Abstract
Notch is a critical regulator of kidney development, but the pathway is mostly silenced once kidney maturation is achieved. Recent reports demonstrated increased expression of Notch receptors and ligands both in acute and chronic kidney injury. In vivo studies indicated that Notch activation might contribute to regeneration after acute kidney injury; on the other hand, sustained Notch expression is causally associated with interstitial fibrosis and glomerulosclerosis. This review will summarize the current knowledge on the role of the Notch signaling with special focus on kidney fibrosis.
Keywords: glomerulosclerosis, Notch, tubulointerstitial fibrosis
The terms ‘chronic kidney disease' (CKD) and its most severe form, ‘end-stage renal disease', describe the progressive functional decline of the kidney.1 Histological lesions that are associated with CKD are collectively described as fibrosis. Kidney fibrosis is characterized by five distinct changes: glomerulosclerosis, interstitial fibrosis, tubular atrophy, peritubular capillary loss, and inflammation. Different insults can initiate fibrosis, such as epithelial injury, persistent inflammation, or progressive capillary loss. As the functions of different cell types are interrelated in the kidney, these events will feed into each other as the fibrosis progresses. For example, capillary rarefaction will cause tubular atrophy, and loss of tubular epithelial cells will further fuel further capillary loss. These fibrotic changes in the intersitium (termed ‘interstitial fibrosis') are currently used as critical pathologic diagnostic criteria in determining the severity of CKD. However, the key process that causes the functional decline of the kidney is unclear. As the primary functions of the kidney (filtration, reabsorption, and secretion) are performed by epithelial cells, we believe that the loss of functional epithelium and tubular atrophy must be the most critical contributors to loss of renal function. Mouse models further support the role of epithelial cells in interstitial fibrosis development. For example, genetic manipulation of the signaling pathway only in epithelial cells has a profound effect on interstitial fibrosis development. Here, we will examine kidney fibrosis and Notch signaling with respect to the renal epithelial cell.
NOTCH SIGNALING: THE BUILDING BLOCKS
In mammals, there are four Notch receptors (Notch 1–4) and two classes of canonical ligands, Jagged (Jag1, 2) and Delta-like ligand (Dll1, 3, and 4).2 The canonical Notch signaling pathway is initiated when the extracellular domain of a Notch receptor binds to a Notch ligand in a trans-interaction (receptor and ligand on opposite cells).3 The Notch protein is proteolytically cleaved on the extracellular face by ADAM/TACE proteases and on the intracellular side of the plasma membrane by γ-secretase. The extracellular cleavage product remains bound to the ligand-presenting cell to be endocytosed. The intracellular part, known as the Notch intracellular domain (NICD), translocates to the nucleus where it complexes with transcription cofactors, such as RBPj and Mastermind-like proteins, to alter gene expression. The most commonly recognized Notch target genes are the helix-loop-helix proteins of the Hey/Hes family. Hey and Hes operate primarily as repressive transcription factors.4 Although altered expression of the Hey/Hes genes are occasionally reported independently of Notch, their expression patterns are consistent enough to be routinely utilized as proof-of-function for Notch signaling.
Post-translational modification of Notch is equally critical for its function.5, 6 O-fucosylation or O-glycosylation via fringe proteins (lunatic, radical, and manic) regulates the specificity of Notch receptor-ligand binding. For example, modification of Notch by manic fringe confers an increased affinity for Dll1 and a decreased affinity for Jag1, essentially allowing Dll1 to outcompete Jag1 for the Notch receptor. Whether or how resultant signaling differs with engagement of one ligand over another is still undergoing evaluation in the field.
When compared with other signaling pathways, several unique features of Notch signaling become apparent. Notch signaling requires the interaction of at least two different cell types. The cell containing the Notch receptor directs neighboring cells to either inhibit Notch ligand/receptor expression (lateral inhibition) or to establish a small population of adjacent Notch signaling cells (inductive signaling). Both types of signaling result in segregated cell populations by establishing boundaries between homogeneous cell types and/or developing heterogeneous cell populations within tissues. These features give the Notch pathway a unique and critical role in distinguishing cell types and deciding cell fates during development, differentiation, and disease pathogenesis.
NOTCH ACTIVITY IS CRITICAL FOR KIDNEY DEVELOPMENT
Notch has an important role in kidney development. The variable severity of developmental defects resulting from loss of different Notch receptors or ligands further illustrates that, despite utilization of the same signaling architecture, these are not redundant genes. Global Notch3 and Notch4 knockout animals are viable with minimal, if any, phenotypic changes. Mice with a global Notch1 deletion are embryonic lethal; however, deletion of Notch1 from renal epithelial precursors has no effect on kidney development. On the other hand, ablation of only Notch2 from epithelial renal precursors severely compromises renal development with loss of proximal epithelium including podocytes and proximal tubules.7 With respect to Notch ligands, both Dll1 and Jagged1 are expressed in the developing kidneys. Gene-deletion studies indicate that Jagged1 is the dominant ligand. The primary role of Notch in kidney development appears to be in deciding proximal epithelial fate, as genetic overexpression of Notch is sufficient to direct cells to proximal tubule and glomerular epithelial fates.
Human genetic studies support animal model observations. Autosomal dominant mutations of JAGGED1 were identified as a cause of Alagille Syndrome.8 Alagille syndrome was first described as a collection of disorders including craniofacial/skeletal abnormalities, cardiac malformation, and hepatic ductal hyperplasia.9 Recent studies indicate that 40–60% of Alagille patients have some type of renal involvement as well.10 NOTCH2 mutations have been associated with an Alagille-like phenotype in patients, who also present with renal abnormalities.11 In summary, although many Notch ligands and receptors are expressed during development, it seems that Jagged1/Notch2 is the dominant axis for proximal epithelial specification.
PUTATIVE ROLE OF NOTCH IN ACUTE KIDNEY INJURY
The renal epithelium can fully regenerate after an episode of an acute insult. Using a rat model of ischemia-reperfusion injury, increased expression of Dll1 and Hes1 mRNA and proteins along with processed Notch2 was noted by Kobayashi et al.12 Gupta et al.13 also described the increased expression of Notch ligand Dll4 during regeneration after acute renal failure. Treatment of rats with recombinant Dll4 improved the recovery after the kidney injury. Incubation of renal tubule cells with Dll1 in vitro stimulated epithelial cell proliferation, indicating a potential beneficial role for Dll1/Notch signaling in epithelial cell recovery.14 In vivo studies using the gamma secretase complex inhibitor (GSI) to reduce Notch signaling in the setting of acute kidney injury showed mixed outcome. Huang et al.15 found that GSI treatment ameliorated the severity of tubular damage after renal ischemia-reperfusion injury in rats, whereas Chen et al.16 described that GSI treatment delayed functional renal recovery in mice.
The mechanism of renal regeneration after an acute injury is a hotly debated issue. The specific question is whether it occurs from a specialized stem/progenitor compartment or via dedifferentiation and redifferentiation of existing epithelial cells. As previously noted, Notch has a role in directing cells to a proximal tubule fate during development, making Notch an attractive candidate for regulating proximal tubule regeneration. In addition, Notch has a critical role in maintaining the stem cell compartment in various organs. To further dissect Notch's potential role in maintaining a renal stem cell reserve, the Romagnani group isolated CD133/CD24 double-positive cells from human kidneys. They showed that under the right conditions in vitro, these putative renal progenitor cells exhibit high Notch activity17, 18 and differentiate into podocytes and tubule cells. According to their recent studies, these progenitor cells can replace podocytes after podocyte loss, and this differentiation into podocytes depends on the Jagged1/Notch2 axis.17
Parietal epithelial cells (PEC) have also emerged as important putative podocyte progenitors. In a recent study that used a transgenic podocyte-depletion system, increased Notch signaling was described in PECs after podocyte ablation.19 In this model, parietal cells proliferated and became hyperplastic after severe podocyte loss. Increased Notch1 and Jagged1 were also detected by immunohistochemistry in hyperplastic PEC. In vitro studies indicated that Notch is responsible for transforming quiescent PECs into activated parietal cells. Treating these mice with GSI to block Notch signaling decreased parietal cell expansion. In summary, recent reports indicate that Notch is expressed in tubular epithelial cells during acute injury and likely has a role in glomerular and tubular epithelial regeneration. Future lineage tagging and genetic-deletion experiments will hopefully establish the precise role and mechanism of Notch-mediated glomerular and tubular epithelial repair.
SUSTAINED NOTCH EXPRESSION IN TUBULAR EPITHELIAL CELLS IS ASSOCIATED WITH KIDNEY FIBROSIS
In 2010, a data set comparing Notch expression in kidney biopsy samples from patients with various acquired renal diseases demonstrated upregulation of the cleaved (active) forms of either Notch1 and/or Notch2 in 9 of 10 examined disease types.20 Tubule-specific Notch1 expression correlated both with tubulointerstitial fibrosis (TIF) and renal function. Interestingly, no change in Notch expression was observed in hypertensive renal disease cases. In a different study, increased transcript expression and coregulation of Jag1 and Notch1 with Gremlin and other transforming growth factor-β (TGF-β)-related transcripts were also observed in the tubule compartments of diabetic kidney disease samples.21 In mice, Notch1, 2, 3 and 4 and Jag1 were all found to be increased in models of TIF.22, 23
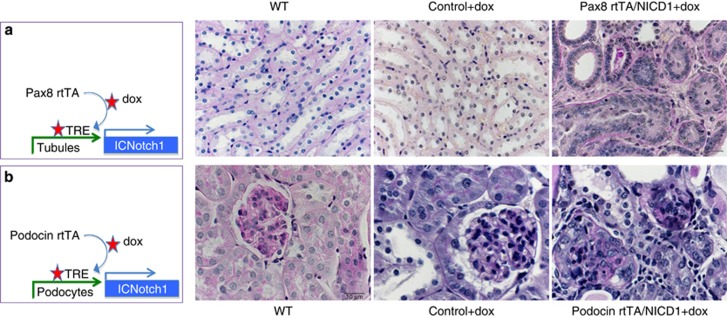
The increased expression of Notch ligands and receptors both in patients and mouse models of TIF have prompted investigators to examine the role of Notch in TIF development. In vivo studies have been performed using pharmacological inhibitors, genetic deletion, and transgenic overexpression. Inhibition of Notch via blockade of the γ-secretase cleavage was associated with improved histological parameters of fibrosis and decreased expression of profibrotic molecules both in the folic acid-induced and in the ureteral obstruction-induced kidney fibrosis models. A mouse model with genetic ablation of the Notch signaling transcriptional co-factor Rbpj was studied.22 Mice without proximal tubule Notch signaling (Rbpjfl/fl/PEPCKCre mice) showed no phenotypic abnormalities at baseline, indicating that Notch is dispensable for proximal tubule maintenance. On the other hand, folic acid-induced injury in Rbpjfl/fl/PEPCKCre mice resulted in significantly less TIF development than in wild-type animals, indicating the important contribution of Notch signaling to TIF pathogenesis. In a different set of experiments, mice with tubule epithelial-specific inducible, active, cleaved Notch1 (Pax8rtTA/ICNotch1) were engineered (Figure 1). These animals developed the full spectrum of severe TIF, including tubular degeneration, interstitial fibrosis, and inflammation. These changes were so extensive that the resultant kidney failure was lethal to these animals within 5 weeks. These studies indicated that Notch signaling is both sufficient and necessary for TIF and highlighted the fact that Notch could be an important therapeutic target for TIF. Unfortunately, global inhibition of Notch signaling is associated with severe gastrointestinal side effects.24 Therefore defining the role of specific Notch ligands and receptors is the next critical task.
Figure 1.
Sustained expression of the Notch1 intracellular domain is sufficient to cause tubulointerstitial fibrosis and glomerulosclerosis. (a) Pax8rtTA animals express the reverse tetracycline transactivator under the Pax8 (tubule epithelial cell specific) promoter. Animals were crossed with tetracycline-inducible (tet-o) NICD1 mice (Pax8rtTA/NICD1, schematic far left panel). Upon doxycycline (dox) administration animals express the Notch intracellular domain in tubule epithelial cells. From left to right micrographs are representative of periodic acid–Schiff (PAS)-stained sections from: wild type (WT), Pax8rtTA with dox, and Pax8rtTA/NICD with dox-fed mice harvested at day 28 post induction. Note the significant tubulointerstitial fibrosis in double-transgenic mice after dox treatment. (b) Tet-o-NICD1 mice were crossed with podocin rtTA animals for podocyte-specific expression of Notch1 (podocin rtTA/NICD1, schematic far left panel). From left to right, micrographs are representative PAS-stained sections from: WT, dox-treated podocin rtTA, and dox-treated podocin rtTa/NICD mice harvested at day 10 post induction. Note the significant glomerulosclerosis and proteinaceous casts. Scale bar=10 μm.
Studies performed by the Mertens group indicated that Notch3 might be the receptor isoform responsible for kidney fibrosis.23 Notch3 was markedly upregulated in patients and mouse models of TIF, and global knockout of Notch3 conferred protection from ureteral obstruction-induced TIF in mice.23 Interestingly, loss of Notch3 resulted in significantly lower levels of Notch1, Jagged1, and Hey-L mRNA expression in response to injury, suggesting that the different receptors may interact in a positive-feedback loop to induce TIF. In summary, multiple studies indicate that sustained expression of Notch in differentiated tubular epithelial cells induce TIF.
SUSTAINED EXPRESSION OF NOTCH IN PODOCYTES IS ASSOCIATED WITH GLOMERULOSCLEROSIS
Microarray studies published by the Cohen and the ERCB (European Renal cDNA Bank) groups described increased transcript levels of Jagged2 and Notch3 in glomeruli of patients with immunoglobulin A, lupus, and membranous nephropathy.23 Immunohistochemistry studies performed on human kidney tissue samples by Murea et al.20 indicated that there was a statistically significant positive correlation between podocyte Notch1 expression and glomerulosclerosis and proteinuria. Upregulation of cleaved Notch1 was detected in podocytes of both diabetic and focal segmental glomerulosclerosis (FSGS) mouse models. Glomeruli of mice with HIV-associated collapsing FSGS showed increased Notch1 and Notch4 levels.25
To understand whether Notch has a role in glomerular disease development, a podocyte-specific, tetracycline-inducible, NICD1-overexpressing mouse was created. When the expression of ICNotch1 was induced in 4-week-old mice, we observed a marked induction of proteinuria followed by mesangial expansion and matrix deposition (Figure 1b). Histological analysis indicated that increased Notch1 signaling in podocytes alone was sufficient to induce glomerulosclerosis (FSGS-like phenotype).26 Animals developed the full spectrum of fibrosis including tubular atrophy, interstitial fibrosis, and inflammatory cell influx. Mechanistic studies showed that expression of Notch1 in mature podocytes caused cellular dedifferentiation and ultimately apoptosis.27
In a different study, ICNotch1 was expressed in podocytes prior to full completion of differentiation.28 Proteinuria and glomerulosclerosis were observed within 2 weeks. Histological lesions observed in these animals were more similar to those seen with collapsing FSGS, for example, as seen in HIV-associated nephropathy. Podocyte proliferation was found to be responsible for the resultant phenotype in this model. The cause for these minor phenotypic differences observed between the two ICNotch1 expression studies might be related to the developmental stage at the time of ICNotch 1 induction, signal intensity (level of expression), or the presence of other signaling molecules.
To further evaluate whether Notch activation contributes to glomerulosclerosis, mice with podocyte-specific Notch signaling deletion (using the Rbpj conditional allele) were developed. These animals showed marked protection from diabetes-induced albuminuria and mesangial expansion. In the absence of Notch signaling, podocytes were protected from hyperglycemia-induced apoptosis. Interestingly, a recent study also suggested that expression of Notch promotes nephrin endocytosis from the slit diaphragm, resulting in foot process alterations and increased proteinuria.29 As nephrin is an important pro-survival molecule in podocytes, Notch-mediated nephrin loss might further contribute to podocyte loss via apoptosis.
Mouse genetic studies (mentioned above) have also propelled investigators to examine whether pharmacological blockade of Notch signaling can be beneficial for the treatment of glomerular diseases. Our lab found that GSI very effectively decreased proteinuria and protected podocytes in a puromycin aminonucletide-induced nephrotic syndrome. Sharma et al.30 published that GSI also reduced albuminuria and histological lesions in a mouse model of HIV-associated nephropathy. Lin et al.31 described that GSI treatment ameliorated changes of diabetic kidney disease in a rat model of diabetes. GSIs also mitigated signs of systemic lupus and its renal complications.32 In summary, both human and mouse models indicate that the reactivation of Notch signaling in differentiated podocytes is associated with albuminuria and glomerulosclerosis development. Although current GSIs exhibited undesirable off-target effects in human clinical trials, with our current and expanding understanding of Notch in the kidney it seems that design of a kidney-specific Notch inhibitor is still within the realm of therapeutic possibility.
THE NOTCH INTERACTOME
Notch likely acts in concert with multiple other pathways to regulate kidney fibrosis. The renin–angiotensin–aldosterone system has a key role in fibrosis development, and indeed, the current treatment of kidney fibrosis relies on blockers of the renin–angiotensin system. Notch interacts with the renin–angiotensin–aldosterone system on multiple levels. Notch can directly bind to the renin promoter to regulate renin expression, and the plasticity of renin-expressing cells directly depends on Rbpj expression.33, 34 In addition, in vitro treatment of renal epithelial cells with angiotensin induces the expression of the Notch ligand Jagged1 and the activation of Notch.35, 36, 37
One of the most explored partners of Notch signaling is the TGF-β pathway. TGF-β is known to regulate multiple aspects of fibrosis, including tubular epithelial cell death, transdifferentiation, and collagen expression. In keratinocytes, TGF-β can directly regulate Notch target genes Hes and Hey.38 In diverse epithelial cells, TGF-β increases the expression of the Notch ligand Jag1 to induce the activation of Notch signaling.21 Indeed, a similar phenomenon was observed in both podocytes and tubular epithelial cells.22 Treatment of cultured podocytes or tubular epithelial cells with TGF-β induced the expression of Jag1 and led to downstream activation of Notch signaling. Surprisingly, significant increases in Notch expression further stimulates the expression of TGF-β, creating a positive-feedback loop.27, 39 This positive-feedback regulatory loop can potentially explain why Notch inhibitors have such profound effects on TIF development.
In endothelial cells, vascular endothelial growth factor is an important regulator of Notch signaling. Again, this mainly occurs via regulation of expression of the ligand, which in this case is Dll4. The vascular endothelial growth factor/Dll4 interaction is critical for determination of the leading tip cell during angiogenesis.40 As vascular endothelial growth factor is particularly highly expressed in the podocytes,41 it would be interesting to examine whether Dll4 regulation also occurs in podocytes.
During development, Notch interacts with many other key developmental pathways including Wnt42 and Hedgehog.43 These pathways are also reactivated in tissue fibrosis and will likely also interact with Notch in this context.
CONCLUSIONS
Understanding tissue fibrosis is critical to the development of therapies for CKD/end-stage renal disease. An obstacle to fibrosis prevention has been that many of the pathways utilized by fibrosis are the same pathways engaged during the processes of development, physiologic repair, and regeneration. In this context Notch, TGF-β1, Wnt and Hedgehog signaling have come into focus as key regulatory targets.
The Jagged1/Notch2 axis is critical for proximal tubule and glomerular epithelial fate determination during kidney development. The Notch pathway is reactivated in these cells both in patients and animal models of acute and chronic kidney injury. Short-term expression of Notch signaling during the recovery phase of acute injury could be beneficial by directing cells toward proximal tubule fate. The prolonged expression of Notch proteins is detrimental to epithelial cells and contributes to fibrosis. Although Notch is able to guide progenitor and undifferentiated cells to make fate decisions during development and differentiation, terminally differentiated cells are unable to tolerate Notch expression. Understanding molecular switches that control Notch activity will be an important future step.
Importantly, manipulation of Notch signaling only in epithelial cells has been shown to be sufficient to induce and prevent fibrosis development in mice, highlighting the key role of epithelial cells in fibrosis. Although Notch alone did not transform renal epithelial cells into activated myofibroblasts in vivo, it is likely that Notch is an important regulator of epithelial–interstitial cell interactions. Defining the critical signaling pathways in epithelial–interstitial cell interaction at baseline and in fibrosis will be an important next step.
In summary, defining Notch signaling during development, repair, regeneration, and fibrosis will likely enhance our understanding of CKD, which may lead to the future prevention of this condition.
Acknowledgments
Work in the Susztak Laboratory is supported by NIH RO1, JDRF, and ADA. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association. JT has received grant support from the National Natural Sciences Foundation of China (ref. no. 81170665).
KS serves on the advisory board for Abbvie and has received grant support from Boehringer Ingelheim, National Institutes of Health, and American Diabetes Association. The remaining authors declared no competing interests.
References
- USRDS: the United States renal data system. Am J Kidney Dis. 2003;42 (Suppl 5:1–230. [PubMed] [Google Scholar]
- Bonegio R, Susztak K. Notch signaling in diabetic nephropathy. Exp Cell Res. 2012;318:986–992. doi: 10.1016/j.yexcr.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Cagan R. Notch on the cutting edge. Trends Genet. 1997;13:465–467. doi: 10.1016/s0168-9525(97)01318-8. [DOI] [PubMed] [Google Scholar]
- Ilagan MX, Lim S, Fulbright M, et al. Real-time imaging of notch activation with a luciferase complementation-based reporter. Sci Signal. 2011;4:rs7. doi: 10.1126/scisignal.2001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vooijs M, Liu Z, Kopan R. Notch: architect, landscaper, and guardian of the intestine. Gastroenterology. 2011;141:448–459. doi: 10.1053/j.gastro.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Sirin Y, Susztak K. The story of Notch and chronic kidney disease. Curr Opin Nephrol Hypertens. 2011;20:56–61. doi: 10.1097/MNH.0b013e3283414c88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HT, Kim M, Valerius MT, et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- Alagille D, Odievre M, Gautier M, et al. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86:63–71. doi: 10.1016/s0022-3476(75)80706-2. [DOI] [PubMed] [Google Scholar]
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A. 2012;158A:85–89. doi: 10.1002/ajmg.a.34369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath BM, Bauer RC, Loomes KM, et al. NOTCH2 mutations in Alagille syndrome. J Med Genet. 2012;49:138–144. doi: 10.1136/jmedgenet-2011-100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Kageyama R. Hes1 regulates embryonic stem cell differentiation by suppressing Notch signaling. Genes Cells. 2010;15:689–698. doi: 10.1111/j.1365-2443.2010.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Li S, Abedin MJ, et al. Effect of Notch activation on the regenerative response to acute renal failure. Am J Physiol Renal Physiol. 2010;298:F209–F215. doi: 10.1152/ajprenal.00451.2009. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Terada Y, Kuwana H, et al. Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int. 2008;73:1240–1250. doi: 10.1038/ki.2008.74. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhou Q, Veeraragoo P, et al. Notch2/Hes-1 pathway plays an important role in renal ischemia and reperfusion injury-associated inflammation and apoptosis and the gamma-secretase inhibitor DAPT has a nephroprotective effect. Ren Fail. 2011;33:207–216. doi: 10.3109/0886022X.2011.553979. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen JK, Conway EM, et al. Survivin mediates renal proximal tubule recovery from AKI. J Am Soc Nephrol. 2013;24:2023–2033. doi: 10.1681/ASN.2013010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagni L, Ballerini L, Angelotti ML, et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. 2010;28:1674–1685. doi: 10.1002/stem.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti ML, Ronconi E, Ballerini L, et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012;30:1714–1724. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- Matsusaka T, Xin J, Niwa S, et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16:1013–1023. doi: 10.1681/ASN.2004080720. [DOI] [PubMed] [Google Scholar]
- Murea M, Park JK, Sharma S, et al. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney Int. 2010;78:514–522. doi: 10.1038/ki.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhan KC, Faherty N, Murray G, et al. Jagged/Notch signalling is required for a subset of TGFbeta1 responses in human kidney epithelial cells. Biochim Biophys Acta. 2010;1803:1386–1395. doi: 10.1016/j.bbamcr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Bielesz B, Sirin Y, Si H, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djudjaj S, Chatziantoniou C, Raffetseder U, et al. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J Pathol. 2012;228:286–299. doi: 10.1002/path.4076. [DOI] [PubMed] [Google Scholar]
- Garber K. Notch emerges as new cancer drug target. J Natl Cancer Inst. 2007;99:1284–1285. doi: 10.1093/jnci/djm148. [DOI] [PubMed] [Google Scholar]
- Sharma M, Callen S, Zhang D, et al. Activation of Notch signaling pathway in HIV-associated nephropathy. AIDS. 2010;24:2161–2170. doi: 10.1097/QAD.0b013e32833dbc31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Susztak K. Repair problems in podocytes: Wnt, Notch, and glomerulosclerosis. Semin Nephrol. 2012;32:350–356. doi: 10.1016/j.semnephrol.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- Waters AM, Wu MY, Onay T, et al. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19:1139–1157. doi: 10.1681/ASN.2007050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Wu MY, Huang YW, et al. Notch promotes dynamin-dependent endocytosis of nephrin. J Am Soc Nephrol. 2012;23:27–35. doi: 10.1681/ASN.2011010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Magenheimer LK, Home T, et al. Inhibition of Notch pathway attenuates the progression of human immunodeficiency virus-associated nephropathy. Am J Physiol Renal Physiol. 2013;304:F1127–F1136. doi: 10.1152/ajprenal.00475.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Wang FS, Hsu YC, et al. Modulation of notch-1 signaling alleviates vascular endothelial growth factor-mediated diabetic nephropathy. Diabetes. 2010;59:1915–1925. doi: 10.2337/db09-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen T, Grammatikos AP, Hedrich CM, et al. cAMP-responsive element modulator alpha (CREMalpha) contributes to decreased Notch-1 expression in T cells from patients with active systemic lupus erythematosus (SLE) J Biol Chem. 2012;287:42525. doi: 10.1074/jbc.M112.425371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Glenn ST, Jones CA, et al. Activation of the rat renin promoter by HOXD10.PBX1b.PREP1, Ets-1, and the intracellular domain of notch. J Biol Chem. 2005;280:20860–20866. doi: 10.1074/jbc.M414618200. [DOI] [PubMed] [Google Scholar]
- Castellanos Rivera RM, Monteagudo MC, Pentz ES, et al. Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics. 2011;43:1021–1028. doi: 10.1152/physiolgenomics.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshizaka M, Takemoto M, Sato S, et al. An angiotensin II type 1 receptor blocker prevents renal injury via inhibition of the Notch pathway in Ins2 Akita diabetic mice. Exp Diabetes Res. 2012;2012:159874. doi: 10.1155/2012/159874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoz C, Rodrigues-Diez R, Benito-Martin A, et al. Angiotensin II contributes to renal fibrosis independently of Notch pathway activation. PloS One. 2012;7:e40490. doi: 10.1371/journal.pone.0040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozasa Y, Akazawa H, Qin Y, et al. Notch activation mediates angiotensin II-induced vascular remodeling by promoting the proliferation and migration of vascular smooth muscle cells. Hypertens Res. 2013;36:859–865. doi: 10.1038/hr.2013.52. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Cermak L, Soto-Nieves N, et al. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan T, Murea M, Susztak K. The pathogenic role of Notch activation in podocytes. Nephron Exp Nephrol. 2009;111:e73–e79. doi: 10.1159/000209207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofaro B, Shi Y, Faria M, et al. Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development. 2013;140:1720–1729. doi: 10.1242/dev.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Stolz DB, Kiss LP, et al. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian SL, Penchev RR, St-Jacques B, et al. Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol. 2012;180:1441–1453. doi: 10.1016/j.ajpath.2011.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]