Abstract
Toll-like receptors (TLRs) are a conserved family of pattern recognition receptors that play a fundamental role in the innate immune system by triggering proinflammatory signaling pathways in response to microbial pathogens through exogenous pathogen-associated molecular patterns or tissue injury through endogenous danger-associated molecular patterns. In the kidney, TLRs are widely expressed in a variety of cell types. Emerging evidence demonstrates the participation of TLRs in the activation of these cells during renal fibrosis. This review highlights the role of TLRs and their endogenous ligands in the pathogenesis of renal fibrosis using ureteral obstruction and diabetic nephropathy as models of chronic kidney disease.
Keywords: diabetic nephropathy, pro-fibrotic, proinflammatory, renal fibrosis, Toll-like receptors, unilateral ureteral obstruction
Renal fibrosis is a wound healing/scarring response following kidney injury that occurs in many forms of chronic kidney disease (CKD). It is also a crucial determinant underlying the progression from CKD to end-stage renal disease. After over a decade of intense study, it is now known that the interstitial accumulation of extracellular matrix (ECM) proteins is not only the aftermath of fibroblast activation, but also a process contributed to by other intrinsic renal cells, including tubular epithelial cells (TECs), mesangial cells, endothelial cells and infiltrating macrophages.1, 2 Following kidney injury, resident fibroblasts are activated by various pro-inflammatory and pro-fibrotic stimuli. Activated fibroblasts, also called myofibroblasts, produce excessive ECM proteins that accumulate in the interstitium, and therefore they are considered as the key mediator of renal fibrosis.3
Despite the fact that most myofibroblasts are derived from local resident fibroblasts, recent studies demonstrated that these ECM-producing cells might originate from bone marrow through differentiation, and from TECs and endothelial cells via a process of epithelial-to-mesenchymal transition and endothelial-to-mesenchymal transition, respectively.4, 5 Although there is an ongoing debate about the existence of epithelial-to-mesenchymal transition in vivo,6 these studies illustrated the contribution of TECs in fibrogenesis through a paracrine mechanism. TECs in co-culture with cortical fibroblasts secreted transforming growth factor-β (TGF-β) and the AB-heterodimer of platelet-derived growth factor (PDGF-AB), which in turn stimulated fibroblast proliferation and total collagen synthesis.7 The production of insulin-like growth factor binding protein from fibroblasts was also enhanced in the presence of TEC-conditioned medium; thus, these cells could modulate the proliferative response during repair.8
Regardless of the primary insult leading to renal fibrosis, chronic inflammation appears to be a critical process heralding fibrogenesis. Elevated levels of inflammatory markers were associated with an increased risk of developing CKD.9 Induction of various pro-inflammatory cytokines (interleukin (IL)-6, IL-8, IL-10, chemokine (C-C motif) ligand 2, and tumor necrosis factor-α) and adhesion molecules (intercellular adhesion molecule-1 and vascular cell adhesion molecule-1) attracted the transmigration of macrophages and T cells from the circulation to the interstitium, thereby further enhancing the inflammatory state.10
Toll-like receptors (TLRs) are a conserved family of pattern recognition receptors that play a fundamental role in innate immunity. TLRs mediate host cell inflammatory response by recognizing pathogen-associated molecular patterns with an extracellular domain comprising leucine-rich repeats and triggering the intracellular signal transduction through a cytoplasmic Toll/IL-1 receptor-like domain. In addition, TLRs are involved in non-infectious inflammatory disease, in which they are activated by endogenous danger-associated molecular patterns that are released from injured tissue.11 Activation of TLR pathways has been implicated in various renal diseases, including acute kidney injury, ischemia–reperfusion injury, allograft rejection and immune complex nephritis,12, 13 in which they participate in the induction of acute inflammation and early tubular injury. Emerging evidence suggests that TLRs may participate in the pathogenesis of renal fibrosis.
THE EXPRESSION OF TLRs DURING CHRONIC KIDNEY INJURY
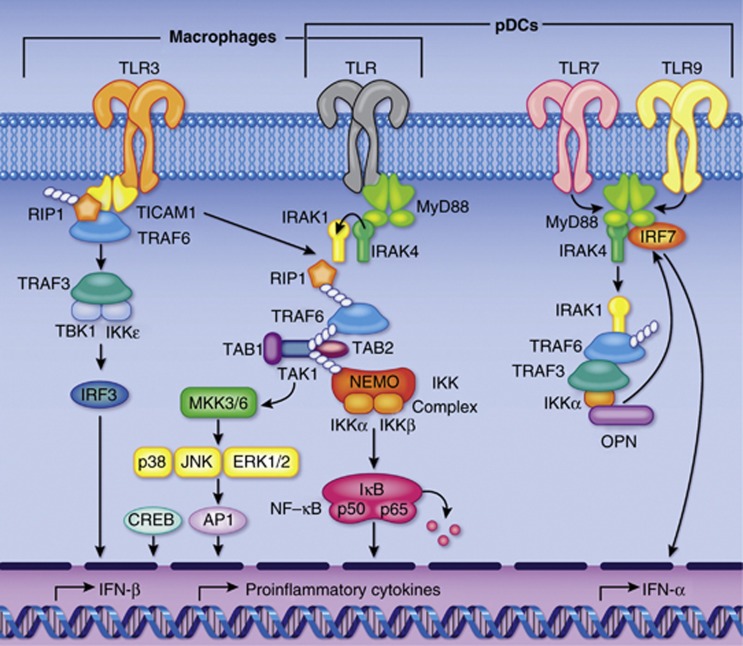
TLRs are important in innate immunity and are widely distributed on myeloid cells. To date, 10 human TLRs (TLR1–10) have been identified and demonstrated to initiate proinflammatory signaling pathways via the adaptor protein MyD88,11 except TLR3, which triggers via TRIF/TICAM-1. Other TLR adaptors include TRIF (TIR-domain-containing adaptor-inducing interferon-β), TIRAP (TIR-domain-containing adaptor protein), and TRAM (TRIF-related adaptor molecule).14 TLRs utilize these adaptor proteins to transmit signals downstream, leading to the activation of nuclear factor-κB, mitogen-activated protein kinases, JNKs (c-Jun NH2-terminal kinases), p38, ERKs and IRF (Figure 1).15
Figure 1.
MyD88-dependent and -independent Toll-like receptor (TLR) signaling pathways. All TLRs (except for TLR3) signal through the adaptor protein MyD88 to activate nuclear factor (NF)-κB and mitogen-activated protein kinases for the induction of proinflammatory cytokines. TLR3 signals through TRIF (TIR-domain-containing adaptor-inducing interferon-β)/TICAM1 (TIR-containing adaptor molecule 1). Other TLR adaptors include TRIF, TIRAP (TIR-domain-containing adaptor protein), and TRAM (TRIF-related adaptor molecule). (Reprinted with permission from Moresco et al.,15 with permission from Elsevier.) IKK, inhibitor of κB-kinase; IRAK, interleukin-1 receptor-associated kinase; NEMO, NF-κB essential modulator; pDCs, plasmacytoid dentritic cells; RIP1, receptor-interacting protein-1; TAB-1, TAK1-binding protein; TAK, TGF-β activated kinase; TRAF, tumor necrosis factor receptor-associated factor.
So far, most of the TLR studies associated with chronic renal injury have focused on TLR2 and TLR4 (Table 1). In the kidney, interstitial and glomerular macrophages express TLR1, 2, 4, and 6, and dendritic cells express TLR4, 7, 8, and 9. TLR2 and TLR4 are upregulated in monocytes, and TLR4 is upregulated in neutrophils of end-stage renal disease patients. The alteration of TLR expression in immune cells might contribute to the increased susceptibility to microbial infection and prevailing inflammation in these patients.16 This notion is supported by the observation that TLR2 expression on monocytes was associated with the inflammatory response of patients with stage 3–4 CKD.17
Table 1. TLR2 and TLR4 expression in association with chronic kidney diseases.
| TLR | Disease | Cell type |
|---|---|---|
| TLR2 | ESRD | Monocyte16 |
| Stage 3–4 CKD | Monocyte17 | |
| Obstructive hydronephrosis | Macrophage and myofibroblast20 | |
| IgA nephropathy | Macrophage and myofibroblast20 | |
| Type 1 DN | Endothelial cell and mesangial cell18; monocyte41; tubular cell44 | |
| UUO | Tubular cell20 | |
| TLR4 | ESRD | Neutrophil and monocyte16 |
| Type 2 DN | Proximal tubular cell19 | |
| Type 1 DN | Tubular cell20; monocyte41 |
Abbreviations: CKD, chronic kidney disease; DN, diabetic nephropathy; ESRD, end-stage renal disease; IgA, immunoglobulin A; TLR, Toll-like receptor; UUO, unilateral ureteral obstruction.
In addition to myeloid cells, TLRs are also expressed in intrinsic renal cells. TECs and mesangial cells express TLR1, 2, 3, 4, and 6, and podocytes express TLR1, 2, 3, 4, 5, 6, and 10. Immunohistochemical studies on human renal biopsies demonstrated the upregulation of TLR218 in the glomerular endothelial and mesangial area and TLR419 expression in the tubules of patients with diabetic nephropathy (DN) compared with normal renal sections. Increased expression of TLR2 was also observed in interstitial myofibroblasts, tubules, and macrophages of the kidney sections from patients with obstructive hydronephrosis and IgA nephropathy.20 Although TLRs are present in both immune and renal cells, it is likely that TLR signaling predominates in intrinsic renal cells rather than leukocytes. For example TLR4-deficient mice engrafted with competent leukocytes showed less tubular damage than wild-type mice reconstituted with TLR4-deficient bone marrow cells.21
ENDOGENOUS TLR LIGANDS RELEASED DURING CHRONIC KIDNEY INJURY
Over the last decade, numerous endogenous ligands have been identified for the activation of TLRs during kidney injury, and some of them have been shown to be closely associated with renal fibrosis.
High-mobility-group box 1 (HMGB1)
HMGB1 protein belongs to a family of non-histone chromosomal proteins that were first identified to bind to DNA and regulate gene transcription. It is also a secreted protein from activated macrophages and dendritic cells during infection and from damaged cells during tissue injury. Increasing evidence supports that extracellular HMGB1 is a key endogenous ligand of TLR signaling that is involved in the initiation of renal inflammation and the subsequent development of progressive renal fibrosis.
Both experimental and clinical models of renal fibrosis have demonstrated that a high HMGB1 level is associated with the development of progressive CKD, including unilateral ureteral obstruction (UUO) injury,20 5/6 nephrectomy,22 and DN.18, 19, 23 Extracellular HMGB1 binds to TLR2 and TLR424 to elicit inflammatory responses via a nuclear factor-κB-dependent pathway. Anti-HMGB1 treatment has been shown to attenuate the severity of sepsis in CKD.22 HMGB1 has also been demonstrated to induce epithelial-to-mesenchymal transition in human proximal TECs.25
Heat shock proteins (HSPs)
HSPs were originally characterized as intracellular chaperone proteins that are involved in protein folding and stabilization. Interestingly, HSPs also interact with TLRs during the maturation of immune cells26 and during induction and termination of cytokine secretion. HSP60 and HSP70 are the two best-known HSPs that bind to TLR2 and TLR4 in inflamed tissue. An early study on DN revealed that HSP60 and HSP70 proteins were significantly induced in lymphocytes of type 2 diabetic patients.27 Together, the serum level of HSP60 and HSP70 was shown to correlate with the increase of TLR2 and TLR4 expression in monocytes of type 2 diabetic patients.23 This supports the role of HSPs as endogenous ligands for TLR signaling.
ECM degradation product
Progressive renal fibrosis results in increasing matrix turnover and excessive production of ECM degradation products such as fibrinogen, biglycan, heparin sulfate, hyaluroran, and fibronectin, which have been shown to interact with TLR4.28, 29
THE ROLES OF TLRs IN PROGRESSIVE RENAL FIBROSIS
Unilateral ureteral obstruction
UUO is a well-characterized rodent model of progressive renal fibrosis. The diseased kidney exhibits significant inflammation, tubular injury, and even cell death at the first week of obstruction. Release of proinflammatory cytokines such as IL-1, tumor necrosis factor-α, and chemokine (C-C motif) ligand 2 from activated renal cells recruits leukocytes to the damaged tubule that in turn sustains and amplifies inflammation. In fact, secretion of TGF-β1 from both infiltrating leukocytes and intrinsic renal cells plays a crucial role at the onset of UUO as TGF-β1 induces the production of ECM proteins through the activation of fibroblasts and the process of epithelial-to-mesenchymal transition.30
There is strong evidence for a role of TLRs in obstructive nephropathy. TLR2 was markedly upregulated in the obstructed kidney after UUO injury.20, 31 Expression of TLR2 was rapidly increased as early as 2 days after surgery, while other TLRs (TLR3, 4, 7, and 9) were only induced 6 days after surgery. This implied the involvement of TLR2 signaling in the early phase of UUO injury. Indeed, the distribution pattern of TLR2 protein was found to be in line with that of the endogenous ligands (e.g. HMGB1 and biglycan) at the apical side of the tubule, suggesting the activation of TLR2 signaling at this site. Results from TLR2−/− mice studies support the idea that TLR2 initiates renal inflammation during the early stage of UUO injury because there was reduced chemokine expression (CXCL1 and chemokine (C-C motif) ligand 2) and leukocyte infiltration compared to wild type.20 Although TLR2 has also been demonstrated to mediate fibrinogen-induced proliferation of fibroblasts,32 there is no conclusive evidence to support the role of TLR2 in the progression of fibrosis. There was no difference in ECM accumulation between TLR2−/− and TLR2+/+ mice at the later stage of UUO although less myofibroblasts and reduced MMPs and TIMPs were shown in TLR2−/− mice.20 Similar findings from a subsequent study also demonstrated that the development of postobstructive renal interstitial fibrosis and tubular atrophy was independent of TLR2-induced MyD88 signaling transduction.31
Expression of TLR4 was progressively upregulated after UUO injury. In contrast to TLR2, no significant difference was shown in the proinflammatory response and macrophage infiltration between TLR4−/− and TLR4+/+ mice. Instead, TLR4-deficient mice were protected from renal fibrosis with reduced α-SMA protein expression and less fibroblast accumulation.33 Both in vivo and in vitro data demonstrated that IL-18 induced pro-fibrotic changes via TLR4 signaling during UUO and in TECs,34 suggesting the role of TLR4 as a molecular link between inflammation and fibrosis. It has been proposed that TLR4 promotes renal fibrosis by altering the susceptibility of renal cells towards TGF-β signaling. Upon stimulation by TGF-β, TECs and myofibroblasts from TLR4−/− mice produced less collagen type I mRNA than wild-type cells, which was later found to be associated with upregulation of Bambi, the negative regulator of TGF-β signaling.35 On the other hand, TLR2 and TLR4 were not found to be involved in renal injury following UUO in another study, in which there was no significant difference in collagen IV deposition and macrophage infiltration between TRL2−/−, TLR4−/−, and wild-type mice.36 This contradictory finding may be due to the C57BL/6 strain-dependent resistance to UUO.
The role of TLR9 is complicated in the pathogenesis of renal diseases,. On one hand, TLR9 stimulation by its ligand, CpG DNA, aggravated renal injury in mice with IgA nephropathy.37 Conversely, TLR9-deficient MRL/1pr mice exhibited more severe glomerular and interstitial lesions compared to wild-type mice. Study on renal fibrosis in the UUO model found that TLR9 protected renal tissue from obstructive damage as mice treated with CpG-ODN showed less renal inflammation and fibrosis, which was accompanied by significant reduction in ERK, Smad3, and Stat3 activity.38
DIABETIC NEPHROPATHY
DN, the most common cause of end-stage renal disease in the developed world, is characterized by the thickening of glomerular and tubular basement membrane and excessive mesangial matrix expansion. Poor glycemic control is a major risk factor for the development of DN. However, it does not account for all the pathophysiological changes observed in the diseased kidney. Research in the past few years have suggested that inflammatory processes may be important in the development and progression of DN.39
Activation of TLR signaling contributes to increased production of proinflammatory mediators, and the sustained chronic inflammatory state associated with diabetes.40 Clinical studies showed a significant increase in TLR2 and TLR4 protein, which correlated with the upregulation of their ligand and MyD88-dependent nuclear factor-κB expression in monocytes from patients with type 141, 42 and type 2 diabetes.23 In addition, stimulation of human monocytes with TLR2 and TLR4 ligands enhanced the production of different cytokines, accounting for the inflammatory state in diabetes. In human renal biopsy studies, sections from diagnosed DN patients also showed an increased expression of TLR218 and TLR419 in the tubules compared to sections from normal kidneys. The positive correlation between tubular TLR4 expression and serum HbA1c level implicated that high glucose might be a critical determinant for TLR expression. Indeed, results from an in vitro study confirmed that high glucose induced TLR2 and TLR4 expression in human monocytes43 and TLR4 expression in proximal TECs19 via a PKC-dependent pathway.
Similar to the model of UUO, TLR2 plays a pro-inflammatory role in DN. In one study, short-term exposure to high glucose for 3 days induced both TLR4 and TLR2, but only TLR2 overexpression was sustained upon prolonged exposure for up to 7 days, suggesting a predominant role of TLR2 in mediating chronic inflammation in DN.44 An animal model of type 1 diabetes supported the progressive induction of TLR2, together with MyD88-nuclear factor-κB expression in the diabetic kidney of rats,18 while knockout of TLR2 attenuated macrophage-mediated inflammation, albuminuria, and podocyte loss in STZ-induced DN mice.45
Recent evidence has suggested that activation of the TLR4 signaling pathway is associated not only with inflammation but also with insulin resistance.46 Insulin signaling is important for normal renal function in glomeruli and tubules.47 Mice with altered insulin signaling in podocytes developed significant albuminuria and renal injury resembling the histological features in DN.48 Blockade of TLR4 signaling in macrophages also improved insulin resistance and reduced albuminuria in db/db mice. Consistent with these observations, there was reduced intrarenal expression of pro-fibrotic molecules including TGF-β, PAI-1, collagen IV, and phosphorylated smad2/3 in the diabetic kidney, while TLR4 signaling was inhibited.49 More recently, our group has demonstrated the pro-fibrotic role of TLR4 in a model of STZ-induced DN in eNOS−/− mice, in which the TLR4 antagonist CRX-526 reduced renal cortical TGF-β, osteopontin production, and collagen deposition, and improved renal function.50
CONCLUSION
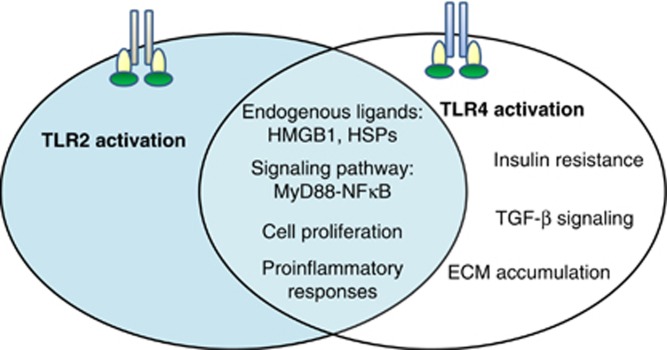
Renal fibrosis features prominently as an irreversible process of tissue damage in most forms of progressive CKD. Activation of TLR signaling, especially in intrinsic renal cells, is involved in the initiation of the innate immune response to various exogenous and endogenous danger signals. However, aberrant activation of this signaling pathway may lead to chronic inflammation and progression to renal fibrosis. There is strong evidence that TLR2 and TLR4 play distinct roles in the pathogenesis of renal fibrosis; TLR2 initiates proinflammatory responses, whereas TLR4 mediates both proinflammatory and pro-fibrotic pathways (Figure 2). Thus, targeting TLR signaling may confer a novel therapeutic paradigm for renal fibrosis and end-stage renal disease of diverse origins.
Figure 2.
Role of Toll-like receptor (TLR)2 and TLR4 in renal fibrosis. During chronic kidney injury, TLR2 initiates the proinflammatory pathway via MyD88-nuclear factor (NF)-κB signal transduction in response to endogenous ligands, while TLR4 activates both proinflammatory and pro-fibrotic pathways. ECM, extracellular matrix; HMGB1, high-mobility-group box 1; HSPs, heat shock proteins; TGF-β, transforming growth factor-β.
Acknowledgments
This work was supported by a General Research Fund from the Research Grants Council of Hong Kong (grant no. HKU 779611M) and the National Basic Research Program of China 973 program no. 2012CB517600 (no. 2012CB517606). WHY is supported by an Endowment Fund established for the Yu Professorship in Nephrology awarded to SCWT, and by a donation from Hong Kong Concrete and Continental Cement and Gypsum Co Ltd. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
All the authors declared no competing interests.
References
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris AB, Colvin RB. Renal interstitial fibrosis: mechanisms and evaluation. Curr Opin Nephrol Hypertens. 2012;21:289–300. doi: 10.1097/MNH.0b013e3283521cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011;92:158–167. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy. J Clin Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Saunders HJ, Baxter RC, et al. Paracrine stimulation of human renal fibroblasts by proximal tubule cells. Kidney Int. 1998;54:747–757. doi: 10.1046/j.1523-1755.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Saunders HJ, Brew BK, et al. Human renal fibroblasts modulate proximal tubule cell growth and transport via the IGF-I axis. Kidney Int. 1997;52:1486–1496. doi: 10.1038/ki.1997.479. [DOI] [PubMed] [Google Scholar]
- Shankar A, Sun L, Klein BE, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 2011;80:1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatr Nephrol. 2009;24:1445–1452. doi: 10.1007/s00467-008-1046-0. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Gluba A, Banach M, Hannam S, et al. The role of Toll-like receptors in renal diseases. Nat Rev Nephrol. 2010;6:224–235. doi: 10.1038/nrneph.2010.16. [DOI] [PubMed] [Google Scholar]
- Robson MG. Toll-like receptors and renal disease. Nephron Exp Nephrol. 2009;113:e1–e7. doi: 10.1159/000228077. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Mori K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Gollapudi P, Yoon JW, Gollapudi S, et al. Leukocyte toll-like receptor expression in end-stage kidney disease. Am J Nephrol. 2010;31:247–254. doi: 10.1159/000276764. [DOI] [PubMed] [Google Scholar]
- Koc M, Toprak A, Arikan H, et al. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol Dial Transplant. 2011;26:955–963. doi: 10.1093/ndt/gfq500. [DOI] [PubMed] [Google Scholar]
- Li F, Yang N, Zhang L, et al. Increased expression of toll-like receptor 2 in rat diabetic nephropathy. Am J Nephrol. 2010;32:179–186. doi: 10.1159/000317023. [DOI] [PubMed] [Google Scholar]
- Lin M, Yiu WH, Wu HJ, et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23:86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans JC, Butter LM, Pulskens WP, et al. The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS One. 2009;4:e5704. doi: 10.1371/journal.pone.0005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Chen G, Wyburn KR, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelahavanichkul A, Huang Y, Hu X, et al. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int. 2011;80:1198–1211. doi: 10.1038/ki.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Devaraj S, Park S, et al. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861–868. doi: 10.2337/dc09-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- Lynch J, Nolan S, Slattery C, et al. High-mobility group box protein 1: a novel mediator of inflammatory-induced renal epithelial-mesenchymal transition. Am J Nephrol. 2010;32:590–602. doi: 10.1159/000320485. [DOI] [PubMed] [Google Scholar]
- Valentinis B, Capobianco A, Esposito F, et al. Human recombinant heat shock protein 70 affects the maturation pathways of dendritic cells in vitro and has an in vivo adjuvant activity. J Leukoc Biol. 2008;84:199–206. doi: 10.1189/jlb.0807548. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Sapienza M, et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12:299–306. doi: 10.1379/CSC-270.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GB, Brunn GJ, Kodaira Y, et al. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XM, Huang XR, Xiao J, et al. Diverse roles of TGF-beta receptor II in renal fibrosis and inflammation in vivo and in vitro. J Pathol. 2012;227:175–188. doi: 10.1002/path.3976. [DOI] [PubMed] [Google Scholar]
- Skuginna V, Lech M, Allam R, et al. Toll-like receptor signaling and SIGIRR in renal fibrosis upon unilateral ureteral obstruction. PLoS One. 2011;6:e19204. doi: 10.1371/journal.pone.0019204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen I, Susnik N, Inhester T, et al. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int. 2011;80:1035–1044. doi: 10.1038/ki.2011.214. [DOI] [PubMed] [Google Scholar]
- Campbell MT, Hile KL, Zhang H, et al. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J Surg Res. 2009;168:e61–e69. doi: 10.1016/j.jss.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum KK, Zhang H, Hile KL, et al. Profibrotic effect of interleukin-18 in HK-2 cells is dependent on stimulation of the Toll-like receptor 4 (TLR4) promoter and increased TLR4 expression. J Biol Chem. 2012;287:40391–40399. doi: 10.1074/jbc.M112.402420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulskens WP, Rampanelli E, Teske GJ, et al. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol. 2010;21:1299–1308. doi: 10.1681/ASN.2009070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury P, Sacks SH, Sheerin NS. Endogenous ligands for TLR2 and TLR4 are not involved in renal injury following ureteric obstruction. Nephron Exp Nephrol. 2010;115:e122–e130. doi: 10.1159/000313493. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Suzuki Y, Narita I, et al. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 2008;19:2384–2395. doi: 10.1681/ASN.2007121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin BM, Wang XX, Jin W, et al. Activation of Toll-like receptor 9 attenuates unilateral ureteral obstruction-induced renal fibrosis. Acta Pharmacol Sin. 2010;31:1583–1592. doi: 10.1038/aps.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero A, Mora C, Muros M, et al. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci (Lond) 2009;116:479–492. doi: 10.1042/CS20080394. [DOI] [PubMed] [Google Scholar]
- Nogueira-Machado JA, Volpe CM, Veloso CA, et al. HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Exp Opin Ther Targets. 2011;15:1023–1035. doi: 10.1517/14728222.2011.575360. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Dasu MR, Rockwood J, et al. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93:578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Dasu MR, Park SH, et al. Increased levels of ligands of Toll-like receptors 2 and 4 in type 1 diabetes. Diabetologia. 2009;52:1665–1668. doi: 10.1007/s00125-009-1394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Devaraj S, Zhao L, et al. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57:3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudaliar H, Pollock C, Komala MG, et al. The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am J Physiol Renal Physiol. 2013;305:F143–F154. doi: 10.1152/ajprenal.00398.2012. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Tobias P, Kasinath BS, et al. Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol. 2011;31:1796–1804. doi: 10.1161/ATVBAHA.111.228924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Sears DD. TLR4 and insulin resistance. Gastroenterol Res Pract. 2010;2010:pii 212563. doi: 10.1155/2010/212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale LJ, Coward RJ. The insulin receptor and the kidney. Curr Opin Nephrol Hypertens. 2013;22:100–106. doi: 10.1097/MNH.0b013e32835abb52. [DOI] [PubMed] [Google Scholar]
- Welsh GI, Hale LJ, Eremina V, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha JJ, Hyun YY, Lee MH, et al. Renal protective effects of toll-like receptor 4 signaling blockade in type 2 diabetic mice. Endocrinology. 2013;154:2144–2155. doi: 10.1210/en.2012-2080. [DOI] [PubMed] [Google Scholar]
- Mansouri L, Paulsson JM, Moshfegh A, et al. Leukocyte proliferation and immune modulator production in patients with chronic kidney disease. PLoS One. 2013;8:e73141. doi: 10.1371/journal.pone.0073141. [DOI] [PMC free article] [PubMed] [Google Scholar]