Abstract
There is a close spatial and temporal relationship between macrophage accumulation and active renal fibrosis in human and experimental kidney disease. Different subtypes of macrophages have been identified. Pro-inflammatory M1-type macrophages can cause acute tissue injury, whereas pro-fibrotic M2-type macrophages can drive the fibrotic response during ongoing tissue injury. Macrophages induce fibrosis through the recruitment, proliferation, and activation of fibroblasts. In addition, there is accumulating evidence that supports a direct fibrotic role for macrophages via transition into myofibroblasts in a process termed macrophage–myofibroblast transition (MMT). Co-expression of macrophage and myofibroblast antigens identifies the MMT process both in human and experimental fibrotic kidney disease. This co-expression identifies a bone marrow–derived monocyte/macrophage source for a substantial proportion of the myofibroblast population present during renal fibrosis. This postulated MMT pathway represents a new mechanism linking macrophage-rich acute inflammation with the progression to myofibroblast accumulation and renal fibrosis. Further studies are required to identify the molecular mechanisms regulating the MMT process, which macrophage populations can undergo MMT, and to define the functional contribution of MMT to active collagen deposition during renal fibrosis.
Keywords: fibrosis, M1 and M2 macrophages, MMT, myofibroblast, transition
Macrophage heterogeneity
Cells of the monocyte/macrophage lineage are highly heterogeneous because of the fact that their functional responses are strongly influenced by the local microenvironment. In the setting of tissue injury, blood monocytes are recruited to the site of damage and then they undergo differentiation in response to the microenvironment that may include oxidative stress, hypoxia, toxins, or activation via triggering of damage or pathogen-associated molecular pattern receptors.1 The current paradigm of distinct macrophage subtypes has been further delineated after extensive in vitro investigation of both classically activated M1-type pro-inflammatory macrophages and the different subtypes of M2-type alternatively activated macrophages. It has now been established that interleukin-4 (IL-4) and IL-13 induce M2a ‘wound-healing' macrophages, immune complexes plus lipopolysaccharide induce M2b, and IL-10, transforming growth factor-β1 (TGF-β1), and glucocorticoids induce M2c ‘regulatory macrophages'.2 Whereas this is a relatively simplistic view of macrophage heterogeneity, it has provided a useful model for exploring the function of macrophages in different homeostatic and pathologic settings.
PRO-INFLAMMATORY M1-TYPE MACROPHAGES CAUSE TISSUE DAMAGE RESULTING IN RENAL FIBROSIS
Glomerular and interstitial macrophage infiltration is a common feature in most forms of glomerulonephritis. Renal biopsy studies have identified prominent infiltration of M1-type pro-inflammatory macrophages in rapidly progressive glomerulonephritis on the basis of their production of pro-inflammatory cytokines (IL-1, tumour necrosis factor-α, and macrophage migration inhibitory factor), and expression of myeloid-related proteins 8 and 14 and sialoadhesin.3 Macrophage infiltration and local proliferation correlate with the severity of glomerular and tubulointerstitial damages, and renal function impairment, and is prognostic of disease progression.4, 5, 6 Furthermore, the tight colocalization of proliferative macrophages and alpha smooth muscle actin (α-SMA) + myofibroblasts in areas of severe renal damage suggests a close link between macrophages and renal fibrosis in chronic kidney disease.7
Infiltration of M1-type pro-inflammatory macrophages is also evident in animal models of crescentic glomerulonephritis. Reversal of glomerular macrophage infiltrate abolishes the upregulation of molecules involved in the M1 response (tumour necrosis factor-α, inducible nitric oxide synthase, and matrix metalloproteinase-12) and prevents crescent formation, and the development of glomerulosclerosis and tubulointerstitial fibrosis yet without abrogation of heavy proteinuria.8 Furthermore, the c-jun amino terminal kinase signaling pathway has been identified as a key mechanism in the M1-type pro-inflammatory macrophage response in this disease model. Interestingly, blockade of c-jun amino terminal kinase signaling did not prevent glomerular macrophage infiltration; however, these macrophages failed to mount the characteristic M1 response in this model and thus were unable to cause renal injury.9 Whereas these studies can establish a role for M1-type pro-inflammatory macrophages in acute renal injury, they cannot establish whether macrophages have a direct role in renal fibrosis that develops as a secondary response to initial tissue injury.
ALTERNATIVELY ACTIVATED MACROPHAGES PROMOTE RENAL FIBROSIS
If chronic immune injury and inflammation persists as is evident in many types of kidney diseases, then infiltrating macrophages can adopt injurious M1-type response or a pro-fibrotic M2-type response. This heterogeneity makes it difficult to delineate the specific role of macrophages in renal fibrosis.
There are a number of mechanisms by which macrophages promote renal fibrosis. Macrophages can promote the formation of a provisional extracellular matrix containing fibrin, fibrinogen, and fibronectin that promotes the recruitment of fibroblasts and their activation to become myofibroblasts. In addition, macrophages can induce the recruitment and proliferation of fibroblasts by secreting factors such as platelet-derived growth factor, fibroblast growth factor-2, TGF-β1, connective tissue growth factor, and galectin-3. Besides secreting latent TGF-β1, macrophages produce factors that can activate latent TGF-β1 such as metalloproteases and thrombospondin. However, a recent study using conditional deletion of TGF-β1 in macrophages found that macrophages are not a functionally important source of this key pro-fibrotic factor during renal fibrosis.10
In human and experimental kidney diseases, there is a close spatial and temporal association between macrophage infiltration and active glomerular and interstitial fibrosis.4, 5 In new onset IgA nephropathy, M2-type CD163+ and CD204+ macrophages expressing the pro-fibrotic connective tissue growth factor are prominent in areas of active fibrosis containing myofibroblasts.11 In a different setting, M2-type CD163+ and CD206+ macrophages are increased within peritoneal dialysis effluents during episodes of peritonitis. These M2-type macrophages produce CCL18 that can promote fibroblast proliferation, and higher levels of CCL18 production was associated with functional deficiency and fibrosis of the peritoneal membrane.12
As rat crescentic anti-GBM glomerulonephritis progresses from an aggressive inflammatory injury to a chronic fibrotic disease, there is a change in the macrophage infiltrate from an M1-type pro-inflammatory to a predominant alternatively activated M2 phenotype.13 Selective deletion of the macrophage infiltrate during this chronic phase of disease significantly reduced glomerular and interstitial fibrosis, protected against further peritubular capillary loss, and improved renal function.13 Further evidence for a pro-fibrotic role for M2-type macrophages in renal disease comes from a study in which steroid treatment of rat Thy-1 nephritis induced an M2-like macrophage phenotype, which failed to modify mesangial hypercellularity and exacerbated global glomerulosclerosis.14
The most commonly studied model of interstitial fibrosis is unilateral ureteric obstruction (UUO) in which the macrophage infiltrate has a predominant M2 phenotype. The standard UUO model has an important advantage in that the renal insult is irreversible, and therefore any beneficial effects of therapeutic agents cannot be attributed to suppressing the injury driving the fibrotic response. A variety of depletion/blocking strategies have been used to reduce macrophage infiltration in the UUO model, including clodronate liposomes, diphtheria-based deletion of CD11b+ cells, and blockade of CCL2/CCR2 and CCR1, which caused a reduction in renal fibrosis.15, 16, 17, 18 However, not all macrophage depletion strategies resulted in a reduction in fibrosis in this model. Reversal of the macrophage infiltrate with inhibition of c-fms kinase failed to affect fibrosis,19 whereas cyclophosphamide-based leukocyte depletion increased fibrosis in the later stage of this model, which was ameliorated by adoptive transfer of macrophages.20 These discrepancies may simply reflect the heterogeneity of the macrophage population such that different macrophage subsets are deleted (and remain) following the different blockade/depletion strategies used. This is exemplified by a recent study in which depletion of CD11b+ cells gave a different outcome in a model of renal ischemia-reperfusion injury compared with macrophage depletion by clodronate liposomes.21
In a mouse model of adriamycin-induced nephropathy, exogenously administered IL-4/IL-13-induced M2a macrophages suppressed renal injury caused by endogenous M1-type pro-inflammatory macrophages in a mouse model of Adriamycin-induced nephropathy. This is in contrast to the pro-fibrotic role ascribed to endogenous M2 macrophages and thus identifies a therapeutic potential of exogenous M2-type macrophages.22 This observation suggests that there may be important functional differences between systemic versus locally induced M2-type macrophages in terms of regulatory and pro-fibrotic responses, which requires further investigation.
MONOCYTE/MACROPHAGES AS PRECURSORS OF MYOFIBROBLASTS IN RENAL FIBROSIS
The source of myofibroblasts in renal fibrosis is a highly controversial issue. Several different cellular origins have been proposed, including resident fibroblasts, resident pericytes, epithelial-to-myofibroblast transition, and endothelial cell-to-myofibroblast transition.23, 24, 25, 26, 27 In addition, there is evidence that bone marrow–derived cells can also give rise to myofibroblasts. This is largely based on studies in which the induction of renal fibrosis in chimeric mice with a green fluorescence protein (GFP)–expressing bone marrow compartment results in the presence of GFP+ α-SMA+ myofibroblasts in the fibrosing kidney,25, 28 as well as the identification of recipient-derived myofibroblasts in chronic kidney allograft nephropathy.29 A leukocyte origin or, more specifically, a monocyte/macrophage origin, for bone marrow–derived myofibroblasts has been proposed, although other studies have failed to support these findings.30, 31
Fibrocytes in renal fibrosis
Fibrocytes were first identified as a bone marrow–derived circulating blood cell with fibroblast properties characterized by its distinctive phenotype (collagen+ vimentin+ CD34+), its rapid entry from blood into subcutaneously implanted wound chambers, and by its presence in connective tissue scars.32 However, it has been difficult to delineate the relationship of fibrocytes to specific leukocyte lineages, and studies have failed to develop a consensus for expression of particular macrophage markers by fibrocytes. Indeed, fibrocyte populations are now most commonly defined as cells co-expressing the leukocyte common antigen, CD45, and collagen I. One study using flow cytometry analysis of cells isolated from the obstructed mouse kidney found that the majority of CD45+ leukocytes expressed both collagen I and α-SMA. Furthermore, production of the chemokine CXCL16 by tubular epithelial cells was shown to be required for the recruitment of these bone marrow–derived CD45+ col I+ α-SMA+ cells into the obstructed kidney and the development of renal fibrosis.33 A second study identified fibrocytes as bone marrow–derived cells that mature in the spleen and have a phenotype distinct from that of circulating monocytes. Furthermore, splenic fibrocytes can migrate to the kidney following ureter obstruction and promote renal fibrosis.34
Macrophage–myofibroblast transition (MMT) in renal fibrosis
An alternative explanation for the presence of CD45+ col I+ cells in the fibrosing kidney is that these are infiltrating monocytes from the bone marrow that have undergone transition into collagen-producing myofibroblasts within the local microenvironment of the injured kidney—most probably driven by TGF-β1. Thus, the question is whether cells leaving the bone marrow have already acquired a fibroblast phenotype, or is it the case that infiltrating monocytes can encounter a pro-fibrotic microenvironment and a subset of these monocytes undergoes transition into myofibroblasts? This will be a difficult question to tease out. However, there is clear evidence that cultured macrophages can be induced to develop into collagen-producing α-SMA+ myofibroblast-type cells.35 In addition, collagen-producing cells with macrophage characteristics have been identified in the peritoneum of schistosome-infected mice and in fibrosing peritoneal capsule during the foreign body response.36, 37
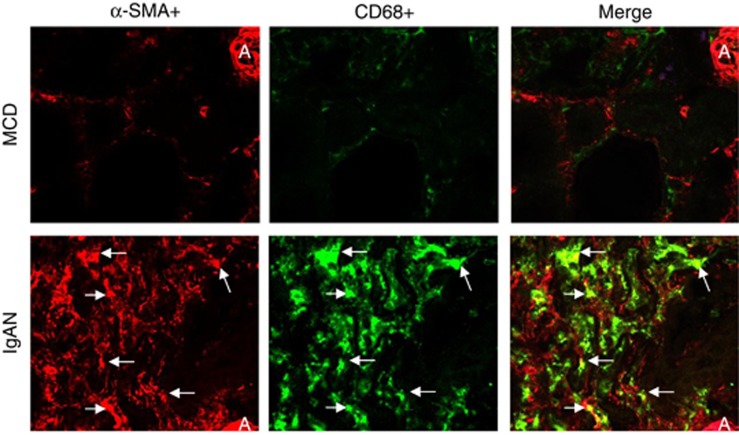
We have now termed the process whereby infiltrating bone marrow–derived monocytes undergo local transition into myofibroblasts within the injured kidney as MMT. Using confocal microscopy, we have identified transitional cells co-expressing both macrophage (CD68+) and myofibroblast (α-SMA+) markers in patients with progressive renal fibrosis; however, this was absent in those without renal fibrosis such as minimal change disease (Figure 1). To establish whether these MMT cells are of bone marrow origin, we performed a cell-tracing study in the UUO model in which lethally irradiated mice were reconstituted with GFP+ bone marrow. Numerous GFP+ cells migrated into the obstructed kidney, but not the sham-operated kidney. In addition, many of the α-SMA+ myofibroblasts present in the obstructed kidney co-expressed F4/80 and GFP, indicating a bone marrow macrophage origin (Figure 2). In contrast, no MMT cells and no renal fibrosis were evident in the sham-operated kidney. However, one limitation of such an approach is that macrophages and myofibroblasts are often in very close proximity within active fibrotic lesions and may not be easy to be distinguished in the confocal image. Thus, we used flow cytometry to analyse individual cells isolated from the obstructed wild-type kidney. This approach verified the presence of a substantial population of cells co-expressing macrophage (F4/80+) and myofibroblast (α-SMA+) markers in the fibrosing mouse kidney (Figure 3). Taken together, these preliminary observations from human and experimental kidney disease suggest that bone marrow–derived monocytes can undergo MMT locally within the injured kidney and that this may have a significant role in progressive renal fibrosis.
Figure 1.
Evidence for macrophage-myofibropblast transition (MMT) in human fibrotic kidney disease. Confocal microscopy reveals that severe renal fibrosis in a patient with IgA nephropathy (IgAN) is associated with numerous MMT cells identified by co-expression of alpha smooth muscle action (α-SMA) (red) and CD68 (green). Such MMT cells are absent in a case of minimal change disease (MCD). A, arteriole; arrows identify α-SMA+CD68+ double-positive MMT cells. Magnification: × 400.
Figure 2.
Evidence for bone marrow-derived macrophage-myofibropblast transition (MMT) in the mouse model of unilateral ureteric obstruction (UUO). A day-7 UUO study was performed in chimeric mice with a green fluorescence protein (GFP)+ bone marrow compartment. Confocal microscopy showed that the severe renal fibrosis on day-7 UUO is associated with numerous α-SMA+F4/80+GFP+ MMT cells in the obstructed kidney but not in the sham-operated kidney. An MMT cell is illustrated in the inserted picture of each panel. Magnification: × 400.
Figure 3.
Two-color flow cytometry detects macrophage-myofibropblast transition (MMT) cells in wild-type mice in the unilateral ureteric obstruction (UUO) model. Two-color immunofluorescence staining show that the majority of alpha smooth muscle action (α-SMA)+ myofibroblasts co-express the macrophage F4/80 antigen in a single-cell suspension of the obstructed kidney prepared by enzyme digestion. However, this is not the case in the sham-operated kidney.
Further studies are required to define whether MMT represents an important mechanism in renal fibrosis. In particular, we need to (1) identify whether MMT is a common feature in different types of chronic kidney diseases in both human and animals; (2) examine whether MMT occurs in both glomerular and interstitial compartments; (3) quantify MMT as a source of myofibroblasts in different types of kidney diseases; (4) use macrophage lineage–tracing technologies to identify MMT and to determine which cells in the monocyte/macrophage lineage have the potential to undergo MMT; (5) define the stimuli required to induce MMT and the mechanisms by which this operates; and (6) use conditional gene deletion to determine whether MMT cells make a significant contribution to collagen deposition in renal fibrosis.
In conclusion, macrophages are a heterogeneous population that has an important role in kidney homeostasis but can also be activated to cause renal injury, or promote chronic fibrosis, when there is an ongoing renal insult. There are a number of mechanisms by which macrophages can promote renal fibrosis, including the potential for direct MMT. Selective targeting of the macrophage pro-fibrotic response may be a fruitful approach for the treatment of chronic fibrotic kidney disease.
Acknowledgments
This study was supported in part by grants from the Research Grant Council of Hong Kong (RGC GRF 468711, CUHK5/CRF/09, and CUHK3/CRF/12R); the Focused Investment Schemes A and B from the Chinese University of Hong Kong; and Major State Basic Research Development Program of China (973 program, no. 2012CB517700). This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
DN-P has received consulting fees and grant support from Gilead Sciences. The remaining authors declared no competing interests.
References
- Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Ma FY, Ikezumi Y, Nikolic-Paterson DJ. Macrophage signaling pathways: a novel target in renal disease. Sem Nephrol. 2010;30:334–344. doi: 10.1016/j.semnephrol.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Eardley KS, Kubal C, Zehnder D, et al. The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int. 2008;74:495–504. doi: 10.1038/ki.2008.183. [DOI] [PubMed] [Google Scholar]
- Nikolic-Paterson DJ, Atkins RC. The role of macrophages in glomerulonephritis. Nephrol Dialysis Transplant. 2001;16 (Suppl 5:3–7. doi: 10.1093/ndt/16.suppl_5.3. [DOI] [PubMed] [Google Scholar]
- Yang N, Isbel NM, Nikolic-Paterson DJ, et al. Local macrophage proliferation in human glomerulonephritis. Kidney Int. 1998;54:143–151. doi: 10.1046/j.1523-1755.1998.00978.x. [DOI] [PubMed] [Google Scholar]
- Yang N, Wu LL, Nikolic-Paterson DJ, et al. Local macrophage and myofibroblast proliferation in progressive renal injury in the rat remnant kidney. Nephrol Dialysis Transplant. 1998;13:1967–1974. doi: 10.1093/ndt/13.8.1967. [DOI] [PubMed] [Google Scholar]
- Han Y, Ma FY, Tesch GH, et al. c-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-GBM glomerulonephritis in the rat. Lab Invest. 2011;91:978–991. doi: 10.1038/labinvest.2011.61. [DOI] [PubMed] [Google Scholar]
- Ma FY, Flanc RS, Tesch GH, et al. Blockade of the c-Jun amino terminal kinase prevents crescent formation and halts established anti-GBM glomerulonephritis in the rat. Lab Invest. 2009;89:470–484. doi: 10.1038/labinvest.2009.2. [DOI] [PubMed] [Google Scholar]
- Tan RJ, Liu Y. Macrophage-derived TGF-beta in renal fibrosis: not a macro-impact after all. Am J Physiol Renal Physiol. 2013;305:F821–F822. doi: 10.1152/ajprenal.00356.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezumi Y, Suzuki T, Karasawa T, et al. Identification of alternatively activated macrophages in new-onset paediatric and adult immunoglobulin A nephropathy: potential role in mesangial matrix expansion. Histopathology. 2011;58:198–210. doi: 10.1111/j.1365-2559.2011.03742.x. [DOI] [PubMed] [Google Scholar]
- Bellon T, Martinez V, Lucendo B, et al. Alternative activation of macrophages in human peritoneum: implications for peritoneal fibrosis. Nephrol Dialysis Transplant. 2011;26:2995–3005. doi: 10.1093/ndt/gfq771. [DOI] [PubMed] [Google Scholar]
- Han Y, Ma FY, Tesch GH, et al. Role of macrophages in the fibrotic phase of rat crescentic glomerulonephritis. Am J Physiol Renal Physiol. 2013;304:F1043–F1053. doi: 10.1152/ajprenal.00389.2012. [DOI] [PubMed] [Google Scholar]
- Ikezumi Y, Suzuki T, Karasawa T, et al. Contrasting effects of steroids and mizoribine on macrophage activation and glomerular lesions in rat thy-1 mesangial proliferative glomerulonephritis. Am J Nephrol. 2010;31:273–282. doi: 10.1159/000279163. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Vielhauer V, Frink M, et al. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest. 2002;109:251–259. doi: 10.1172/JCI14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Wada T, Furuichi K, et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto K, Machida Y, Uchida J, et al. Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci. 2009;111:285–292. doi: 10.1254/jphs.09227fp. [DOI] [PubMed] [Google Scholar]
- Ma FY, Liu J, Kitching AR, et al. Targeting renal macrophage accumulation via c-fms kinase reduces tubular apoptosis but fails to modify progressive fibrosis in the obstructed rat kidney. Am J Physiol Renal Physiol. 2009;296:F177–F185. doi: 10.1152/ajprenal.90498.2008. [DOI] [PubMed] [Google Scholar]
- Nishida M, Okumura Y, Fujimoto S, et al. Adoptive transfer of macrophages ameliorates renal fibrosis in mice. Biochem Biophys Res Commun. 2005;332:11–16. doi: 10.1016/j.bbrc.2005.04.083. [DOI] [PubMed] [Google Scholar]
- Ferenbach DA, Sheldrake TA, Dhaliwal K, et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–933. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang YP, Zheng G, et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekema M, Harmsen MC, van Luyn MJ, et al. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol. 2007;18:165–175. doi: 10.1681/ASN.2005070730. [DOI] [PubMed] [Google Scholar]
- Grimm PC, Nickerson P, Jeffery J, et al. Neointimal and tubulointerstitial infiltration by recipient mesenchymal cells in chronic renal-allograft rejection. N Engl J Med. 2001;345:93–97. doi: 10.1056/NEJM200107123450203. [DOI] [PubMed] [Google Scholar]
- Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roufosse C, Bou-Gharios G, Prodromidi E, et al. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J Am Soc Nephrol. 2006;17:775–782. doi: 10.1681/ASN.2005080795. [DOI] [PubMed] [Google Scholar]
- Bucala R, Spiegel LA, Chesney J, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Chen G, Lin SC, Chen J, et al. CXCL16 recruits bone marrow-derived fibroblast precursors in renal fibrosis. J Am Soc Nephrol. 2011;22:1876–1886. doi: 10.1681/ASN.2010080881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich B, Schmidbauer K, Rodriguez Gomez M, et al. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int. 2013;84:78–89. doi: 10.1038/ki.2013.84. [DOI] [PubMed] [Google Scholar]
- Pilling D, Gomer RH. Differentiation of circulating monocytes into fibroblast-like cells. Methods Mol Biol. 2012;904:191–206. doi: 10.1007/978-1-61779-943-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand S, Godoy M, Semal P, et al. Transdifferentiation of macrophages into fibroblasts as a result of Schistosoma mansoni infection. Int J Dev Biol. 1992;36:179–184. [PubMed] [Google Scholar]
- Mooney JE, Rolfe BE, Osborne GW, et al. Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response. Am J Pathol. 2010;176:369–380. doi: 10.2353/ajpath.2010.090545. [DOI] [PMC free article] [PubMed] [Google Scholar]