Abstract
Interstitial fibrosis represents the final common pathway of any form of progressive renal disease. The severity of tubular interstitial damage is highly correlated to the degree of decline of renal function, even better than the glomerular lesions do. Angiotensin II (Ang II), the main effector of the renin–angiotensin system, is a critical promoter of fibrogenesis. It represents a nexus among glomerular capillary hypertension, barrier dysfunction, and renal tubular injury caused by abnormally filtered proteins. Transforming growth factor (TGF)-β1 and reactive oxygen species (ROS) are the key mediators of the pro-fibrotic effect of Ang II causing apoptosis and epithelial-to-mesenchymal transition of the renal tubular epithelium. Recent studies link fibrosis to changes of microRNA (miRNA) modulated by Ang II through TGF-β1, unraveling that antifibrotic action of Ang II antagonism is attributable to epigenetic control of fibrosis-associated genes. Other mechanisms of Ang II-induced fibrosis include ROS-dependent activation of hypoxia-inducible factor-1. Finally, Ang II via angiotensin type 1 receptor regulates the activation and transdifferentiation of pericytes and fibrocytes into scar-forming myofibroblasts. Detachment and phenotypic changes of the former can lead to the loss of peritubular capillaries and also contribute to hypoxia-dependent fibrosis.
Keywords: angiotensin II, fibrosis, hypoxia-inducible factor, microRNA, reactive oxygen species, transforming growth factor-β
Independently of the primary insult, progression of chronic kidney disease (CKD) to end-stage renal failure is the common outcome. The rate of decline of renal function varies among nephropathies and for the same disease in different individuals1 ‘…the functional disturbances known to occur in human renal failure are precisely those that occur in animal experiment as a result of reduction in the amount of functioning renal substance—that is, loss of nephron.' (Robert Platt, Lumleian Lecture to the Royal College of Physicians, London 1952).2 After 30 years of Platt's observation, Brenner and colleagues, while deeper investigating the structural and functional renal adaptation to nephron loss in the model of renal ablation in the rat,3 identified glomerular capillary hypertension as a leading cause of progressive deterioration of remaining nephrons. Mathematical modeling of the size-selectivity function of the glomerular membrane suggested that elevated transcapillary hydraulic pressure increased the population of large unselective pores perforating the glomerular membrane by a mechanism at least partly mediated by angiotensin II (Ang II).4 Mechanical strain, as results of glomerular hypertension, upregulates angiotensin type 1 receptor (AT1R) and increases the production of Ang II in cultured podocytes.5 Loss of podocyte–podocyte contact and increased albumin permeability induced by Ang II in these cells6 translate in the glomerular sieving dysfunction observed in proteinuric nephropathies. The proteinuric ultrafiltrate represents the way for spreading the disease from the glomerulus to the tubulointerstitial compartment.
ANG II-INDUCED PROTEINURIA AND INTERSTITIAL FIBROSIS
Ang II-induced proteinuria is a leading cause of CKD progression. Proteins abnormally filtered through the glomerular capillary have intrinsic toxicity on the proximal tubule contributing to the development of interstitial inflammation, fibrosis, and ultimately to kidney dysfunction.
Proximal tubular cells exposed in vitro to albumin undergo apoptosis through a mechanism involving reduced expression of its receptor megalin. Furthermore, overload of plasma proteins stimulates proximal tubular cells to synthesize and release pro-inflammatory substances including Monocyte Chemoattractant Protein-1 (MCP-1/CCL2), Regulated upon Activation, Normal T-cell Expressed and Secreted (RANTES/CCL5), fractalkine/CX3CL1 that are potent chemoattractants for monocytes/macrophages, and T lymphocytes.
Changes observed in vitro parallel those obtained in proteinuric models in vivo. They showed apoptosis-induced glomerular–tubule disconnection and inflammation via release of cytokine including MCP-1 from activated proximal tubular cells.7 A similar inflammatory pathway occurs in patients with severe proteinuria in whom renal upregulation of MCP-1, RANTES, and osteopontin, mainly in tubular epithelial cells, correlates with the severity of the disease.8, 9 Both injured tubules and inflammatory cells enhance fibrogenesis by activating interstitial fibroblasts via a paracrine mechanism that involves the release of transforming growth factor (TGF)-β1, the most potent inducer of epithelial-to-mesenchymal transition (EMT). Albumin induces the upregulation of TGF-β receptor type II expression in proximal tubular cells and makes them more susceptible to the matrix-stimulatory actions of TGF-β. Accumulation of extracellular matrix (ECM) proteins by proximal tubular cells occurs concomitantly with the induction of tissue inhibitors of metalloproteinases, (TIMP)-1 and TIMP-2, in response to albumin.7 Filtered C3 is also recognized as a major promoter of injury in proteinuric nephropathies as highlighted by cross-transplantation experiments employing C3-deficient mice and wild-type littermates. Mechanism of injury induced by C3 is attributed to the formation of the C5b-9 membrane attack complex.10
Dendritic cells, the main professional antigen-presenting cell population of the kidney, accumulate in the renal parenchyma in proteinuric nephropathies in the vicinity of proximal tubular cells. Epithelial–dendritic cell interaction leads to albumin processing into antigenic peptides that activate immune cells. Overall proteinuric state and inflammatory environment enable dendritic cells to become immunogenic towards normally ignored self-antigens providing a link among proteinuria, autoimmunity, and renal disease progression.11
Targeting proteinuria by angiotensin-converting enzyme (ACE) inhibitors alone or as combined therapy prevents glomerular–tubule disconnection and atrophy and ameliorates interstitial inflammation and fibrosis.12
ANG II-INDUCED INTERSTITIAL FIBROSIS IS MEDIATED BY TGF-β
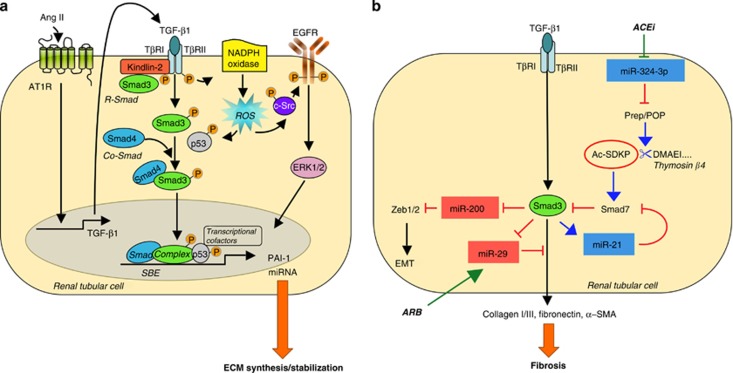
Besides protein load, Ang II directly stimulates the TGF-β1 gene, protein expression in proximal tubular cells,13 and triggers the production of plasminogen activator inhibitor-1 (PAI-1), ECM synthesis and deposition in the interstitial space.14, 15 TGF-β1 signaling includes Smad and non-Smad pathways for the activation of its major effector and downstream target PAI-1. Kindlin-2, a β-integrin adaptor protein, has been recently discovered to work as a TGF-β type I receptor/Smad3 adapter protein that amplifies profibrogenic effects of TGF-β1 through Smad3 signaling in tubular cells in vitro and in vivo16 (Figure 1).
Figure 1.
Transforming growth factor (TGF)-β1 mediates angiotensin II (Ang II)-induced renal fibrosis. (a) Smad and non-Smad signaling contribute to the transcription of TGF-β1 target genes. Ang II via angiotensin type 1 receptor (AT1R) upregulates TGF-β1 expression. The growth factor binds to TGF-β receptor II (TβRII), which activates TβRI resulting in the phosphorylation of R-Smads (that is, Smad3). Kindlin-2 mediates the interaction of TβRI with Smad3 favoring Smad3 activation. The phosphorylated Smad3 binds to the Co-Smad, Smad4 forming the Smad complex that translocates into the nucleus and binds to Smad-binding elements (SBE) in promoter regions of target genes (that is, plasminogen activator inhibitor-1 (PAI-1), microRNAs (miRNAs)). TGF-β1 also triggers reactive oxygen species (ROS) that activate epidermal growth factor receptor (EGFR) signaling and p53, which in turn interacts with pSmad3 and other transcriptional cofactors sustaining gene induction. (b) TGF-β1 promotes fibrosis through the Smad3-dependent regulation of miRNA. Upregulation of miR-324-3p that represses Prep-dependent synthesis of the antifibrotic peptide N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) amplifies TGF-β1 signaling.
The non-Smad pathway involves reactive oxygen species (ROS)-dependent-c-Src-mediated activation of epidermal growth factor receptor and downstream signaling cascade.17 ROS also maintain receptor-activated Smads in a phoshorylated state and are crucial for p53 activation that interacts with Smads and transcriptional cofactors forming transcriptionally active multiprotein complexes important for maximal PAI-1 induction17 (Figure 1). Smad and non-Smad signal integration contributes to renal fibrosis in unilateral ureteral obstruction (UUO) and in diabetic nephropathy.18
Control of ECM accumulation through the inhibition of TGF-β1/PAI-1 has been proposed as the underlying mechanism of the therapeutic effect of Ang II blockade in several models of progressive nephropathies.19 In Munich Wistar Fromter (MWF) rats with advanced nephropathy, ACE inhibition induces regression of glomerular lesions and prevents worsening of interstitial changes through significant reduction of TGF-β1 expression.20 Moreover, add-on anti-TGF-β antibody to ACE inhibitor attenuates interstitial inflammation and accumulation of type III collagen and abrogates tubular damage in rats with overt diabetic nephropathy.21 Such studies paved the way for the use of fresolimumab, a human monoclonal antibody, which neutralizes the mammalian isoforms of TGF-β, in patients with primary focal segmental glomerulosclerosis.22
CROSS-TALK BETWEEN ANG II AND TGF-β IN RENAL FIBROSIS: MICRORNAS, POTENTIAL TARGETS OF THE ANTIFIBROTIC EFFECT OF ANG II ANTAGONISM
MicroRNAs (miRNA) are a class of short, single-stranded noncoding RNAs of ∼20–22 nucleotides in length that act as post-transcriptional repressors. Tubulointerstitial fibrosis has been recently linked to the loss or activation of those miRNA. In this context, we have recently discovered miR-324-3p as a new mediator of renal fibrosis.23 MiR-324-3p, identified as the most highly expressed miRNA in microdissected glomeruli from MWF rats with advanced nephropathy, localizes to the glomerulus and, most abundantly, to cortical tubules. The downstream target of miR-324-3p is prolyl endopeptidase (Prep), also known as prolyl oligopeptidase, a serine protease that is involved in the metabolism of Ang-I/Ang II into Ang-(1–7) and in the synthesis of the antifibrotic peptide N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP). Transfection of tubular epithelial cells with miR-324-3p results in downregulation of Prep expression and activity and increases susceptibility to developing pro-fibrotic phenotype in response to TGF-β1, suggesting a feedback loop sustaining fibrosis (Figure 1). In MWF rats, overexpression of miR-324-3p is associated with reduced expression of Prep in both glomeruli and tubular epithelium in fibrotic areas. Unbalance between miR-324-3p and Prep is normalized by ACE inhibitor that also increases urine and plasma Ac-SDKP levels, hence attenuating tubulointerstitial fibrosis. These data underline how dysregulation of the miR-324-3p and its target Prep greatly limits the activation of the Ac-SDKP antifibrotic pathway in the kidney, sensitizing MWF rats to Ang II and TGF-β, which in turn favor progressive renal fibrosis. Ac-SDKP that is formed from its precursor, thymosin β4, by Prep-mediated proteolytic activity has an important role in collagen balance, as a decrease in endogeneous levels of the peptide in the heart and kidneys promotes organ fibrosis.24 Exogenous administration of thymosin β4 plus prolyl oligopeptidase inhibitor exacerbates both early and late interstitial fibrosis in UUO mice through a PAI-1-dependent effect. Conversely, Ac-SDKP administration decreases both early and late fibrosis and inflammation lessening PAI-1 and TGF-β1 activation.25 Increased Ac-SDKP levels, secondary to modulation of the miR-324-3p/Prep pathway by ACE inhibitors, represent a mechanism complementary to Ang II inhibition, whereby these drugs protect the kidney from pro-fibrotic stimuli such as TGF-β1 (Figure 1). In addition, inhibition of TGF-β/Smad signaling, through the upregulation of Smad7, underscores the antifibrotic and anti-inflammatory effects of Ac-SDKP in progressive renal disease.26 TGF-β1 has been recently shown to promote fibrosis by upregulating pro-fibrotic miR-21 and downregulating antifibrotic miR-29 and miR-200 (Figure 1). Smad3-mediated overexpression of miR-21 increases the expression of ECM proteins, collagen 1, and fibronectin, and the α-smooth muscle actin in renal tubular cells and in fibrotic kidneys from animals with UUO.27, 28 Conversely, inhibition of miR-21 halts the progression of renal fibrosis in established obstructive nephropathy27, 28 and in type 2 diabetes.29 The favorable effect of miR-21 inhibition was attributed to restoring Smad7, an inhibitor of the TGF-β/Smad pathway capable of reducing the expression of pro-inflammatory and fibrotic markers.29 Altogether, these lines of evidence suggest that miR-21 mediates renal fibrosis in both diabetic and non-diabetic experimental nephropathies acting in a feed-forward loop that amplifies TGF-β signaling. The translational relevance of such findings rests on a recent study showing that circulating miR-21 levels were associated with the severity of the kidney fibrosis and renal function decline in renal transplant patients.30
Both miR-29 and -200 families are targets of TGF-β signaling. Progressive renal fibrosis in obstructive nephropathy is associated with the downregulation of miR-29 in fibrotic kidneys that acts as a downstream inhibitor for TGF-β1-induced expression of collagen type I and III by renal tubular cells.31 TGF-β1 downregulates the expression of the miR-29 family (miR-29a, -29b, and -29c) in proximal tubular epithelial cells, podocytes, and mesangial cells in culture, and this effect results in increased expression of collagen I, III, and IV.32 Low expression of miR-29a and miR-29c characterizes advanced non-diabetic and diabetic experimental nephropathies. In the latter model, treatment with the Rho-associated kinase inhibitor fasudil or the angiotensin receptor blocker losartan ameliorates proteinuria, structural lesions, and interstitial ECM accumulation by restoring miR-29a and -29c expression.32 As far as the miR-200 family, two of its members, miR-200a and miR-141, are downregulated at the early phase of UUO.33 In vitro, TGF-β1 induces downregulation of miR-200 family that represses E-cadherin repressors resulting in EMT of renal tubular epithelial cells.33 Moreover, in experimental diabetes, overexpression of both TGF-β1 and TGF-β2 in the kidney cortex parallels the downregulation of miR-200a and increases protein expression of collagen type IV and fibronectin.34
ANG II-INDUCED RENAL FIBROSIS: ROLE OF ROS AND HYPOXIA
ROS are important mediators of Ang II-induced EMT and apoptosis leading to renal fibrosis. In tubular epithelial cells, stably expressing AT1R, Ang II promotes ROS-dependent activation of Src kinase that phosphorylates caveolin-1 and epidermal growth factor receptor leading to extracellular signal-regulated kinase activation and ultimately to EMT. Prolonged epidermal growth factor receptor/extracellular signal-regulated kinase signaling is inhibited by silencing NADPH oxidase (Nox)4, a member of the Nox family in mitochondria, which modulates the generation of ROS.35 Nox4 is the major source of ROS in the kidney cortex of db/db mice36 to the extent that overexpression of catalase, a scavenger of H2O2, in renal proximal tubules of db/db mice attenuates interstitial fibrosis and tubular apoptosis.37, 38 Excess generation of mitochondrial ROS is limited by Sirt3, the main mitochondrial NAD-dependent deacetylase, through the activation of antioxidant enzymes.39 Conversely, deletion of Sirt3 in mouse embryo fibroblasts gives rise to higher ROS, increases cell proliferation, and hypoxia-inducible factor (HIF)-1α transcriptional activity.40 Sirt3 expression is downregulated by Ang II, via AT1R as documented in mice lacking AT1R having however increased Sirt3 levels in the kidney. These mice are protected from aging-induced oxidative damage and live longer than wild-type littermates.41
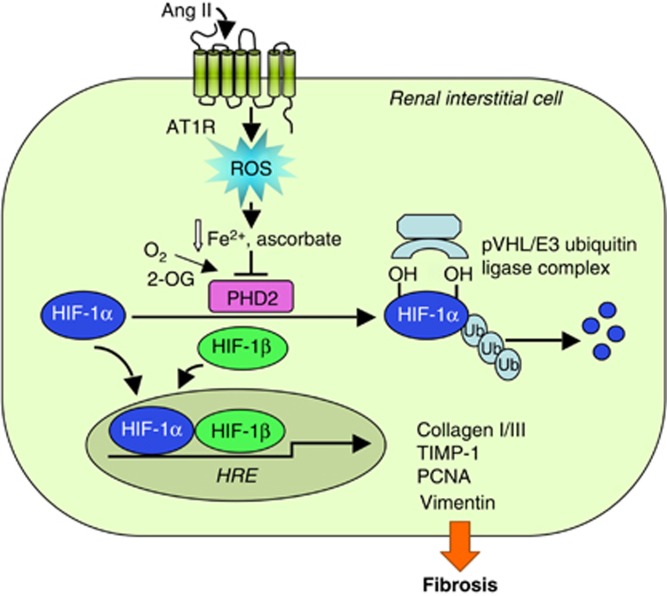
ROS are involved in the activation of HIF-1, a heterodimeric transcription factor that has a key role in cellular adaptation to different oxygen concentrations. Under normoxia, the oxygen-sensitive HIF-1α subunit is hydroxylated at specific proline residues by prolyl-4-hydroxylase domain (PHD) proteins, enabling interaction with von Hippel–Lindau-E3 ubiquitin ligase complex that targets HIF-1α for proteasomal degradation. Ang II activates HIF-1α through the generation of ROS that increase HIF-1α translation and inactivate PHD promoting HIF-1α stabilization under nonhypoxic conditions42 (Figure 2). A recent study has demonstrated a functional link between the activation of HIF-1α and the pro-fibrotic effects of Ang II in renal medullary interstitial cells.43 The mechanism whereby Ang II stabilizes HIF-1α in these cells is dependent on H2O2-mediated inhibition of PHD2 activity, the predominant PHD in the kidney. Gene silencing of HIF-1α blocks Ang II-induced transcription of pro-fibrotic markers TIMP-1 and collagen I/III as well as of proliferating nuclear antigen and vimentin (Figure 2). In vivo, kidneys infused with Ang II show positive staining for both HIF-1α and α-smooth muscle actin in renal medullary interstitial cells.43 Altogether, these findings suggest that Ang II-induced interstitial fibrosis mediated by HIF-1α requires cell transdifferentiation possibly including EMT. Interestingly, targeting Ang II signaling by angiotensin receptor blocker valsartan reduces renal expression of HIF-1α and its target gene TIMP-1 and attenuates interstitial fibrosis in streptozotocin-diabetic rats.44
Figure 2.
Reactive oxygen species (ROS) mediate angiotensin II (Ang II)-induced renal interstitial fibrosis via hypoxia-inducible factor-1α (HIF-1α). Under normoxia, prolyl-4-hydroxylase domain 2 (PHD2) hydroxylates HIF-1α at specific proline residues allowing for recognition by pVHL/E3 ubiquitin ligase complex that ubiquinates and targets HIF-1α for proteasomal degradation. Ang II-induced H2O2, by limiting the availability of ferrous iron and ascorbate, inactivates PHD2 promoting HIF-1α stabilization. HIF-1α interacts with the HIF-1β forming an active heterodimer that translocates into the nucleus and binds to hypoxia-response elements (HRE) in promoter regions of the target genes (collagen I/III, TIMP-1, proliferating nuclear antigen (PCNA), vimentin). 2-OG, 2-oxoglutarate; Ub, ubiquitin.
Activation of the HIF system induces a cell type-dependent molecular response that has an impact on different disease outcomes. Activation of HIF-1 signaling in renal epithelial cells by hypoxia also promotes fibrosis.45 Increased expression of HIF-1 and its target genes has been detected in fibrotic areas of renal tissues microdissected from patients with diabetic and IgA nephropathy.45 Of note, ACE has been identified as a novel target of HIF-1α that binds and transactivates the ACE promoter directly. Increased Ang II in turn downregulates ACE2 protein expression as observed in later stages of hypoxia.46 This mechanism, underlying vessel remodeling in hypoxic pulmonary hypertension, can be envisaged as a functional loop to sustain Ang II-driven renal fibrosis.
Ang II causes hypoxia of the tubulointerstitium by inducing structural and functional changes of microvascular endothelium (reviewed in Nangaku and Fujita47). Loss of peritubular capillaries by Ang II is associated with tubulointerstitial fibrosis, events that well correlate with residual renal function in patients with CKD. Decline in renal oxygenation may manifest before microvascular rarefaction as a consequence of low peritubular capillary blood flow because of vasoconstrictor effect of Ang II on glomerular efferent arterioles.47 Targeting Ang II attenuates tubular hypoxia and interstitial inflammation and fibrosis in models of CKD.48, 49 Preservation of the structural integrity and luminal patency of peritubular capillaries is part of the protective effect that improves renal oxygenation.48 Increased renal cortical microvascular pO250 and decreased oxygen consumption factored by sodium transport have been claimed as additional mechanisms of renoprotection by Ang II blockade that enhance renal blood flow and glomerular filtration rate.51
CONTRIBUTION OF ANG II TO THE ACTIVATION OF FIBROBLAST–MYOFIBROBLAST PRECURSORS IN RENAL FIBROSIS
Proliferating fibroblasts and, their contractile and potentially invasive subtype, myofibroblasts are the main sources of interstitial ECM deposition during fibrogenesis. The origin of these activated cells is still a matter of debate. Evidence was provided both for EMT in tubular epithelium52 and endothelial-mesenchymal transition.53 However, fate mapping studies, using reporter genes that track fibrillar collagen-producing cells, identified pericytes rather than the epithelium as a source of scar-forming myofibroblasts in animal models of CKD.54 Pericytes are mesenchyme-derived mural cells located to the abluminal side of endothelial cells in the microvasculature. Pericyte–endothelial cell cross-talk is crucial for maintenance of stability of kidney peritubular microvasculature. Upon kidney injury, pericytes detach from endothelial cells and migrate into the interstitial space where they transdifferentiate into myofibroblasts driving fibrosis, dysangiogenesis, and ultimately microvasculature rarefaction.54
Ang II, via AT1R, stimulates migration of retinal microvascular pericytes through a mechanism involving platelet-derived growth factor-BB and TGF-β1, but not MMPs.55 TGF-β1 signaling from the injured tubular epithelium also activates pericyte–myofibroblast transition during kidney fibrosis as it occurs after UUO.54 Moreover, in the remnant kidney model upregulated TGF-β1 mRNA expression in proximal tubular cells, busy to reabsorb excess proteins, parallel peritubular accumulation of α-smooth muscle actin-positive myofibroblasts and inflammatory cells.56 Treatment with an ACE inhibitor abrogates TGF-β1-mediated induction of myofibroblast formation by limiting excess protein accumulation and interstitial inflammatory cell infiltration.56 Consistently, a combined treatment with an ACE inhibitor and an endothelin A receptor antagonist abrogates tubular damage, interstitial inflammation, and fibrosis by normalizing tubular expression of TGF-β1 and preserving peritubular capillary structure in advanced diabetic nephropathy.57
Besides pericytes, circulating bone marrow–derived fibrocytes are precursors of fibrogenic myofibroblasts. They develop as mature cells in the bone marrow and migrate as pre-differentiated collagen-producing cells into the injured kidney through a chemokine receptor-dependent mechanism.58
Ang II activates fibrocytes by increasing pro α1 chain of type I collagen and TGF-β1 mRNA expression. Infusion of Ang II in mice promotes progressive renal fibrosis by increasing the number of fibrocytes both in the kidney and in the bone marrow. These effects are reduced by AT1R blockade with valsartan and exacerbate in AT2R-null mice. Deficiency of AT2R signaling also worsens, whereas valsartan improves interstitial fibrosis in UUO mice by differently modulating the number of fibrocytes in the bone marrow and their recruitment and activation in the injured kidney.59 Blocking fibrocyte to myofibroblast differentiation by angiotensin receptor blocker might have therapeutic potential in patients with CKD where the number of fibrocytes infiltrating the renal interstitium correlates with the severity of tubulointerstitial lesions.59
CONCLUSIONS
Targeting Ang II by AT1R blockers or ACE inhibitors as mono or multimodal therapy halts the progression of CKDs toward end-stage renal failure. Regression of proteinuria confers protection from tubular injury, interstitial inflammation, and fibrosis in advanced stage of the disease. TGF-β1 and ROS are the key mediators of the pro-fibrotic effects of Ang II that involve activation and transdifferentiation of renal resident or circulating bone marrow–derived fibrogenic precursor cells and the regulation of intracellular pathways responsible for induction and stabilization of ECM proteins. Among signaling, miRNA and the PHD-HIF axis have been identified as new potential therapeutic targets of Ang II antagonism.
Acknowledgments
We thank Dr Antonella Piccinelli for advice in preparing the figures. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
GR has consultancy agreements with Alexion Pharmaceuticals, Reata Pharmaceuticals, Bayer Healthcare, and Novartis Pharma and is a member of the Abbvie Atrasentan Steering Committee; GR does not accept personal remuneration; compensations are paid to his institution for research and educational activities. GR has received lecture fees from Novartis Pharma. AB has received consulting fees from AbbVie and Chiesi Farmaceutici. The remaining author declared no competing financial interests.
References
- Jones RH, Hayakawa H, Mackay JD, et al. Progression of diabetic nephropathy. Lancet. 1979;1:1105–1106. doi: 10.1016/s0140-6736(79)91788-4. [DOI] [PubMed] [Google Scholar]
- Platt R. Structural and functional adaptation in renal failure. Br Med J. 1952;1:1372–1377. doi: 10.1136/bmj.1.4773.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter TH, Olson JL, Rennke HG, et al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- Bohrer MP, Deen WM, Robertson CR, et al. Mechanism of angiotensin II-induced proteinuria in the rat. Am J Physiol. 1977;233:F13–F21. doi: 10.1152/ajprenal.1977.233.1.F13. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Petermann AT, Hiromura K, et al. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- Macconi D, Abbate M, Morigi M, et al. Permselective dysfunction of podocyte-podocyte contact upon angiotensin II unravels the molecular target for renoprotective intervention. Am J Pathol. 2006;168:1073–1085. doi: 10.2353/ajpath.2006.050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbate M, Macconi D, Remuzzi G, et al. Role of proteinuria in the progression of renal diseaseIn: Alpern RJ, Caplan MJ, Moe OW (eds)Seldin and Giebisch's The Kidney—Physiology and Pathophysiology5th edn.Academic Press: Oxford, UK; 20132961–2983. [Google Scholar]
- Mezzano SA, Droguett MA, Burgos ME, et al. Overexpression of chemokines, fibrogenic cytokines, and myofibroblasts in human membranous nephropathy. Kidney Int. 2000;57:147–158. doi: 10.1046/j.1523-1755.2000.00830.x. [DOI] [PubMed] [Google Scholar]
- Mezzano SA, Barria M, Droguett MA, et al. Tubular NF-kappaB and AP-1 activation in human proteinuric renal disease. Kidney Int. 2001;60:1366–1377. doi: 10.1046/j.1523-1755.2001.00941.x. [DOI] [PubMed] [Google Scholar]
- Abbate M, Zoja C, Corna D, et al. Complement-mediated dysfunction of glomerular filtration barrier accelerates progressive renal injury. J Am Soc Nephrol. 2008;19:1158–1167. doi: 10.1681/ASN.2007060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macconi D, Chiabrando C, Schiarea S, et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol. 2009;20:123–130. doi: 10.1681/ASN.2007111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Gagliardini E, Remuzzi A, et al. Angiotensin-converting enzyme inhibition prevents glomerular-tubule disconnection and atrophy in passive Heymann nephritis, an effect not observed with a calcium antagonist. Am J Pathol. 2001;159:1743–1750. doi: 10.1016/s0002-9440(10)63021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert C, Brodbeck K, Klopfer K, et al. Angiotensin II induces human TGF-beta 1 promoter activation: similarity to hyperglycaemia. Diabetologia. 2002;45:890–898. doi: 10.1007/s00125-002-0843-4. [DOI] [PubMed] [Google Scholar]
- Wolf G, Zahner G, Schroeder R, et al. Transforming growth factor beta mediates the angiotensin-II-induced stimulation of collagen type IV synthesis in cultured murine proximal tubular cells. Nephrol Dial Transplant. 1996;11:263–269. doi: 10.1093/oxfordjournals.ndt.a027251. [DOI] [PubMed] [Google Scholar]
- Fintha A, Sebe A, Masszi A, et al. Angiotensin II activates plasminogen activator inhibitor-I promoter in renal tubular epithelial cells via the AT1 receptor. Acta Physiol Hung. 2007;94:19–30. doi: 10.1556/APhysiol.94.2007.1-2.4. [DOI] [PubMed] [Google Scholar]
- Wei X, Xia Y, Li F, et al. Kindlin-2 mediates activation of TGF-beta/Smad signaling and renal fibrosis. J Am Soc Nephrol. 2013;24:1387–1398. doi: 10.1681/ASN.2012101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarakoon R, Overstreet JM, Higgins PJ. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–268. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarakoon R, Overstreet JM, Higgins SP, et al. TGF-beta1 —> SMAD/p53/USF2 --> PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res. 2012;347:117–128. doi: 10.1007/s00441-011-1181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macconi D. Targeting the renin angiotensin system for remission/regression of chronic kidney disease. Histol Histopathol. 2010;25:655–668. doi: 10.14670/HH-25.655. [DOI] [PubMed] [Google Scholar]
- Remuzzi A, Gagliardini E, Sangalli F, et al. ACE inhibition reduces glomerulosclerosis and regenerates glomerular tissue in a model of progressive renal disease. Kidney Int. 2006;69:1124–1130. doi: 10.1038/sj.ki.5000060. [DOI] [PubMed] [Google Scholar]
- Benigni A, Zoja C, Corna D, et al. Add-on anti-TGF-beta antibody to ACE inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol. 2003;14:1816–1824. doi: 10.1097/01.asn.0000074238.61967.b7. [DOI] [PubMed] [Google Scholar]
- Trachtman H, Fervenza FC, Gipson DS, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macconi D, Tomasoni S, Romagnani P, et al. MicroRNA-324-3p promotes renal fibrosis and is a target of ACE inhibition. J Am Soc Nephrol. 2012;23:1496–1505. doi: 10.1681/ASN.2011121144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavasin MA, Liao TD, Yang XP, et al. Decreased endogenous levels of Ac-SDKP promote organ fibrosis. Hypertension. 2007;50:130–136. doi: 10.1161/HYPERTENSIONAHA.106.084103. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Chun B, Potthoff SA, et al. Thymosin beta4 and its degradation product, Ac-SDKP, are novel reparative factors in renal fibrosis. Kidney Int. 2013;84:1165–1175. doi: 10.1038/ki.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata M, Taniguchi H, Koya D, et al. N-acetyl-seryl-aspartyl-lysyl-proline ameliorates the progression of renal dysfunction and fibrosis in WKY rats with established anti-glomerular basement membrane nephritis. J Am Soc Nephrol. 2006;17:674–685. doi: 10.1681/ASN.2005040385. [DOI] [PubMed] [Google Scholar]
- Zhong X, Chung AC, Chen HY, et al. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol. 2011;22:1668–1681. doi: 10.1681/ASN.2010111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau BN, Xin C, Hartner J, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4:121ra118. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Chung AC, Chen HY, et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56:663–674. doi: 10.1007/s00125-012-2804-x. [DOI] [PubMed] [Google Scholar]
- Glowacki F, Savary G, Gnemmi V, et al. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS ONE. 2013;8:e58014. doi: 10.1371/journal.pone.0058014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Chung AC, Huang XR, et al. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Komers R, Carew R, et al. Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol. 2012;23:252–265. doi: 10.1681/ASN.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Jiang L, Zhou Y, et al. The miR-200 family regulates TGF-beta1-induced renal tubular epithelial to mesenchymal transition through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J Physiol Renal Physiol. 2012;302:F369–F379. doi: 10.1152/ajprenal.00268.2011. [DOI] [PubMed] [Google Scholar]
- Wang B, Koh P, Winbanks C, et al. miR-200a Prevents renal fibrogenesis through repression of TGF-beta2 expression. Diabetes. 2011;60:280–287. doi: 10.2337/db10-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen JK, Harris RC. Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol Cell Biol. 2012;32:981–991. doi: 10.1128/MCB.06410-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeek M, Callera G, Montezano A, et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2010;299:F1348–F1358. doi: 10.1152/ajprenal.00028.2010. [DOI] [PubMed] [Google Scholar]
- Brezniceanu ML, Liu F, Wei CC, et al. Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes. 2008;57:451–459. doi: 10.2337/db07-0013. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lo CS, Chenier I, et al. Overexpression of catalase prevents hypertension and tubulointerstitial fibrosis and normalization of renal angiotensin-converting enzyme-2 expression in Akita mice. Am J Physiol Renal Physiol. 2013;304:F1335–F1346. doi: 10.1152/ajprenal.00405.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Guarente L. The SirT3 divining rod points to oxidative stress. Mol Cell. 2011;42:561–568. doi: 10.1016/j.molcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Emerling BM, Ricoult SJ, et al. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–2996. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benigni A, Corna D, Zoja C, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten DA, Lafleur VN, Robitaille GA, et al. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Mol Biol Cell. 2010;21:3247–3257. doi: 10.1091/mbc.E10-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tang L, Zhu Q, et al. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int. 2011;79:300–310. doi: 10.1038/ki.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Yi R, Yang B, et al. Valsartan inhibited HIF-1alpha pathway and attenuated renal interstitial fibrosis in streptozotocin-diabetic rats. Diabetes Res Clin Pract. 2012;97:125–131. doi: 10.1016/j.diabres.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wu Y, Zhao M, et al. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- Nangaku M, Fujita T. Activation of the renin-angiotensin system and chronic hypoxia of the kidney. Hypertens Res. 2008;31:175–184. doi: 10.1291/hypres.31.175. [DOI] [PubMed] [Google Scholar]
- Manotham K, Tanaka T, Matsumoto M, et al. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277–1288. doi: 10.1097/01.asn.0000125614.35046.10. [DOI] [PubMed] [Google Scholar]
- Wiggins KJ, Tiauw V, Zhang Y, et al. Perindopril attenuates tubular hypoxia and inflammation in an experimental model of diabetic nephropathy in transgenic Ren-2 rats. Nephrology (Carlton) 2008;13:721–729. doi: 10.1111/j.1440-1797.2008.01008.x. [DOI] [PubMed] [Google Scholar]
- Norman JT, Stidwill R, Singer M, et al. Angiotensin II blockade augments renal cortical microvascular pO2 indicating a novel, potentially renoprotective action. Nephron Physiol. 2003;94:p39–p46. doi: 10.1159/000071289. [DOI] [PubMed] [Google Scholar]
- Deng A, Arndt MA, Satriano J, et al. Renal protection in chronic kidney disease: hypoxia-inducible factor activation vs. angiotensin II blockade. Am J Physiol Renal Physiol. 2010;299:F1365–F1373. doi: 10.1152/ajprenal.00153.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanholle G, Ligresti G, Gharib SA, et al. Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol. 2013;304:C591–C603. doi: 10.1152/ajpcell.00414.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal JA, Scicli GM, Carbini LA, et al. Angiotensin II stimulates migration of retinal microvascular pericytes: involvement of TGF-beta and PDGF-BB. Am J Physiol Heart Circ Physiol. 2002;282:H739–H748. doi: 10.1152/ajpheart.00656.2001. [DOI] [PubMed] [Google Scholar]
- Abbate M, Zoja C, Rottoli D, et al. Proximal tubular cells promote fibrogenesis by TGF-beta1-mediated induction of peritubular myofibroblasts. Kidney Int. 2002;61:2066–2077. doi: 10.1046/j.1523-1755.2002.00380.x. [DOI] [PubMed] [Google Scholar]
- Gagliardini E, Corna D, Zoja C, et al. Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol. 2009;297:F1448–F1456. doi: 10.1152/ajprenal.00340.2009. [DOI] [PubMed] [Google Scholar]
- Reich B, Schmidbauer K, Rodriguez Gomez M, et al. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int. 2013;84:78–89. doi: 10.1038/ki.2013.84. [DOI] [PubMed] [Google Scholar]
- Wada T, Sakai N, Sakai Y, et al. Involvement of bone-marrow-derived cells in kidney fibrosis. Clin Exp Nephrol. 2011;15:8–13. doi: 10.1007/s10157-010-0372-2. [DOI] [PubMed] [Google Scholar]