged chemists to devise new and improved macrolide derivatives to combat infections caused by drug-resistant bacteria. Two key advances led to the development of the “ketolide” antibiotics, which retain antibacterial activity against many macrolide-resistant organisms. In 1988, William Baker and colleagues at Abbott Laboratories developed a synthetic sequence for introduction of a C11–C12 cyclic carbamate, to which a range of aryl-alkyl side chains could be attached (Scheme 7).[67] Soon after discovery of this sequence, Abbott scientists reported that many of these compounds were active against macrolide-resistant bacteria.[68] It was recently established by X-ray crystallography[69] that the arylalkyl sidechain of the ketolides reaches into an adjacent (novel) binding site within the bacterial ribosome where it makes several additional contacts, accounting for the increased potency of this class. It should be noted that the pioneering crystallographic studies of Yonath, Ramakrishnan, Steitz, as well as other ribosomologists, whose work has produced detailed molecular views of dozens of ribosome-targeting antibiotics bound to their common molecular target, has provided an extraordinarily powerful tool informing antibiotics discovery, broadly speaking.[70,71]
Scheme 7.
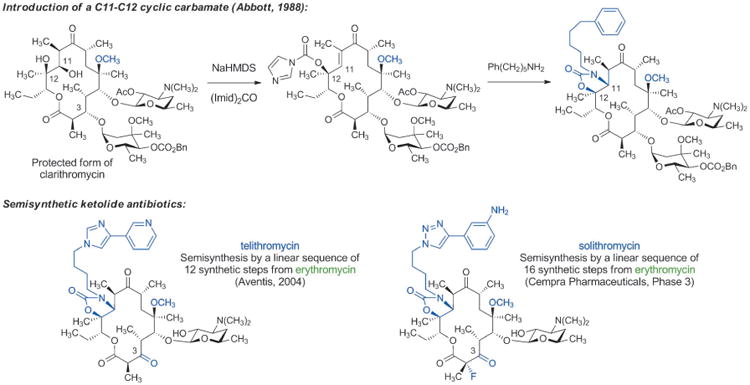
Chemical innovations enable development of semisynthetic ketolide antibiotics.
Previously it had been observed that some forms of macrolide resistance are not induced in the presence of certain natural and non-natural macrolides lacking the l-cladinose sugar (normally attached to the C3-hydroxy group),[72] however advancement of this insight was impeded by the accompanying misperception that l-cladinose was required for activity. The combination of Abbott's chemical innovations and replacement of the l-cladinose sugar with a C3-keto group enabled the development of the “ketolide” antibiotics, which possess excellent activity against many macrolide-resistant organisms.[73] The FDA approved the first commercial ketolide antibiotic, telithromycin (Aventis, Scheme 7) in 2004. Although use of this drug has been greatly curtailed due to evidence of liver toxicity (thought to be caused by its 3-pyridyl function),[74] the innovations that led to its development have revitalized innovation in macrolide discovery and have provided a number of new clinical candidates for the treatment of bacterial infections. It is worth noting, however, that semisyntheses of telithromycin and solithromycin (a ketolide being developed by Cempra Pharmaceuticals, currently undergoing phase III clinical trials)[75] require linear sequences of 12 and 16 synthetic steps, respectively, from their common starting material, the fermentation product erythromycin.
4. Fully Synthetic Antibacterials, 1940-Present
Despite the advent of semisynthesis in the postwar period and its continued widespread application to the present day, fully synthetic approaches to antibacterial drug discovery (which began with the arsenicals and sulfa drugs, as discussed in the introduction) have also led to important new classes of antibiotics and large numbers of approved drugs. The most widely appreciated examples may be the quinolones, carbapenems, and oxazolidinones, but the development of these families occurred well after the discovery of four other important fully synthetic antibacterials—chloramphenicol, metronidazole, trimethoprim, and fosfomycin.
4.1. Amphenicols, Trimethoprim, and Nitroimidazoles
The next antibiotic manufactured by a fully synthetic route after the sulfa drugs was chloramphenicol, a natural product first isolated in 1947 from a culture of Streptomyces venezuelae by John Ehrlich and collaborators at Parke, Davis & Co. and shown to have broad spectrum activity (Scheme 8).[76] Chloramphenicol is a rare case of a natural product that is more economical to produce on industrial scale by chemical synthesis rather than fermentation (another example is thienamycin, the precursor to imipenem). A practical, fully synthetic route to chloramphenicol was developed by John Controulis, Mildred Rebstock, and Harry Crooks at Parke, Davis & Co.[77] and this drug was approved in 1949. Millions of patients were treated with the new antibiotic before reports of rare but fatal aplastic anemia began to emerge.[78] This and other adverse effects, combined with the development of other broad-spectrum antibiotics, led to reduced use of chloramphenicol in the clinic; however, as the result of its ease of manufacture and low cost it is still produced on a massive scale and is widely employed in developing countries, and it remains a component of the WHO Model List of Essential Medicines.[79] A structural analog of chloramphenicol with similar antibacterial activity—thiamphenicol—was first synthesized in 1952 (Figure 5).[80] The replacement of the nitro group in chloramphenicol with a methanesulfonyl group increased potency and avoided the fatal aplastic anemia, rendering the class safer for use in humans.
Scheme 8.
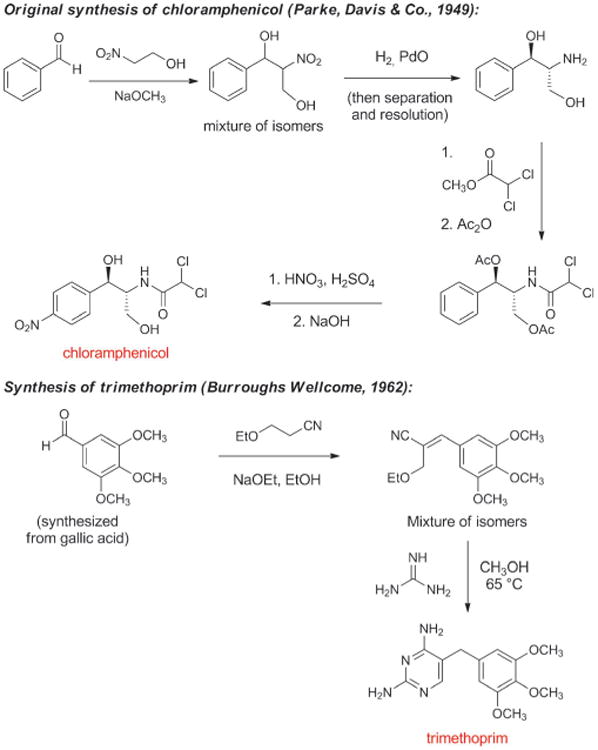
Chemical synthesis of chloramphenicol and trimethoprim.
Figure 5.
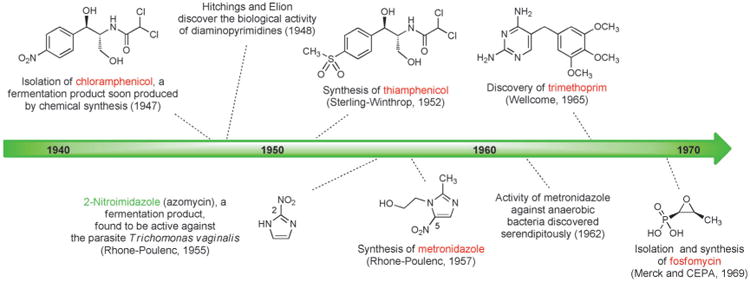
Milestones in the development of fully synthetic antibacterials, 1940–1969.
Contemporaneously with the development of chloramphenicol, George Hitchings, Gertrude Elion, and colleagues at Wellcome Research Laboratories discovered that synthetic analogs of purine and pyrimidine bases inhibited growth of the benign bacterium Lactobacillus casei (their initial test organism) as well as pathogenic bacteria.[81] As Hitchings described in his 1988 Nobel Lecture, their research program was designed to “explore nucleic acid biosynthesis in a new and revealing way by employing synthetic analogs of the purine and pyrimidine bases in a system utilizing these heterocyclic compounds for biosynthesis.”[82] It was soon established that the diaminopyrimidine structural class acted as inhibitors of dihydrofolate reductase, an enzyme found in both bacteria and eukaryotes, whose function is to catalyze the conversion of folic acid (vitamin B9) to tetrahydrofolate, which is essential for cell division. Synthesis and biological testing of various diaminopyrimidines led to the discovery in the early 1960s of trimethoprim (Scheme 8), a potent and highly selective inhibitor of the bacterial form of dihydrofolate reductase.[83] Diaminopyrimidines and sulfonamides (which inhibit an earlier step in tetrahydrofolate biosynthesis) had previously been found to act synergistically in vitro, and trimethoprim was initially only developed in combination with sulfamethoxazole (a therapy known as co-trimoxazole or Bactrim, approved in 1969).[84] Subsequent clinical studies questioned the importance of this synergy and trimethoprim is now also available as a single agent.[85] As with chloramphenicol, the low cost of trimethoprim makes it a particularly attractive treatment option in developing countries. It is reported that more than 1000 tonnes are produced annually in India alone.[86]
Another class of fully synthetic antibacterials developed in this period was the nitroimidazoles. In 1953 Hamao Umezawa and colleagues at the University of Tokyo isolated 2-nitroimidazole (azomycin, Figure 5),[87] a fermentation product which was subsequently found by researchers at Rhône–Poulenc in Paris to be active against Trichomonas vaginalis, the causative parasite of trichomoniasis.[84] Azomycin was toxic and difficult to prepare by chemical synthesis (surprising, given its simple structure), but synthesis and evaluation of a variety of nitroimidazoles led to the discovery in 1957 of a fully synthetic 5-nitroimidazole, metronidazole, which became the first effective drug for the treatment of trichomoniasis (1959, Rhône–Poulenc). Three years later, in 1962, a woman receiving metronidazole for this indication reported an unexpected side effect to her dentist: clearance of her gum infection.[88] This serendipitous discovery eventually led to the use of metronidazole (Flagyl) for the treatment of infections caused by a variety of anaerobic bacteria (including C. difficile), for which it is still prescribed today despite a range of adverse effects.
In 1969, David Hendlin (Merck), Justo M. Mata (Compañía Española de la Penicilina y Antibioticos, CEPA), and coworkers described the isolation of fosfomycin from three strains of Streptomyces.[89] This very polar small molecule exhibited bactericidal activity against both Gram-positive and Gram-negative bacteria, which was found to be due to disruption of cell-wall biosynthesis. In a concurrent publication, Burton Christensen and coworkers (Merck) described the racemic synthesis and resolution of fosfomycin,[90] adaptations of which are still used for large-scale production.[91] Fosfomycin is most commonly prescribed today for urinary-tract infections, conveniently administered as a single-dose treatment.[92]
4.2. Fully Synthetic Quinolone Antibacterials
The first quinolone antibacterial was discovered in the early 1960s by George Lescher and co-workers at Sterling-Winthrop Research Institute when a by-product from an earlier synthesis of the antimalarial drug chloroquine was included in a new screening program. The quinolone by-product exhibited modest activity against Gram-negative bacteria and subsequent synthesis of many similar compounds led to the discovery of nalidixic acid, a 1,8-naphthyridine, which became the first clinically approved antibiotic in this family (Figure 6).[93] Nalidixic acid was widely used in the 1960s and 1970s for the treatment of urinary tract infections caused by Gram-negative pathogens, however this compound's lack of activity against both Gram-positive bacteria and strains of Pseudomonas aeruginosa, as well as its significant side effects, necessitated the development of more effective agents.
Figure 6.
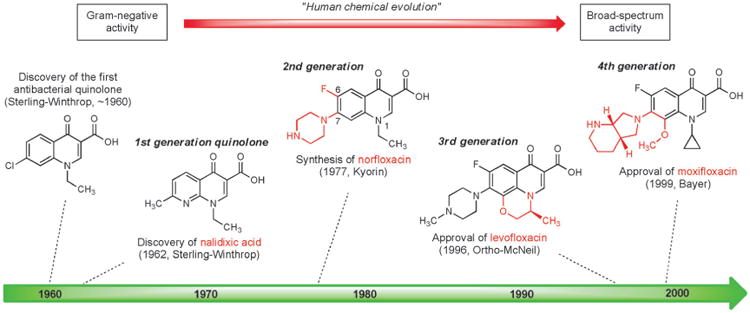
Milestones in the development of fully synthetic quinolone antibacterials, 1960–1999.
Quinolones are more difficult to synthesize in the laboratory than sulfanilamides, but they are nevertheless easily constructed by short synthetic routes. It has been estimated that more than 10 000 quinolones or structurally-related agents have been synthesized as part of quinolone antibacterial research and development, resulting in the approval of more than 25 fully synthetic antibiotics of this class.[94] A major advance came in 1977 when Hiroshi Koga and collaborators at the Kyorin Company in Japan first synthesized norfloxacin (Figure 6),[95] which incorporated both a fluorine atom at C6 and a piperazine substituent at C7.[96] Norfloxacin exhibited greatly improved Gram-negative activity and modest activity against Gram-positive bacteria. Replacement of the N1 ethyl group of norfloxacin with a cyclopropyl substituent produced ciprofloxacin, which received FDA approval in 1987 and became the first quinolone antibiotic to be used for treatment of respiratory tract, skin and joint infections, including infections caused by Pseudomonas aeruginosa.
As with the human evolutionary processes described above for the development of semisynthetic antibiotics, each generation of fully synthetic quinolones has retained key structural features which were the product of prior medicinal chemistry efforts while incorporating new elements to further expand utility. Third- and fourth-generation quinolones such as levofloxacin[97] (Hayakawa and coworkers, Daiichi Seiyaku, approved 1996) and moxifloxacin (Klaus Grohe and coworkers, Bayer, approved in 1999)[98] have improved pharmacokinetic properties and have demonstrated stronger activity against anaerobes and Gram-positive bacteria. Although quinolones are one of the most commonly prescribed classes of antibiotics, they are also associated with a wide variety of adverse side effects.[99] Discovery and development of new fully synthetic quinolone antibiotics remains an active area of research.[100]
The introduction of third- and fourth-generation quinolones advanced the human chemical evolution of this family of antibacterials by transforming molecules that targeted Gram-negative bacteria alone to create broad-spectrum agents. This process mirrors the development of semisynthetic cephalosporins (discussed above), which were selectively active against Gram-positive bacteria until Gram-negative activity was engineered into them through strategic exploration of chemical space. Medicinal chemists have repeatedly proven their ability to shift the activity spectrum of antibacterial agents (Gram-positive to Gram-negative, or vice-versa), suggesting that molecules possessing Gram-positive activity should not be disregarded as potential starting points for the development of new antibacterials with Gram-negative activity (currently an even more pressing clinical need). Similarly, chemists have shown many times over that acquired resistance mechanisms to a class of antibiotics can often be defeated by further structural optimization.
4.3. Fully Synthetic Routes to β-Lactams Finally Become Sufficiently Practical for Commercial Production
From the early 1900s until 1980, all antibacterial agents developed and then manufactured using fully synthetic approaches had very simple structures (from the standpoint of chemical synthesis). All but two of them—chloramphenicol and thiamphenicol—were achiral molecules. The development of fully synthetic β-lactams in the 1980s and early 1990s marked a dramatic leap forward in the complexity of antibacterial molecules that could be manufactured practically on an industrial scale using fully synthetic approaches. A prodigious amount of effort has been devoted to the development of fully synthetic routes to a wide variety of natural and non-natural β-lactams. For a comprehensive list of fully synthetic β-lactams that have been investigated and a full account of β-lactam development more broadly, we direct readers to the relevant chapter in Antibiotic Discovery and Development.[101] Our discussion here will focus on those fully synthetic β-lactams that have achieved clinical importance.
The success of cephalosporin antibiotics stimulated great interest in the design and synthesis of cephalosporin analogs with modified core structures. In 1974 Cama, Christensen, and Guthikonda at Merck reported fully synthetic routes to “carbacephalosporin” and “oxacephalosporin” analogs replacing the sulfur atom within the bicyclic core of cephalothin (a first-generation cephalosporin), by a carbon atom and an oxygen atom, respectively (Figure 7).[102] Crucially, these fully synthetic analogs exhibited biological activity that was comparable to cephalothin.
Figure 7.
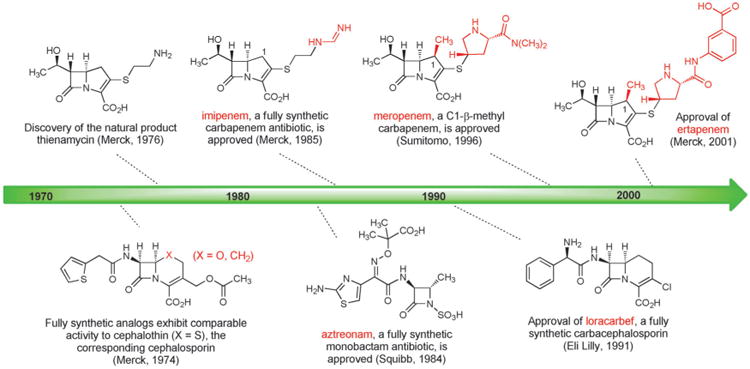
Milestones in the development of fully synthetic β-lactam antibiotics.
The most significant advances that followed were the discovery and development of the carbapenems, but two other innovations flowed more directly from this early work. The idea of replacing the sulfur atom in the cephalosporin core with an oxygen atom was adopted by Mitsuru Yoshioka, Teruji Tsuji, Wataru Nagata, and colleagues at Shionogi Research Laboratories who developed a semisynthetic route from penicillins to oxacephalosporins, including the antibiotic latamoxef,[103] which was approved in the early 1980s but subsequently discontinued following several fatal cases of coagulopathy. Building upon Merck's original synthesis of a carbacephalosporin and other important synthetic precedents,[104] Leland Weigel and collaborators at Eli Lilly developed a kilogram-scale synthesis of loracarbef, a fully synthetic carbacephalosporin which received FDA approval in 1991.[105] Although clinical use of loracarbef was discontinued in the U.S. in 2006, the impressive body of work that led to the discovery and development of this compound still represents a significant achievement in the history of fully synthetic β-lactam antibacterials (Figure 7).
In 1976 scientists at Merck isolated thienamycin from fermentation broths of the soil bacterium Streptomyces cattleya.[106] Thienamycin was the first natural “carbapenem” antibiotic—penems are a group of bicyclic β-lactam structures with a “right-hand,” five-membered ring that contains a carbon-carbon double bond (penicillins are “penams,” with a carbon-carbon single bond in the corresponding position); in carbapenems the sulfur atom within this ring is replaced by a carbon atom. Thienamycin was found to be a broad-spectrum antibiotic, with exceptional activity against both Gram-positive and Gram-negative organisms, including strains of Pseudomonas aeruginosa and organisms with acquired β-lactamase resistance mechanisms. Thienamycin also proved to be chemically unstable because of a propensity for intermolecular reaction of the amine function of one thienamycin molecule with the β-lactam of another.[107] This instability made thienamycin unsuitable for commercial development, but W. J. Leanza and colleagues at Merck found that transformation of the amine group to an N-formimidoyl group led to significantly more stable compound, the highly active antibiotic imipenem (Figure 7).[108] However, thienamycin was extremely difficult to isolate and purify from complex fermentation mixtures, leading Thomas Salzmann and collaborators at Merck to initiate development of a practical, fully synthetic route to this exciting new class of β-lactams (Scheme 9).[109]
Scheme 9.
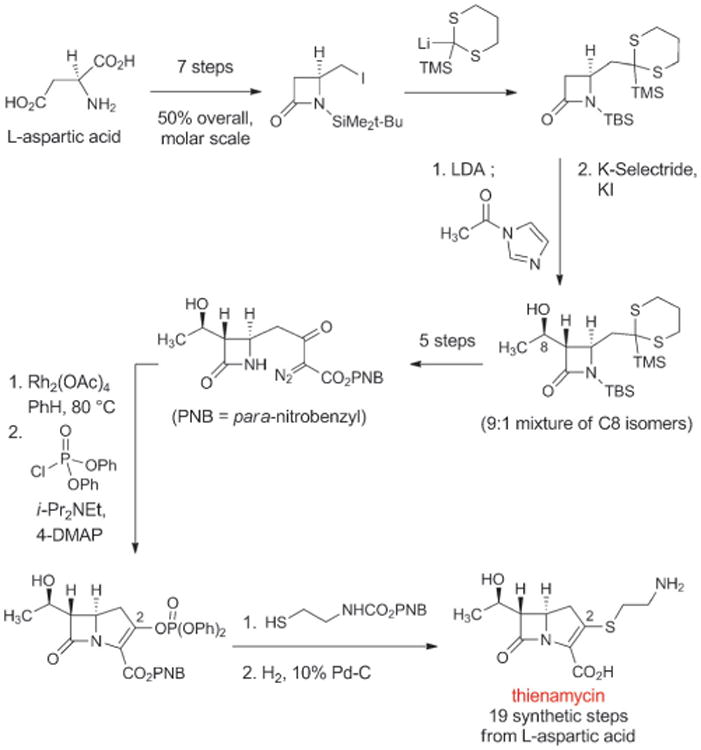
A fully synthetic route to the natural carbapenem thienamycin, the precursor to the fully synthetic antibiotic imipenem.
The strategy behind the original Merck synthesis was to defer introduction of the C2-cysteamine side chain until late in the synthesis, enabling a series of analogs with structural variations in the thiol side-chain to be prepared. In the key step of the synthesis the bicyclic carbapenem core is formed in quantitative yield by rhodium-catalyzed cyclization of a diazo keto ester (Scheme 9). Merck chemists D. G. Melillo and I. Shinkai built upon this original work in their second-generation fully synthetic route to thienamycin.[110] These impressive achievements were the driving force behind the development of imipenem, the thienamycin derivative that in 1985 became the first carbapenem to be approved for clinical use (Figure 7). Imipenem remained an essential last line of defense against a number of serious infections for decades after its introduction, but it also suffered from significant limitations. Imipenem is rapidly inactivated by human renal dehydropeptidase-1, so it must be administered in combination with cilastatin, an inhibitor of this enzyme.[111] Furthermore, its relatively poor hydrolytic stability (though not to the same extent as thienamycin) necessitated four-times daily dosing.
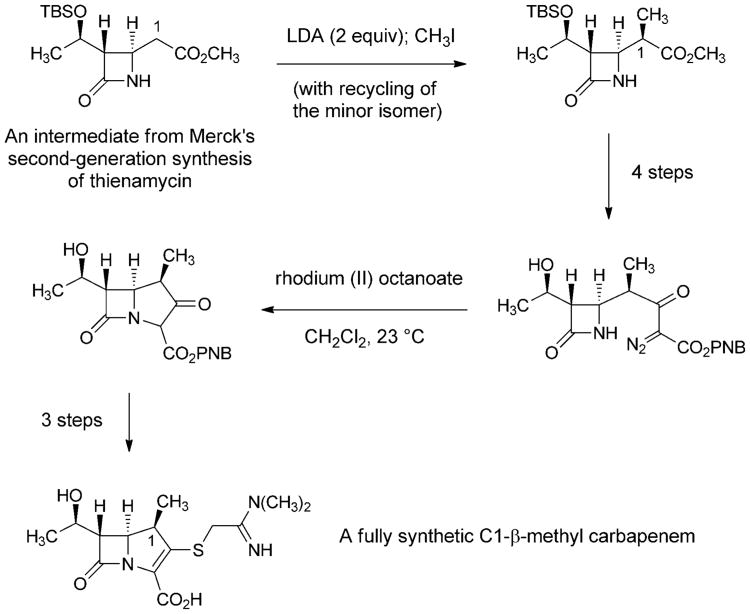
The search for carbapenems with a broad antibacterial spectrum but better pharmacokinetics than imipenem led to another key innovation by David Shih and colleagues at Merck—the introduction of a C1-β-methyl substituent into the carbapenem core (Scheme 10).[112] The C1-methyl group was introduced by alkylation of an intermediate from their second-generation fully synthetic route to thienamycin. The C1-β-methyl product was advanced to the corresponding fully synthetic carbapenems, which were found to be resistant to renal dehydropeptidase and active against a broad spectrum of bacterial pathogens. This innovative modification to the carbapenem core was then adopted by chemists at Sumitomo Pharmaceuticals, leading them to discover meropenem, the first C1-β-methyl carbapenem to receive clinical approval (1996, Figure 7).[113] Further improvements were subsequently made (frequent dosing is still required with meropenem), leading to the 2001 approval of another fully synthetic C1-β-methyl carbapenem, ertapenem (Merck).[114]
Scheme 10.
Key steps of a fully synthetic route to a C1-β-methyl carbapenem (Merck, 1984).
Carbapenems are not the only fully synthetic β-lactams that have become important antibiotics. In 1981, two research groups independently reported the isolation of monocyclic β-lactam (“monobactam”) natural products from different bacterial strains.[115] The promising Gram-negative activity of some of these compounds and the relative simplicity of their core structures (compared with bicyclic β-lactams such as penicillins and cephalosporins) led Breuer, Denzel, Treuner, and collaborators at Squibb to develop fully synthetic routes to various monobactam analogs.[116] The result of this work was the discovery of aztreonam (approved 1984, Figure 7), the only commercially available monobactam and an important antibiotic for the treatment of infections caused by Gram-negative bacteria. The development of new, fully synthetic monobactams continues to be an active area of pharmaceutical research.[117]
4.4. Fully Synthetic Oxazolidinone Antibacterials, a New Structural Class of Antibiotics
The oxazolidinones provide further examples of antibacterials discovered and developed using fully synthetic approaches. The antibacterial properties of the oxazolidinone structural class were first recognized in 1984 by Andrew Slee and collaborators at DuPont while investigating compounds for the treatment of plant diseases caused by microbial pathogens. The DuPont group synthesized a number of oxazolidinones that were active against streptococci and staphylococci (including methicillin-resistant Staphylococcus aureus, MRSA),[118] but subsequent animal studies revealed significant bone marrow toxicity. Recognizing the potential of this compound class, Steven J. Brickner and colleagues at Upjohn initiated a research program to find potent oxazolidinones that were safe for human use, leading to the discovery and approval in 2000 of linezolid (Figure 8), the first commercial oxazolidinone and the first antibacterial from a novel structural class in almost 40 years (the last was nalidixic acid).[8b,119] Linezolid is an essential last line of defense for treatment of infections caused by Gram-positive bacteria such as MRSA and vancomycin-resistant enterococci (VRE), but long-term use can cause serious adverse effects such as bone marrow suppression. The development of next-generation oxazolidinones is an exciting area of research, now informed by an X-ray crystal structure of linezolid bound to its target, the large subunit of the bacterial ribosome.[120, 121]
Figure 8.
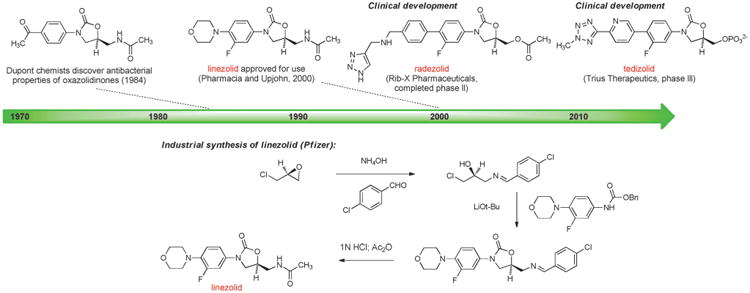
Development of fully synthetic oxazolidinone antibacterials.
4.5. A Fully Synthetic Platform for the Discovery and Development of Novel Tetracycline Antibiotics
The important if rather obvious lesson from the sulfa drugs, quinolones, carbapenems, and oxazolidinones is that when chemists are able to access antibiotic classes by diversifiable, fully synthetic routes, their ability to modify the structures at will is transformative, leading to new, more powerful, and safer drugs. Looking at the complete set of fully synthetic antibacterials in clinical use today, the carbapenems developed in the 1980s and early 1990s (imipenem and meropenem) stand out as the most challenging structures to be successfully manufactured by fully synthetic routes. At the time our laboratory undertook the development of a practical synthetic route to tetracycline antibiotics in the mid-1990s, all clinically approved tetracyclines were fermentation products or had been derived from them by semisynthesis. For six decades semisynthetic modification of tetracyclines had been limited largely to just three positions (C6, C7, and C9) and substitutions at C7 and C9 were highly constrained by lack of chemical enablement. This is undoubtedly a contributing factor to the stark disparity in the number of approved tetracyclines (fewer than 10 in the US since 1949) versus the numbers of approved quinolones (> 40) and beta-lactams (> 50). From the time that the structures of the tetracycline antibiotics were first elucidated by Woodward and collaborators in 1953,[122] laboratories throughout the world had worked to develop routes to prepare existing and novel members of the class. The Woodward, Shemyakin, and Muxfeldt groups reported remarkable advances for their time with their successful constructions of sancycline (25 synthetic steps, 0.002% yield), tetracycline (yield not reported), and oxytetracycline (22 steps, 0.06% yield), respectively, but these routes were lengthy and impractical to scale (though it should be noted that the Muxfeldt approach was for a time adapted by researchers at Merck in Germany for the preparation of fully synthetic 6-thiatetracycline, an antibiotic candidate that was abandoned during clinical development due to liver toxicity).[123] Interestingly, each group had employed a “left-to-right” or D→A mode of construction, which was not ideal from the standpoint of drug discovery, since substitution of the D ring proves to be remarkably fruitful for the development of novel antibiotics, especially those with improved activities against tetracycline-resistant microorganisms, whereas most substitutions of the A ring diminish or abolish antibiotic activity.
In 2005, after more than 10 years of research on the problem, our laboratory reported that tetracyclines could be assembled in three steps from two relatively simple building blocks—a “left-side” D-ring precursor and a “right-side” AB-ring precursor (Scheme 11).[124] The identification of a practical route to the AB enone was the most time-consuming aspect of the problem. We have since described different, more practical component-based routes to the AB enone,[125] one of which has been adapted to prepare >50 kg of this key intermediate. In the AB+D approach, the C ring of tetracyclines is formed by a stereocontrolled Michael-Claisen cyclization reaction that forms two carbon-carbon bonds and two stereogenic centers in one operation.[124] This transformation has proven to be remarkably robust, is effective with a broad range of D-ring precursors, and has been executed on kilogram scale in >90% yield.[126] The cyclization products are transformed into fully synthetic tetracyclines by two or three “deprotection” steps that unveil much of the polar functionality that had long hampered semi-synthetic innovation. A key enablement in this regard was the development of the benzyloxyisoxazole function to protect the A-ring of tetracyclines, reported by Stork and Hagedorn in 1978.[127]
Scheme 11.
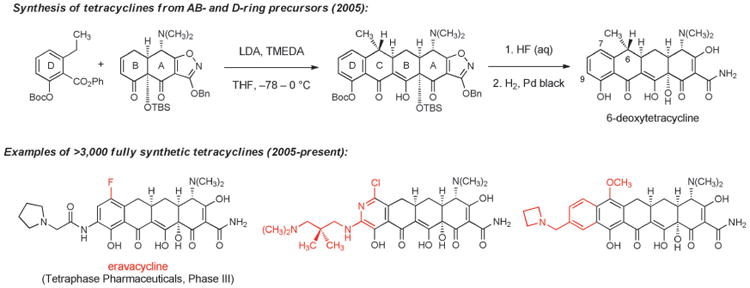
Development of fully synthetic tetracycline antibacterials (novel structural features that could not be introduced by semisynthesis are highlighted in red).
The development of a highly diversifiable and scalable synthesis of tetracyclines, broadly defined, has led to a dramatic expansion of the chemical space accessible to medicinal chemists. Positions that had not been previously modified, such as C5[128] and C8, have now been explored extensively, and a broad array of substituents that were previously inaccessible at other positions have been introduced. More than 3000 diverse, fully synthetic antibiotic candidates have been made and tested since 2005 at a small biotechnology company, Tetraphase Pharmaceuticals, which was founded specifically to commercialize the tetracycline technology platform.[126,129] The majority of these fully synthetic compounds are active in antimicrobial assays; those with most promising activities, either as broad-spectrum antibiotics or Gram-negative focused agents, have entered the path toward clinical development. The most advanced fully synthetic clinical candidate is eravacycline, which is currently in phase III clinical trials as a broad-spectrum antibiotic for life-threatening complicated intra-abdominal infections, with planned advancement into phase III trials for complicated urinary tract infections this year. Eravacycline is the first 7-fluorotetracycline to enter clinical trials. It is characterized by a unique combination of potent broad-spectrum activity, favorable pharmacokinetics, low incidence of adverse events, and it is the first glycylcycline with demonstrated oral activity. The frontispiece of this Review depicts results from a microdilution assay of eravacycline and earlier tetracyclines against a lethal strain (a clinical isolate, exhibiting mortality in approximately half of patients with bloodstream infections) of Acinetobacter baumannii that is highly resistant to carbapenems (ertapenem, imipenem, and meropenem MICs > 32 μgmL−1), fluoroquinolones (levofloxacin MIC > 32 μgmL−1), aminoglycosides (gentamycin MIC > 32 μgmL−1), and piperacillin/tazobactam (MIC > 128 μgmL−1).[130]
The synthetic platform that enabled both the rapid preparation of a diverse library of fully synthetic tetracyclines (broadly defined) and cost effective, multi-kilo-scale synthesis of eravacycline exemplifies the power of a highly modular, convergent synthetic strategy. It is estimated that the cost of dispensing eravacycline into a sterile vial for IV administration will exceed that of the drug substance, making clear that fully synthetic pathways to complex molecular scaffolds need not be prohibitive with respect to cost-of-goods. From the standpoint of a researcher in Massachusetts, with access to large quantities of the AB precursor from a vendor, the route to new tetracycline antibiotics appears as a 3–4 step process: coupling, followed by 2–3 steps for deprotection.[131] From the standpoint of the commercial vendor responsible for synthesizing the AB precursor, the route appears as a 5-step sequence beginning from two simple fragments of similar synthetic complexity.[125a] The component-based approach makes possible a division of complexity into approachable subunits, much like the supply chains that enable commercial production of cell phones, personal computers, and aircraft. It allows for multiplicative expansion of structural diversity by component modification, and accelerates development of the route overall by independent evolution of component syntheses. We believe that with greater emphasis on highly convergent, component-based processes, access to an array of heretofore-inaccessible antibiotics platforms can be achieved.
5. Chemical Synthesis as a Path Forward
“The chemists will fasten on the molecule and modify it, as they have done with the sulfanilamide molecule in the last 5 years, so that derivatives of penicillin will appear more powerful, or with wider applications, and diseases now untouched will be conquered.”
(Alexander Fleming, 1943, to the Royal Society)[23]
A broader interpretation of Fleming's statement might hold that few if any antibacterial natural products cannot be improved as human therapeutics by chemical modification. The 100-year history of antibiotics discovery and development began with the clinical deployment of the arsenicals and sulfa drugs, molecules derived not from natural products but fully synthetic compound collections. Since then, one constant driver of progress in antibacterial therapy has been the expansion of accessible chemical space around (and within) natural and non-natural molecular scaffolds known to possess antibacterial properties. Repeatedly, chemical synthesis has led to antibiotics with increased potencies, improved safety profiles, and extended spectrums of activity, especially toward bacteria with acquired resistance mechanisms.
The development of fully synthetic β-lactams such as imipenem, loracarbef and meropenem in the 1980s and early 1990s marked a dramatic leap in the complexity of antibacterial molecules that could be produced on an industrial scale using fully synthetic approaches. Thirty years on, we believe that the power of modern chemical synthesis can make possible the development of practical, flexible routes to substantially more complex antibacterial molecules—molecules based on existing natural product scaffolds that would not have been feasible for commercial synthesis in the past. Should this be true, this path forward surely offers a very high probability of delivering multiple new antibiotics to society and, as such, may provide the engine of innovation that is so desperately needed. Complex natural antibacterials for which practical, readily diversifiable synthetic routes have not yet been devised are underutilized resources that present major opportunities for future innovation. While we strongly believe that a sustained and focused effort to develop practical routes for the synthesis of yet unsolved natural antibiotics scaffolds provides an extraordinary opportunity to restock the dwindling antibiotic pipeline in the near term (5–20 years), we do not suggest that this is the only path forward, for to do so would ignore the successes that led to the inception of the field (organoarsenicals, sulfa drugs) and, later, other life-saving antibiotics (trimethoprim, the quinolones, the oxazolidinones).
Previous sections make evident that some of the greatest advances in antibacterial drug discovery arose only with the development of scalable, fully synthetic routes. Often these achievements served to re-define the boundaries of what was commonly perceived to be practically accessible by synthesis (the potent oncology drug Halaven, manufactured by Eisai Co. using chemical innovations originating from the Kishi laboratory, probably defines the farthest limit of what may be synthesized on large scale today; the typically higher dosages of antibiotics define a somewhat more constraining environment). We have chosen the natural products in Figure 9 and 10 with the view that they may now fall within the realm of synthetic feasibility, especially with emphasis on proper design strategy (convergent assembly of components of similar synthetic complexity), and giving due consideration to their antibacterial activities. This selection is neither complete nor is it static—new antibacterial natural products will undoubtedly be discovered, though admittedly the pace of discovery has slowed. What follows is a brief discussion of the historical advances thus far (largely through semisynthesis) with focus on the potential for fully synthetic platform technologies to define future advances.
Figure 9.
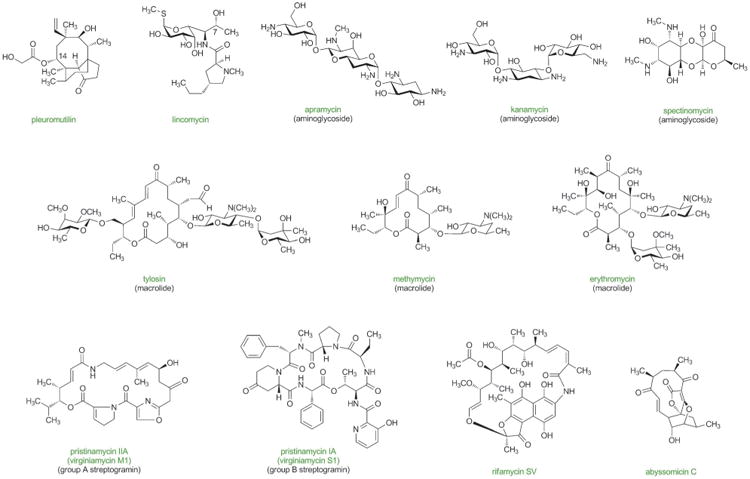
Antibacterial natural products with potential for improvement for human use through the development of practical, fully synthetic routes.
Figure 10.
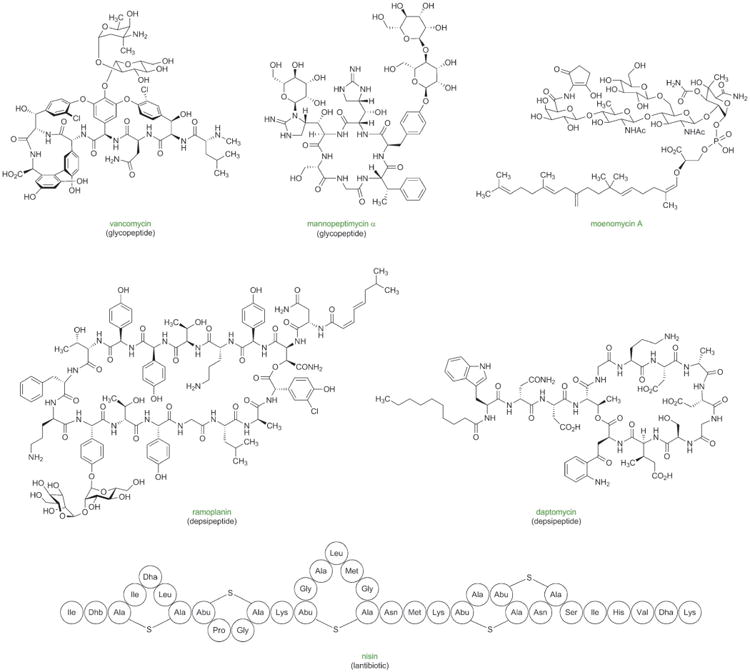
Antibacterial natural products with potential for improvement for human use through the development of practical, fully synthetic routes.
5.1. Pleuromutilins
The tricyclic natural product pleuromutilin was discovered in 1951 from a fungal culture and was found to be active primarily against Gram-positive bacteria.[132] Since then more than 1,200 derivatives of pleuromutilin have been prepared by semisynthesis, including retapamulin (GSK, approved in 2007 for the topical treatment of skin infections, the only pleuromutilin currently approved for human use) and BC-3781 (Nabriva Therapeutics, phase II clinical trial completed in 2011).[133] Like many antibiotics currently in clinical development, the pleuromutilins are inhibitors of bacterial protein synthesis, and X-ray crystallographic studies have revealed details of their binding to the 50S subunit of the bacterial ribosome.[134] The structurally complex core of pleuromutilins and the majority of the substituents on its periphery would be extremely difficult to modify by semisynthesis, as evidenced by the fact that the vast majority of the >1200 semisynthetic analogs synthesized to date are variant solely within the C14 sidechain.[133] Recent studies have suggested that the primary binding interactions between pleuromutilin antibiotics and the ribosome are localized within the polycyclic core.[134] The development of a modular synthetic platform to pleuromutilins (broadly defined), one linking components of similar structural complexity by a short and convergent route, would hold enormous potential for antibiotics discovery, we believe. No fewer than three fully synthetic routes to pleuromutilin have been published, all remarkable achievements, but the linear routes employed would be challenging to scale and do not lend themselves to rapid analog synthesis.[135] Both the Zard[136] and Sorensen[137] groups have reported abbreviated routes to simplified pleuromutilin analogs; in the latter work compounds with activity against M. tuberculosis were identified.[137b] Densely functionalized, stereochemically complex polycyclic targets such as pleuromutilin are among the most challenging types of targets to prepare by highly convergent, component-based synthetic routes, but the return on investment for a successful platform technology could be substantial in terms of new antibiotics generated.
5.2. Lincosamides
Lincomycin was first reported by Mason, Dietz, and DeBoer at UpJohn in 1962 and was launched commercially in 1964.[138] In 1965 Birkenmeyer and Kagan at UpJohn announced that they had prepared a semisynthetic lincosamide, as the new antibiotic class was known, by invertive replacement of the 7-hydroxy group of lincomycin with chloride.[139] The new semisynthetic antibiotic, “clindamycin,” was more potent, had a broader spectrum of activity, and in a marked advance, was orally bioavailable. Clindamycin has been used since 1968 for the treatment of infections caused by aerobic and anaerobic Gram-positive bacteria, including strains of methicillin-resistant Staphylococcus aureus.[140] Clindamycin remains an essential life-saving antibiotic, but is burdened with promotion of C. difficile infections, as well as growing resistance.[141] It is possible to hydrolyse (and reform) the central amide bond that links two molecular “halves” of similar sizes, an amino sugar component and a substituted proline residue. This has fueled bursts of somewhat limited structural variation of both components by semisynthesis over the years. For example, in the 1980s Birkenmeyer, Zurenko and collaborators at UpJohn replaced the pyrrolidine ring with a substituted piperidine ring and so obtained “pirlimycin,” which they launched for the treatment of mastitis in veterinary medicine.[142] Other semisynthetic modifications have been described, some as recently as 2013,[143] as well as fully synthetic approaches,[144] but data available in the public domain suggests that more systematic exploration of the entire molecule has yet to be conducted. A fully synthetic platform technology targeting novel lincosamides could well provide new opportunities for antibiotic discovery. Such a program would be informed by detailed crystallographic studies of lincomycin and clindamycin bound to their molecular target, the 50S subunit of the bacterial ribosome.[69e]
5.3. Aminoglycosides
The aminoglycosides are a structurally diverse family of natural and semisynthetic antibiotics that have been used primarily for the treatment of infections caused by Gram-negative bacteria.[145] Significant advances have been made to overcome developing resistance to aminoglycosides, including the semisynthetic incorporation of a 2-hydroxy-4-amino-butyroyl sidechain within the approved antibiotic amikacin to prevent enzymatic inactivation of the drug, a problem common to natural aminoglycosides (such as the amikacin parent kanamycin). Heinz Moser and collaborators at Achaogen are developing a next-generation semisynthetic amino-glycoside, plazomicin, which exhibits activity against many aminoglycoside-resistant bacteria and recently completed a successful phase II clinical trial evaluating its efficacy in complicated urinary-tract infections (CUTIs).[146] The structurally unique aminoglycoside apramycin has recently been found to have greatly decreased ototoxicity relative to other aminoglycosides, as well as a favorable activity profile, and has potential for further development.[147]
The widespread prevalence of multidrug-resistant Neisseria gonorrhoeae has led to renewed interest in the antibiotic spectinomycin, which is broadly effective in this pathogen, including strains resistant to many other antibiotics.[148] Spectinomycin inhibits translation by binding to the ribosomal protein S5,[149] and several mechanisms of resistance are characterized on the molecular level. Fully synthetic approaches to spectinomycin have been reported,[150] but further structural exploration could increase the potential of this class to defeat developing resistance in N. gonorrhoeae and perhaps other microorganisms.
While further semisynthetic innovations may be possible, the density of heteroatoms within the aminoglycosides has allowed only a few select positions of these scaffolds to be investigated thoroughly.[151] Although fully synthetic approaches to the aminoglycosides have been reported,[152] we believe that the diverse group of natural products that define this family offers untapped potential for the development of practical, fully synthetic routes that harness the power of modern chemical synthesis. In light of the potential for new aminoglycosides to address the growing threat of Gram-negative infections, further advances in this area would be especially welcome.
5.4. Macrolides
Since the discovery of erythromycin over 60 years ago, the macrolides have proven to be safe and effective antibiotics for the treatment of respiratory infections caused by Gram-positive bacteria. Rising resistance both nosocomially and in the community has rendered older macrolides ineffective. To date, all macrolides approved or in development for use in humans are derived by linear semisynthetic modifications of erythromycin (vide supra). Analysis of more recent clinical candidates raises the question of whether semisynthesis of macrolides may be reaching its practical limits. Whereas the most recently approved macrolide, telithromycin, is manufactured from erythromycin by a linear sequence of 12 steps, the current phase III clinical candidate solithromycin requires a linear sequence of 16 steps (10 steps from the semisynthetic drug clarithromycin).[75b] We believe, and our current research aims to demonstrate, that shorter fully synthetic sequences are feasible and extraordinarily more powerful. Thus far, four groups have reported fully synthetic routes to active macrolide antibiotics (which is to say glycosylated macrolides).[153] These pioneering investigations include classic studies in complex molecule synthesis, but it is fair to say that highly practical routes are not yet at hand.
There is great need for more effective treatments for resistant strains of S. pneumoniae (frontline treatment for which is the macrolide antibiotic azithromycin). Over 1.2 million antibiotic-resistant bacterial pneumococcal infections occur annually in the United States, causing at least 7000 deaths.[2] Molecular details of the binding of macrolides to ribosomes of pathogenic organisms have recently been revealed,[69d,e] providing a wealth of information for the design of future macrolide antibiotics. Structure activity relationships of semisynthetic macrolides, and consideration of the diversity of natural macrolides erythromycin (a 14-membered macrolactone) and tylosin (a 16-membered macrolactone), known to share a common binding site on the bacterial ribosome,[69e, 154] make clear that the macrolides are surprisingly permissive of structural variation, suggesting that there is enormous opportunity for future drug discovery.[71] What is very much needed is synthetic chemistry that will broadly enable the exploration of novel macrolides, especially with variations of the many positions of the molecule that have not previously been feasible due to the limitations of semisynthesis. Ideally, this discovery chemistry would also be scalable, to provide new drug substances in quantity by an economically viable process. These are challenging standards to meet, but we believe lie within reach in the near term.
5.5. Streptogramins
The streptogramin antibiotics are products of actinomycetes and comprise two structurally distinct subclasses (group A and group B) that act synergistically against a variety of Gram-positive bacteria, including multidrug-resistant pathogens. The flagship members of this class are virginiamycin M1 (group A, also known as pristinamycin IIA, osteogrycin A, mykamycin A, vernamycin A, and PA114a) and virginiamycin S1 (group B, also known as pristinamycin IA). The virginiamycins are poorly soluble in water, making them poor candidates for intravenous formulations. Semisynthetic engineering at Rhône–Poulenc led to the development of dalfopristin and quinupristin,[155] which had greatly increased water solubility while maintaining synergistic activity, and these were approved as a combination intravenous therapy for the treatment of vancomycin-resistant E. faecium in 1999 under the trade name Synercid. Both group A and group B streptogramins inhibit protein synthesis by binding to the 50S subunit of the bacterial ribosome, and exhibit bactericidal activity when used together, usually in a 70:30 ratio (A:B). The molecular mechanism of binding and the origin of the synergistic effect have been elucidated through high-resolution crystal structures of these antibiotics bound to the ribosomes of H. marismortui and D. radiodurans.[69a,b,156] This more complete understanding of the binding mode of the streptogramin antibiotics, which has only arisen in the past decade, provides vital insights for molecular-level drug design. Practical, de novo syntheses of the streptogramin subclasses based on these insights would enable access to analogs to overcome developing resistance. [157] Some progress has already been made to this end: fully synthetic routes to group A[158] and group B[159] streptogramins have been reported, but improvements would be necessary to access large numbers of analogs or to achieve commercial scalability.
5.6. Ansamycins
The ansamycin natural product rifamycin SV was isolated from a soil sample from the French Riviera in 1957, and was found to be extremely active against Gram-positive and, to a lesser extent, Gram-negative bacteria.[160] After two years of semisynthetic work, Piero Sensi and collaborators at the Dow-Lepetit Research Laboratories developed rifampicin (also known as rifampin), an analog suitable for use in humans that was launched in 1967 for treatment of tuberculosis.[161, 162] The rifamycins bind to bacterial RNA polymerase and prevent extension of RNA through a steric occlusion mechanism, and high-resolution crystal structures of rifampicin bound to its target have been published.[163] Resistance develops very quickly to this class of antibacterials, primarily due to mutations in the target that affect the binding site, and for this reason use of rifampicin is restricted to combination tuberculosis therapy and for prophylaxic treatment for Neisseria meningitides.[164] Future drug design in the rifamycin class, informed by detailed knowledge of target binding and resistance mechanisms, could greatly benefit from a versatile, scalable, fully synthetic platform. Thus far, the pioneering synthetic work of the Kishi group (1980), followed in 1990 by a synthesis reported by the Tatsuta group, stand as the only fully synthetic routes to rifamycins.[165]
5.7. Abyssomicins
Abyssomicin C was reported in 2004 and was found to inhibit growth of a number of antibiotic-resistant pathogens including MRSA and VRSA by interfering with bacterial p-aminobenzoic acid production (part of the tetrahydrofolate synthesis pathway).[166] Fermentative production of abyssomicin C has not yet succeeded in producing quantities that would be necessary for commercialization. The density of sensitive functional groups within the abyssomicins renders them difficult targets (the Sorensen, Nicolaou, and Saicic groups have reported fully synthetic routes).[167] Though extremely challenging, a platform solution for the synthesis of abyssomicins (broadly defined) would be a welcome advance.
5.8. Glycopeptides
The glycopeptide natural product vancomycin remains a critical component of the modern antibacterial arsenal for the treatment of serious infections caused by Gram-positive bacteria, including MRSA. The glycopeptides disrupt bacterial cell-wall biosynthesis, by increasingly well-understood mechanisms.[168] The emergence of vancomycin-resistant enterococci (VRE) and vancomycin-resistant Staphlococcus aureus (VRSA) has stimulated development of next-generation semisynthetic antibiotics in this class: telavancin (Theravance Inc., approved in 2009),[169] oritavancin (originally developed by Robin Cooper and collaborators at Eli Lilly,[170] currently owned by The Medicines Company, approved by the FDA in May 2014),[171] and dalbavancin (originally developed by Gianpaolo Candiani and collaborators at Biosearch Italia,[172] currently being developed by Durata Therapeutics, phase III).[173] Extraordinary efforts focused on the development of fully synthetic routes to glycopeptide antibiotics have led to highly innovative solutions by the Evans,[174] Nicolaou,[175] and Boger[176] groups. In an impressive series of follow-up studies, the Boger group designed and synthesized a number of fully synthetic vancomycin analogs, one of which exhibited a 2000-fold increase in potency compared to vancomycin in a multidrug-resistant strain of E. faecalis.[177]
The mannopeptimycins were first isolated from a strain of Streptomyces hygroscopicus in the 1950s, but were not further explored until the early 2000s when Wyeth re-investigated shelved fractions of their own natural product collection that had previously been discarded due to a narrow spectrum of activity.[178] These glycopeptide antibiotics target the membrane-bound precursor lipid II, but bind at a different location than vancomycin, and are effective against vancomycin-resistant strains.[179] Semisynthetic modifications have enabled the development of analogs with improved safety and increased activity.[180]
The sheer size of the glycopeptides, their high degree of concatenation, and attendant issues of atropisomerism conspire to define targets that may lie beyond practical large-scale synthesis; however, in light of the successes of pioneering investigators, perhaps the challenge should no longer be considered insurmountable.
5.9. Depsipeptides
Depsipeptide antibiotics comprise polypeptides that contain one or more ester linkages. Ramoplanin, a structurally complex member of this class, successfully completed phase II clinical trials in 2006 for C. difficile-related diarrhea (where its lack of oral bioavailability is an advantage), but further clinical evaluation has not been reported.[6] Structural optimization of this class for systemic application could greatly expand its utility. Boger and Walker employed a fully synthetic approach to prepare ramoplanins and developed biochemical assays to probe the role of each amino acid residue in the binding of ramoplanin to its molecular targets, the cell-wall biosynthesis precursors Lipids I and II.[181]
The depsipeptide natural product daptomycin, developed at Eli Lilly[182] and licensed to Cubist Pharmaceuticals, was approved in 2003 and marketed under the trade name Cubicin for the treatment of serious infections caused by Gram-positive bacteria, including MRSA, VRSA, and VRE.[183] Daptomycin is bactericidal by a unique mode of action: it inserts into the bacterial cell membrane and forms aggregates that cause cell wall and cell membrane defects, leading to cell death.[184] The molecular size and complexity of daptomycin has limited the number of analogs accessible by semisynthesis,[185] the range of which has been supplemented by chemoenzymatic synthesis[186] and genetic engineering of the biosynthetic pathway.[187] Recently, the Li group reported the first fully synthetic route to daptomycin by using a combination of solid- and liquid-phase peptide synthesis.[188]
5.10. Moenomycin
The Kahne and Walker groups have developed a fully synthetic route to the complex natural product moenomycin A (an extremely potent antibiotic that disrupts cell-wall biosynthesis, but whose poor oral bioavailability and long serum half-life render it unsuitable for clinical use).[189] They also employed a chemoenzymatic route to synthesize truncated moenomycin analogs, enabling identification of other compounds capable of binding to bacterial peptidoglycan glycosyltransferases (the target of moenomycin).[190] The moenomycin class of antibiotics represents a substantial challenge for platform synthesis, but the pioneering studies by the Kahne and Walker groups suggest that such an approach could be a reasonable path forward.
5.11. Lantibiotics
The lantibiotics are polypeptide antibacterials that are internally crosslinked with one or more lanthionine residues, each comprising two alanine units connected by a thioether at their β-carbons. This class of antibiotics has found widespread use in food preservation, and two distinct modes of action for the lantibiotics have been characterized: inhibition of cell-wall biosynthesis,[191] and aggregation with Lipid II to form pores in the cell membrane.[192] Novacta Biosystems and Wellcome Trust have developed a lantibiotic, NVB302, that is currently undergoing phase I clinical trials for infections of the gut caused by C. difficile.[6] The recent chemical syntheses of the lantibiotics reported by the van der Donk and Vederas groups are highly noteworthy contributions and will undoubtedly prove informative in further optimization of the class and evasion of known modes of resistance and inactivation, such as oxidation of the sulfur-linked lanthionine residues.[193]
6. Outlook
Alexander Fleming presciently predicted in 1948 that virtually no antibiotic that humans develop would be impervious to the power of evolution; bacteria, with doubling times of minutes to hours, have a decided advantage in the contest. A prudent society would strive to restock the armamentarium of antibiotics and would recognize that this will likely require continuous effort, certainly for decades in the foreseeable future. It is conceivable that mankind will one day regain the upper hand in the battle against infectious disease, but premature declarations of victory now seem absurd: “It is time to close the book on infectious disease, declare the war on pestilence won and shift national resources to such chronic problems as cancer and heart disease.” (William Stewart, U.S. Surgeon General, 1969.)[194]
As we consider strategies for the discovery of new medicines to treat infections caused by multidrug-resistant bacteria, we should not abandon the core scientific approaches that have produced our current armamentarium of antibiotics. Although much of the “low-hanging fruit” in certain classes of antibacterials may have already been harvested, this does not mean that chemical synthesis has suddenly become a blunt tool in antibiotics discovery and development. New compound classes, new antibacterial targets, and wholly original approaches to antibiotics discovery will always be desirable, but we must also be guided by what has worked in the past. Are there lessons to be drawn from the antibiotics that arose during the past several hundred million years of natural selection? We believe that nature has both shown us primary targets, the ribosome and the bacterial cell wall (natural inhibitors of RNA polymerase, DNA gyrase, and tetrahydrofolate biosynthesis also occur), and provided the lead matter to pursue them. These “old” antibacterial targets may be less fashionable than new targets (the quest for which has been famously unsuccessful),[7k, 195] but they have not become “bad” targets and if we lose focus on them we do so at our own peril. The majority of naturally occurring antibiotics are structurally highly complex molecules requiring sophisticated and energetically costly biosynthetic pathways, raising the interesting question of why such complexity evolved if simple, less costly solutions were feasible and equally viable or superior. If the question is even meaningful it is possible that its answer(s) may not be relevant to human medicine (e.g., complexity might be rooted in self-protection mechanisms of the producing strains), but intuitively it seems that we must embrace (rather than recoil from) this structural complexity in antibiotic synthesis moving forward if we are to compete successfully. The best targets and the best discovery approaches are those that work. The strategy that we advocate—the development of practical, fully synthetic routes to complex antibacterial molecules—is not a new one. It is a tried-and-tested strategy whose perceived constraints (molecular size and complexity, gestation period, scalability) need to be re-evaluated to account for the power of modern chemical synthesis. Free from out-dated constraints, we believe this approach could be applied broadly for years to come.
We conclude by raising an important question: In what environment(s) will this research take place on a scale that is likely to produce significant results? Not in the pharmaceutical industry at its current levels of antibiotics R&D funding and with likely time frames of 2 to 10 years for the development of practical, fully synthetic approaches to complex antibacterials. Certainly not in U.S. academic chemistry laboratories, facing a current pay-line of 6% for funding from NIAID (National Institute of Allergy and Infectious Diseases) and a prevailing mindset that practical innovations should occur only in industry. It is no coincidence that the academic pioneers of antibacterial chemotherapy—Ehrlich, Domagk, Florey, and Waksman—all had close ties with industry partners. A group of small biopharmaceutical firms shoulder the responsibility for most of the antibacterials currently in clinical development, but these few companies lack the resources to produce an entirely new pipeline by themselves. It is evident that the current problem is as much (or more) one of economics as one of science. Not infrequently, successfully launched antibiotics do not generate enough revenue to cover their research and development costs. Pricing for life-saving, curative antibiotic therapies of < 14 days duration is but a tiny fraction of that for current oncology drugs. Beyond this, if the funding system for antibiotics research is not strengthened, if the attitude that academia is not the place for practical innovations persists, and if pharmaceutical companies (and venture capitalists) refuse to prime the pump independently, then the consequences for society could be dire.
Acknowledgments
Alastair and Celine Mactaggart have recognized the critical importance of antibiotics research to society and through their generosity have enabled us to sustain our research efforts to discover new antibiotics in the absence of U.S. government grant support. For this we are extremely grateful. Colleagues Stuart Schreiber, Robin Cooper, Joyce Sutcliffe, and Daniel Kahne have immeasurably improved the quality of this article with their insightful commentary, but bear no responsibility for any of its inaccuracies or deficiencies. Corey Fyfe, Trudy Grossman, and Joyce Sutcliffe of Tetraphase Pharmaceuticals are thanked for the experimental results depicted in the frontispiece graphic. I.B.S. thanks the NIH for a postdoctoral fellowship (GM099233).
Biographies

Peter M. Wright was born and raised in Southport, U.K. He studied chemistry at St. John's College, University of Oxford, under the guidance of Prof. George W.J. Fleet, and conducted research with Prof. Timothy J. Donohoe. He then moved to the U.S. to pursue graduate studies with Prof. Andrew G. Myers at Harvard University, working on the synthesis of tetracycline antibiotics. He is now an Associate at McKinsey & Company in New York City.

Ian B. Seiple received his B.Sc. from the University of California at Berkeley under the tutelage of Prof. Dirk Trauner, and in 2006 joined the laboratory of Prof. Phil S. Baran at the Scripps Research Institute as an NSF Predoctoral Fellow. During his graduate studies he developed fully synthetic routes to pyrrole-imidazole alkaloids, including palau'amine. He is currently an NIH postdoctoral fellow in the laboratory of Prof. Andrew G. Myers at Harvard University.

Andrew G. Myers received his B.Sc. from the Massachusetts Institute of Technology in 1981, and went on to pursue graduate studies with Prof. EliasJ. Corey at Harvard University. He started his independent career at the California Institute of Technology in 1987, and moved to Harvard University in 1998, where he is currently Amory Houghton Professor of Chemistry. His research program involves the synthesis and study of complex molecules of importance in biology and human medicine. He and his students have also developed methods of general utility in the construction of complex molecules.
References
- 1.World Economic Forum. Global Risks Report 2013. [accessed October, 2013]; http://www.weforum.org/reports/global-risks-2013-eighth-edition.
- 2.CDC's Antibiotic Resistance Threats in the United States, 2013. [accessed October, 2013]; http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- 3.Boucher HW, Talbot GH, Benjamin DK, Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D. Clin Infect Dis. 2013;56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Center for Disease Dynamics, Economics & Policy, Resistance Map. [accessed October, 2013]; http://www.cddep.org/map.
- 5.a) For an example of carbapenem resistance conferred by New Delhi metallo-β-lactamases (NDMs, also known as plasmid-encoding, carbapenem-resistant metallo-β-lactamases, PCMs), see: Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2.; b) see also: Moellering RC., Jr N Engl J Med. 2010;363:2377–2379. doi: 10.1056/NEJMp1011715.; c) For an example of a Klebsiella pneumoniae carbapenemase, see: Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA N. C. S. P. Group. Sci Transl Med. 2012;4:148ra116. doi: 10.1126/scitranslmed.3004129.
- 6.For a recent (September 2013) review of antibiotics in the clinical pipeline, see: Butler MS, Blaskovich MA, Cooper MA. J Antibiot. 2013;66:571–591. doi: 10.1038/ja.2013.86.
- 7.a) Labischinski H. Int J Med Microbiol. 2001;291:317–318. doi: 10.1078/1438-4221-00172. [DOI] [PubMed] [Google Scholar]; b) Chopra I, Hesse L, O'Neill AJ. J Appl Microbiol. 2002;92:4S–15S. [PubMed] [Google Scholar]; c) Rachakonda S, Cartee L. Curr Med Chem. 2004;11:775–793. doi: 10.2174/0929867043455774. [DOI] [PubMed] [Google Scholar]; d) Overbye KM, Barrett JF. Drug Discovery Today. 2005;10:45–52. doi: 10.1016/S1359-6446(04)03285-4. [DOI] [PubMed] [Google Scholar]; e) Butler MS, Buss AD. Biochem Pharmacol. 2006;71:919–929. doi: 10.1016/j.bcp.2005.10.012. [DOI] [PubMed] [Google Scholar]; f) Fischbach MA, Walsh CT. Science. 2009;325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) BrötzOesterhelt H, Sass P. Future Microbiol. 2010;5:1553–1579. doi: 10.2217/fmb.10.119. [DOI] [PubMed] [Google Scholar]; h) Moellering RC., Jr Int J Antimicrob Agents. 2011;37:2–9. doi: 10.1016/j.ijantimicag.2010.08.018. [DOI] [PubMed] [Google Scholar]; i) Silver LL. Clin Microbiol Rev. 2011;24:71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Lewis K. Nat Rev Drug Discovery. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]; k) Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Nat Rev Drug Discovery. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 8.a) For a review of the early syntheses of glycopeptide antibiotics, see: Nicolaou KC, Boddy CNC, Bräse S, Winssinger N. Angew Chem. 1999;111:2230–2287. doi: 10.1002/(sici)1521-3773(19990802)38:15<2096::aid-anie2096>3.0.co;2-f.Angew Chem Int Ed. 1999;38:2096–2152. doi: 10.1002/(sici)1521-3773(19990802)38:15<2096::aid-anie2096>3.0.co;2-f.Barbachyn MR, Ford CW. Angew Chem. 2003;115:2056–2070.Angew Chem Int Ed. 2003;42:2010–2023.Angew Chem. 2003;115:2056–2070.; c) For an excellent earlier review of antibacterial natural products complimentary to this Review but from the perspective of medicinal chemists, see: von Nussbaum F, Brands M, Hinzen B, Weigand S, Habich D. Angew Chem. 2006;118:5194–5254. doi: 10.1002/anie.200600350.Angew Chem Int Ed. 2006;45:5072–5129. doi: 10.1002/anie.200600350.Angew Chem. 2006;118:5194–5254.Nicolaou KC, Chen JS, Edmonds DJ, Estrada AA. Angew Chem. 2009;121:670–732. doi: 10.1002/anie.200801695.Angew Chem Int Ed. 2009;48:660–719. doi: 10.1002/anie.200801695.O'Connell KMG, Hodgkinson JT, Sore HF, Welch M, Salmond GPC, Spring DR. Angew Chem. 2013;125:10904–10932. doi: 10.1002/anie.201209979.Angew Chem Int Ed. 2013;52:10706–10733. doi: 10.1002/anie.201209979.
- 9.Walsh C. Antibiotics: Actions, Origins, Resistance. ASM; Washington, DC: 2003. [Google Scholar]
- 10.Dougherty TJ, Pucci MJ. Antibiotic Discovery and Development. Springer; Amsterdam: 2012. [Google Scholar]
- 11.Béchamp A. Ann Chim Phys. 1854;42:186–196. [Google Scholar]
- 12.a) Drews J. Nat Rev Drug Discovery. 2004;3:797–801. doi: 10.1038/nrd1498. [DOI] [PubMed] [Google Scholar]; b) Kaufmann SHE. Nat Rev Drug Discovery. 2008;7:373. doi: 10.1038/nrd2582. [DOI] [PubMed] [Google Scholar]
- 13.Guttmann P, Ehrlich P. Berl Klin Wochenschr. 1891;28:953–956. [Google Scholar]
- 14.a) Steverding D. Parasites Vectors. 2008;1:3. doi: 10.1186/1756-3305-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Steverding D. Parasites Vectors. 2010;3:15. doi: 10.1186/1756-3305-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Thomas HW. Br Med J. 1905;1:1140–1143. doi: 10.1136/bmj.1.2317.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Riethmiller S. Bull Hist Chem. 1999;23:28–33. [Google Scholar]
- 16.Ehrlich P, Bertheim A. Ber Dtsch Chem Ges. 1907;40:3292–3297. [Google Scholar]
- 17.Baumler E. Paul Ehrlich, Scientist for Life. Holmes & Meier; New York: 1984. [Google Scholar]
- 18.Bosch F, Rosich L. Pharmacology. 2008;82:171–179. doi: 10.1159/000149583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrlich P, Bertheim A. Ber Dtsch Chem Ges. 1912;45:756–766.; b) The chemical structure of salvarsan has been revised: Lloyd NC, Morgan HW, Nicholson BK, Ronimus RS. Angew Chem. 2005;117:963–966. doi: 10.1002/anie.200461471.Angew Chem Int Ed. 2005;44:941–944. doi: 10.1002/anie.200461471.
- 20.Raiziss GW, Gavron JL. ACS Monograph Series. New York: 1923. p. 509. [Google Scholar]
- 21.Podolsky ML. Cures Out of Chaos. Harwood Academic; Newark: 1997. [Google Scholar]
- 22.Lesch JE. The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine. Oxford University Press; New York: 2007. [Google Scholar]
- 23.For an excellent text on the discovery and development of penicillins, see: Sheehan JC. The Enchanted Ring, The Untold Story of Penicillin. MIT Press; Cambridge: 1982.
- 24.According to various sources, Churchill could have been treated with sulfapyridine, sulfathiazole, or sulfadiazine. The likelihood that the life-saving drug was sulfapyridine is discussed in ref. [22], p. 159.
- 25.Interestingly, the molecular basis for some of the side effects of sulfa drugs has only recently been elucidated: Haruki H, Pedersen MG, Gorska KI, Pojer F, Johnsson K. Science. 2013;340:987–991. doi: 10.1126/science.1232972.
- 26.Fleming A. Br J Exp Pathol. 1929;10:226–236. [Google Scholar]
- 27.Chain E, Florey HW, Gardner AD, Heatley NG, Jennings MA, Orr-Ewing J, Sanders AG. Lancet. 1940;2:226–228. [Google Scholar]
- 28.Abraham EP, Chain E, Fletcher CM, Gardner AD, Heatley NG, Jennings MA, Florey HW. Lancet. 1941;2:177–188. [PubMed] [Google Scholar]
- 29.Lye SC. From Molds to Molecules The Development of Tetracycline Antibiotics and the Transformation of the Pharmaceutical Industry, 1943 - 1963. Harvard University; Harvard: 1998. [Google Scholar]
- 30.Pfizer's development of deep-fermentation techniques was recognized as a National Historic Chemical Landmark by the American Chemical Society in 2008. [accessed December, 2013]; http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/penicillin.html.
- 31.du Vigneaud V, Carpenter FH, Holley RW, Livermore AH, Rachele JR. Science. 1946;104:431–450. doi: 10.1126/science.104.2706.431. [DOI] [PubMed] [Google Scholar]
- 32.Clarke HTL, Johnson JR, Robinson R. The Chemistry of Penicillin. Princeton University Press; Princeton: 1949. [Google Scholar]
- 33.Woodward RB. Perspectives in Organic Chemistry. Interscience; New York: 1956. [Google Scholar]
- 34.New York Times. 1946 Jul 5;17:8. [Google Scholar]
- 35.Sheehan JC, Buhle EL, Corey EJ, Laubach GD, Ryan JJ. J Am Chem Soc. 1950;72:3828–3829. [Google Scholar]
- 36.Sheehan JC, Hess GP. J Am Chem Soc. 1955;77:1067–1068. [Google Scholar]
- 37.Sheehan JC, Henery-Logan KR. J Am Chem Soc. 1957;79:1262–1263. [Google Scholar]
- 38.a) Sheehan JC, Henery-Logan KR. J Am Chem Soc. 1959;81:5838–5839. [Google Scholar]; b) Sheehan JC, Henery-Logan KR. J Am Chem Soc. 1962;84:2983–2990. [Google Scholar]; c) Hamilton-Miller JMT. Int J Antimicrob Agents. 2008;31:189–192. doi: 10.1016/j.ijantimicag.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Taylor DW. Nature. 1959;183:257. doi: 10.1038/183257a0. [DOI] [PubMed] [Google Scholar]
- 40.Peck RL, Hoffhine CE, Jr, Folkers K. J Am Chem Soc. 1946;68:1390–1391. doi: 10.1021/ja01211a513. [DOI] [PubMed] [Google Scholar]
- 41.Bartz QR, Controulis J, Crooks HM, Jr, Rebstock MC. J Am Chem Soc. 1946;68:2163–2166. [PubMed] [Google Scholar]
- 42.Welch H. Principles and Practice of Antibiotic Therapy. Blakiston; New York: 1954. [Google Scholar]
- 43.a) Duggar BM. Ann N Y Acad Sci. 1948;51:177–181. doi: 10.1111/j.1749-6632.1948.tb27262.x. [DOI] [PubMed] [Google Scholar]; b) Duggar BM. US2482055. Aureomycin and preparation of same. 1949 Sep 13;
- 44.a) Finlay AC, Hobby GL, P'an SY, Regna PP, Routien JB, Seeley DB, Shull GM, Sobin BA, Solomons IA, Vinson JW, Kane JH. Science. 1950;111:85. [Google Scholar]; b) Sobin BA, Finlay AC. US2516080. Terramycin and its Production. 1950 Jul 18;
- 45.For analyses of the different physicochemical properties of antibacterials with activity against Gram-positive and Gram-negative bacteria, see: O'Shea R, Moser HE. J Med Chem. 2008;51:2871–2878. doi: 10.1021/jm700967e.
- 46.Conover LH, Moreland WT, English AR, Stephens CR, Pilgrim FJ. J Am Chem Soc. 1953;75:4622–4623. [Google Scholar]
- 47.Minieri PP, Sokol H, Firman MC. US2734018. Tetracycline and chlortetracycline. 1956
- 48.Newton GGF, Abraham EP. Nature. 1955;175:548. doi: 10.1038/175548a0.; b) A number of years after they had isolated cephalosporin C, Abraham and Newton established its chemical structure: Abraham EP, Newton GGF. Biochem J. 1961;79:377–393. doi: 10.1042/bj0790377.
- 49.a) Abraham EP, Newton GGF. GB810196. Cephalosporin C. 1959; b) Loder B, Newton GGF, Abraham EP. Biochem J. 1961;79:408–416. doi: 10.1042/bj0790408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morin CRB, Jackson BG, Flynn EH, Roeske RW. J Am Chem Soc. 1962;84:3400–3401. [Google Scholar]
- 51.Chauvette RR, Flynn EH, Jackson BG, Lavagnino ER, Morin RB, Mueller RA, Pioch RP, Roeske RW, Ryan CW, Spencer JL, Van HE. J Am Chem Soc. 1962;84:3401–3402. [Google Scholar]
- 52.O'Callaghan CH, Sykes RB, Griffiths A, Thornton JE. Antimicrob Agents Chemother. 1976;9:511–519. doi: 10.1128/aac.9.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.a) O'Callaghan CH, Acred P, Harper PB, Ryan DM, Kirby SM, Harding SM. Antimicrob Agents Chemother. 1980;17:876–883. doi: 10.1128/aac.17.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Verbist L, Verhaegen J. Antimicrob Agents Chemother. 1980;17:807–812. doi: 10.1128/aac.17.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kessler RE, Bies M, Buck RE, Chisholm DR, Pursiano TA, Tsai YH, Misiek M, Price KE, Leitner F. Antimicrob Agents Chemother. 1985;27:207–216. doi: 10.1128/aac.27.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garau J, Wilson W, Wood M, Carlet J. Clin Micribiol Infect. 1997;3:s87–s101. [Google Scholar]
- 56.a) Basilea news release on the approval of ceftobiprole in Europe on October 23, 2013. [accessed January, 2014]; http://www.basilea.com/News-and-Media/Basileas-antibiotic-ceftobiprole-obtains-regulatory-approval-in-Europe-for-pneumonia/d32d8fcb-a9df-6a85-069f-9ef7ed599a8e.; b) Hebeisen P, Heinze-Krauss I, Angehrn P, Hohl P, Page MGP, Then RL. Antimicrob Agents Chemother. 2001;45:825–836. doi: 10.1128/AAC.45.3.825-836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.a) Stephens CR, Murai K, Rennhard HH, Conover LH, Brunings KJ. J Am Chem Soc. 1958;80:5324–5325. [Google Scholar]; b) McCormick JRD, Jensen ER, Miller PA, Doerschuk AP. J Am Chem Soc. 1960;82:3381–3388. [Google Scholar]; c) Blackwood RK, Stephens CR. J Am Chem Soc. 1962;84:4157–4159. [Google Scholar]; d) Stephens CR, Jr, Beereboom JJ, Rennhard HH, Gordon PN, Murai K, Blackwood RK, von Wittenau MS. J Am Chem Soc. 1963;85:2643–2652. [Google Scholar]
- 58.a) Martell MJ, Jr, Boothe JH. J Med Chem. 1967;10:44–46. doi: 10.1021/jm00313a009. [DOI] [PubMed] [Google Scholar]; b) Zambrano RT. US3483251A. Antibacterial alkylamino tetracyclines. 1969; c Church RFR, Schaub RE, Weiss MJ. J Org Chem. 1971;36:723–725. doi: 10.1021/jo00804a025. [DOI] [PubMed] [Google Scholar]
- 59.a) Sum PE, Lee VJ, Testa RT, Hlavka JJ, Ellestad GA, Bloom JD, Gluzman Y, Tally FP. J Med Chem. 1994;37:184–188. doi: 10.1021/jm00027a023. [DOI] [PubMed] [Google Scholar]; b) Sum PE, Petersen P. Bioorg Med Chem Lett. 1999;9:1459–1462. doi: 10.1016/s0960-894x(99)00216-4. [DOI] [PubMed] [Google Scholar]
- 60.a) Bergeron J, Ammirati M, Danley D, James L, Norcia M, Retsema j, Strick CA, Su WG, Sutcliffe J, Wondrack L. Antimicrob Agents Chemother. 1996;40:2226–2228. doi: 10.1128/aac.40.9.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bauer G, Berens C, Projan SJ, Hillen W. J Antimicrob Chemother. 2004;53:592–599. doi: 10.1093/jac/dkh125. [DOI] [PubMed] [Google Scholar]
- 61.a) Wu YJ. Curr Pharm Des. 2000;6:181–223. doi: 10.2174/1381612003401316. [DOI] [PubMed] [Google Scholar]; b) Ma X, Ma S. Curr Med Chem. 2011;18:1993–2015. doi: 10.2174/092986711795590075. [DOI] [PubMed] [Google Scholar]
- 62.Woodward RB. Angew Chem. 1957;69:50–58. [Google Scholar]
- 63.a) Kurath P, Jones PH, Egan RS, Perun TJ. Experientia. 1971;27:362. doi: 10.1007/BF02137246. [DOI] [PubMed] [Google Scholar]; b) Cachet T, Van den Mooter G, Hauchecorne R, Vinckier C, Hoogmartens J. Int J Pharm. 1989;55:59–65. [Google Scholar]
- 64.a) Morimoto S, Takahashi Y, Watanabe Y, Omura S. J Antibiot. 1984;37:187–189. doi: 10.7164/antibiotics.37.187. [DOI] [PubMed] [Google Scholar]; b) Morimoto S, Misawa Y, Adachi T, Nagate T, Watanabe Y, Omura S. J Antibiot. 1990;43:286–294. doi: 10.7164/antibiotics.43.286. [DOI] [PubMed] [Google Scholar]
- 65.a) Kobrehel G, Radobolja G, Tamburasev Z, Djokic S. DE3012533A1. 11-Aza-4-0-cladinosyl-6-0-desosaminyl-15-ethyl-7,13,14-trihy-droxy-3,5,7,9,12,14-hexamethyloxacyclopentadecan-2-one derivatives as well as process for their production. 1980; b) Djokic S, Kobrehel G, Lazarevski G, Lopotar N, Tamburasev Z, Kamenar B, Nagl A, Vickovic I. J Chem Soc Perkin Trans 1. 1986:1881–1890. [Google Scholar]; c) Djokic S, Kobrehel G, Lopotar N, Kamenar B, Nagl A, Mrvos D. J Chem Res Synop. 1988:152–153. [Google Scholar]
- 66.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM, Engl N. J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833.Knirsch CA, Chandra R. N Engl J Med. 2012;367:772–775. doi: 10.1056/NEJMc1207269.; c) For a response from the FDA, see: http://www.fda.gov/Drugs/DrugSafety/ucm304372 (accessed October, 2013).
- 67.Baker WR, Clark JD, Stephens RL, Kim KH. J Org Chem. 1988;53:2340–2345. [Google Scholar]
- 68.Fernandes PB, Baker WR, Freiberg LA, Hardy DJ, McDonald EJ. Antimicrob Agents Chemother. 1989;33:78–81. doi: 10.1128/aac.33.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.a) Hansen JL, Moore PB, Steitz TA. J Mol Biol. 2003;330:1061–1075. doi: 10.1016/s0022-2836(03)00668-5. [DOI] [PubMed] [Google Scholar]; b) Tu D, Blaha G, Moore PB, Steitz TA. Cell. 2005;121:257–270. doi: 10.1016/j.cell.2005.02.005. [DOI] [PubMed] [Google Scholar]; c) Bulkley D, Innis CA, Blaha G, Steitz TA. Proc Natl Acad Sci USA. 2010;107:17158–17163. doi: 10.1073/pnas.1008685107. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JHD, Mankin AS. Antimicrob Agents Chemother. 2010;54:4961–4970. doi: 10.1128/AAC.00860-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Dunkle JA, Xiong L, Mankin AS, Cate JH. Proc Natl Acad Sci USA. 2010;107:17152–17157. doi: 10.1073/pnas.1007988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.a) Yonath AE. Nobel Lecture. [accessed January, 2014];2009 Dec 8; http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2009/yonath-lecture.html.; b) Ramakrishnan V. Nobel Lecture. [accessed January, 2014];2009 Dec 8; http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2009/ramakrishnan-lecture.html.; c) Steitz TA. Nobel Lecture. [accessed January, 2014];2009 Dec 8; http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2009/steitz-lecture.html.
- 71.For a review of the ribosome as a target for antibiotics with an excellent summary of crystal structure data up to 2005, see: Poehlsgaard J, Douthwaite S. Nat Rev Microbiol. 2005;3:870–881. doi: 10.1038/nrmicro1265.
- 72.Allen NE. Antimicrob Agents Chemother. 1977;11:669–674. doi: 10.1128/aac.11.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonnefoy A, Girard AM, Agouridas C, Chantot JF. J Antimicrob Chemother. 1997;40:85–90. doi: 10.1093/jac/40.1.85. [DOI] [PubMed] [Google Scholar]
- 74.a) Clay KD, Hanson JS, Pope SD, Rissmiller RW, Purdum PP, Banks PM. Ann Intern Med. 2006;144:415–420. doi: 10.7326/0003-4819-144-6-200503210-00121. [DOI] [PubMed] [Google Scholar]; b) Bertrand D, Bertrand S, Neveu E, Fernandes P. Antimicrob Agents Chemother. 2010;54:5399–5402. doi: 10.1128/AAC.00840-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.a) Putnam SD, Castanheira M, Moet GJ, Farrell DJ, Jones RN. Diagn Microbiol Infect Dis. 2010;66:393–401. doi: 10.1016/j.diagmicrobio.2009.10.013. [DOI] [PubMed] [Google Scholar]; b) Pereira DE, Fernandes PB. WO2010048600A1. Preparation of aminodeoxy glycoside macrolides and ketolides as antibiotics for the treatment of gastrointestinal resistant diseases. 2010
- 76.a) Ehrlich J, Bartz QR, Smith RM, Joslyn DA. Science. 1947;106:417. doi: 10.1126/science.106.2757.417. [DOI] [PubMed] [Google Scholar]; b) Bartz QR. J Biol Chem. 1948;172:445–450. [PubMed] [Google Scholar]
- 77.Controulis J, Rebstock MC, Crooks HM., Jr J Am Chem Soc. 1949;71:2463–2468. [Google Scholar]
- 78.a) Rich ML, Ritterhoff RJ, Hoffmann RJ. Ann Intern Med. 1950;33:1459–1467. doi: 10.7326/0003-4819-33-6-1459. [DOI] [PubMed] [Google Scholar]; b) Sneader W. Drug Discovery A History. Wiley; Hoboken: 2005. [Google Scholar]
- 79. [accessed December, 2013];Word Health Organization Model List of Essential Medicines. (18th). http://www.who.int/medicines/publications/essentialmedicines/en/
- 80.Cutler RA, Stenger RJ, Suter CM. J Am Chem Soc. 1952;74:5475–5481. [Google Scholar]
- 81.a) Hitchings GH, Elion GB, Van der Werff H, Falco EA. J Biol Chem. 1948;174:765–766. [PubMed] [Google Scholar]; b) Hitchings GH, Elion GB, Falco EA, Russell PB, Van der Werff H. Ann N Y Acad Sci. 1950;52:1318–1335. doi: 10.1111/j.1749-6632.1950.tb54032.x. [DOI] [PubMed] [Google Scholar]
- 82.Hitchings George H. Nobel Lecture. [accessed January, 2014];1988 Dec 8; http://www.nobelprize.org/nobel_prizes/medicine/laureates/1988/hitchings-lecture.html.
- 83.a) Stenbuck P, Hood HM. US3049544. 2,4-Diamino-5-benzylpyrimidines. 1962; b) Burchall JJ, Hitchings GH. Mol Pharmacol. 1965;1:126–136. [PubMed] [Google Scholar]; c) Burchall JJ. J Antimicrob Chemother. 1979;5:3–14. doi: 10.1093/jac/5.supplement_b.3. [DOI] [PubMed] [Google Scholar]
- 84.Greenwood D. Antimicrobial Drugs: Chronicle of a Twentieth Century Medical Triumph. Oxford University Press; Oxford: 2008. pp. 312–315. [Google Scholar]
- 85.Masters PA, O'Bryan TA, Zurlo J, Miller DQ, Joshi N. Arch Intern Med. 2003;163:402–410. doi: 10.1001/archinte.163.4.402. [DOI] [PubMed] [Google Scholar]
- 86.Executive Summary on Trimethoprim, Department of Scientific and Industrial Research, Ministry of Science and Technology of India. [accessed December, 2013]; http://www.dsir.gov.in/reports/techreps/tsr142.pdf.
- 87.a) Maeda K, Osato T, Umezawa H. J Antibiot Ser A. 1953;6:182. [PubMed] [Google Scholar]; b) Nakamura S. Pharm Bull. 1955;3:379–383. doi: 10.1248/cpb1953.3.379. [DOI] [PubMed] [Google Scholar]
- 88.Shinn DLS. Lancet. 1962;279:1191. [Google Scholar]
- 89.Hendlin D, Stapley EO, Jackson M, Wallick H, Miller AK, Wolf FJ, Miller TW, Chaiet L, Kahan FM, Foltz EL, Woodruff HB, Mata JM, Hernandez S, Mochales S. Science. 1969;166:122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 90.Christensen BG, Leanza WJ, Beattie TR, Patchett AA, Arison BH, Ormond RE, Kuehl FA, Jr, Albers-Schonberg G, Jardetzky O. Science. 1969;166:123–125. doi: 10.1126/science.166.3901.123. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Z, Tang J, Wang X, Shi H. J Mol Catal A. 2008;285:68–71. [Google Scholar]
- 92.Estebanez A, Pascual R, Gil V, Ortiz F, Santibanez M, Barba CP. Eur J Clin Microbiol Infect Dis. 2009;28:1457–1464. doi: 10.1007/s10096-009-0805-6. [DOI] [PubMed] [Google Scholar]
- 93.Lesher GY, Froelich EJ, Gruett MD, Bailey JH, Brundage RP. J Med Pharm Chem. 1962;5:1063–1065. doi: 10.1021/jm01240a021. [DOI] [PubMed] [Google Scholar]
- 94.Gootz TD, Brighty KE. In: The Quinolones. 2nd. Andriole VT, editor. Academic Press; 1998. p. 30. [Google Scholar]
- 95.Koga H, Itoh A, Murayama S, Suzue S, Irikura T. J Med Chem. 1980;23:1358–1363. doi: 10.1021/jm00186a014. [DOI] [PubMed] [Google Scholar]
- 96.The fluorine substituent was later found to increase affinity of quinolones for their antibacterial target (DNA gyrase) and significantly improve cellular penetration: Domagala JM, Hanna LD, Heifetz CL, Hutt MP, Mich TF, Sanchez JP, Solomon M. J Med Chem. 1986;29:394–404. doi: 10.1021/jm00153a015.
- 97.Hayakawa I, Atarashi S, Yokohama S, Imamura M, Sakano K, Furukawa M. Antimicrob Agents Chemother. 1986;29:163–164. doi: 10.1128/aac.29.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petersen U, Schenke T, Krebs A, Grohe K, Schriewer M, Haller I, Metzger KG, Endermann R, Zeiler HJ. US4990517A. Preparation of 7-(1-pyrrolidinyl)-3-quinolonecarboxylic acids and naphthyridone-3-carboxylic acid analogs as antibacterial agents and feed additives. 1991
- 99.Pépin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S, Leblanc M, Rivard G, Bettez M, Primeau V, Nguyen M, Jacob CÉ, Lanthier L. Clin Infect Dis. 2005;41:1254–1260. doi: 10.1086/496986.Etminan M, Forooghian F, Brophy JM, Bird ST, Maberley D. JAMA J Am Med Assoc. 2012;307:1414–1419. doi: 10.1001/jama.2012.383.; c) For a discussion of the side effects of fluoroquinolones, see: http://well.blogs.nytimes.com/2012/09/10/popular-antibiotics-may-carry-serious-side-effects/ (accessed October, 2013).
- 100.Lemaire S, Tulkens PM, Van BF. Antimicrob Agents Chemother. 2011;55:649–658. doi: 10.1128/AAC.01201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Page MGP. In: Antibiotic Discovery and Development. Dougherty TJ, Pucci MJ, editors. Springer; Amsterdam: 2012. pp. 79–118. [Google Scholar]
- 102.a) Cama LD, Christensen BG. J Am Chem Soc. 1974;96:7582–7583. doi: 10.1021/ja00831a049. [DOI] [PubMed] [Google Scholar]; b) Guthikonda RN, Cama LD, Christensen BG. J Am Chem Soc. 1974;96:7584. doi: 10.1021/ja00831a050. [DOI] [PubMed] [Google Scholar]; c Firestone RA, Fahey JL, Maciejewicz NS, Patel GS, Christensen BG. J Med Chem. 1977;20:551–556. doi: 10.1021/jm00214a018. [DOI] [PubMed] [Google Scholar]
- 103.a) Yoshioka M, Tsuji T, Uyeo S, Yamamoto S, Aoki T, Nishitani Y, Mori S, Satoh H, Hamada Y, Ishitobi H, Nagata W. Tetrahedron Lett. 1980;21:351–354. [Google Scholar]; b) Tsuji T, Satoh H, Narisada M, Hamashima Y, Yoshida T. J Antibiot. 1985;38:466–476. doi: 10.7164/antibiotics.38.466. [DOI] [PubMed] [Google Scholar]
- 104.a) Hatanaka M, Ishimaru T. Tetrahedron Lett. 1983;24:4837–4838. [Google Scholar]; b) Evans DA, Sjogren EB. Tetrahedron Lett. 1985;26:3787–3790. [Google Scholar]; c) Ogasa T, Saito H, Hashimoto Y, Sato K, Hirata T. Chem Pharm Bull. 1989;37:315–321. doi: 10.1248/cpb.37.315. [DOI] [PubMed] [Google Scholar]; d) Bodurow CC, Boyer BD, Brennan J, Bunnell CA, Burks JE, Carr MA, Doecke CW, Eckrich TM, Fisher JW, Gardner JP, Graves BJ, Hines P, Hoying RC, Jackson BG, Kinnick MD, Kochert CD, Lewis JS, Luke WD, Moore LL, Morin JM, Jr, Nist RL, Prather DE, Sparks DL, Vladuchick WC. Tetrahedron Lett. 1989;30:2321–2324. [Google Scholar]
- 105.Frazier JW, Staszak MA, Weigel LO. Tetrahedron Lett. 1992;33:857–860. [Google Scholar]
- 106.a) Tally FP, Jacobus NV, Gorbach SL. Antimicrob Agents Chemother. 1978;14:436–438. doi: 10.1128/aac.14.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kahan JS, Kahan FM, Goegelman R, Currie SA, Jackson M, Stapley EO, Miller TW, Miller AK, Hendlin D, Mochales S, Hernandez S, Woodruff HB, Birnbaum J. J Antibiot. 1979;32:1–12. doi: 10.7164/antibiotics.32.1. [DOI] [PubMed] [Google Scholar]
- 107.Geddes AM, Stille W. Rev Infect Dis. 1985;7:S353–356. doi: 10.1093/clinids/7.supplement_3.s353. [DOI] [PubMed] [Google Scholar]
- 108.Leanza WJ, Wildonger KJ, Miller TW, Christensen BG. J Med Chem. 1979;22:1435–1436. doi: 10.1021/jm00198a001. [DOI] [PubMed] [Google Scholar]
- 109.Salzmann TN, Ratcliffe RW, Christensen BG, Bouffard FA. J Am Chem Soc. 1980;102:6161–6163. doi: 10.1098/rstb.1980.0037. [DOI] [PubMed] [Google Scholar]
- 110.a) Melillo DG, Shinkai I, Liu T, Ryan K, Sletzinger M. Tetrahedron Lett. 1980;21:2783–2786. [Google Scholar]; b) Melillo DG, Cvetovich RJ, Ryan KM, Sletzinger M. J Org Chem. 1986;51:1498–1504. [Google Scholar]
- 111.Norrby SR, Alestig K, Bjoernegaard B, Burman LA, Ferber F, Huber JL, Jones KH, Kahan FM, Kahan JS, Kropp H, Meisinger MAP, Sundelof JG. Antimicrob Agents Chemother. 1983;23:300–307. doi: 10.1128/aac.23.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shih DH, Baker F, Cama L, Christensen BG. Heterocycles. 1984;21:29–40. [Google Scholar]
- 113.Fukasawa M, Sumita Y, Harabe ET, Tanio T, Nouda H, Kohzuki T, Okuda T, Matsumura H, Sunagawa M. Antimicrob Agents Chemother. 1992;36:1577–1579. doi: 10.1128/aac.36.7.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williams JM, Brands KMJ, Skerlj RT, Jobson RB, Marchesini G, Conrad KM, Pipik B, Savary KA, Tsay FR, Houghton PG, Sidler DR, Dolling UH, DiMichele LM, Novak TJ. J Org Chem. 2005;70:7479–7487. doi: 10.1021/jo0501442. [DOI] [PubMed] [Google Scholar]
- 115.a) Imada A, Kitano K, Kintaka K, Muroi M, Asai M. Nature. 1981;289:590–591. doi: 10.1038/289590a0. [DOI] [PubMed] [Google Scholar]; b) Sykes RB, Cimarusti CM, Bonner DP, Bush K, Floyd DM, Georgopapadakou NH, Koster WH, Liu WC, Parker WL, Pincipe PA, Rathnum ML, Slusarchyk WA, Trejo WH, Wells JS. Nature. 1981;291:489–491. doi: 10.1038/291489a0. [DOI] [PubMed] [Google Scholar]
- 116.a) Breuer H, Cimarusti CM, Denzel T, Koster WH, Slusarchyk WA, Treuner UD. J Antimicrob Chemother. 1981;8:21–28. doi: 10.1093/jac/8.suppl_e.21. [DOI] [PubMed] [Google Scholar]; b) Cimarusti CM, Bonner DP, Breuer H, Chang HW, Fritz AW, Floyd DM, Kissick TP, Koster WH, Kronenthal D, Massa F, Müller RH, Pluscec J, Slusarchyk WA, Sykes RB, Taylor M, Weaver ER. Tetrahedron. 1983;39:2577–2589. [Google Scholar]
- 117.a) Page MGP, Dantier C, Desarbre E. Antimicrob Agents Chemother. 2010;54:2291–2302. doi: 10.1128/AAC.01525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mitton-Fry MJ, Arcari JT, Brown MF, Casavant JM, Finegan SM, Flanagan ME, Gao H, George DM, Gerstenberger BS, Han S, Hardink JR, Harris TM, Hoang T, Huband MD, Irvine R, Lall MS, Megan LM, Li C, Lin J, McCurdy SP, Mueller JP, Mullins L, Niosi M, Noe MC, Pattavina D, Penzien J, Plummer MS, Risley H, Schuff BP, Shanmugasundaram V, Starr JT, Sun J, Winton J, Young JA. Bioorg Med Chem Lett. 2012;22:5989–5994. doi: 10.1016/j.bmcl.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 118.a) Slee AM, Wuonola MA, McRipley RJ, Zajac I, Zawada MJ, Bartholomew PT, Gregory WA, Forbes M. Antimicrob Agents Chemother. 1987;31:1791–1797. doi: 10.1128/aac.31.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gregory WA, Brittelli DR, Wang CLJ, Wuonola MA, McRipley RJ, Eustice DC, Eberly VS, Slee AM, Forbes M, Bartholomew PT. J Med Chem. 1989;32:1673–1681. doi: 10.1021/jm00128a003. [DOI] [PubMed] [Google Scholar]
- 119.Brickner SJ, Hutchinson DK, Barbachyn MR, Manninen PR, Ulanowicz DA, Garmon SA, Grega KC, Hendges SK, Toops DS, Ford CW, Zurenko GE. J Med Chem. 1996;39:673–679. doi: 10.1021/jm9509556. [DOI] [PubMed] [Google Scholar]
- 120.For an X-ray crystal structure of linezolid bound to the large subunit of the bacterial ribosome, see: Ippolito JA, Kanyo ZF, Wang D, Franceschi FJ, Moore PB, Steitz TA, Duffy EM. J Med Chem. 2008;51:3353–3356. doi: 10.1021/jm800379d.
- 121.a) Zhou J, Bhattacharjee A, Chen S, Chen Y, Duffy E, Farmer J, Goldberg J, Hanselmann R, Ippolito JA, Lou R, Orbin A, Oyelere A, Salvino J, Springer D, Tran J, Wang D, Wu Y, Johnson G. Bioorg Med Chem Lett. 2008;18:6175–6178. doi: 10.1016/j.bmcl.2008.10.011. [DOI] [PubMed] [Google Scholar]; b) Locke JB, Finn J, Hilgers M, Morales G, Rahawi S, Kedar GC, Picazo JJ, Im W, Shaw KJ, Stein JL. Antimicrob Agents Chemother. 2010;54:5337–5343. doi: 10.1128/AAC.00663-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Prokocimer P, Bien P, DeAnda C, Pillar CM, Bartizal K. Antimicrob Agents Chemother. 2012;56:4608–4613. doi: 10.1128/AAC.00458-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.a) Stephens CR, Conover LH, Hochstein FA, Regna PP, Pilgrim FJ, Brunings KJ, Woodward RB. J Am Chem Soc. 1952;74:4976–4977. [Google Scholar]; b) Hochstein FA, Stephens CR, Conover LH, Regna PP, Pasternack R, Gordon PN, Pilgrim FJ, Brunings KJ, Woodward RB. J Am Chem Soc. 1953;75:5455–5475. [Google Scholar]
- 123.a) Gurevich AI, Karapetyan MG, Kolosov MN, Korobko VG, Onoprienko VV, Popravko SA, Shemyakin MM. Tetrahedron Lett. 1967:131–134. doi: 10.1016/s0040-4039(00)90501-x. [DOI] [PubMed] [Google Scholar]; b) Korst JJ, Johnston JD, Butler K, Bianco EJ, Conover LH, Woodward RB. J Am Chem Soc. 1968;90:439–457. [Google Scholar]; c) Muxfeldt H, Hardtmann G, Kathawala F, Vedejs E, Mooberry JB. J Am Chem Soc. 1968;90:6534–6536. doi: 10.1021/ja01025a063. [DOI] [PubMed] [Google Scholar]; d) Muxfeldt H, Haas G, Hardtmann G, Kathawala F, Mooberry JB, Vedejs E. J Am Chem Soc. 1979;101:689–701. doi: 10.1021/ja01025a063. [DOI] [PubMed] [Google Scholar]; e) Kirchlechner R, Rogalski W. Tetrahedron Lett. 1980;21:247–250. [Google Scholar]
- 124.Charest MG, Lerner CD, Brubaker JD, Siegel DR, Myers AG. Science. 2005;308:395–398. doi: 10.1126/science.1109755. [DOI] [PubMed] [Google Scholar]
- 125.a) Brubaker JD, Myers AG. Org Lett. 2007;9:3523–3525. doi: 10.1021/ol071377d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kummer DA, Li D, Dion A, Myers AG. Chem Sci. 2011;2:1710–1718. doi: 10.1039/C1SC00303H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun C, Wang Q, Brubaker JD, Wright PM, Lerner CD, Noson K, Charest M, Siegel DR, Wang YM, Myers AG. J Am Chem Soc. 2008;130:17913–17927. doi: 10.1021/ja806629e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stork G, Hagedorn AA., III J Am Chem Soc. 1978;100:3609–3611. [Google Scholar]
- 128.Wright PM, Myers AG. Tetrahedron. 2011;67:9853–9869. doi: 10.1016/j.tet.2011.09.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.a) Clark RB, He M, Fyfe C, Lofland D, O'Brien WJ, Plamondon L, Sutcliffe JA, Xiao XY. J Med Chem. 2011;54:1511–1528. doi: 10.1021/jm1015389. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sun C, Hunt DK, Clark RB, Lofland D, O'Brien WJ, Plamondon L, Xiao XY. J Med Chem. 2011;54:3704–3731. doi: 10.1021/jm1015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.The microtiter broth assay that appears on the frontispiece was performed by Trudy Grossman and Corey Fyfe at Tetraphase Pharmaceuticals. The assay was performed following Clinical Laboratory Standards with the exception that white-walled microtiter plates (rather than clear) were used. The MIC is defined as the minimum inhibitory growth or the concentration of compound where there is no visible growth. Growth in this context produces a completely white well, whereas no growth results in brown broth.
- 131.Ronn M, Zhu Z, Hogan PC, Zhang WY, Niu J, Katz CE, Dunwoody N, Gilicky O, Deng Y, Hunt DK, He M, Chen CL, Sun C, Clark RB, Xiao XY. Org Process Res Dev. 2013;17:838–845. [Google Scholar]
- 132.Kavanagh F, Hervey A, Robbins WJ. Proc Natl Acad Sci USA. 1951;37:570–574. doi: 10.1073/pnas.37.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Novak R. Ann N Y Acad Sci. 2011;1241:71–81. doi: 10.1111/j.1749-6632.2011.06219.x. [DOI] [PubMed] [Google Scholar]
- 134.a) Schlünzen F, Pyetan E, Fucini P, Yonath A, Harms JM. Mol Microbiol. 2004;54:1287–1294. doi: 10.1111/j.1365-2958.2004.04346.x. [DOI] [PubMed] [Google Scholar]; b) Davidovich C, Bashan A, Auerbach-Nevo T, Yaggie RD, Gontarek RR, Yonath A. Proc Natl Acad Sci USA. 2007;104:4291–4296. doi: 10.1073/pnas.0700041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.a) Gibbons EG. J Am Chem Soc. 1982;104:1767–1769. [Google Scholar]; b) Boeckman RK, Jr, Springer DM, Alessi TR. J Am Chem Soc. 1989;111:8284–8286. [Google Scholar]; c) Fazakerley NJ, Helm MD, Procter DJ. Chem Eur J. 2013;19:6718–6723. doi: 10.1002/chem.201300968. [DOI] [PubMed] [Google Scholar]
- 136.Bacqué E, Pautrat F, Zard SZ. Org Lett. 2003;5:325–328. doi: 10.1021/ol027312m. [DOI] [PubMed] [Google Scholar]
- 137.a) Liu J, Lotesta SD, Sorensen EJ. Chem Commun. 2011;47:1500–1502. doi: 10.1039/c0cc04077k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lotesta SD, Liu J, Yates EV, Krieger I, Sacchettini JC, Freundlich JS, Sorensen EJ. Chem Sci. 2011;2:1258–1261. doi: 10.1039/C1SC00116G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.a) Mason DJ, Dietz A, DeBoer C. Antimicrob Agents Chemother. 1963:554–559. [Google Scholar]; b) Hoeksema H, Bannister B, Birkenmeyer RD, Kagan F, Magerlein BJ, MacKellar FA, Schroeder W, Slomp G, Herr RR. J Am Chem Soc. 1964;86:4223–4224. [Google Scholar]
- 139.Birkenmeyer RD, Kagan F. J Med Chem. 1970;13:616–619. doi: 10.1021/jm00298a007. [DOI] [PubMed] [Google Scholar]
- 140.Daum RS. N Engl J Med. 2007;357:380–390. doi: 10.1056/NEJMcp070747. [DOI] [PubMed] [Google Scholar]
- 141.Lewis JS, II, Jorgensen JH. Clin Infect Dis. 2005;40:280–285. doi: 10.1086/426894. [DOI] [PubMed] [Google Scholar]
- 142.Birkenmeyer RD, Kroll SJ, Lewis C, Stern KF, Zurenko GE. J Med Chem. 1984;27:216–223. doi: 10.1021/jm00368a020. [DOI] [PubMed] [Google Scholar]
- 143.a) O'Dowd H, Lewis JG, Trias J, Asano R, Blais J, Lopez SL, Park CK, Wu C, Wang W, Gordeev MF. Bioorg Med Chem Lett. 2008;18:2645–2648. doi: 10.1016/j.bmcl.2008.03.032. [DOI] [PubMed] [Google Scholar]; b) Umemura E, Wakiyama Y, Kumura K, Ueda K, Masaki S, Watanabe T, Yamamoto M, Hirai Y, Fushimi H, Yoshida T, Ajito K. J Antibiot. 2013;66:195–198. doi: 10.1038/ja.2012.107. [DOI] [PubMed] [Google Scholar]
- 144.a) Magerlein BJ. Tetrahedron Lett. 1970:33–36. doi: 10.1016/s0040-4039(01)87558-4. [DOI] [PubMed] [Google Scholar]; b) Magerlein BJ. J Med Chem. 1972;15:1255–1259. doi: 10.1021/jm00282a013. [DOI] [PubMed] [Google Scholar]; c) Larson ER, Danishefsky S. J Am Chem Soc. 1983;105:6715–6716. [Google Scholar]; d) Knapp S, Kukkola PJ. J Org Chem. 1990;55:1632–1636. [Google Scholar]
- 145.a) Krueger RJ. J Med Chem. 2007;50:5243. [Google Scholar]; b Becker B, Cooper MA. ACS Chem Biol. 2013;8:105–115. doi: 10.1021/cb3005116. [DOI] [PubMed] [Google Scholar]
- 146.a) Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias RD, Lopez S, Gomez M, Wlasichuk KB, Diokno R, Miller GH, Moser HE. Antimicrob Agents Chemother. 2010;54:4636–4642. doi: 10.1128/AAC.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Armstrong ES, Miller GH. Curr Opin Microbiol. 2010;13:565–573. doi: 10.1016/j.mib.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 147.Matt T, Ng CL, Lang K, Sha SH, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, Perez-Fernandez D, Vasella A, Ramakrishnan V, Schacht J, Boettger EC. Proc Natl Acad Sci USA. 2012;109:10984–10989. doi: 10.1073/pnas.1204073109.b) We are thankful to Dr. Robin Cooper (Rigel Pharmaceuticals) for suggesting the inclusion of apramycin.
- 148.For a recent summary of spectinomycin resistance in N gonorrhoeae, see: Unemo M, Golparian D, Skogen V, Olsen AO, Moi H, Syversen G, Hjelmevoll SO. Antimicrob Agents Chemother. 2013;57:1057–1061. doi: 10.1128/AAC.01775-12.
- 149.Ramakrishnan V, White SW. Nature. 1992;358:768–771. doi: 10.1038/358768a0. [DOI] [PubMed] [Google Scholar]
- 150.a) Hanessian S, Roy R. J Am Chem Soc. 1979;101:5839–5841. [Google Scholar]; b) White DR, Birkenmeyer RD, Thomas RC, Mizsak SA, Wiley VH. Tetrahedron Lett. 1979:2737–2740. [Google Scholar]; c) Hanessian S, Roy R. Can J Chem. 1985;63:163–172. [Google Scholar]; d) Cuny E, Lichtenthaler FW. Tetrahedron: Asymmetry. 2006;17:1120–1124. [Google Scholar]
- 151.Houghton JL, Green KD, Chen W, Garneau-Tsodikova S. ChemBioChem. 2010;11:880–902. doi: 10.1002/cbic.200900779. [DOI] [PubMed] [Google Scholar]
- 152.a) Greenberg WA, Priestley ES, Sears PS, Alper PB, Rosenbohm C, Hendrix M, Hung SC, Wong CH. J Am Chem Soc. 1999;121:6527–6541. [Google Scholar]; b) Busscher GF, Rutjes FPJT, Van DFL. Chem Rev. 2005;105:775–791. doi: 10.1021/cr0404085. [DOI] [PubMed] [Google Scholar]
- 153.Woodward RB, Au-Yeung BW, Balaram P, Browne LJ, Ward DE, Card PJ, Chen CH, et al. J Am Chem Soc. 1981;103:3213–3215.Woodward RB, Logusch E, Nambiar KP, Sakan K, Ward DE, Au-Yeung BW, Balaram P, Browne LJ, Card PJ, Chen CH, Chenevert RB, Fliri A, Frobel K, Gais HJ, Garratt DG, Hayakawa K, Heggie W, Hesson DP, Hoppe D, Hoppe I, Hyatt JA, Ikeda D, Jacobi PA, Kim KS, Kobuke Y, Kojima K, Krowicki K, Lee VJ, Leutert T, Malchenko S, Martens J, Matthews RS, Ong BS, Press JB, RajanBabu TV, Rousseau G, Sauter HM, Suzuki M, Tatsuta K, Tolbert LM, Truesdale EA, Uchida I, Ueda Y, Uyehara T, Vaselia AT, Vladuchick WC, Wade PA, Williams RM, Wong HNC. J Am Chem Soc. 1981;103:3210–3213.Woodward RB, Logusch E, Nambiar KP, Sakan K, Ward DE, Auyeung BW, Balaram P, Browne LJ, Card PJ, Chen CH, Chenevert RB, Fliri A, Frobel K, Gais HJ, Garratt DG, Hayakawa K, Heggie W, Hesson DP, Hoppe D, Hoppe I, Hyatt JA, Ikeda D, Jacobi PA, Kim KS, Kobuke Y, Kojima K, Krowicki K, Lee VJ, Leutert T, Malchenko S, Martens J, Matthews RS, Ong BS, Press JB, Rajanbabu TV, Rousseau G, Sauter HM, Suzuki M, Tatsuta K, Tolbert LM, Truesdale EA, Uchida I, Ueda Y, Uyehara T, Vasella AT, Vladuchick WC, Wade PA, Williams RM, Wong HNC. J Am Chem Soc. 1981;103:3215–3217.Breton P, Hergenrother PJ, Hida T, Hodgson A, Judd AS, Kraynack E, Kym PR, Lee WC, Loft MS, Yamashita M, Martin SF. Tetrahedron. 2007;63:5709–5729.Kim HC, Kang SH. Angew Chem. 2009;121:1859–1861.Angew Chem Int Ed. 2009;48:1827–1829. doi: 10.1002/anie.200805334.Velvadapu V, Paul T, Wagh B, Glassford I, DeBrosse C, Andrade RB. J Org Chem. 2011;76:7516–7527. doi: 10.1021/jo201319b. The Journal of organic chemistry; Velvadapu V, Paul T, Wagh B, Klepacki D, Guvench O, Mackerell A, Jr, Andrade RB. ACS Med Chem Lett. 2011;2:68–72. doi: 10.1021/ml1002184.Velvadapu V, Glassford I, Lee M, Paul T, Debrosse C, Klepacki D, Small MC, Mackerell AD, Jr, Andrade RB. ACS Med Chem Lett. 2012;3:211–215. doi: 10.1021/ml200254h.Wagh B, Paul T, Glassford I, Debrosse C, Klepacki D, Small MC, Mackerell AD, Jr, Andrade RB. ACS Med Chem Lett. 2012;3:1013–1018. doi: 10.1021/ml300230h.Wagh B, Paul T, DeBrosse C, Klepacki D, Small MC, MacKerell AD, Andrade RB. ACS Med Chem Lett. 2013;4:1114–1118. doi: 10.1021/ml400337t.
- 154.a) For a crystal structure of tylosin bound to the large subunit of a bacterial ribosome, see: Hansen JL, Ippolito JA, Ban N, Nissen P, Moore PB, Steitz TA. Mol Cell. 2002;10:117–128. doi: 10.1016/s1097-2765(02)00570-1. b) Interestingly, the 12-membered macrolactone methymycin has been proposed to bind at a different site in the bacterial ribosome: Auerbach T, Mermershtain I, Bashan A, Davidovich C, Rozenberg H, Sherman DH, Yonath A. Biotechnologia. 2009:24–35.
- 155.a) Barriere JC, Bouanchaud DH, Harris NV, Paris JM, Rolin O, Smith C. Program Abstr 30th Intersci Conf Antimicrob Agents Chemother. 1990 doi: 10.1093/jac/30.suppl_a.1. abstract 768. [DOI] [PubMed] [Google Scholar]; b) Fass RJ. Antimicrob Agents Chemother. 1991;35:553–559. doi: 10.1128/aac.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Harms JM, Schlunzen F, Fucini P, Bartels H, Yonath A. BMC Biol. 2004;2:4. doi: 10.1186/1741-7007-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thal LA, Zervos MJ. J Antimicrob Chemother. 1999;43:171–176. doi: 10.1093/jac/43.2.171. [DOI] [PubMed] [Google Scholar]
- 158.a) Schlessinger RH, Li YJ. J Am Chem Soc. 1996;118:3301–3302. [Google Scholar]; b) Tavares F, Lawson JP, Meyers AI. J Am Chem Soc. 1996;118:3303–3304. [Google Scholar]; c) Ghosh AK, Liu W. J Org Chem. 1997;62:7908–7909. doi: 10.1021/jo971616i. [DOI] [PubMed] [Google Scholar]; d) Breuilles P, Uguen D. Tetrahedron Lett. 1998;39:3149–3152. [Google Scholar]; e) Entwistle DA. Synthesis. 1998:603–612. [Google Scholar]; f) Dvorak CA, Schmitz WD, Poon DJ, Pryde DC, Lawson JP, Amos RA, Meyers AI. Angew Chem. 2000;112:1730–1732. doi: 10.1002/(sici)1521-3773(20000502)39:9<1664::aid-anie1664>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2000;39:1664–1666. doi: 10.1002/(sici)1521-3773(20000502)39:9<1664::aid-anie1664>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; g) Mortensen MS, Osbourn JM, O'Doherty GA. Org Lett. 2007;9:3105–3108. doi: 10.1021/ol071145e. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Wu J, Panek JS. Angew Chem. 2010;122:6301–6304. [Google Scholar]; Angew Chem Int Ed. 2010;49:6165–6168. doi: 10.1002/anie.201002220. [DOI] [PubMed] [Google Scholar]
- 159.a) Kessler H, Kuehn M, Loeschner T. Liebigs Ann Chem. 1986:21–31. [Google Scholar]; b) Shaginian A, Rosen MC, Binkowski BF, Belshaw PJ. Chem Eur J. 2004;10:4334–4340. doi: 10.1002/chem.200400267. [DOI] [PubMed] [Google Scholar]
- 160.Sensi P, Margalith P, Timbal MT. Farmaco Sci. 1959;14:146–147. [PubMed] [Google Scholar]
- 161.a) Furesz S, Arioli V, Pallanza R. Antimicrob Agents Chemother. 1965;5:770–777. [PubMed] [Google Scholar]; b) Maggi N, Pallanza R, Sensi P. Antimicrob Agents Chemother. 1965;5:765–769. [PubMed] [Google Scholar]; c) Maggi N, Pasqualucci CR, Ballotta R, Sensi P. Chemotherapy. 1966;11:285–292. doi: 10.1159/000220462. [DOI] [PubMed] [Google Scholar]
- 162.For a review on the development of rifampicin, see: Sensi P. Rev Infect Dis. 1983;5:S402–S406. doi: 10.1093/clinids/5.supplement_3.s402.
- 163.a) Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA. Cell. 2001;104:901–912. doi: 10.1016/s0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]; b) Artsimovitch I, Vassylyeva MN, Svetlov D, Svetlov V, Perederina A, Igarashi N, Matsugaki N, Wakatsuki S, Tahirov TH, Vassylyev DG. Cell. 2005;122:351–363. doi: 10.1016/j.cell.2005.07.014. [DOI] [PubMed] [Google Scholar]; c) Ho MX, Hudson BP, Das K, Arnold E, Ebright RH. Curr Opin Struct Biol. 2009;19:715–723. doi: 10.1016/j.sbi.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.a) Floss HG, Yu TW. Chem Rev. 2005;105:621–632. doi: 10.1021/cr030112j. [DOI] [PubMed] [Google Scholar]; b) Aristoff PA, Garcia GA, Kirchhoff PD, Showalter HDH. Tuberculosis. 2010;90:94–118. doi: 10.1016/j.tube.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 165.a) Iio H, Nagaoka H, Kishi Y. J Am Chem Soc. 1980;102:7965–7967. [Google Scholar]; b) Nagaoka H, Rutsch W, Schmid G, Iio H, Johnson MR, Kishi Y. J Am Chem Soc. 1980;102:7962–7965. [Google Scholar]; c) Nakata M, Akiyama N, Kamata J, Kojima K, Masuda H, Kinoshita M, Tatsuta K. Tetrahedron. 1990;46:4629–4652. [Google Scholar]; d) Nakata M, Akiyama N, Kojima K, Masuda H, Kinoshita M, Tatsuta K. Tetrahedron Lett. 1990;31:1585–1588. [Google Scholar]
- 166.Riedlinger J, Reicke A, Zahner H, Krismer B, Bull AT, Maldonado LA, Ward AC, Goodfellow M, Bister B, Bischoff D, Sussmuth RD, Fiedler HP. J Antibiot. 2004;57:271–279. doi: 10.7164/antibiotics.57.271. [DOI] [PubMed] [Google Scholar]
- 167.a) Zapf CW, Harrison BA, Drahl C, Sorensen EJ. Angew Chem. 2005;117:6691–6695. doi: 10.1002/anie.200502119. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:6533–6537. doi: 10.1002/anie.200502119. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Harrison ST. Angew Chem. 2006;118:3334–3338. [Google Scholar]; Angew Chem Int Ed. 2006;45:3256–3260. [Google Scholar]; c) Nicolaou KC, Harrison ST. J Am Chem Soc. 2007;129:429–440. doi: 10.1021/ja067083p. [DOI] [PubMed] [Google Scholar]; d) Bihelovic F, Saicic RN. Angew Chem. 2012;124:5785–5789. doi: 10.1002/anie.201108223. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:5687–5691. doi: 10.1002/anie.201108223. [DOI] [PubMed] [Google Scholar]; e) Bihelovic F, Karadzic I, Matovic R, Saicic RN. Org Biomol Chem. 2013;11:5413–5424. doi: 10.1039/c3ob40692j. [DOI] [PubMed] [Google Scholar]
- 168.Kahne D, Leimkuhler C, Lu W, Walsh C. Chem Rev. 2005;105:425–448. doi: 10.1021/cr030103a. [DOI] [PubMed] [Google Scholar]
- 169.Corey GR, Stryjewski ME, Weyenberg W, Yasothan U, Kirkpatrick P. Nat Rev Drug Discovery. 2009;8:929–930. doi: 10.1038/nrd3051. [DOI] [PubMed] [Google Scholar]
- 170.Cooper RDG, Snyder NJ, Zweifel MJ, Staszak MA, Wilkie SC, Nicas TI, Mullen DL, Futler TF, Rodriguez MJ, Huff BE, Thompson RC. J Antibiot. 1996;49:575–581. doi: 10.7164/antibiotics.49.575. [DOI] [PubMed] [Google Scholar]
- 171.Mendes RE, Woosley LN, Farrell DJ, Sader HS, Jones RN. Antimicrob Agents Chemother. 2012;56:1639–1642. doi: 10.1128/AAC.06067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Candiani G, Abbondi M, Borgonovi M, Romano G, Parenti F. J Antimicrob Chemother. 1999;44:179–192. doi: 10.1093/jac/44.2.179. [DOI] [PubMed] [Google Scholar]
- 173.Chen AY, Zervos MJ, Vazquez JA. Int J Clin Pract. 2007;61:853–863. doi: 10.1111/j.1742-1241.2007.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.a) Evans DA, Wood MR, Trotter BW, Richardson TI, Barrow JC, Katz JL. Angew Chem. 1998;110:2864–2868. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2700::AID-ANIE2700>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:2700–2704. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2700::AID-ANIE2700>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; b) Evans DA, Katz JL, Peterson GS, Hintermann T. J Am Chem Soc. 2001;123:12411–12413. doi: 10.1021/ja011943e. [DOI] [PubMed] [Google Scholar]
- 175.a) Nicolaou KC, Natarajan S, Li H, Jain NF, Hughes R, Solomon ME, Ramanjulu JM, Boddy CNC, Takayanagi M. Angew Chem. 1998;110:2872–2878. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2708::AID-ANIE2708>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:2708–2714. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2708::AID-ANIE2708>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Jain NF, Natarajan S, Hughes R, Solomon ME, Li H, Ramanjulu JM, Takayanagi M, Koumbis AE, Bando T. Angew Chem. 1998;110:2879–2881. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2714::AID-ANIE2714>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:2714–2716. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2714::AID-ANIE2714>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]; c) Nicolaou KC, Takayanagi M, Jain NF, Natarajan S, Koumbis AE, Bando T, Ramanjulu JM. Angew Chem. 1998;110:2881–2883. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2717::AID-ANIE2717>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 1998;37:2717–2719. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2717::AID-ANIE2717>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]; d) Nicolaou KC, Mitchell HJ, Jain NF, Winssinger N, Hughes R, Bando T. Angew Chem. 1999;111:253–255. [Google Scholar]; Angew Chem Int Ed. 1999;38:240–244. [Google Scholar]; e) Nicolaou KC, Mitchell HJ, Jain NF, Bando T, Hughes R, Winssinger N, Natarajan S, Koumbis AE. Chem Eur J. 1999;5:2648–2667. [Google Scholar]
- 176.a) Boger DL, Miyazaki S, Kim SH, Wu JH, Castle SL, Loiseleur O, Jin Q. J Am Chem Soc. 1999;121:10004–10011. [Google Scholar]; b) Boger DL, Kim SH, Miyazaki S, Strittmatter H, Weng JH, Mori Y, Rogel O, Castle SL, McAtee JJ. J Am Chem Soc. 2000;122:7416–7417. [Google Scholar]; c) Boger DL, Kim SH, Mori Y, Weng JH, Rogel O, Castle SL, McAtee JJ. J Am Chem Soc. 2001;123:1862–1871. doi: 10.1021/ja003835i. [DOI] [PubMed] [Google Scholar]
- 177.a) Crowley BM, Boger DL. J Am Chem Soc. 2006;128:2885–2892. doi: 10.1021/ja0572912. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xie J, Pierce JG, James RC, Okano A, Boger DL. J Am Chem Soc. 2011;133:13946–13949. doi: 10.1021/ja207142h. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) James RC, Pierce JG, Okano A, Xie J, Boger DL. ACS Chem Biol. 2012;7:797–804. doi: 10.1021/cb300007j. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Xie J, Okano A, Pierce JG, James RC, Stamm S, Crane CM, Boger DL. J Am Chem Soc. 2012;134:1284–1297. doi: 10.1021/ja209937s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.He H, Williamson RT, Shen B, Graziani EI, Yang HY, Sakya SM, Petersen PJ, Carter GT. J Am Chem Soc. 2002;124:9729–9736. doi: 10.1021/ja020257s. [DOI] [PubMed] [Google Scholar]
- 179.Singh MP, Petersen PJ, Weiss WJ, Janso JE, Luckman SW, Lenoy EB, Bradford PA, Testa RT, Greenstein M. Antimicrob Agents Chemother. 2003;47:62–69. doi: 10.1128/AAC.47.1.62-69.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.a) Dushin RG, Wang TZ, Sum PE, He H, Sutherland AG, Ashcroft JS, Graziani EI, Koehn FE, Bradford PA, Petersen PJ, Wheless KL, How D, Torres N, Lenoy EB, Weiss WJ, Lang SA, Projan SJ, Shlaes DM, Mansour TS. J Med Chem. 2004;47:3487–3490. doi: 10.1021/jm049765y. [DOI] [PubMed] [Google Scholar]; b) He H, Shen B, Petersen PJ, Weiss WJ, Yang HY, Wang TZ, Dushin RG, Koehn FE, Carter GT. Bioorg Med Chem Lett. 2004;14:279–282. doi: 10.1016/j.bmcl.2003.09.071. [DOI] [PubMed] [Google Scholar]; c) Petersen PJ, Wang TZ, Dushin RG, Bradford PA. Antimicrob Agents Chemother. 2004;48:739–746. doi: 10.1128/AAC.48.3.739-746.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Weiss WJ, Murphy T, Lenoy E, Young M. Antimicrob Agents Chemother. 2004;48:1708–1712. doi: 10.1128/AAC.48.5.1708-1712.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.a) Jiang W, Wanner J, Lee RJ, Bounaud PY, Boger DL. J Am Chem Soc. 2002;124:5288–5290. doi: 10.1021/ja020237q. [DOI] [PubMed] [Google Scholar]; b) Chen L, Yuan Y, Helm JS, Hu Y, Rew Y, Shin D, Boger DL, Walker S. J Am Chem Soc. 2004;126:7462–7463. doi: 10.1021/ja047879t. [DOI] [PubMed] [Google Scholar]; c) Walker S, Chen L, Hu Y, Rew Y, Shin D, Boger DL. Chem Rev. 2005;105:449–475. doi: 10.1021/cr030106n. [DOI] [PubMed] [Google Scholar]; d) Nam J, Shin D, Rew Y, Boger DL. J Am Chem Soc. 2007;129:8747–8755. doi: 10.1021/ja068573k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.DeBono M, Abbott BJ, Krupinski BM, Molloy RM, Berry DR, Counter FT, Howad LC, Ott JL, Hamill RL. Program Abstr 24th Intersci Conf Antimicrob Agents Chemother. 1984 Abstract #1077. [Google Scholar]
- 183.Steenbergen JN, Alder J, Thorne GM, Tally FP. J Antimicrob Chemother. 2005;55:283–288. doi: 10.1093/jac/dkh546. [DOI] [PubMed] [Google Scholar]
- 184.Pogliano J, Pogliano N, Silverman JA. J Bacteriol. 2012;194:4494–4504. doi: 10.1128/JB.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.a) Hill J, Siedlecki J, Parr I, Morytko M, Yu X, Zhang Y, Silverman J, Controneo N, Laganas V, Li T, Lai JJ, Keith D, Shimer G, Finn J. Bioorg Med Chem Lett. 2003;13:4187–4191. doi: 10.1016/j.bmcl.2003.07.019. [DOI] [PubMed] [Google Scholar]; b) He Y, Li J, Yin N, Herradura PS, Martel L, Zhang Y, Pearson AL, Kulkarni V, Mascio C, Howland K, Silverman JA, Keith DD, Metcalf CA. Bioorg Med Chem Lett. 2012;22:6248–6251. doi: 10.1016/j.bmcl.2012.08.013. [DOI] [PubMed] [Google Scholar]; c) Yoganathan S, Yin N, He Y, Mesleh MF, Gu YG, Miller SJ. Org Biomol Chem. 2013;11:4680–4685. doi: 10.1039/c3ob40924d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Grünewald J, Sieber SA, Mahlert C, Linne U, Marahiel MA. J Am Chem Soc. 2004;126:17025–17031. doi: 10.1021/ja045455t. [DOI] [PubMed] [Google Scholar]
- 187.Nguyen KT, Ritz D, Gu JQ, Alexander D, Chu M, Miao V, Brian P, Baltz RH. Proc Natl Acad Sci USA. 2006;103:17462–17467. doi: 10.1073/pnas.0608589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lam HY, Zhang Y, Liu H, Xu J, Wong CTT, Xu C, Li X. J Am Chem Soc. 2013;135:6272–6279. doi: 10.1021/ja4012468. [DOI] [PubMed] [Google Scholar]
- 189.Taylor JG, Li X, Oberthuer M, Zhu W, Kahne DE. J Am Chem Soc. 2006;128:15084–15085. doi: 10.1021/ja065907x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gampe CM, Tsukamoto H, Doud EH, Walker S, Kahne D. J Am Chem Soc. 2013;135:3776–3779. doi: 10.1021/ja4000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bierbaum G, Sahl HG. Curr Pharm Biotechnol. 2009;10:2–18. doi: 10.2174/138920109787048616. [DOI] [PubMed] [Google Scholar]
- 192.Breukink E, Wiedemann I, Kraaij Cv, Kuipers OP, Sahl HG, de Kruijff B. Science. 1999;286:2361–2364. doi: 10.1126/science.286.5448.2361. [DOI] [PubMed] [Google Scholar]
- 193.a) Ross AC, McKinnie SMK, Vederas JC. J Am Chem Soc. 2012;134:2008–2011. doi: 10.1021/ja211088m. [DOI] [PubMed] [Google Scholar]; b) Knerr PJ, van der Donk WA. J Am Chem Soc. 2013;135:7094–7097. doi: 10.1021/ja4014024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.We express our gratitude to Mark Noe of Pfizer, Inc. for this quote, and many helpful conversations concerning antibiotics research.
- 195. [accessed December, 2013];Anacor Pharmaceuticals announces that GSK has discontinued clinical development of GSK2251052. http://investor.anacor.com/releasedetail.cfm?ReleaseID=711456.