Abstract
Renal fibrosis is the hallmark of chronic kidney disease progression and is characterized by an exaggerated wound-healing process with the production of renal scar tissue. It comprises both the glomerular and the tubulointerstitial compartments. Among the factors that contribute to kidney fibrosis, the members of the platelet-derived growth factor (PDGF) family are among the best characterized ones. They appear to be the key factors in driving renal fibrosis, independent of the underlying kidney disease. The PDGF family consists of four isoforms (PDGF-A, -B, -C, and -D) and two receptor chains (PDGFR-α and -β), which are constitutively or inducibly expressed in most renal cells. These components have an irreplaceable role in kidney development by recruitment of mesenchymal cells to the glomerular and tubulointerstitial compartments. They further regulate multiple pathophysiologic processes including cell proliferation, cell migration, expression and accumulation of extracellular matrix, production and secretion of pro- and anti-inflammatory mediators, vascular permeability, and hemodynamics. This review provides a brief update on the role of different PDGF isoforms in the development of glomerulosclerosis and tubulointerstitial fibrosis, newly identified endogeneous PDGF antagonists, and resulting potential therapies.
Keywords: extracellular matrix, glomerulosclerosis, mesangial cell, mesenchymal cells, myofibroblast
Nearly all progressive renal diseases funnel into renal fibrosis as a final common pathway and subsequently lead to end-stage renal disease. Apart from the medical consequences, this is accompanied by an immense economic and social burden. Fibrotic kidneys are characterized by glomerulosclerosis, tubular atrophy and dilatation, tubulointerstitial fibrosis, and rarefaction of glomerular as well as peritubular capillaries.1 Similarities between the fibrogenic mechanisms, independent of the underlying disease and by involving nearly all renal cells, make these processes an attractive therapeutic target to halt or even induce regression of renal fibrosis. Meanwhile, a huge number of molecules with pro- or antifibrotic properties in the kidney have been identified.2 In numerous studies performed during the last 15–20 years, it has been established that platelet-derived growth factors (PDGFs) have a crucial role in driving these processes that ultimate lead to fibrosis, especially in the kidney but also in other organs.3 Moreover, PDGFs and their receptors have been demonstrated to be important in embryonic development, angiogenesis, and hematopoiesis.4 They have also been implicated in many other diseases, such as autocrine and paracrine PDGF signaling in cancer.5 Besides its role in fibrotic diseases, PDGFs drive pathological mesenchymal responses in vascular disorders and retinal diseases.4 Therein, the main cellular processes regulated by PDGFs are proliferation, differentiation, and migration of PDGF receptor (PDGFR)-expressing mesenchymal cells in physiological and pathological processes.
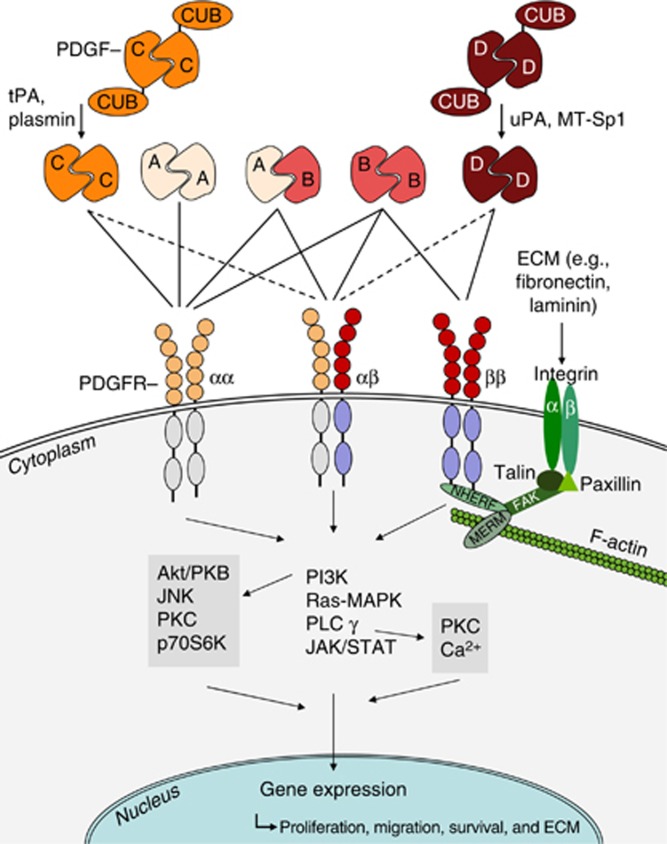
The PDGFs consist of highly conserved disulfide-linked homo- and heterodimeric growth factors (Figure 1, PDGF-AA, -AB, -BB, -CC, and -DD), which are structurally and functionally related to a larger growth factor family including vascular endothelial growth factor.3, 6 All isoforms are produced as inactive precursors. However, in contrast to PDGF-A and -B, which are activated already intracellularly by furin-like proteases, the isoforms PDGF-C and -D are secreted in a latent form with an N-terminal CUB (complement C1r/C1s, Uegf, Bmp1) domain.4 The CUB domain needs to be extracellularly cleaved before the ligand can bind and activate its receptor. For CUB cleavage, different proteases have been identified, for example, the tissue-type plasminogen activator for PDGF-C or urokinase-type plasminogen activator for PDGF-D, but also others may be of physiological importance (Figure 1).3 PDGFRs are composed of PDGFR-α and/or -β chains, which dimerize upon ligand-binding (Figure 1). Whereas PDGF-AA binds to the PDGFR-αα-dimer only, PDGF-BB is a ligand for all receptors. The core domain of PDGF-CC binds to PDGFR-αα and -αβ, whereas PDGF-DD predominantly binds to PDGFR-ββ. Downstream signaling events for activated PDGFRs are similar, but not identical. PDGF binding results in autophosphorylation of the cytoplasmic tyrosine kinase domain of the PDGF receptor chains and subsequently recruits adaptor proteins carrying SH2 and SH3 domains to this site. Downstream signaling occurs mainly via the JAK/STAT, phosphoinositide 3-kinase, PLC-γ, or RAS–MAPK (mitogen-activated protein kinase) pathways, promoting gene expression and mediating the biological functions of the PDGF isoforms, for example, proliferation, migration, and survival. A highly relevant pro-fibrotic cross-talk is the cooperation of PDGF and integrin signaling. Na+/H+ exchanger regulatory factors were shown to link PDGFR-β with focal adhesion kinase and the cortical actin cytoskeleton, thereby enhancing PDGF-induced MAPK signaling. It is suggested that specificity of PDGFR signaling is achieved through a combination of cell type–specific expression and differential engagement of further downstream signaling pathways. For more details concerning PDGFR signaling, the reader is referred to Figure 1 and other reviews.3, 4, 7
Figure 1.
Simplified scheme of the main molecules involved in PDGF–PDGFR interactions. PDGF -AA, -AB, and -BB are secreted in an active form, whereas PDGF-CC and -DD have to be proteolytically cleaved to allow binding of the ligands to their receptors. Proteases known to split off the PDGF-CUB domains are tPA or plasmin (PDGF-CC) and uPA or MT-Sp1 (PDGF-DD). For simplification of the scheme, many other regulatory processes, for example, processes limiting the PDGF response, are not included but mentioned in the text. Also not shown are the transactivation processes of PDGFRs without ligand binding by, for example, G-protein-coupled receptors. Akt/PKB, protein kinase B; CUB, complement C1r/C1s, Uegf, Bmp1; ECM, extracellular matrix; FAK, focal adhesion kinase; JAK, janus kinase; JNK, c-Jun amino-terminal kinase; MAPK, mitogen-activated protein kinase; MERM, merlin and ezrin/radixin/moezin family of cytoskeletal linkers; MT-Sp1, Matriptase; NHERF, Na+/H+ exchanger regulatory factor; p70S6K, ribosomal protein S6 kinase beta-1; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; PI3K, phosphatidyl-inositol-3-kinase; PKC, protein kinase C; PLC-γ, phospholipase C-γ RAS, rat sarcoma; STAT, signal transducers and activators of transcription; tPA, tissue-type plasminogen activator; uPA, urokinase-type plasminogen activator. Figure with kind permission from Springer Science+Business Media (license date 12 September 2013) Ostendorf et al.3
All PDGF isoforms and their receptors are expressed early during kidney development, and all PDGF-A-, -B-, -C-, PDGFR-β-, or PDGFR-α-deficient mice die prenatally or very early during postnatal life.6 For PDGF-C-deficient mice, this phenotype depends on the genetic background. Only double PDGF-A/C-deficient mice reproduce the phenotype of PDGFR-alpha-null mice, which is characterized by the lack of the renal interstitial mesenchyme. In contrast, PDGF-B- and PDGFR-β-deficient mice are mainly characterized by dysfunctional glomeruli with a specific lack of the mesangium. PDGF-D-deficient mice have not been described so far (reviewed in Floege et al.6).
RENAL EXPRESSION OF PDGFs AND THEIR RECEPTORS IN HEALTH AND DISEASE
The expression of PDGF ligands and receptors in normal postnatal human and rodent kidneys is well documented in many studies and has been previously reviewed by us in detail.6 Both receptor chains are constitutively expressed by mesangial cells, fibroblasts, and vascular smooth muscle cells, but not by epithelial cells, such as visceral epithelial cells (podocytes) and tubular cells. Focal PDGFR-β expression, however, was reported for parietal epithelial cells,6 and we demonstrated PDGFR-α expression by glomerular endothelial cells in vitro.8 Renal expression of the PDGF ligands is less well defined, which is because of the variability of immunohistochemical approaches, species differences, and lack of reporter mice. Constitutive PDGF-A expression is detected in podocytes and epithelial cells of the distal nephron, whereas low levels of PDGF-B may be present in mesangial cells in normal mature glomeruli. PDGF-C and -D expression patterns reveal differences between species, for example, in rats PDGF-C is localized to arterial smooth muscle cells and epithelial cells of the collecting duct,9 whereas in humans it has been localized to parietal epithelial cells, distal tubular epithelial cells, and arterial endothelial cells.10 In humans, renal PDGF-D is constitutively expressed in podocytes and vascular smooth muscle cells, whereas expression in the rat kidney is restricted to vascular smooth muscle cells. PDGF-D expression in the mouse glomerulus is limited to mesangial cells.11, 12, 13
During kidney disease, de novo or upregulated renal expression of all PDGF isoforms and their receptors has been described in nearly all rodent kidney injury models and their corresponding human renal diseases.6 PDGF-A is upregulated in mesangial cells in models of mesangioproliferative glomerulonephritis and in endothelial cells and smooth muscle actin cells during human vascular transplant rejection.6 PDGF-C is inducibly expressed by interstitial cells and macrophages, explicitly at sites of tubulointerstitial fibrosis.9, 10 The ligand expression therefore localizes near to those areas with increased PDGFR-α expression, that is, tubulointerstitial smooth muscle cells and myofibroblasts.6, 9, 10 Infiltrating, PDGF-C-expressing macrophages have been shown to be important for glomerular crescent formation in experimental lupus nephritis, and induced upregulation of PDGF-C in mesangial cells and podocytes has been reported for several human and experimental renal diseases.6, 14 Many studies describe the upregulation and/or de novo expression of PDGF-B in mesangial cells, podocytes, vascular smooth muscle cells, tubular cells, and interstitial cells in animal models and human renal diseases, whereas PDGF-D as the second high-affinity ligand of PDGFR-β is less well characterized.6 A de novo expression of PDGF-D, however, was demonstrated in tubulointerstitial cells in fibrotic areas of experimental and human obstructive nephropathy, also correlating with induced PDGF-B- and PDGFR-β expression.13 Overexpression of PDGF-D has been further demonstrated in alpha smooth muscle actin-expressing cells of arterial neointimas and by interstitial cells during human chronic allograft nephropathy.15 In the acute phase of experimental mesangioproliferative glomerulonephritis, PDGF-D is upregulated in the mesangium,12 and IgA nephropathy patients have elevated systemic PDGF-DD levels.16 Upregulation of PDGFR-β during experimental and human renal diseases has been detected in mesangial cells, parietal epithelial cells, endothelial cells, tubular cells, and interstitial cells.6
ENDOGENEOUS SILENCING OF PDGFR SIGNALING
The main PDGF/PDGFR signaling pathways are briefly outlined in Figure 1 and reviewed in detail elsewhere.3, 4, 7 Once activated, the fine tuning and efficacy of PDGFR signaling is modulated through several regulatory processes and endogenous antagonists (also reviewed in van Roeyen et al.17). These processes operate on several levels. First, by activation/inactivation of the ligands, for example, PDGF-C can be downregulated by its own CUB domain. Once cleaved, CUB can bind to the protease tissue-type plasminogen activator and inhibits further PDGF-C activation.17 Proteases such as matriptase and factor VII-activating protease cleave PDGF-D and PDGF-B within their growth factor domain, respectively, and inhibit the binding of these ligands to their receptors. Furthermore, the binding of PDGF to the α2-macroglobulin receptor/low-density lipoprotein receptor-related protein leads to inactivation of the ligands by lysosomal proteinases.17 Second, direct interactions with PDGFRs further modify signaling. For example, extracellular SPARC (secreted protein, acidic and rich in cysteine/BM40/osteonectin) directly inhibits the binding of PDGF-BB and -AB to their receptors,18 and extracellular protein laminin α4 has been demonstrated to be an inhibitor of PDGFR-β mRNA expression in mesangial cells.19 Finally, PDGFR-β signaling is switched off by the autoinhibitory activity of the C-terminal region of the cytoplasmic receptor domain in the absence of its ligand.20 Third, endogeneous regulation of PDGFR signaling can occur via interference with PDGFR-induced downstream signaling pathways, for example, with the MAPK pathway or the phosphoinositide 3-kinase pathway (reviewed in van Roeyen et al.17).
Two recently identified endogenous inhibitors of PDGF-B/D signaling are the nephroblastoma-overexpressed gene (NOV, CCN3) and growth arrest–specific protein-1 (GAS1). Both have been identified as PDGF-B/D-downregulated proteins and both are overexpressed in growth-arrested mesangial cells.21, 22 NOV/CCN3 expression in human necrotizing glomerulonephritis correlated negatively with glomerular cell proliferation.21 Importantly, our recent studies with systemic overexpression of NOV and soluble GAS1 in rats with mesangioproliferative glomerulonephrits (anti-Thy 1.1 model) demonstrated the therapeutic potential of both inhibitors by limiting mesangial cell activation, proliferation, and production of extracellular matrix in this model.22, 23 In addition, NOV overexpression at the same time led to an accelerated glomerular endothelial recovery in the acute phase of the disease.23 At which level NOV and GAS1 act on PDGF/PDGFR signaling is not conclusively resolved. However, NOV overexpression in the anti-Thy 1.1 model in vivo reduced glomerular transcript and protein expression of PDGFR-β, whereas overexpression of soluble GAS1 in mesangial cells reduced the expression of PDGF-B transcripts.22, 23
PDGFS AND RENAL FIBROSIS
Both high-affinity ligands of PDGFR-β, PDGF-B, and -D hold central roles for the activation of the mesangium and the development of glomerulosclerosis (that is, ‘glomerular fibrosis'). This has been impressively documented in many in vivo studies, for example, by infusion of recombinant PDGF-BB in healthy rats and in rats with pre-existing minor subclinical mesangial injury, by infusion of subclinical dosages of PDGF-BB in hyperglycemic rats or by in vivo gene transfer of PDGF-B cDNA in healthy rats and mice.6 Furthermore, at very high dosages, PDGF-BB infusion into rats induced renal tubulointerstitial cell proliferation, myofibroblast formation, and fibrosis.24 Similarly, transfection of mice with adenoviral constructs encoding PDGF-D led to severe mesangial proliferative glomerulopathy, and transgenic mice overexpressing PDGF-D specifically in podocytes developed mesangioproliferative lesions, crescentic glomerulonephritis, and substantial tubulointerstitial damage.25, 26 Vice versa, specific interventions in PDGF-B/-D-mediated PDGFR-β signaling reduced mesangial cell proliferation and matrix accumulation in the rat anti-Thy 1.1 mesangioproliferative glomerulonephritis without effects on glomerular TGF-β signaling following PDGF-B antagonism. PDGF-B therefore may act downstream or independent of TGF-β and obviously represents a specific target to treat mesangioproliferative disorders with subsequent fibrotic changes (reviewed in Ostendorf et al.3, 6 Floege et al.3, 6). Importantly, in a progressive variant of the anti-Thy 1.1 glomerulonephritis model, in rats, transient and specific blockade of either PDGF-B or PDGF-D during the acute mesangioproliferative phase prevented the development of renal failure and glomerular as well as tubulointerstitial fibrosis at late stages of the disease.3, 6 At least PDGF-D has been demonstrated to have also a direct pro-fibrotic effect on the cortical tubulointerstitium, possibly by miR-200-regulated tubular epithelial cell dedifferentiation.27, 28 In contrast to the beneficial effects of PDGF-B antagonism in most models of renal disease, the nonspecific PDGF antagonist trapidil worsened experimental acute kidney injury, which points to a potential role of PDGF-B in the repair of acute renal cell damage.3, 6 In terms of fibrotic changes following ischemia/reperfusion-induced acute kidney injury in mice, one study reported beneficial effects by treatment with the tyrosine kinase inhibitor imatinib with only limited specificity for PDGFRs.29 Further studies using more specific approaches in different phases of acute kidney injury are needed to further clarify a potential safety issue connected with PDGF antagonism in this disease. Reduction of renal fibrosis has been demonstrated in mice with unilateral ureteral obstruction (UUO)-induced renal fibrosis following treatment with PDGFR-α and/or PDGFR-β-antibodies, interestingly without additive effects.29 PDGF receptor tyrosine kinase blockers such as the above-mentioned imatinib have often been evaluated in models of renal diseases; however, these inhibitors normally exhibit only restricted specificity for PDGF receptors.6 Despite their beneficial effects on glomerular and tubulointerstitial changes in several animal models, for example, experimental mesangioproliferative glomerulonephritis, diabetic nephropathy, lupus nephritis, chronic allograft nephropathy, and unilateral ureteral obstruction, multitargeted tyrosine kinase receptor inhibitors have potential nephrotoxic or, as has been demonstrated for imatinib, significant myocardial or hematopoietic side effects. At present, this prevents the widespread and long-term clinical use of such reagents to treat renal fibrosis.6 In renal fibrosis induced by UUO in rats, it has been demonstrated that most of the antifibrotic effects of imatinib result from the blocking of c-Abl kinase, rather than from blocking PDGF receptors.30 Transgenic mice that constitutively overexpress soluble PDGFR-β (that is, a PDGF-B and -D antagonist) during late embryogenesis show no phenotype, and mice with postnatal deletion of PDGFR-β, suggesting that PDGF-B and -D are not required during normal adult life.3, 6 These data indicate that specific antagonism of PDGF-B and -D, with the aim of halting the progression of glomerulosclerosis and tubulointerstitial fibrosis, may be both safe and effective.
Cell types involved in renal interstitial and glomerular fibrotic lesions, mainly myofibroblasts, mesangial cells, or smooth muscle cells, overexpress PDGFR-α that binds PDGF-A, -B, and -C with high affinity.3, 6 Application of PDGF-A chain antisense oligonucleotides improved arterial and renal damages in stroke-prone spontaneously hypertensive rats, suggesting a certain pro-fibrotic potential of PDGF-A.31 However, administration of even very high doses of PDGF-A (5 mg/kg) in healthy rats had no effect on renal pathology, which rather points to a minor role of this ligand in renal fibrosis.24 In contrast, our studies in mice support a distinct role of PDGF-C for the development of tubulointerstitial fibrosis.9, 10, 32, 33 The neutralizing of PDGF-C in mice with UUO-induced renal fibrosis using specific antibodies strikingly reduced tubulointerstitial fibrotic lesions and myofibroblast activation.32 A similarly reduced renal fibrosis was obtained in PDGF-C-deficient mice, when compared with wild-type littermates in the same model.32 In contrast to the different roles of PDGF-A and PDGF-C for the development of renal fibrosis, a recent proteomic approach to identify differentially regulated proteins in cultured rat renal fibroblasts following stimulation with either PDGF-A or -C did not yield gross differences.34 Surprisingly, and in contrast to the results in kidney fibrosis, PDGF-C neutralization or deficiency did not protect mice from bile duct ligation-induced liver fibrosis.33 Further analyses revealed a compensatory hepatic upregulation and activation of PDGFR-β in bile duct ligation upon PDGF-C neutralization, which obviously accounts for the observed differences between the kidney and the liver.33 By infusion of recombinant PDGF-C- or PDGF-C-neutralizing antibodies, PDGF-C has been further identified as a potent pro-angiogenic factor, having an important role in glomerular capillary healing, for example, in the early mesangiolytic phase of mesangioproliferative glomerulonephritis in rats and in a model of thrombotic microangiopathy in mice.8 It is presently unknown whether PDGF-C also affects peritubular capillaries. In contrast to PDGFR-β signaling, the absence of mesangial pathology upon PDGF-C infusion in the rat anti-Thy 1.1 model or upon systemic overexpression of PDGF-C in mice demonstrate that PDGFR-α signaling is obviously of minor relevance for the activation of the mesangium.8, 25
PERSPECTIVES
Among all known growth factor systems, the PDGF/PDGFR system is now among the best characterized in human and experimental renal diseases. On the basis of the wealth of existing studies and databases, it becomes clear that PDGFs and their receptors hold the key functions in the development of glomerulosclerosis and tubulointerstitial fibrosis, which clearly justifies proceeding with further clinical trials. Consequently, the specific targeting of PDGF-B- and/or -D-mediated activation of the mesangium, for example, in IgA nephropathy or diabetic nephropathy, is the next step. A phase-I study of a human monoclonal anti-PDGF-D antibody in healthy volunteers was safe and well tolerated.35 In addition, the basis for trials targeting particular PDGF-B, -C, and -D in tubulointerstitial fibrosis is steadily increasing. For the latter, reliable and non-invasive imaging techniques and suitable biomarkers need to be developed. We believe that specific blockade of PDGFs is preferable to more nonspecific antagonists such as tyrosine kinase blockers. Although many of these have already been approved for cancer treatment, they have a rather broad mode of action and correspondingly frequent side effects.
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft to TO, PB, CRCvR, and JF (SFB/TRR57: P17, P25; DFG: BO3755/2-1; DFG: RO4036/1) and from the Interdisciplinary Centre for Clinical Research within the Faculty of Medicine at the RWTH Aachen University to TO and JF (E6-10). This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
CRCvR and JF hold a patent on the use of anti-PDGF antibodies in nephritis. The remaining authors declared no competing interests.
References
- Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–656. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- Boor P, Sebekova K, Ostendorf T, et al. Treatment targets in renal fibrosis. Nephrol Dial Transplant. 2007;22:3391–3407. doi: 10.1093/ndt/gfm393. [DOI] [PubMed] [Google Scholar]
- Ostendorf T, Eitner F, Floege J. The PDGF family in renal fibrosis. Pediatr Nephrol. 2012;27:1041–1050. doi: 10.1007/s00467-011-1892-z. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jechlinger M, Sommer A, Moriggl R, et al. Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest. 2006;116:1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Boor P, van Roeyen CR, Kunter U, et al. PDGF-C mediates glomerular capillary repair. Am J Pathol. 2010;177:58–69. doi: 10.2353/ajpath.2010.091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitner F, Ostendorf T, Van Roeyen C, et al. Expression of a novel PDGF isoform, PDGF-C, in normal and diseased rat kidney. J Am Soc Nephrol. 2002;13:910–917. doi: 10.1681/ASN.V134910. [DOI] [PubMed] [Google Scholar]
- Eitner F, Ostendorf T, Kretzler M, et al. PDGF-C expression in the developing and normal adult human kidney and in glomerular diseases. J Am Soc Nephrol. 2003;14:1145–1153. doi: 10.1097/01.asn.0000062964.75006.a8. [DOI] [PubMed] [Google Scholar]
- Changsirikulchai S, Hudkins KL, Goodpaster TA, et al. Platelet-derived growth factor-D expression in developing and mature human kidneys. Kidney Int. 2002;62:2043–2054. doi: 10.1046/j.1523-1755.2002.00662.x. [DOI] [PubMed] [Google Scholar]
- Ostendorf T, van Roeyen CR, Peterson JD, et al. A fully human monoclonal antibody (CR002) identifies PDGF-D as a novel mediator of mesangioproliferative glomerulonephritis. J Am Soc Nephrol. 2003;14:2237–2247. doi: 10.1097/01.asn.0000083393.00959.02. [DOI] [PubMed] [Google Scholar]
- Taneda S, Hudkins KL, Topouzis S, et al. Obstructive uropathy in mice and humans: potential role for PDGF-D in the progression of tubulointerstitial injury. J Am Soc Nephrol. 2003;14:2544–2555. doi: 10.1097/01.asn.0000089828.73014.c8. [DOI] [PubMed] [Google Scholar]
- Triantafyllopoulou A, Franzke CW, Seshan SV, et al. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci USA. 2010;107:3012–3017. doi: 10.1073/pnas.0914902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Changsirikulchai S, Hudkins KL, et al. Identification of platelet-derived growth factor D in human chronic allograft nephropathy. Hum Pathol. 2008;39:393–402. doi: 10.1016/j.humpath.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boor P, Eitner F, Cohen CD, et al. Patients with IgA nephropathy exhibit high systemic PDGF-DD levels. Nephrol Dial Transplant. 2009;24:2755–2762. doi: 10.1093/ndt/gfp152. [DOI] [PubMed] [Google Scholar]
- van Roeyen CR, Ostendorf T, Floege J. The platelet-derived growth factor system in renal disease: an emerging role of endogenous inhibitors. Eur J Cell Biol. 2012;91:542–551. doi: 10.1016/j.ejcb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Raines EW, Lane TF, Iruela-Arispe ML, et al. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci USA. 1992;89:1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrass CK, Hansen KM, Patton BL. Laminin alpha4-null mutant mice develop chronic kidney disease with persistent overexpression of platelet-derived growth factor. Am J Pathol. 2010;176:839–849. doi: 10.2353/ajpath.2010.090570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara F, Bishayee S, Heldin CH, et al. Autoinhibition of the platelet-derived growth factor beta-receptor tyrosine kinase by its C-terminal tail. J Biol Chem. 2004;279:19732–19738. doi: 10.1074/jbc.M314070200. [DOI] [PubMed] [Google Scholar]
- van Roeyen CR, Eitner F, Scholl T, et al. CCN3 is a novel endogenous PDGF-regulated inhibitor of glomerular cell proliferation. Kidney Int. 2008;73:86–94. doi: 10.1038/sj.ki.5002584. [DOI] [PubMed] [Google Scholar]
- van Roeyen CR, Zok S, Pruessmeyer J, et al. Growth arrest-specific protein 1 is a novel endogenous inhibitor of glomerular cell activation and proliferation. Kidney Int. 2013;83:251–263. doi: 10.1038/ki.2012.400. [DOI] [PubMed] [Google Scholar]
- van Roeyen CR, Boor P, Borkham-Kamphorst E, et al. A novel, dual role of CCN3 in experimental glomerulonephritis: pro-angiogenic and antimesangioproliferative effects. Am J Pathol. 2012;180:1979–1990. doi: 10.1016/j.ajpath.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Tang WW, Ulich TR, Lacey DL, et al. Platelet-derived growth factor-BB induces renal tubulointerstitial myofibroblast formation and tubulointerstitial fibrosis. Am J Pathol. 1996;148:1169–1180. [PMC free article] [PubMed] [Google Scholar]
- Hudkins KL, Gilbertson DG, Carling M, et al. Exogenous PDGF-D is a potent mesangial cell mitogen and causes a severe mesangial proliferative glomerulopathy. J Am Soc Nephrol. 2004;15:286–298. doi: 10.1097/01.asn.0000108522.79652.63. [DOI] [PubMed] [Google Scholar]
- van Roeyen CR, Eitner F, Boor P, et al. Induction of progressive glomerulonephritis by podocyte-specific overexpression of platelet-derived growth factor-D. Kidney Int. 2011;80:1292–1305. doi: 10.1038/ki.2011.278. [DOI] [PubMed] [Google Scholar]
- Boor P, Konieczny A, Villa L, et al. PDGF-D inhibition by CR002 ameliorates tubulointerstitial fibrosis following experimental glomerulonephritis. Nephrol Dial Transplant. 2007;22:1323–1331. doi: 10.1093/ndt/gfl691. [DOI] [PubMed] [Google Scholar]
- Kong D, Li Y, Wang Z, et al. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Chang FC, Wu CF, et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- Wang S, Wilkes MC, Leof EB, et al. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005;19:1–11. doi: 10.1096/fj.04-2370com. [DOI] [PubMed] [Google Scholar]
- Kishioka H, Fukuda N, Wen-Yang H, et al. Effects of PDGF A-chain antisense oligodeoxynucleotides on growth of cardiovascular organs in stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2001;14:439–445. doi: 10.1016/s0895-7061(00)01290-5. [DOI] [PubMed] [Google Scholar]
- Eitner F, Bucher E, van Roeyen C, et al. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J Am Soc Nephrol. 2008;19:281–289. doi: 10.1681/ASN.2007030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin IV, Borkham-Kamphorst E, Zok S, et al. Platelet-derived growth factor (PDGF)-C neutralization reveals differential roles of PDGF receptors in liver and kidney fibrosis. Am J Pathol. 2013;182:107–117. doi: 10.1016/j.ajpath.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Seikrit C, Henkel C, van Roeyen CR, et al. Biological responses to PDGF-AA versus PDGF-CC in renal fibroblasts. Nephrol Dial Transplant. 2013;28:889–900. doi: 10.1093/ndt/gfs509. [DOI] [PubMed] [Google Scholar]
- Hawthorne T, Giot L, Blake L, et al. A phase I study of CR002, a fully-human monoclonal antibody against platelet-derived growth factor-D. Int J Clin Pharmacol Ther. 2008;46:236–244. doi: 10.5414/cpp46236. [DOI] [PubMed] [Google Scholar]