Abstract
Tubular injury has a major etiological role in fibrosis. For many years, this relationship has been dominated by the perception that epithelial cells are transformed into myofibroblasts that proliferate and generate fibrotic matrix—the so-called epithelial-to-mesenchymal transition. Here we focus on mechanisms by which injury to the tubule results in fibrosis because of paracrine mechanisms. Specific injury to the proximal tubule results in inflammation, reversible injury, and adaptive repair if the insult is mild, self-limited in time, and occurs in a background of a normal kidney. Repeated injury, in contrast, leads to maladaptive repair with sustained tubule injury, chronic inflammation, proliferation of interstitial myofibroblasts, vascular rarefaction, interstitial fibrosis, and glomerular sclerosis. During the maladaptive repair process after the renal insult, many tubular cells become arrested in the G2/M phase of the cell cycle. This results in activation of the DNA repair response with the resultant synthesis and secretion of pro-fibrotic factors. Pharmacologic interventions that enhance the movement through G2/M or facilitate apoptosis of cells that otherwise would be blocked in G2/M may reduce the development of fibrosis after kidney injury and reduce the progression of chronic kidney disease.
Keywords: acute kidney injury, apoptosis, chronic kidney disease, G2/M arrest, mitotic stress
There has been accumulating epidemiologic evidence of a causal connection between the clinical syndrome of acute kidney injury (AKI) and the subsequent development of chronic kidney disease (CKD), a hallmark of which is kidney fibrosis. Chronic processes that originate in the glomerulus or the tubulointerstitium lead to the loss of tubules, nephron atrophy, and fibrosis. In many experimental systems, it is not easy to fully characterize the effects of tubular injury as the processes of induced injury or toxins used are somewhat nonspecific and affect other tissue compartments, such as the vasculature and glomeruli, in addition to the tubules. There are a number of excellent, recent reviews on the role of acute tubular epithalial injury in progression of CKD.1, 2 In this mini review, we will focus on the consequences of highly specific injury to the proximal tubule of the kidney and the role of cell cycle arrest in the fibrotic response, which results from paracrine actions of the injured epithelial cells.
THE CONSEQUENCES OF HIGHLY SPECIFIC PROXIMAL TUBULAR INJURY ON THE INTERSTITIUM, MICROVESSELS, AND GLOMERULI OF THE KIDNEY
We generated bigenic Six2-GFPCre+;iDTR+ (DTRrec) mice in which simian diphtheria toxin receptor (DTR) expression was limited to the descendents of the metanephric mesenchyme.3 With low sublethal doses of DT, we were able to limit injury to the early proximal tubular S1 and S2 segments. DT crosses the rodent glomerular barrier into the urinary space, thus exposing the proximal tubular epithelium to the higher toxin concentrations as water is reabsorbed. Although there are DT receptors in other areas of the nephron that are derived from the metanephric mesenchyme, DT molecules that bound to DT receptors are internalized4 and hence removed from the tubular lumen. Therefore, they cannot affect subsequent parts of the nephron. With higher doses of DT, this specificity to the early parts of the proximal tubule is increasingly lost and there is damage to more distal parts of the nephron. With careful examination of the kidneys from DT-treated animals using light and electron microscopy, there were no podocyte abnormalities or other glomerular tuft pathology over the first 7 days after DT administration, although these cells derive from the metanephric mesenchyme. The reasons for the absence of podocyte injury in this model may include an insufficient deletion frequency of the STOP cassette in podocyte progenitor cells, or a lower expression strength of the Gt (ROSA)26Sor promoter, poor cell-surface expression of DTR, and/or a lower overall susceptibility to DT in murine podocytes as compared with renal tubular cells.
Administration of sublethal DT doses resulted in tubular injury with continued urine output and transient albuminuria, which is common in tubular injury. With one dose of DT there was a significant inflammatory response with attraction of inflammatory cells, including neutrophils, macrophages, and T lymphocytes. This was accompanied by proliferation and a focal moderate increase in cell numbers in the interstitium. There was a 2.5-fold (day 1) and a 14-fold (day 3) increase in tissue intercellular adhesion molecule expression, potentially reflecting endothelial cell injury secondary to the tubular injury.
Repair of the kidney after a single dose of DT was adaptive with few longer-term sequelae. There was a very robust proliferative response of the proximal tubule cells to replace the cells that had died as a result of the DT. Ultimately, the inflammation resolved and there was little, if any, residual interstitial inflammation, expansion, or matrix deposition. By contrast, after three doses of DT, administered at weekly intervals, there was maladaptive repair over time with development of a chronic interstitial infiltrate, increased myofibroblast proliferation, tubulointerstitial fibrosis, and tubular atrophy as well as an increase in serum creatinine (0.6±0.1 vs. 0.18±0.02 mg/dl in control mice) by week 5, 2 weeks after the last dose in the thrice-treated animals. There was a dramatic increase in the number of interstitial cells that expressed the PDGF receptor β+ (pericytes/perivascular fibroblasts), α-smooth actin muscle+ (myofibroblasts), FSP-1/S100A4+ (fibroblast-specific protein-1), and F4/80+ (macrophages). In addition, there was vascular rarefaction with loss of CD31/PECAM+ endothelial cells and focal global and segmental glomerulosclerosis. The extent of glomerulosclerosis strongly correlated with the degree of interstitial fibrosis/tubular atrophy and amount of albuminuria. The kidneys also had increased mRNA levels of the pro-fibrotic cytokine transforming growth factor-β1 (TGF-β1), collagen-1α1, and fibronectin. KIM-1 protein levels were elevated in both kidney sections and the urine of animals injected thrice with DT when compared with controls or animals treated only once with DT.
Thus, recurrent specific tubular injury leads to a pattern very typical of CKD in humans: tubular atrophy, interstitial chronic inflammation and fibrosis, vascular rarefaction, and glomerulosclerosis. The mechanisms involved in the development of glomerulosclerosis evoked by primary tubular injury may be multifactorial. Damage to nephron segments may lead to sloughing of cells into the lumen and tubular obstruction with progressive narrowing of the early proximal tubule near the glomerular tuft that can lead to a sclerotic atubular glomerulus similar to those which are seen with ureteral obstruction.5 There may be paracrine signaling from injured and regenerating/undifferentiated epithelium to directly have an impact on the glomerulus. Alternatively, a progressive tubulointerstitial reaction originating around atrophic and undifferentiated tubules may directly encroach upon the glomerular tuft. The loss of interstitial capillaries may lead to a progressive reduction of glomerular blood flow with ischemia to the glomerulus and to the kidney regions perfused by the post-glomerular capillaries. This speaks to the fact that primary tubular injury can trigger a response that adversely affects multiple compartments of the kidney and leads to a positive feedback process involving loss of capillaries, glomerulosclerosis, persistent ischemia, tubular atrophy, increased fibrosis, and ultimately kidney failure.
DETERMINATION OF WHETHER REPAIR IS ADAPTIVE OR MALADAPTIVE
Epithelial injury is usually heterogeneous with some nephrons being injured while adjacent ones are spared. This could be related to variability in vascular perfusion, as a given nephron does not have a single source for perfusion but is generally perfused by a number of post-glomerular capillary beds.6 Even within a given area of the nephron, cells can show signs of severe injury, whereas other adjacent cells appear to be unaffected. This cellular heterogeneity could relate to intrinsic stochastic differences in survival characteristics related to variability in preconditioning or senescence characteristics that evolve from unequal proliferative histories over time.
Injury to the kidney triggers a repair response that can be adaptive, leading to restoration of the normal epithelium, or maladaptive, leading to fibrosis and CKD (Figure 1). With adaptive repair, the injury is associated with a profound proliferative response of cells within the proximal tubule that have survived the insult. Using transplantation approaches to replace the mouse bone marrow with donor cells from a mouse in which each cell was labeled with green fluorescent protein, we demonstrated that cells restoring the integrity of the proximal tubule after ischemia did not derive from the bone marrow.7 Using genetic fate-mapping techniques, we generated transgenic mice, in which 94–95% of tubular epithelial cells, but no interstitial cells, were labeled with either β-galactosidase (lacZ) or red fluorescent protein. After injury, there was no dilution of the cell-fate marker, indicating that the cells that replaced the damaged cells derived from the resident proximal tubule cells that survived the ischemic insult.8 There is no evidence for any specialized progenitor cell population within the proximal tubule.9 During the fate-mapping experiments and in greatly expanded studies by Humphreys et al.10 it became clear that none of the labeled tubular cells entered the interstitium even with severe injury, leading to the conclusion that the expansion of the interstitial myofibroblasts did not occur because of EMT.
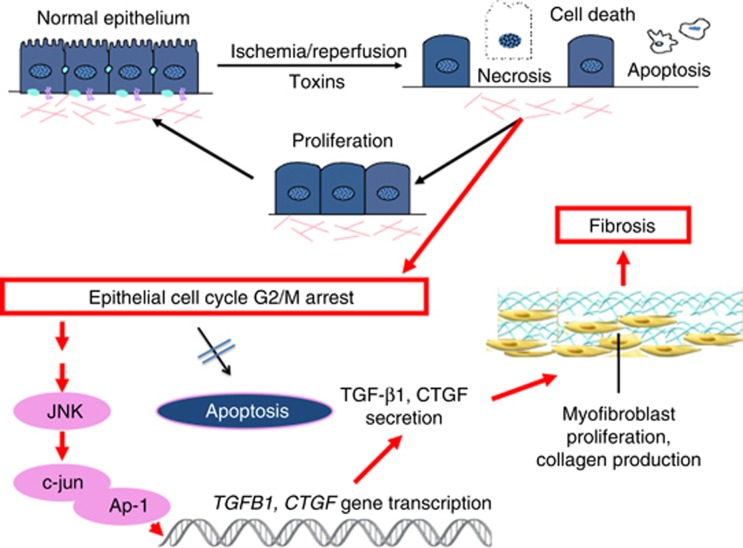
Figure 1.
Adaptive and maladaptive repair after injury. After mild injury self-limited over time and to a kidney that has not sustained prior insults of significance, damaged proximal tubule cells are replaced by surviving proximal tubule cells that dedifferentiate, proliferate, and restore the normal epithelium. By contrast, if the injury is more severe, repeated, or to a kidney that is abnormal at baseline, then the cells that are stimulated to proliferate cannot complete the cell cycle efficiently and often are arrested in G2/M. Apoptosis of these cells may be blocked so that they continue to survive. They then turn on signaling pathways involving Jun N-terminal kinase (JNK), and activate pro-fibrotic gene transcription. This sets off a sequence of events resulting in vascular rarefaction, myofibroblast proliferation, and deposition of collagens and other matrix components in the interstitium.
Whereas there has been a good deal of emphasis on the adverse effects of apoptosis of the epithelial cell, one might postulate that apoptosis is not necessarily maladaptive but rather a way to eliminate the damaged cell that cannot effectively participate in the adaptive repair response but rather generates pro-inflammatory and pro-fibrotic paracrine factors. In this construct, factors that prevent the cell removal may be important contributors to the fibrotic process.
GENERAL CHARACTERISTICS OF SENESCENT CELLS
There are a number of mechanisms by which paracrine effects mediated by a damaged kidney epithelium can lead to changes in the interstitium. In our opinion, an important mechanism relates to arrest of the cell cycle in damaged cells that cannot undergo adaptive repair. This mechanism is closely related to sensescence and aging. Senescence is a cell-fate decision when cell cycle arrest becomes permanent. Although this is generally the case, some senescent cells that do not express p16INK4a can resume growth after inactivation of p53.11 Senescence has been well studied in response to DNA damage where some cell types undergo apoptosis, whereas others, particularly epithelial lineages, undergo a senescence response. Stromal lineages can also undergo senescence.12 The senescent cell can have dramatic effects on its local environment. It induces a complex inflammatory response that has been termed the senescence-associated secretory phenotype.13 Finally, senescent cells secrete factors such as matrix metalloproteinase-3 which favor extracellular matrix remodeling, promote defects in epithelial cell differentiation, and stimulate cancer cell growth.13
G2/M ARREST AFTER AKI IS AN IMPORTANT MEDIATOR OF CKD
Is there evidence that senescence of renal tubular cells participates in a causal way in the fibrotic response to severe or repeated injury to the kidney? We have identified a role for the cell cycle in maladaptive repair after AKI and proposed that epithelial cell cycle arrest can lead to fibrosis progression, thus providing a link between acute injury and CKD (Figure 2).14 As stated previously, after mild tubular injury, survivor epithelial cells enter into the cell cycle and proliferate to regenerate a structurally and functionally repaired nephron.8, 9 This repair process can be maladaptive however, especially if the injury is more severe, involves DNA damage, or occurs on the backdrop of chronic injury and baseline increased cell senescence. Maladaptive repair will lead to incomplete structural and functional restoration of kidney tissue with persistent low-grade inflammation, activation of perivascular fibroblasts (pericytes),10 accumulation of myofibroblasts, vascular rarefaction,3 intermittent chronic ischemia, increased production of interstitial matrix, and development of fibrosis. Indeed, under particularly stressful conditions, some tubular cells stay arrested in the G2/M phase leading to the production of pro-fibrotic factors such as connective tissue growth factor (CTGF) and TGF-β1.14 The percentage of cells that have entered the cell cycle that undergo G2/M arrest correlates with the extent of development of fibrosis.14
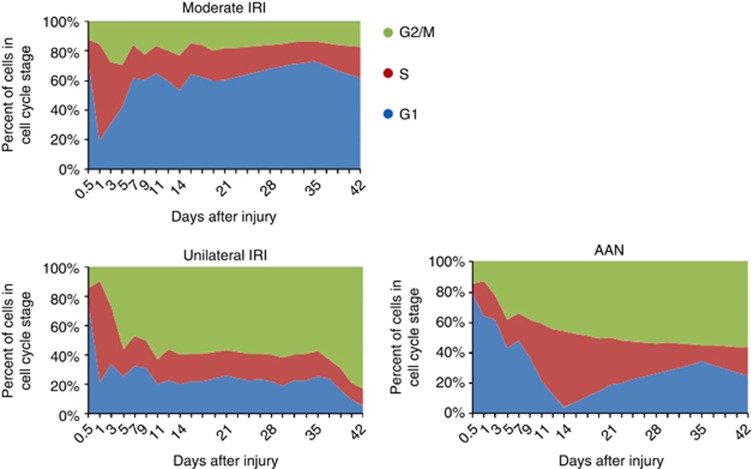
Figure 2.
In vivo cell cycle analysis in acute kidney injury (AKI). Proximal tubule cell cycle distribution (G1, S, and G2/M) of cells in the cell cycle over 42 days after moderate ischemia reperfusion injury (IRI; top left), unilateral ischemia/reperfusion (bottom left), and after administration of aristolochic acid (bottom right; n=3 mice of each time point in each group). AAN, aristolochic acid nephropathy. (Modified from Yang et al.14)
Other laboratories have recently reported data that corroborate our findings that G2/M arrest in the kidney tubular epithelial cell is a prominent feature of the maladaptive repair process resulting in fibrosis.15, 16, 17, 18 Cianciolo Cosentino et al.16 reported that a histone acetylase inhibitor could reduce the number of cells in G2/M arrest and reduce post-injury tubular atrophy and interstitial fibrosis. Jenkins et al.18 suggest that miR-192 has an important role in aristolochic acid–induced G2/M arrest.
We established a method to characterize the cell cycle profile of tubular epithelial cells in vivo at various times after an acute insult in five models of kidney injury. One model involved moderate ischemia reperfusion injury (IRI) with repair without fibrosis. Four models led to fibrosis: severe IRI, unilateral IRI, acute aristolochic acid toxic nephropathy, and unilateral ureteral obstruction (UUO). The development of fibrosis and the production of pro-fibrotic cytokines in each of these four models correlated with the arrest of many proximal tubule epithelial cells in the G2/M phase of the cell cycle (Figure 2). These cells were stimulated to enter the cell cycle because of injury to the nephron.
TGF-β1 and CTGF were both upregulated in production by the G2/M-arrested kidney tubular cells in vivo and in aristolochic acid-treated cells in vitro, a model which was established to explore mechanisms in more detail. TGF-β1 is a well-established driver of fibrosis, and CTGF partially mediates the downstream pro-fibrotic effects of TGFβ1 on fibronectin and collagen-IV secretion but had no effects on TGF-β1-mediated interleukin-8 and monocyte chemoattractant protein-1 production by human proximal tubule cells.19 TGF-β1 potentiates myofibroblast proliferation, extracellular matrix synthesis, and inhibition of collagenases in multiple organs. CTGF also acts in a TGFβ1-independent manner to promote the development of fibrosis in multiple organs, including the kidney.19
Few cell cycle regulators have been studied during the recovery phase after AKI. Among them, p21 (CIP1/WAF1) was one the first proteins explored. Early after AKI, p21, which is downstream of p53, is upregulated both at the mRNA and protein levels in the kidney.20 Interestingly, the effect of p21 seems to be different during AKI or CKD progression. Indeed, p21 seems to be protective during AKI, as assessed by the more pronounced kidney dysfunction, more severe kidney tissue damage, and the higher rate of mortality rate observed in p21−/− mice compared with wild-type mice.21 However, p21−/− mice developed less pronounced histological lesions after subtotal nephrectomy with enhanced tubular proliferation compared with wild-type mice.22 p53, which regulates the transcription of p21, is also upregulated in the kidney after AKI, and its inhibition or gene deletion reduces kidney lesions.14, 23 In contrast to the moderate amount of information related to p53 and p21, very few data are available regarding other proteins that regulate the cell cycle. After ischemic injury, mRNA and protein levels of cyclins D1, D3, and B, mRNA level of cyclin A, protein levels, and the activities of CDK4 and CDK2 increase with a temporal relationship consistent with tubular cell proliferation.24 The precise role of each cyclin and its regulators during AKI deserves a good deal more investigation.
The protein kinases Chk1 and Chk2 regulate the G2/M checkpoint and, when activated, induce arrest. Chk1 and Chk2 are downstream of the ATM–ATR (ataxia telangiectasia, mutated-ATM and Rad3-related) pathways. Chk1 and Chk2 might be exclusively ATR and ATM substrates, respectively. BRCA and p53 are phosphorylated by both kinases.25
Although DNA damage can activate the ATM–ATR pathways, non-DNA-damaging conditions, such as hypoxia and reoxygenation, can also activate these pathways. ATM is phosphorylated on ser1981 and activated in tubular cells after severe IRI, unilateral IRI, acute aristolochic acid toxic nephropathy, and UUO mice and in human proximal tubule cells in culture exposed to aristolochic acid. There were increased levels of p-Chk2 and p-p53. There was no increase in p-ATR by either immunostaining or western blotting in kidney injury models. KU-55933 (an ATM inhibitor) reduced the fraction of aristolochic acid–treated renal epithelial cells in culture that were in G2/M by ∼50% and reduced the mRNA levels of TGFB1, CTGF, COL4A1, and COL1A1 by ∼50%. ATM short hairpin RNA transfection of HK-2 cells and LLC-PK1 cells treated with aristolochic acid resulted in marked reductions in TGF-β1 and CTGF mRNA levels.
In light of our findings, a number of other studies can be seen to be supportive of our conclusions that proximal tubule G2/M arrest leads to fibrosis. The greater hyperplastic response and protection against renal dysfunction or interstitial fibrosis after partial renal ablation in p21−/− mice may be related to a reduced amount of cell cycle arrest.22 Stmn, (stathmin)-deficient mice have a defect in proper formation of the mitotic spindle and a propensity toward G2/M arrest after injury. These mice show delayed recovery, prolonged tubular proliferation, and interstitial fibrosis after ischemic-reperfusion injury.26 It is possible that normal nonfibrotic repair depends on efficient dedifferentiation of the surviving epithelial cells, which is dependent upon reduced p53–p21 pathway activation. If this pathway is activated, G2/M arrest occurs, reducing the efficiency of normal repair.
INEFFICIENT REMOVAL OF DAMAGED CELLS CONTRIBUTES TO THE DEVELOPMENT OF CKD?
There is precedent for this concept of a beneficial effect of removal of damaged cells. Senescent cells accumulate with aging, and it has been proposed that they are maladaptive because of the factors they secrete.13 In a very interesting experiment, Baker et al.27 created a mouse with a novel transgene, INK-ATTAC, which allowed the investigators to inducibly delete cells that expressed p16INK4a, a senescence marker, in the BubR1 progeroid mouse background. Life-long removal of senescent cells delayed tissue dysfunction characteristic of this mouse in adipose tissue, eye, and skeletal muscle. If senescent cells have an important role in fibrosis of the kidney, then these observations in the progeroid mouse provide a clear link between aging and the increased propensity for CKD. The CKD that develops as a consequence of repeated injury, sometimes documented by bouts of AKI in the native kidney or chronic injury to the renal allograft often undocumented, can be considered a form of accelerated aging.
Apoptosis of epithelial cells has been associated with fibrosis in the kidney. In each of the AKI models we used in our study,14 whether resulting in fibrosis or not, there was an increase in apoptosis after injury. It is of interest that in moderate IRI, where there was very little, if any, fibrosis development, there was more apoptosis than in the aristolochic acid–treated mice. On the other hand, there was much more pro-fibrogenic gene expression, protein production and fibrosis in the aristolochic acid–treated mice where there was less apoptosis. This provides additional evidence that perhaps apoptosis is adaptive after injury and cells that undergo cell cycle arrest and do not progress to apoptosis are pro-fibrogenic and maladaptive.
URINARY BIOMARKER EVIDENCE FOR CELL CYCLE ARREST AFTER AKI
A recent report Kashani et al.28 identified two urinary proteins chosen for their ability to identify patients who develop AKI using the RIFLE injury/failure (I/F) definitions. The findings were then validated in another cohort. The proteins identified were insulin-like growth factor-binding protein 7 and tissue inhibitor of metalloproteinase-2 (TIMP2) as the best-performing markers in the discovery study (area under the curve=0.77 and 0.75, respectively, for RIFLE-I/F within 12–36 h). Both of these proteins have been associated with cell cycle arrest. Insulin-like growth factor-binding protein 7 induces G1/G2 cell cycle arrest and cellular senescence. It increases the expression of p53 and p21.29 It is also interesting that TIMP2 inhibits endothelial cell proliferation and hence is antiangiogenic, which may contribute to maladaptive repair and vascular rarefaction.30 TIMP2 has recently been reported to promote injury to the kidney in the UUO model through the activation of matrix metalloproteinase-2.31 Tubulointerstitial fibrosis secondary to UUO was reduced in TIMP2−/− mice.
CONCLUSIONS
Our data, and those of other investigators, suggest a new target for prevention and/or treatment of fibrosis and reduction in the epidemic of CKD exacerbated by the rapidly expanding rate of development of AKI. Cells trapped in G2/M may represent a new histological biomarker for CKD progression. Moreover, pharmacologic intervention that leads to an increased number of tubular cells arrested in the G2/M phase after AKI may worsen kidney fibrosis, whereas interventions that enhance the movement through G2/M or facilitate apoptosis of cells that otherwise would be blocked in G2/M may reduce the development of fibrosis after kidney injury.
Acknowledgments
This research was supported by NIH grants DK039773 and DK072381 to JVB. I apologize to our colleagues whose work is not cited due to the length and reference constraints of this mini review. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
JVB has received consulting fees from Astellas, Sanofi, and UCB Celltech, owns stock in Thrasos and Sentien, and received grant support from NovoNordisk and the National Institutes of Health.
References
- Kaissling B, Lehir M, Kriz W. Renal epithelial injury and fibrosis. Biochim Biophys Acta. 2013;1832:931–939. doi: 10.1016/j.bbadis.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Venkatachalam MA, Griffin KA, Lan R, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078–F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol. 2008;19:197–206. doi: 10.1681/ASN.2007080862. [DOI] [PubMed] [Google Scholar]
- Beeuwkes R, III, Bonventre JV. Tubular organization and vascular-tubular relations in the dog kidney. Am J Physiol. 1975;229:695–713. doi: 10.1152/ajplegacy.1975.229.3.695. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Park KM, Hsiao LL, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Valerius MT, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Czerniak S, Dirocco DP, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausejour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35:7475–7484. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Liu N, Tolbert E, et al. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol. 2013;183:160–172. doi: 10.1016/j.ajpath.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, et al. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol. 2013;24:943–953. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Chiang WC, Lai CF, et al. Transforming growth factor beta-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol. 2013;182:118–131. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins RH, Davies LC, Taylor PR, et al. miR-192 induces G/M growth arrest in aristolochic acid nephropathy. Am J Pathol. 2014;184:996–1009. doi: 10.1016/j.ajpath.2013.12.028. [DOI] [PubMed] [Google Scholar]
- Qi W, Chen X, Poronnik P, et al. Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int J Biochem Cell Biol. 2008;40:9–13. doi: 10.1016/j.biocel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Megyesi J, Udvarhelyi N, Safirstein RL, et al. The p53-independent activation of transcription of p21 WAF1/CIP1/SDI1 after acute renal failure. Am J Physiol. 1996;271:F1211–F1216. doi: 10.1152/ajprenal.1996.271.6.F1211. [DOI] [PubMed] [Google Scholar]
- Megyesi J, Andrade L, Vieira JM, Jr., et al. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int. 2001;60:2164–2172. doi: 10.1046/j.1523-1755.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- Megyesi J, Price PM, Tamayo E, et al. The lack of a functional p21(WAF1/CIP1) gene ameliorates progression to chronic renal failure. Proc Natl Acad Sci USA. 1999;96:10830–10835. doi: 10.1073/pnas.96.19.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA, Dagher PC, Sandoval RM, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Kang MJ, Kim W, et al. Renal tubule regeneration after ischemic injury is coupled to the up-regulation and activation of cyclins and cyclin dependent kinases. Kidney Int. 1997;52:706–714. doi: 10.1038/ki.1997.386. [DOI] [PubMed] [Google Scholar]
- Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi K, Revelo MP, Barone S, et al. Stathmin-deficient mice develop fibrosis and show delayed recovery from ischemic-reperfusion injury. Am J Physiol Renal Physiol. 2006;290:F1559–F1567. doi: 10.1152/ajprenal.00424.2005. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatar T, Yang W, Amemiya Y, et al. IGFBP7 reduces breast tumor growth by induction of senescence and apoptosis pathways. Breast Cancer Res Treat. 2012;133:563–573. doi: 10.1007/s10549-011-1816-4. [DOI] [PubMed] [Google Scholar]
- Seo DW, Li H, Guedez L, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- Wang Z, Famulski K, Lee J, et al. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int. 2014;85:82–93. doi: 10.1038/ki.2013.225. [DOI] [PubMed] [Google Scholar]