Abstract
With the escalating cost of monitoring and follow-up required in the care of patients with chronic kidney disease (CKD), biomarkers are increasingly being investigated for their utility in predicting patients most at risk of decline in renal function in order to rationalize and target care. Putative biomarkers have also emerged as treatment targets, with the potential to develop novel therapeutics. However, biomarker studies in CKD are largely derived from single-sample collections in observational or nested case-control studies that are suboptimal in study design, analyses, and end points relevant to confirm the utility of specific biomarkers. It has been demonstrated that biomarker expression may be modified by declining kidney function. Hence, their value in predicting future kidney dysfunction is limited. Therefore, understanding the nature, mechanism of action, and how specific biomarkers interact with the CKD disease process is a crucial step in defining the potential for biomarkers to predict outcome, or alternatively, develop as a therapeutic target. Unlike conventional risk factors that, albeit partly, enable us to distinguish an individual at risk of cardiovascular disease, biomarkers in patients with CKD may not be required to be modifiable either directly or indirectly in the disease process or by therapy. Reproducibility and prospective validation remain major challenges for the burgeoning number of purported biomarkers in patients with CKD. It is highly likely a combination of conventional and novel biomarkers will be needed to accurately predict the risk of end-stage kidney disease. This review will focus on recently identified biomarkers and their utility in predicting progressive kidney fibrosis.
Keywords: biomarkers, chronic kidney disease, diabetic nephropathy, fibrosis, transforming growth factor-β1
Chronic kidney disease (CKD) is recognized as the single biggest risk factor for cardiovascular disease, and the combination of diabetes and kidney disease confers a significant increase in the risk of death.1 The cost of treating end-stage kidney disease (ESKD) poses a significant risk to the community.1, 2 Hence CKD, and in particular diabetic nephropathy as the most common cause of CKD, is a major international health and socioeconomic burden.
Strategies to mitigate the development of diabetic nephropathy are primarily limited to blood pressure optimization and maximizing renin–angiotensin–aldosterone system blockade, but a treatment gap still exists. Hence additional therapies are urgently needed, and the identification of accurate and early predictors to define those at high risk of declining renal function may assist in timely intervention with targeted therapy.
Currently, new biomarkers are largely being developed based on both mechanistic and ‘data trawling' approaches. Validation of potential biomarkers in prospective studies as surrogate end points for hard clinical outcomes is often complicated by the long lag time to so-called ‘hard' renal end points, as evidenced by ESKD. Hence, validated surrogate markers for progressive renal disease are required to facilitate clinical trial programs, potentially yielding novel diagnostics and therapies to aid in prognostication and treatment of patients. This review will focus on current concepts and application of biomarkers, in particular, those relating to the risk of kidney fibrosis and progressive CKD.
WHAT ARE BIOMARKERS?
The term ‘biomarker' has been defined as a ‘quantitative indicator of biologic or pathologic processes that vary continuously with progression of the process'.3 This was further refined by the National Institutes of Health working group report in 2001 and included its role in measuring and monitoring pharmacologic responses to a particular therapeutic intervention.4 Biomarkers used in nephrology can be measured from blood and urine specimens, and more recently from DNA and microRNA analyses, kidney biopsy specimens, and from imaging modalities.
Ideally, biomarker levels vary continuously with the activity or degree of progression of the disease process. Increasingly, biomarkers are measurable intermediary components of molecular or cellular pathways involved in the biologic process, which may be disease specific or relevant to treatment toxicity. As such, single biomarker measurements represent a temporal slice through one of possibly many pathways that converge to define that process and its treatment. For instance, the estimated glomerular filtration rate (eGFR) and albuminuria are generally accepted as ‘standard' biomarkers for the prediction of cardiovascular mortality and risk of ESKD.5 However, the classification inherently implies that those with renal impairment are likely to progress. The challenge is to identify those at risk of complications and progressive renal disease earlier in its development.
WHY DO WE NEED BIOMARKERS IN CKD?
A biomarker is different from a risk factor or susceptibility marker. Most known risk factors for CKD can rarely distinguish patients who will develop CKD from those who will not. Conversely, surrogate end points can also be described as biomarkers that are strongly associated with a distant clinical event. They can reliably substitute for hard clinical end points in predicting clinical benefit, lack of benefit, or harm from a treatment. For that reason, albuminuria has been widely used as a surrogate marker in large-scale clinical trials for both cardiovascular and renal end points to improve clinical trial efficiency and decrease the need for lengthy studies of slowly progressive diseases such as CKD.6 However, the use of albuminuria as a surrogate marker for such end points is controversial.7
Therefore, we need biomarkers that can:
accurately predict an individual's risk of developing CKD or hard renal end points such as ESKD or death,
identify additional risk factors other than known conventional risk factors for CKD,
identify and quantify a pathological process within the kidney, which may or may not be modifiable, and
act as an indicator of treatment response
Emerging technologies in the field of genomics, metabolomics, and proteomics are providing new platforms for biomarker discovery. Although molecular genetic typing of kidney biopsy specimens may eventually replace classical renal pathology in diagnosis of various nephropathies, the vast majority of patients with the potential for development of CKD, or indeed existing CKD, will not undergo a renal biopsy. Hence peripheral blood biomarkers are still required. The role of epigenetic modification is increasingly recognized to be associated with disease phenotype and an area of active investigation, but its predictive association with progression of CKD is as yet unclear.
HOW DO WE IDENTIFY POTENTIAL BIOMARKERS?
Targeted pathophysiologic investigations, especially in animal models of disease, have been the predominant method of biomarker discovery in the past. The primary goal of these studies has been an understanding of disease mechanisms, and biomarker discovery was usually a secondary outcome. A ‘fishing' approach involves exhaustive quantitative analyses of mRNA or proteins in patient samples (tissue, blood, urine) and the use of analytical methods to search for patterns in the resulting large data sets. The major drawback of this approach is data overload, with the potential for overfitting of models, and lack of immediate pathophysiological insight.8 The risk of spurious associations is, therefore, evidently present. However, with the recent advances in urinary proteomic and gene expression arrays, this approach allows rapid screening of huge numbers of possible associations and the increased likelihood of serendipitous discovery.
LIMITATIONS IN THE IDENTIFICATION OF NOVEL BIOMARKERS
Once new target biomarkers are identified, or existing ones are refined, preclinical trials are used to validate them in individuals with or without disease. Subsequently, retrospective case-control studies are used to determine assay sensitivity and specificity. This is followed by prospective screening studies in large cohort studies to determine reproducibility. Finally, the biomarker is validated as a disease-predictive tool in alternative cohort studies or randomized controlled trials. Most biomarker studies are conducted within population-based studies, epidemiological cohorts, or in clinical trials. These investigations are generally designed as either a cohort study or a nested case-control study extracted from a larger cohort. Participants are selected based on criteria including the absence of CKD at baseline and the subsequent outcome based on defined end points of CKD during follow-up. The methodology is usually based on the availability of specimens (blood or urine, single or two time points), and the cost and feasibility of the assay to be performed, which generally is not factored into the cost of the primary study. In either case, multivariate analysis is typically used to control for confounding variables that may alter associations between the biomarkers and risk of progressive CKD.
Validation of the performance of a new biomarker is the most problematic aspect of biomarker development and is best achieved by prospective validation with a separate cohort of patients.
STATISTICAL METHODS FOR DETERMINATION OF BIOMARKER UTILITY
Relative risk indicators, such as odds ratio or risk ratios, are the most frequently used measures of association between a biomarker and outcomes. Owing to the nature of distribution of biomarker levels, which typically overlap in cases and controls, the risk ratios do not provide direct information as to whether a biomarker affects risk prediction.9 Therefore, c-statistics are used to measure risk discrimination of the biomarkers. The c-statistic is more commonly known as the area under the receiver-operating characteristic curve. The c-statistic ranges from 0.5 (no better than random guessing) to 1 (perfect discrimination), and represents the trade-off between sensitivity and specificity of a candidate biomarker.10 An ideal biomarker should provide a c-statistic value over and above that of the conventional risk factor or the gold standard for the outcomes. For example, in the Framingham Offspring Study, the B-type natriuretic peptide level and urinary albumin to creatinine ratio were found to be good predictors of major cardiovascular events. The highest quartile of these markers, by a ‘multimarker' score method, gave a fourfold increase in all-cause mortality and a twofold increase in cardiovascular events. However, the c-statistic for models of major cardiovascular disease events was 0.68 (with age and sex as conventional predictors), with only marginal improvement to 0.7 (with age, sex, and the addition of B-type natriuretic peptide or albumin to creatinine ratio to the predictive equation).11
The net reclassification index (NRI) is a more recent method of analysis that interprets the value of a biomarker by exploring whether the incorporation of a biomarker leads to a change in assigned risk categories.12 The categorical NRI method requires the specification of risk thresholds (such as low, intermediate, and high risk) and whether a biomarker will move individuals across these risk thresholds into different risk categories. The risk reclassification is considered appropriate if persons who are moved to a higher-risk category do in fact have a higher risk for the outcome, or vice versa. Another measure, the integrated discrimination improvement (IDI), may be regarded as a continuous version of the NRI, with reclassification measured in terms of differences in predicted probabilities.13
All methods have limitations. C-statistics do not provide any information on the ability of a model to predict probabilities, which are often important in decision-making in a clinical setting.10 Sensitivity and specificity may vary with disease prevalence and patient characteristics. Hence c-statistics may change depending upon the study population.14 There is also increasing recognition that even modest improvements in the c-statistic require extremely strong associations between a novel biomarker and an outcome, which most biomarkers fail to achieve.15 Similarly, reclassifying an individual using NRI at low risk of disease to an intermediate-risk category may have the same effect upon the NRI as reclassifying an individual at intermediate-risk to a high-risk group. Yet, the clinical implications may be quite different. Similarly, incorrect down-classification of a few individuals at high risk may result in significantly more clinical harm.16
Most importantly, useful biomarkers must demonstrate that they can distinguish between individuals with risks of developing CKD in a clinically meaningful way. Two modifications to the NRI have been attempted to address the above-mentioned limitations. The ‘clinical NRI' examines reclassification only for individuals starting in the intermediate-risk group, with the assumption that these are individuals in whom clinical decision-making is most likely to be altered. The other modification is a ‘weighted NRI', which takes into consideration factors such as perceived costs or savings.13 Other statistical methods such as Akaike's information criterion17 and Schwartz's Bayesian information criterion18 are also used to measure the ‘fitness' of a model, by incorporating existing risk factors with or without the biomarkers in predicting CKD risk. Typically, the lower the Akaike's information criterion and Bayesian information criterion values, the closer the fitness to the model, hence a better marker of risk.
BIOMARKERS OF INTERSTITIAL FIBROSIS
It is well known that the degree and extent of tubulointerstitial fibrosis are accurate predictors of future renal function. Hence markers indicating progressive fibrosis and subsequent risk of CKD progressive are needed.
Transforming growth factor-β1 (TGF-β1) is well known as a pro-fibrotic cytokine that has a pivotal role in kidney fibrosis. TGF-β1 levels can be measured in both serum and urine. In cross-sectional studies, Suthanthiran and et al.19 showed that serum levels of TGF-β1 were higher in African American subjects with ESKD relative to Caucasians, and hypertensive compared with normotensive individuals. Hence they hypothesized that TGF-β1 may be a contributing risk factor for renal disease progression in the African American hypertensive subjects. Increasing evidence suggests circulating TGF-β1 levels are linked to vascular target organ damage in both the diabetic and non-diabetic population.20
Bone morphogenetic protein-7 (BMP-7) is recognized as a natural antagonist to TGF-β1, with antifibrotic and anti-inflammatory properties. Loss of tubular BMP-7 has been observed in experimental models of progressive CKD.21 The role of TGF-β1 and BMP-7 in the pathogenesis of kidney fibrosis has been highlighted by a growing body of research over recent years.22
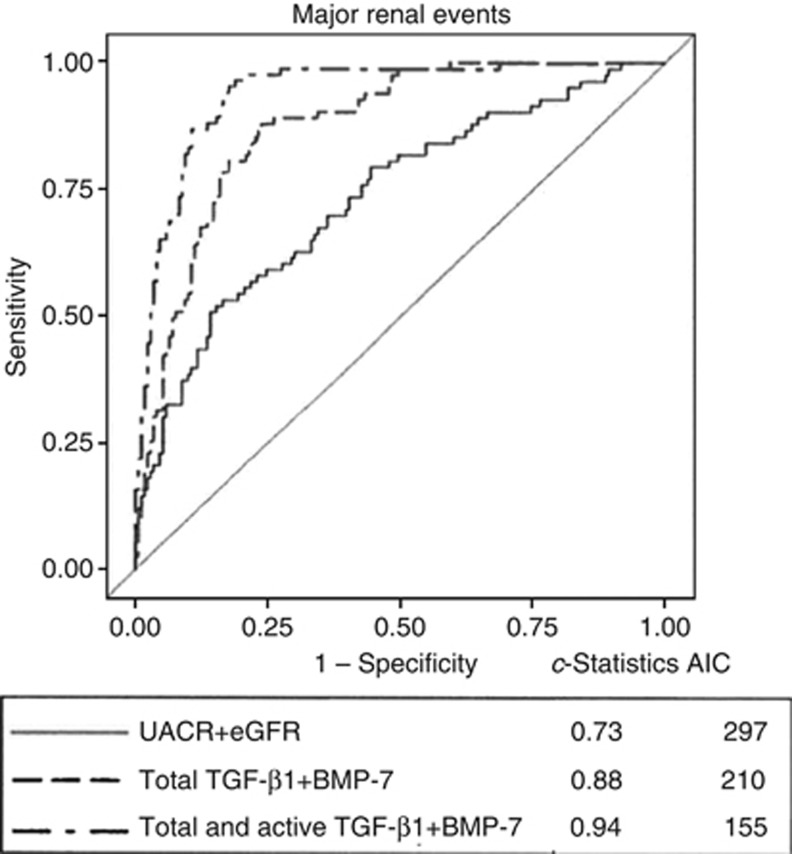
Our group has examined the levels of circulating total and active TGF-β1 and BMP-7 in the baseline blood samples from 281 participants enrolled the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation study. We then determined the utility of these measurements in predicting the development of progressive kidney disease using hard renal end points.23 Using a nested case-control study and propensity-scoring methodology, cases were defined as those who developed a renal end point during the study; i.e., doubling of serum creatinine to at least 200 umol/l, the need for renal replacement therapy, or death due to renal disease. Individuals who developed renal end points had higher total TGFβ1 and lower BMP-7 levels (all P<0.0001) at baseline. A graded increase in risk was observed in individuals with lower BMP-7 levels (odds ratio 24.07, 95% confidence interval (CI) 8.08–72.73, P<0.0001, for the lowest vs. the highest tertile), or higher TGF-β1 levels (odds ratio for the highest vs. the lowest third 8.43, CI 4.03–17.67, P<0.0001). The c-statistic for conventional predictors (i.e., albuminuria and reduced glomerular filtration rate) was 0.73. Using BMP-7, total and active TGF-β1, the receiver-operating characteristic was 0.94 (P<0.0001 vs. conventional predictors)23(Figure 1).
Figure 1.
The comparison of area under the curve (AUC) between urinary albumin creatinine ratio (UACR), estimated glomerular filtration rate (eGFR), and circulating transforming growth factor-β1 (total and active TGFβ1) and bone morphogenetic protein-7 (BMP-7). Adding the biomarkers clearly provided an incremental c-statistic value to that of the known conventional markers. Akaike's information criterion (AIC) showed the lowest value when all markers combined. (Adapted with permission from Wong et al.23)
In summary, we showed that by incorporating total or active TGF-β1, or BMP-7 to the conventional predictors model (urinary albumin to creatinine ratio, eGFR in addition to the clinical parameters, age, sex, body mass index, duration of diabetes mellitus, HbA1c, history of macrovascular events, and retinopathy known as the baseline model) provided a higher predictive value for the development of predefined renal end points. The strongest predictive ability was observed when circulating BMP-7, along with total and active TGF-β1 were incorporated into the baseline model (c-statistic=0.96, P<0.0001 vs. the baseline model), suggestive of an additive effect on CKD risk prediction by these markers. Sensitivity analyses were performed using Akaike's information criterion17 and the Bayesian information criterion,18 which showed the lowest value of Akaike's information criterion and Bayesian information criterion when the cytokines were combined. The results suggest these novel kidney markers are better predictors of renal progression than albuminuria and reduced glomerular filtration rate in patients with type 2 diabetes mellitus. These data further suggest the role of pro-fibrotic and antifibrotic cytokines in maintaining the physiological balance in kidney fibrosis, and translates the pathophysiology of kidney fibrosis to potential clinical practice.
Clearly, validation of these results using these biomarkers in a separate population, with a cost effectiveness assessment, is required. In addition, the results cannot necessarily be extrapolated to cohorts other than patients with diabetes mellitus.
BIOMARKERS OF INFLAMMATION
With better understanding of the molecular mechanisms underpinning CKD, the roles of inflammation and fibrosis in progressive renal functional decline are becoming increasingly inseparable.24 Despite the fact that markers of inflammation are known to be associated with an increased risk of cardiovascular disease, the association of these markers with the onset and progression of kidney disease has remained unclear.
Tumor necrosis factor (TNF)-α is a transmembrane protein that has an essential role in mediating inflammatory processes. TNF-α and its receptors, TNF receptor 1 and TNF receptor 2 are shed from the cell surface, which can be detected in the plasma as free or bound to circulating TNFRs.25 Niewczas et al.26 examined the levels of TNF-α and its receptors in 410 patients with type 2 diabetes in a 12-year prospective study. They reported the cumulative incidence of ESKD for patients in the highest TNF receptor 1 quartile was 54%, but only 3% for the other quartiles (P<0.001). This association with ESKD is seen in both proteinuric and non-proteinuric patients with type 2 diabetes. In a separate study of 628 non-proteinuric and normal renal function patients with type 1 diabetes mellitus,27 the same group reported a cumulative incidence of stage 3 CKD (eGFR<60 ml/min per 1.73 m2) for patients in the highest TNF receptor 2 quartile and they were threefold more likely to experience renal decline than patients in the lower quartiles (hazard ratio 3.0, 95% CI 1.7–5.5), independent of relevant clinical covariates such as age, HbA1c, urinary albumin creatinine ratio, baseline cystatin-C-based eGFR (eGFRcys), blood pressure, or treatment with renin–angiogtensin system inhibitors. Together, these associations point to the involvement of TNFRs and their independent role in leading to early and late renal function loss in both types 1 and type 2 diabetes mellitus. The association of inflammatory biomarkers with progressive renal disease is deserving of future study.
CONCLUSION
Numerous promising biomarkers for risk of CKD progression have been evaluated in the past decade but to date, evidence to support their use in routine clinical practice remains limited. Key limitations limiting the use of current biomarkers include variable and inappropriate analytical methods to define predictive risk and the lack of a validation cohort. Hence, the ability to use these biomarkers to predict CKD risk more accurately than current methodology remains to be tested. However, it is evident that combination of biomarkers with conventional risk factors is necessary to substantially improve risk prediction. Further research is needed to identify new biomarkers that can successfully stratify risk of CKD in a general population, as well as to determine whether management strategies informed by biomarker testing improve the targeting and efficiency of health-care services compared with current ‘standard of care'.
Acknowledgments
This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
The authors declared no competing interests.
References
- Deloitte Access Economics. Two of a Kind http://www.deloitte.com/view/en_AU/au/industries/Lifesciencesandhealth/ , 2011
- USRDS . Cost of ESRD. In: Atlas of CKD & ESRD, Vol 2, Chapter 11. USRDS: Ann Arbor, MI, USA, 2013, pp 325–332.
- Lemley KV. An introduction to biomarkers: applications to chronic kidney disease. Pediatr Nephrol. 2007;22:1849–1859. doi: 10.1007/s00467-007-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravedi P, Ruggenenti P, Remuzzi G. Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol. 2012;8:301–306. doi: 10.1038/nrneph.2012.42. [DOI] [PubMed] [Google Scholar]
- Thompson A. Proteinuria as a surrogate end point—more data are needed. Nat Rev Nephrol. 2012;8:306–309. doi: 10.1038/nrneph.2012.43. [DOI] [PubMed] [Google Scholar]
- Ransohoff DF. Rules of evidence for cancer molecular-marker discovery and validation. Nat Rev Cancer. 2004;4:309–314. doi: 10.1038/nrc1322. [DOI] [PubMed] [Google Scholar]
- Ware JH. The limitations of risk factors as prognostic tools. N Engl J Med. 2006;355:2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
- Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D'Agostino RB, Sr., D'Agostino RB, Jr., et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- Pencina MJ, D'Agostino RB, Sr., Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med. 1997;16:981–991. doi: 10.1002/(sici)1097-0258(19970515)16:9<981::aid-sim510>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Pepe MS, Janes H, Longton G, et al. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- Pepe MS. Problems with risk reclassification methods for evaluating prediction models. Am J Epidemiol. 2011;173:1327–1335. doi: 10.1093/aje/kwr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. "A new look at the statistical model identification". IEEE Trans Automat Contr. 1974;6:716–723. [Google Scholar]
- Schwarz GE. "Estimating the dimension of a model". Ann Stat. 1978;2:461–464. [Google Scholar]
- Suthanthiran M, Gerber LM, Schwartz JE, et al. Circulating transforming growth factor-beta1 levels and the risk for kidney disease in African Americans. Kidney Int. 2009;76:72–80. doi: 10.1038/ki.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitouni K, Harry DD, Nourooz-Zadeh J, et al. Circulating vitamin E, transforming growth factor beta1, and the association with renal disease susceptibility in two racial groups with type 2 diabetes. Kidney Int. 2005;67:1993–1998. doi: 10.1111/j.1523-1755.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- Zeisberg M. Bone morphogenic protein-7 and the kidney: current concepts and open questions. Nephrol Dial Transplant. 2006;21:568–573. doi: 10.1093/ndt/gfk010. [DOI] [PubMed] [Google Scholar]
- Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MG, Perkovic V, Woodward M, et al. Circulating bone morphogenetic protein-7 and transforming growth factor-beta1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int. 2013;83:278–284. doi: 10.1038/ki.2012.383. [DOI] [PubMed] [Google Scholar]
- Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl. 2010;119:S22–S26. doi: 10.1038/ki.2010.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76:262–276. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]
- Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]