Abstract
Wnt/β-catenin signaling is an evolutionarily conserved, highly complex, key developmental pathway that regulates cell fate, organ development, tissue homeostasis, as well as injury and repair. Although relatively silent in normal adult kidney, Wnt/β-catenin signaling is re-activated after renal injury in a wide variety of animal models and in human kidney disorders. Whereas some data point to a protective role of this signaling in healing and repair after acute kidney injury, increasing evidence suggests that sustained activation of Wnt/β-catenin is associated with the development and progression of renal fibrotic lesions. In kidney cells, Wnt/β-catenin promotes the expression of numerous fibrosis-related genes such as Snail1, plasminogen activator inhibitor-1, and matrix metalloproteinase-7. Recent studies also indicate that multiple components of the renin–angiotensin system are the direct downstream targets of Wnt/β-catenin. Consistently, inhibition of Wnt/β-catenin signaling by an assortment of strategies ameliorates kidney injury and mitigates renal fibrotic lesions in various models of chronic kidney disease, suggesting that targeting this signaling could be a plausible strategy for therapeutic intervention. In this mini review, we will briefly discuss the regulation, downstream targets, and mechanisms of Wnt/β-catenin signaling in the pathogenesis of kidney fibrosis.
Keywords: β-catenin, fibroblasts, proteinuria, renal fibrosis, Wnt
Wnt/β-catenin is an evolutionarily conserved signaling pathway that has a fundamental role in regulating a variety of biologic processes such as organ development, tissue homeostasis, and pathogenesis of human diseases. The building blocks of this pathway are exceptionally complex and consist of more than 50 distinctive proteins, which include a large family of secreted ligands, numerous cell membrane receptors and co-receptors, multifaceted intracellular mediators, and several classes of endogenous antagonists.1, 2 Activity of this pathway is indispensable for nephron formation during mammalian development.3 In the adult kidney, Wnt/β-catenin signaling becomes functionally silent after differentiation. However, increasing data have demonstrated that Wnt/β-catenin is re-activated after kidney injury, and it is often intertwined with other pathologic signal pathways.4, 5, 6, 7 Over the past several years, significant progress has been made in investigating the regulation, the downstream targets, and the underlying mechanisms of Wnt/β-catenin signaling in the pathogenesis of various kidney diseases.5, 6, 7 A better understanding of this pathway is critical for developing new therapeutics in the fight against kidney disease.
Wnt/β-CATENIN SIGNALING: COMPONENTS AND REGULATORS
The Wnt family comprises a group of secreted, lipid-modified, signaling glycoproteins. In mammalian systems, there are at least 19 members of the Wnt protein family (for more details, see the Wnt homepage: www.stanford.edu/group/nusselab/cgi-bin/wnt). Wnt proteins transmit their signal across the plasma membrane through interactions with the Frizzled (Fzd) family of proteins and their co-receptors, the low-density lipoprotein receptor–related protein-5 or protein-6 (LRP5/6). The Fzd receptors comprise at least 10 family members, all of which are composed of seven transmembrane domains. The vast majority of Wnts and Fzd receptors are expressed or induced in the kidneys.6 The reasons underlying the simultaneous expression of so many Wnts and their receptors in a given tissue remain elusive.
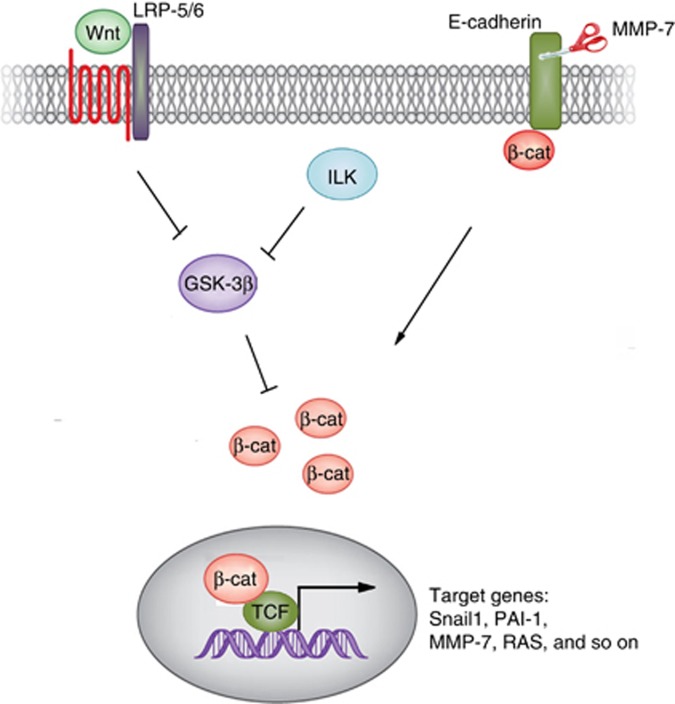
Wnts exert their biological functions via both canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) pathways. In the canonical pathway, LRP5 or LRP6 (LRP5/6) is closely associated with the Fzds and becomes phosphorylated upon binding Wnt ligands.8 This triggers a series of downstream intracellular signaling events involving Disheveled (Dvl), axin, adenomatosis polyposis coli, and glycogen synthase kinase-3β, ultimately resulting in dephophorylation of β-catenin. This leads to the stabilization and nuclear translocation of β-catenin, where it binds to and activates transcription factors of the T-cell factor (TCF) and lymphoid enhancer–binding factor (LEF) families to stimulate the transcription of target genes (Figure 1).9
Figure 1.
Schematic diagram showing canonical Wnt/β-catenin signaling. Wnts bind to their cell membrane receptors and co-receptors, and this triggers a cascade of intracellular signaling events, leading to β-catenin dephosphorylation and stabilization. The stabilized β-catenin then translocates into the nucleus, where it interacts with T-cell factor (TCF)/lymphoid enhancer–binding factor (LEF) transcription factors and drives the transcription of its target genes. Apart from Wnts, integrin-linked kinase (ILK) also leads to β-catenin activation. In addition, MMP-mediated E-cadherin extracellular domain shedding also releases β-catenin, resulting in its activation.
In addition, Wnt proteins may exert their activities through numerous non-canonical intracellular signaling routes. One pathway helps to determine cell polarity during development and involves small Rho GTPases and downstream c-Jun N-terminal kinase activation.10 This pathway still relies upon a cell surface complex involving Wnt, Fzd, and Dvl, demonstrating that Fzd and Dvl can mediate both canonical and non-canonical pathways. Another pathway is dependent upon phospholipase C-mediated increases in intracellular calcium that in turn activates Ca2+/calmodulin-dependent protein kinase, protein kinase C, and nuclear factor of activated T cells.9 This pathway appears to be involved in mesenchymal-to-epithelial transitions during nephrogenesis.11 Other receptors for Wnts are also known to exist, including Ror2 and Ryk, which act through c-Jun N-terminal kinase and Src, respectively.1 The contributions of these non-canonical pathways in kidney disease are an area of active investigation.
Regardless of the end result, signaling through Wnt is tightly controlled in a multitude of ways. There are several secreted antagonists of Wnt signaling, including soluble Frizzled-related proteins (sFRPs), Wnt inhibitory factor, and the family of Dickkopf (DKK) proteins. sFRPs, by virtue of their sequence homology with the Fzd receptors, are capable of binding and sequestering Wnts in the extracellular space to prevent binding to Fzd receptors.8 Wnt inhibitory factor is a lipid-binding protein that binds to Wnt proteins and prevents signaling. Among the different Wnt antagonists, DKK family proteins are unique in that they antagonize canonical signaling by binding and internalizing the LRP5/6 co-receptor that is vital for Wnt signaling through Fzd. However, DKK may also have roles that are Wnt-independent.2 Recent studies suggest that DKK1 blocks growth factor–triggered mitogen-activated protein kinase and c-Jun N-terminal kinase signaling cascades by mechanisms dependent on LRP6 and Wnt ligands but not downstream β-catenin signaling.12 Experimental data now also implicate the antiaging protein Klotho in antagonizing Wnt signaling. Klotho can directly bind various Wnts and can inhibit their activity.13, 14 Finally, not all proteins are negative regulators; the protein Cripto-1 was found to enhance Wnt binding to LRP5/6.15
It is important to point out that β-catenin can be activated by signals other than Wnts. One of the upstream regulators of β-catenin is the integrin-linked kinase (ILK), which is induced by transforming growth factor-β1 (TGF-β1), angiotensin II, integrins, and other fibrogenic cues. Although there is controversy surrounding whether ILK is a true kinase, upregulation of ILK is reported to result in β-catenin stabilization and activation in many cell types including podocytes. We have shown that TGF-β1 activates β-catenin, either through the induction of Wnt1 and ILK expression or through the activation of Akt and p38 MAP kinase.16, 17 Furthermore, matrix metalloproteinase-7 (MMP-7)-mediated degradation of E-cadherin, the well-characterized cell adhesion receptor that can be found associated with β-catenin, leads to β-catenin release and activation.18, 19 Therefore, apart from Wnts, multiple pathogenic cues can lead to β-catenin activation in diseased kidneys (Figure 1).
Wnt/β-CATENIN ACTIVATION AFTER KIDNEY INJURY: TOO MUCH OF A GOOD THING?
Despite being relatively silent in normal adult kidneys, Wnt/β-catenin signaling is re-activated after renal injury. For instance, in acute kidney injury (AKI) induced by folic acid or ischemia-reperfusion injury, β-catenin is highly upregulated in renal tubular epithelial cells.20 As Wnt/β-catenin signaling is essential for proper nephron formation and kidney development, one would speculate that activation of Wnt/β-catenin signaling might be reparative by promoting renal regeneration through recapitulating kidney developmental programs. Indeed, studies show that this upregulation of Wnt/β-catenin is advantageous, as tubule-specific ablation of β-catenin in tubular epithelium was associated with increased renal injury, tubule cell apoptosis, and mortality after either ischemic or toxic AKI.20 Similarly, it was found that Wnt7b originating from macrophages is capable of stimulating renal repair in ischemia-reperfusion injury.21 These results support a protective role for canonical Wnt/β-catenin signaling after AKI.
Sustained activation of Wnt/β-catenin signaling, however, is detrimental and could lead to CKD progression. Chronic and progressive upregulation of β-catenin appears to be a common pathologic feature in a wide variety of fibrotic CKDs such as obstructive nephropathy, diabetic nephropathy, adriamycin (ADR) nephropathy, remnant kidneys after 5/6 nephrectomy, polycystic kidney disease, and chronic allograft nephropathy.5, 6, 7, 22, 23 A comprehensive survey demonstrated that 16 of the 19 Wnts are induced at various time points in the obstructed kidneys after unilateral ureteral obstruction (UUO), suggesting robust activation of Wnt signaling in this model. This induction was deleterious, as inhibition of Wnt/β-catenin signaling by a wide variety of approaches such as DKK1, Klotho, or small molecule β-catenin inhibitor protected against myofibroblast activation and fibrosis.6, 24, 25 Therefore, it appears that the activation of this signaling is reparative in AKI, but sustained activation is detrimental in CKD. It remains unclear whether it is one specific Wnt, or a group of Wnts, that contribute to fibrosis development.
Wnt/β-CATENIN IN KIDNEY FIBROSIS: TARGETS AND MECHANISMS
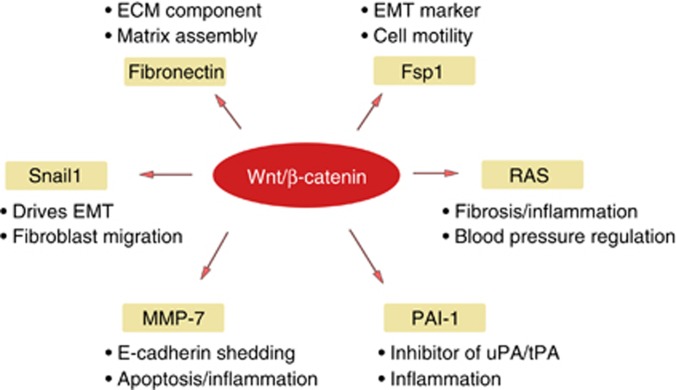
Wnt/β-catenin signaling elicits its actions through induction of its target genes. Given that this signaling is implicated in regulating organ development and oncogenesis, it is not surprising that the well-characterized targets in the literature are predominantly the proliferation-related genes such as c-Myc and cyclin D1.26, 27 Over the last few years, great efforts have been placed on identifying the Wnt/β-catenin unique target genes that are relevant to kidney injury and fibrosis. Several direct targets of Wnt/β-catenin are characterized, and include fibronectin, fibroblast-specific protein 1 (Fsp1), Snail1, MMP-7, plasminogen activator inhibitor-1 (PAI-1), and components of the renin–angiotensin system (RAS) (Figure 2). Of them, fibronectin and Fsp1 have well-known roles in fibrosis, as the former is an extracellular matrix component, whereas Fsp1 is a marker for fibroblasts and myofibroblasts.28 In this section, we will only discuss several recently identified Wnt/β-catenin targets in the setting of fibrotic CKD.
Figure 2.
Wnt/β-catenin signaling promotes renal fibrosis through induction of its target genes. This schematic representation shows several direct targets of Wnt/β-catenin that are relevant to kidney injury and fibrosis. These genes include fibronectin, fibroblast-specific protein 1 (Fsp1), Snail1, matrix metalloproteinase-7 (MMP-7), plasminogen activator inhibitor-1 (PAI-1), and components of the renin–angiotensin system (RAS; arrows). The major functions of these genes are also highlighted. ECM, extracellular matrix; EMT, epithelial–mesenchymal transition; uPA/tPA, urokinase-/tissue-type plasminogen activators.
Snail1 is a key transcription factor that drives epithelial–mesenchymal transition (EMT).29, 30 Snail1 transcriptionally represses E-cadherin expression and leads to the disruption of epithelial cell–cell adhesion, the initial step of EMT.31 Snail1 also induces Id1, a transcription antagonist that has a critical role in facilitating EMT and renal inflammation.32 Both Snail1 and β-catenin are upregulated in the renal tubular epithelium in human and animal fibrotic kidneys, and activation of β-catenin induces Snail1 expression in tubular epithelial cells and glomerular podocytes in vitro.5, 24 Interestingly, during embryologic development, the opposite occurs, as Snail1 is downregulated when mesenchymal cells differentiate into the epithelium. Consistent with these findings, expression of Snail1 and the epithelial marker E-cadherin are mutually antagonistic, and Snail1 dominates in fibrotic disease.33 A number of converging signals can lead to its upregulation, including Wnt/β-catenin, TGF-β, and tumor necrosis factor (TNF-α).23, 26, 27 It is worthwhile to stress that Snail1 is not only a transcriptional target of β-catenin but is also regulated post-translationally by glycogen synthase kinase-3β, the same regulatory protein governing β-catenin activity. Therefore, when Wnt signaling leads to the inhibition of glycogen synthase kinase-3β, both β-catenin and Snail1 can be potentially activated simultaneously, leading to additive or synergistic effects in promoting EMT.33
Another downstream target of Wnt/β-catenin signaling is MMP-7, a secreted zinc- and calcium-dependent endopeptidase that degrades extracellular matrix substrates such as elastin and syndecan. It also cleaves additional substrates such as cell-associated Fas ligand, promotes the release of TNF-α, mediates E-cadherin ectodomain shedding, and activates other proteinases such as pro-MMP-1, -2, and -9.34, 35 MMP-7 is preferentially expressed, at extremely low levels, in the epithelial cells of various normal tissues. Earlier studies documented an increased expression of MMP-7 in polycystic kidney disease, AKI induced by folic acid, and obstructive nephropathy.36 We have shown that MMP-7 expression is controlled by β-catenin in kidney cells in vitro, that it is induced in injured kidneys, and that its level is closely correlated with renal Wnt/β-catenin in various models of CKD and in human kidney biopsies. In fact, urinary MMP-7 level could serve as a surrogate biomarker for predicting the activity of renal Wnt/β-catenin, and it is associated with the severity of renal fibrotic lesions as well.37 Given its ability to act on a broad spectrum of substrates including extracellular matrix, Fas ligand, TNF-α, and E-cadherin, MMP-7 is likely a critical factor in regulating a diverse array of cellular processes including matrix remodeling, apoptosis, inflammation, and EMT.35
PAI-1 is a secreted acute-phase glycoprotein that is normally produced in low levels in the undamaged kidney. However, in various human kidney diseases such as diabetic nephropathy, membranous nephropathy, focal and segmental glomerulosclerosis (FSGS), and crescentic glomerulonephritis, its expression becomes highly upregulated. As its name suggests, it is classically known for its ability to inhibit both tissue-type and urokinase-type plasminogen activators.38 Experimental evidence from animal models has shown that the upregulation of PAI-1 is pro-fibrotic, through mechanisms as diverse as induction of TGF-β and recruitment of inflammatory cells and myofibroblasts.39, 40, 41 The promoter region of PAI-1 contains a TCF/LEF-binding site, suggesting that it is regulated by β-catenin. Indeed, it was found that upregulation of β-catenin via either TGF-β or Wnt stimulation could induce PAI-1 expression in tubular cells, and disruption of the TCF/LEF-binding site abolished this effect. Similarly, blockade of β-catenin signaling also inhibited PAI-1 induction.24, 42 β-Catenin-induced modulation of PAI-1 appears to be an important mechanism leading to fibrosis.
It is well appreciated that RAS upregulation has deleterious effects on the kidney. Whereas RAS is traditionally understood as a hormonal system with involvement of multiple organ systems, it has also been found that the kidney is capable of upregulating RAS components in pathologic conditions (intrarenal RAS).43, 44 RAS upregulation in turn leads to increased reactive oxygen species generation, nuclear factor kappa-B, and TGF-β expression.45, 46 Our studies have indicated that RAS genes possess TCF/LEF-binding sites, are upregulated by β-catenin in vitro and in vivo, and RAS upregulation can be blocked with β-catenin inhibitors (unpublished results). A link between β-catenin and RAS would hold immense promise for future therapeutic applications.
Wnt/β-CATENIN AND PODOCYTE INJURY: IMPLICATIONS FOR GLOMERUOSCLEROSIS
Glomerular fibrosis is often referred to as glomerulosclerosis and is the common outcome of various glomerular diseases such as FSGS and diabetic nephropathy. Recent studies have established that Wnt/β-catenin signaling also has a critical role in promoting podocyte injury and dysfunction, thereby contributing to the pathogenesis of proteinuria and glomerulosclerosis. Both Wnt and β-catenin are specifically activated in glomerular podocytes from patients with FSGS and diabetic nephropathy, suggesting the clinical relevance of the activation of this signal pathway to human proteinuric kidney disorders.22, 47 We further demonstrate that ectopic expression of the Wnt1 gene aggravates podocyte injury and proteinuria in ADR nephropathy, an experimental model for FSGS, whereas blockade of Wnt signaling with its endogenous antagonist DKK1 reduced proteinuria and podocyte lesions. Wnt/β-catenin also mediates TGF-β-induced podocyte injury.16 Furthermore, genetic and pharmacologic activation of β-catenin in podocytes is sufficient for causing proteinuria in mice,5, 22 whereas podocyte-specific ablation of β-catenin protects mice from developing albuminuria after ADR injury.5 Studies also indicate that amelioration of kidney injury and fibrosis by paricalcitol is associated with its ability to inhibit Wnt/β-catenin.48
The mechanisms by which Wnt/β-catenin triggers podocyte injury could be multifactorial. As terminally differentiated cells, podocytes possess little proliferative capacity.49 They often undergo a range of changes in response to injury, including hypertrophy, autophagy, dedifferentiation, detachment, and apoptosis.50 Podocyte depletion contributes to defective glomerular filtration, as a reduction of podocyte numbers in otherwise healthy kidneys induces proteinuria in experimental animal models.51 However, numerous studies indicate that proteinuria precedes podocyte depletion, suggesting that podocyte dysfunction, rather than depletion, may be an initial cause of proteinuria in many circumstances.50
In this context, Wnt/β-catenin may lead to podocyte dysfunction through effects on regulatory molecules such as Snail1, TRPC6 (transient receptor potential cation channel, subfamily C, member 6), angiotensin II type I receptor, and Wilms tumor 1. Just as in tubule cells, β-catenin can induce Snail1 expression, which in turn induces podocyte dedifferentiation and EMT.5 Studies show that induction of Snail1 in podocytes was associated with downregulation of nephrin and P-cadherin.52 TRPC6 is a calcium channel expressed in podocytes, for which mutations have been found in proteinuric renal disease.53, 54 It has been found that high glucose can activate TRPC6 in a Wnt/β-catenin-dependent manner, which provides one explanation for how diabetes causes proteinuria.55 A study also revealed that angiotensin II exposure induces podocyte injury, whereas inhibition of Wnt/β-catenin by DKK1 attenuated this injury.56 Finally, Wilms tumor 1 is a transcription factor that is exclusively expressed in glomerular podocytes in adult kidneys and is critical for maintenance of a differentiated podocyte phenotype, and can become downregulated in podocyte injury. We recently found that Wnt/β-catenin signaling can target Wilms tumor 1 by promoting its protein degradation via an ubiquitin-mediated pathway (unpublished data). Interestingly, during kidney development Wilms tumor 1 expression antagonizes Wnt/β-catenin signaling.57 These findings suggest that there are numerous ways in which Wnt/β-catenin signaling may perturb normal podocyte biology, leading to proteinuria and glomerulosclerosis.
TARGETING Wnt/β-CATENIN: THERAPEUTIC STRATEGIES
In view of the importance of Wnt/β-catenin signaling in renal fibrosis, one would imagine that blockade of this signaling might be beneficial in fibrotic CKD. Indeed, investigators have used a variety of strategies to block this pathway at various steps (Table 1). For instance, sFRP4 was used in a UUO model and resulted in reduction of β-catenin signaling and concomitant decreases in myofibroblast numbers and fibrosis.7 Similarly, Klotho was shown to bind and sequester several Wnts in kidney injury, leading to decreased β-catenin activity and a reduction in both interstitial fibrosis and podocyte injury in different animal models of disease.25 Both Klotho and sFRPs, by virtue of their Wnt-sequestering mechanisms, have the advantage of affecting both canonical and non-canonical Wnt signaling.
Table 1. Therapeutic actions of Wnt/β-catenin inhibitors.
| Inhibitor | Mechanism of action | Model system | Effect | Reference |
|---|---|---|---|---|
| sFRP4 | Binds and sequesters Wnts | UUO | Reduced fibrosis | 7 |
| Klotho | Binds and sequesters Wnts | UUO | Reduced fibrosis | 25 |
| ADR | Reduced proteinuria | |||
| DKK1 | Inhibits LRP5/6 | UUO | Reduced fibrosis | 6 |
| ADR | Reduced proteinuria | 5 | ||
| Ang II | Reduced proteinuria | 56 | ||
| IRI | Reduced fibrosis | 12 | ||
| Paricalcitol | VDR binds and sequesters β-catenin | ADR | Reduced proteinuria and fibrosis | 48 |
| ICG-001 | Inhibits β-catenin/CBP interaction | UUO | Reduced fibrosis | 24 |
Abbreviations: ADR, adriamycin nephropathy; Ang II, angiotensin II-mediated injury; CBP, cyclic AMP response-element-binding protein-binding protein; DKK1, Dickkopf 1; IRI, ischemia/reperfusion injury; UUO, unilateral ureteral obstruction; VDR, vitamin D receptor.
DKK1, as an inhibitor of the LRP5/6 co-receptors, has been used in a number of studies to interrupt canonical Wnt signaling. In murine obstructive injury, DKK1 was capable of inhibiting myofibroblast activation and ultimately fibrosis.6 It was similarly found that DKK1 was protective against podocyte dysfunction and proteinuria induced by ADR.5, 56 However, as DKK1 may elicit actions by other pathways,12 it is unclear whether all of the protective effects of DKK1 are contributable to inhibition of Wnt/β-catenin.
Inhibition of Wnt/β-catenin could be an explanation for the efficacy of existing drugs. In this regard, vitamin D receptor (VDR) agonists, such as paricalcitol, have been shown to have potential renoprotective effects in CKD.58 We showed that paricalcitol prevents podocyte injury and proteinuria in a mouse model of ADR nephropathy. The protective effect of this VDR agonist is at least partially attributable to its inhibition of Wnt/β-catenin signaling. We found that paricalcitol triggered VDR activation and its translocation into the nucleus, where it physically interacts with nuclear β-catenin and sequesters its ability to activate gene transcription.48 Similar VDR/β-catenin interactions have also been shown to occur in tubular cells and can prevent EMT under experimental conditions.59
As many Wnts are induced in the diseased kidney, therapeutic strategies to target each individual Wnt are neither practical nor efficient. This speculation is recently confirmed by genetic approaches, in which Wnt4 gene ablation in mice has no impact on renal fibrosis after UUO.60 However, because all canonical Wnt signaling converges on β-catenin, it could be an ideal target for therapeutic intervention. Tubule cell–specific and podocyte-specific knockout of the β-catenin gene has no overt abnormality,5, 20 suggesting that it is functionally dispensable in the kidney under normal physiological conditions. These data provide a compelling rationale for targeting β-catenin as a novel and effective approach for the treatment of fibrotic CKD.
Recent studies show that ICG-001, a small molecule peptidomimetic, can selectively inhibit β-catenin/TCF-mediated gene transcription. When active β-catenin translocates to the nucleus, it binds to TCF/LEF transcription factors, leading to recruitment of co-activators, including cyclic AMP response-element-binding protein-binding protein (CBP) or its closely related protein p300, which creates a transcriptionally active complex. ICG-001 is unique in that it selectively disrupts the β-catenin/CBP interaction by binding to CBP, rather than β-catenin itself.24 Although CBP and p300 are generally considered indistinguishable in terms of promoting their downstream gene expression, studies indicate that differential co-activator usage results in the selective expression of target genes.61 Whereas β-catenin/p300 signaling is instrumental in initiating normal cellular differentiation, β-catenin/CBP-driven gene transcription is shown to be critical for inducing a dedifferentiated/proliferative state. β-catenin/CBP is also responsible for expression of fibrogenic genes such as MMP-7, Snail1, fibronectin, PAI-1 and Fsp1.62 Our studies show that ICG-001 antagonizes tubular cell EMT in vitro, while ameliorating renal fibrosis in vivo.24 Additional studies should confirm the therapeutic benefits of ICG-001 in broader surveys of fibrotic kidney diseases.
CONCLUSION REMARKS
The panoply of Wnt/β-catenin effects described in this review emphasizes the challenges to understanding the biological impact of this pathway on kidney disease. At this point, additional studies are required to further identify the exact temporal and spatial effects of this signaling in renal injury so as to properly harness therapeutic approaches to effectively treat kidney disorders. It is, for instance, clear that while Wnt/β-catenin is pathologic in the development of interstitial fibrosis and chronic podocyte injuries in CKD, it also appears to be paradoxically protective in AKI. Further, whereas canonical signaling has a clearly defined role in these processes, the relative contributions of non-canonical and non-traditional pathways need to be elucidated in both AKI and CKD. Similarly, the exact contributions of the 19 different Wnts have yet to be fully defined. This could represent a daunting task, as genetic deletion of a particular Wnt may not have a huge impact on the severity of CKD, as many Wnts are simultaneously induced after kidney injury. In conclusion, the understanding of Wnt/β-catenin remains a significant challenge but one that carries immense opportunity for the therapy of kidney diseases.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants DK064005 and DK091239, National 973 Program Grant 2012CB517700, and NSFC grants 81130011 and 81370839. R.J.T was supported by NIH grants (P30DK079307 and T32DK061296) and an American Heart Association Fellow-to-Faculty Transition Grant (13FTF16990086). This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
The authors declared no competing interest.
References
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Barasch J. WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int. 2008;74:1004–1008. doi: 10.1038/ki.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- Dai C, Stolz DB, Kiss LP, et al. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Dai C, Li Y, et al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- Hwang I, Seo EY, Ha H. Wnt/beta-catenin signaling: a novel target for therapeutic intervention of fibrotic kidney disease. Arch Pharm Res. 2009;32:1653–1662. doi: 10.1007/s12272-009-2200-3. [DOI] [PubMed] [Google Scholar]
- Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- Karner CM, Chirumamilla R, Aoki S, et al. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn SF, Webb A, Berry RL, et al. Calcium/NFAT signalling promotes early nephrogenesis. Dev Biol. 2011;352:288–298. doi: 10.1016/j.ydbio.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Johnson BG, Kida Y, et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl Acad Sci USA. 2013;110:1440–1445. doi: 10.1073/pnas.1211179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Satoh M, Nagasu H, Morita Y, et al. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol. 2012;303:F1641–F1651. doi: 10.1152/ajprenal.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Karasawa H, Turbyville T, et al. Cripto-1 enhances the canonical Wnt/beta-catenin signaling pathway by binding to LRP5 and LRP6 co-receptors. Cell Signal. 2013;25:178–189. doi: 10.1016/j.cellsig.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Dai C, Li Y, et al. Canonical Wnt/beta-catenin signaling mediates transforming growth factor-beta1-driven podocyte injury and proteinuria. Kidney Int. 2011;80:1159–1169. doi: 10.1038/ki.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YS, Li Y, Dai C, et al. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 2010;78:363–373. doi: 10.1038/ki.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei JM, Borchert GL, Donald SP, et al. Matrix metalloproteinase(s) mediate(s) NO-induced dissociation of beta-catenin from membrane bound E-cadherin and formation of nuclear beta-catenin/LEF-1 complex. Carcinogenesis. 2002;23:2119–2122. doi: 10.1093/carcin/23.12.2119. [DOI] [PubMed] [Google Scholar]
- Kim H, He Y, Yang I, et al. delta-Catenin promotes E-cadherin processing and activates beta-catenin-mediated signaling: implications on human prostate cancer progression. Biochim Biophys Acta. 2012;1822:509–521. doi: 10.1016/j.bbadis.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Li Y, Lin L, et al. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Li B, Rao S, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Gruenwald A, Suh JH, et al. Wnt/beta-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem. 2011;286:26003–26015. doi: 10.1074/jbc.M111.223164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Toerne C, Schmidt C, Adams J, et al. Wnt pathway regulation in chronic renal allograft damage. Am J Transplant. 2009;9:2223–2239. doi: 10.1111/j.1600-6143.2009.02762.x. [DOI] [PubMed] [Google Scholar]
- Hao S, He W, Li Y, et al. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol. 2011;22:1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li Y, Zhou D, et al. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toualbi K, Guller MC, Mauriz JL, et al. Physical and functional cooperation between AP-1 and beta-catenin for the regulation of TCF-dependent genes. Oncogene. 2007;26:3492–3502. doi: 10.1038/sj.onc.1210133. [DOI] [PubMed] [Google Scholar]
- Koehler A, Schlupf J, Schneider M, et al. Loss of Xenopus cadherin-11 leads to increased Wnt/beta-catenin signaling and up-regulation of target genes c-myc and cyclin D1 in neural crest. Dev Biol. 2013;383:132–145. doi: 10.1016/j.ydbio.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutet A, De Frutos CA, Maxwell PH, et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25:5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Monkawa T, Tsuji M, et al. Snail1 is involved in the renal epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2007;362:63–68. doi: 10.1016/j.bbrc.2007.07.146. [DOI] [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- Li Y, Wen X, Liu Y. Tubular cell dedifferentiation and peritubular inflammation are coupled by the transcription regulator Id1 in renal fibrogenesis. Kidney Int. 2012;81:880–891. doi: 10.1038/ki.2011.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Herreros A, Baulida J. Cooperation, amplification, and feed-back in epithelial-mesenchymal transition. Biochim Biophys Acta. 2012;1825:223–228. doi: 10.1016/j.bbcan.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302:F1351–F1361. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran K, Simon TC, Liapis H, et al. Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int. 2004;65:2212–2222. doi: 10.1111/j.1523-1755.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- He W, Tan RJ, Li Y, et al. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/beta-catenin activity in CKD. J Am Soc Nephrol. 2012;23:294–304. doi: 10.1681/ASN.2011050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006;17:2999–3012. doi: 10.1681/ASN.2006050503. [DOI] [PubMed] [Google Scholar]
- Kitching AR, Kong YZ, Huang XR, et al. Plasminogen activator inhibitor-1 is a significant determinant of renal injury in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:1487–1495. doi: 10.1097/01.asn.0000065550.13931.00. [DOI] [PubMed] [Google Scholar]
- Nicholas SB, Aguiniga E, Ren Y, et al. Plasminogen activator inhibitor-1 deficiency retards diabetic nephropathy. Kidney Int. 2005;67:1297–1307. doi: 10.1111/j.1523-1755.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- Oda T, Jung YO, Kim HS, et al. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int. 2001;60:587–596. doi: 10.1046/j.1523-1755.2001.030002587.x. [DOI] [PubMed] [Google Scholar]
- He W, Tan R, Dai C, et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/beta-catenin signaling. J Biol Chem. 2010;285:24665–24675. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Zhou QG, Nie J, et al. Albumin overload activates intrarenal renin-angiotensin system through protein kinase C and NADPH oxidase-dependent pathway. J Hyperten. 2011;29:1411–1421. doi: 10.1097/HJH.0b013e32834786f0. [DOI] [PubMed] [Google Scholar]
- Freundlich M, Quiroz Y, Zhang Z, et al. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008;74:1394–1402. doi: 10.1038/ki.2008.408. [DOI] [PubMed] [Google Scholar]
- Ng HY, Tain YL, Lee YT, et al. Renin angiotensin system blockade ameliorates lead nephropathy. Biochem Biophys Res Commun. 2013;438:359–363. doi: 10.1016/j.bbrc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- Chen S, Ge Y, Si J, et al. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int. 2008;74:1128–1138. doi: 10.1038/ki.2008.380. [DOI] [PubMed] [Google Scholar]
- Naves MA, Requiao-Moura LR, Soares MF, et al. Podocyte Wnt/ss-catenin pathway is activated by integrin-linked kinase in clinical and experimental focal segmental glomerulosclerosis. J Nephrol. 2012;25:401–409. doi: 10.5301/jn.5000017. [DOI] [PubMed] [Google Scholar]
- He W, Kang YS, Dai C, et al. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W, Shirato I, Nagata M, et al. The podocyte's response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304:F333–F347. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- Brinkkoetter PT, Ising C, Benzing T. The role of the podocyte in albumin filtration. Nat Rev Nephrol. 2013;9:328–336. doi: 10.1038/nrneph.2013.78. [DOI] [PubMed] [Google Scholar]
- D'Agati VD. Podocyte injury in focal segmental glomerulosclerosis: lessons from animal models (a play in five acts) Kidney Int. 2008;73:399–406. doi: 10.1038/sj.ki.5002655. [DOI] [PubMed] [Google Scholar]
- Li Y, Kang YS, Dai C, et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol. 2008;172:299–308. doi: 10.2353/ajpath.2008.070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, Polu KR, Moller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MP, Conlon PJ, Lynn KL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- Li Z, Xu J, Xu P, et al. Wnt/beta-catenin signalling pathway mediates high glucose induced cell injury through activation of TRPC6 in podocytes. Cell Prolif. 2013;46:76–85. doi: 10.1111/cpr.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Xu L, Song Y, et al. Calmodulin-dependent protein kinase II/cAMP response element-binding protein/Wnt/beta-catenin signaling cascade regulates angiotensin II-induced podocyte injury and albuminuria. J Biol Chem. 2013;288:23368–23379. doi: 10.1074/jbc.M113.460394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Yoon SK, Bollig F, et al. A novel Wilms tumor 1 (WT1) target gene negatively regulates the WNT signaling pathway. J Biol Chem. 2010;285:14585–14593. doi: 10.1074/jbc.M109.094334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Borst MH, Hajhosseiny R, Tamez H, et al. Active vitamin D treatment for reduction of residual proteinuria: a systematic review. J Am Soc Nephrol. 2013;24:1863–1871. doi: 10.1681/ASN.2013030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Joo SY, Lee KE, et al. Paricalcitol attenuates 4-hydroxy-2-hexenal-induced inflammation and epithelial-mesenchymal transition in human renal proximal tubular epithelial cells. PLoS ONE. 2013;8:e63186. doi: 10.1371/journal.pone.0063186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRocco DP, Kobayashi A, Taketo MM, et al. Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol. 2013;24:1399–1412. doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Nguyen C, Lee KS, et al. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–3631. doi: 10.1038/sj.onc.1208433. [DOI] [PubMed] [Google Scholar]
- Henderson WR, Jr., Chi EY, Ye X, et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]