Abstract
Advanced oxidation protein products (AOPPs) are the dityrosine-containing and crosslinking protein products formed during oxidative stress by reaction of plasma protein with chlorinated oxidants, and often carried by albumin in vivo. Accumulation of plasma and renal AOPPs is a common pathologic finding in chronic kidney disease (CKD) patients. Moreover, AOPP accumulation is an independent risk factor for cardiovascular events (CVDs) in CKD. Clinical and experimental studies indicate that AOPPs are involved in the structural changes of progressive nephropathies such as glomerulosclerosis, interstitial fibrosis, and tubular atrophy via the redox-dependent pathway. Mounting evidence supports the role of AOPPs as a new class of renal pathogenic mediators in the progression of CKD. This mini review describes the formation of AOPPs, the involvement of AOPPs in CKD pathogenesis, and the underlying mechanisms.
Keywords: AOPPs, CD36, NAD(P)H oxidase, oxidative stress, RAGE
Oxidative stress is prevalent in chronic kidney disease (CKD) patients and is considered to be an important driver of CKD progression and its complications.1 Oxidative stress is defined as an imbalance between an excessive generation of oxidant compounds and reduced antioxidant defense mechanisms. Reactive oxygen species (ROS) are the most important contributor of oxidative stress in biological systems. Increased ROS production in the diseased kidney is primarily driven by activation and upregulation of ROS-producing enzymes such as NAD(P)H oxidase, mitochondrial dysfunction, and endoplasmic reticulum stress. Granata et al.2 demonstrated impaired mitochondrial respiratory machinery in CKD patients (stages 2–3). Moreover, it is well known that enhanced oxidative stress in CKD patients is often accompanied by a decrease in intracellular and circulating antioxidants, such as superoxide dismutase, glutathione peroxidase, and glutathione.1
Extracellular fluids usually contain only minimal amounts of antioxidant enzymes, and therefore plasma proteins are prone to be oxidized by ROS. Accordingly, elevated levels of oxidized protein products, termed ‘advanced oxidation protein products' (AOPPs), are often detected in the plasma of dialysis patients.3, 4 Albumin, the most abundant plasma protein, was identified as the main source of AOPPs in the plasma.5 Size exclusion chromatography of uremic plasma has isolated high-molecular-weight (600 kDa) and low-molecular-weight (80 kDa) AOPPs. The high-molecular-weight AOPPs were mostly formed because of albumin aggregates, likely resulting from disulfide bridges and/or dityrosine crosslinking. The low molecular weight of AOPPs contained albumin in its monomeric form.3
In addition to chronic uremia, AOPP accumulation was also found in preterm hypoxic babies, and in patients with coronary artery disease, diabetes mellitus, systemic sclerosis, chronic rheumatic valve disease, and colorectal cancer among other chronic diseases. These findings underscore the universal pathogenic role of AOPPs. The aim of this mini review is to summarize current findings regarding the role of AOPPs in kidney diseases as well as the underlying mechanisms.
AOPP FORMATION
AOPPs are a family of oxidized, dityrosine-containing, crosslinked protein compounds formed by the reaction of plasma proteins with chlorinated oxidants. In vivo, the generation of chlorinated oxidants is a feature of phagocytic cells that possess myeloperoxidase, the only enzyme that is able to generate a chlorinated oxidant. In vitro, AOPPs can be formed by exposure of albumin to hypochlorous acid. The formation of AOPPs is correlated to the concentration of chlorinated oxidant added, indicating that AOPPs result from the interaction between oxidants and plasma proteins.3 In uremic patients, the oxidative activity of circulating neutrophils corresponded with the concentration of plasma AOPPs,6 suggesting that neutrophils, which possess high content of myeloperoxidase, might be involved in plasma AOPP formation.
CLINICAL RELEVANCE OF AOPPs IN KIDNEY DISEASES
In 1996, Witko-Sarsat et al.3 first reported elevated plasma level of AOPPs in uremic patients. The highest level of AOPPs is detected in patients on maintenance hemodialysis, followed by those on peritoneal dialysis. Notably, patients with advanced chronic renal failure not yet on dialysis had almost three times higher AOPP levels than healthy subjects.
IgA nephropathy (IgAN) is the most common type of primary glomerulonephritis worldwide.7 Its outcome is highly variable between individuals. End-stage renal failure may occur within 5 years of presentation, or conversely, more than 20 years later.7 Therefore, identification of factors predictive of subsequent outcome at an earlier stage of the disease should be of great interest, particularly if these factors can effectively guide treatment decisions.8 A prospective cohort study reveals that elevated plasma level of AOPP at the early stage of IgAN is one of the most potent independent risk factors for poor renal outcome (start of dialysis).9 Consistent with this finding, another study that included 62 IgAN patients with a mean follow-up of 4.46 (range 3–10) years shows that AOPPs are strongly associated with proteinuria at sampling and the rate of renal function decline during follow-up.10 These studies suggest that AOPPs may serve as a surrogate marker for a bad prognosis in IgAN, and elevated plasma level of AOPPs at the early stage of the disease may warrant more aggressive intervention.
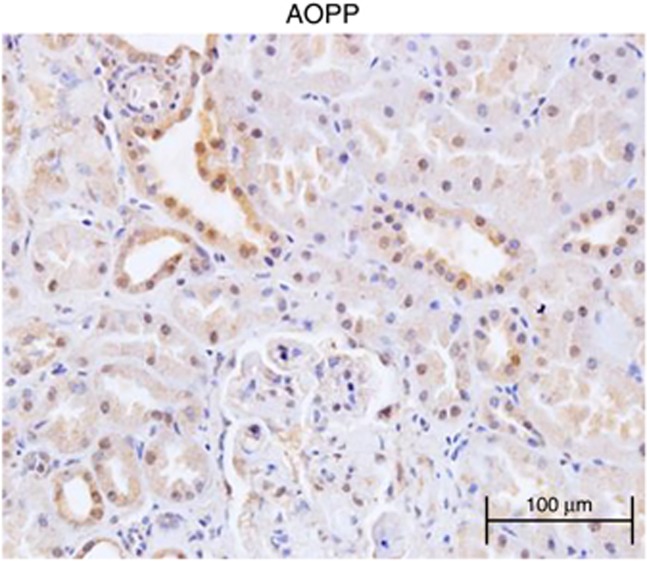
Besides elevated plasma level of AOPPs, increased AOPPs in renal tissues are detected in patients with diabetic nephropathy, membranous nephropathy, and IgAN patients (unpublished data). Figure 1 gives an example of the histological distribution of AOPPs in renal tissue in a patient with IgAN. AOPP accumulation in the kidney appears multifactorial and may be because of trapping of AOPPs from the circulation, increased local generation of new AOPPs, or pathologically decreased AOPP turnover. Renal trapping of AOPPs has been demonstrated by steady intravenous infusion of AOPP-modified albumin into unilateral nephrectomized rats. After 3 weeks of infusion, the AOPP content in the kidneys was significantly increased in both glomeruli and tubules.11
Figure 1.
Renal accumulation of advanced oxidation protein products (AOPPs) in IgA nephropathy. AOPPs were detected predominantly in tubular epithelial cells, podocytes, and mesangial cells, and in some cases, in arterial/arteriolar endothelial cells and interstitial infiltrating cells.
It is believed that ROS increases in patients with acute kidney injury (AKI). A prospective cohort study to compare AOPP levels between AKI and non-AKI patients revealed that the levels of AOPPs are significantly higher in AKI patients than healthy controls. Patients with the most severe AKI (RIFLE class Failure) have markedly elevated AOPP levels compared with RIFLE class Risk and Injury patients.12
ROLE OF AOPPs IN RENAL DAMAGE: CELLULAR AND MOLECULAR MECHANISMS
Clinical studies provide convincing evidence for an association between elevated AOPP levels and kidney injury, suggesting AOPPs may be important predictors of the prognosis of kidney disease. In addition, the pathogenic role of AOPPs in kidney damage is supported by a number of experimental observations.11, 13, 14, 15 Cell culture and animal studies show that most resident renal cells are adversely affected by AOPPs. For example, given the importance of podocytes in maintaining glomerular filtration barrier, we find that chronic loading of AOPPs causes podocyte apoptosis via the p53-Bax apoptotic pathway, leading to proteinuria.13 Moreover, chronic loading of AOPPs causes activation of the renin–angiotensin system (RAS) in tubular cells.11 In addition, AOPPs upregulate the expression of fibronectin and collagen I in cultured mesangial cells via NAD(P)H oxidase-dependent O2 production,15 and when endothelial cells in the kidney are stimulated by AOPPs, the pro-inflammatory mediators such as vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 are released.14 These detrimental cellular events caused by AOPPs ultimately lead to renal function perturbations such as an increase in urinary protein excretion, decreased creatinine clearance, and an aggravated glomerulosclerosis and interstitial fibrosis.16, 17
Oxidative stress and inflammation are inseparable co-stimulators. For instance, by activating NF-κB, a redox-sensitive transcription factor that regulates expression of pro-inflammatory cytokines and chemokines, oxidative stress promotes recruitment and activation of leukocytes and resident cells, thereby eliciting inflammation. It is known that nuclear translocation of NF-κB subunits p50 and p65 is significantly greater in the peripheral mononuclear cells of patients with IgAN than healthy control.18 AOPPs as mediators of inflammation are known to be involved in monocyte activation, and AOPPs formed in vitro are potent mediators of monocyte activation, triggering both their respiratory burst and tumor necrosis factor-α synthesis. Likewise, AOPPs derived from the plasma of hemodialysis patients have the capacity to trigger respiratory burst of monocytes.19 Consistent with clinical finding, animal studies demonstrate that chronic loading of AOPPs in streptozotocin-induced diabetes or unilateral nephrectomy rats results in the activation of NF-κB and in the upregulation of pro-inflammatory and pro-fibrotic factors such as monocyte chemoattractant protein-1 and TGF-β1.17
AOPPs were first recognized as markers of oxidative stress. However, emerging studies reveal that AOPPs are also capable of promoting ROS production, leading to an inexorable positive feedback loop. AOPPs induce intracellular superoxide generation by a mechanism involving NAD(P)H oxidase. AOPPs activate NAD(P)H oxidase through the protein kinase C-dependent pathway that leads to an excessive generation of intracellular superoxide in various renal cells including podocyte, endothelial cells, mesangial cells, and tubular epithelial cells.11, 13, 14, 15 Significantly, the effect of AOPP-modified albumin on ROS generation is 100 times that of native albumin.11 In conditions with increased protein oxidative damage such as CKD and diabetes, increased AOPP-modified albumin in the lumen of renal tubules might cause a more dramatic renal injury than unaltered albumin.
ROLE OF AOPPs IN ATHEROSCLEROSIS OF CKD PATIENTS
In addition to the involvement in renal damage, AOPPs are also implicated in the pathogenesis of atherosclerosis and cardiovascular events (CVDs) in CKD. Descamps-Latscha B et al.20 report that AOPP levels are independent predictors of CVD in non-diabetic CKD patients in the predialysis phase. Our most recent study indicates that the plasma AOPP level is an independent risk factor for CVD in patients on hemodialysis.21
AOPPs may induce CVD through three different mechanisms. First, AOPPs may induce endothelial dysfunction and vascular inflammation. A clinical study showed that AOPPs are independently correlated with flow-mediated dilatation% and nitrate-mediated dilatation%, and therefore can predict endothelial function of peritoneal dialysis patients.22 In vitro studies reveal that AOPPs induce vascular endothelial cells-dysfunction by activating NF-κB and p38 mitogen-activated protein kinase signaling.14 Second, AOPPs are involved in lipid disorders. Karand colleagues23 shows that in vitro generated AOPP-modified albumin binds with high affinity to the high-density lipoprotein (HDL) scavenger receptor class B type I (SR-BI), and competitively inhibits HDL association to SR-BI and thus decreases SR-BI-mediated cholesterol ester uptake and results in depressed plasma clearance of HDL-cholesterol. Third, AOPPs have been shown to inhibit cholesterol efflux from human macrophage foam cells by downregulating the expression of ATP-binding membrane cassette transporter A-1 and liver X receptor α, and thus may promote macrophage foam cell formation.23 Our group found that intravenous infusion of AOPP-modified albumin also significantly increases macrophage infiltration in atherosclerotic plaques in hypercholesterolemic rabbits.24
RECEPTORS FOR AOPPs
The presence of AOPP-modified proteins in tissues and the biological effects associated with their accumulation have stimulated an intensive search for cellular surface molecules that recognize AOPPs and activate downstream cellular responses. Receptor for advanced glycation end products (RAGE) on the surface of endothelial cells was first discovered as a receptor of AOPP-modified albumin.14, 25 Further research also identified CD36 as being capable of binding AOPP-modified albumin.26
In the kidney, physiological expression of RAGE is found on podocyte at a very low concentration but is significantly enhanced by chronic loading of AOPPs.27 The AOPP–RAGE interaction could activate NAD(P)H oxidase leading to ROS production. ROS generated by this mechanism in turn leads to p53 upregulation, Bax and caspase 3 activation, and podocyte apoptosis. Interestingly, although both RAGE and CD36 are found in tubular epithelia, knocking down CD36 by small interfering RNA shows a more dramatic inhibition on AOPP-induced intrarenal RAS activation.11 Similarly to that of RAGE, CD36 mediates AOPP–human serum albumin (HSA)-induced intracellular ROS generation via a mechanism that involves protein kinase C and membrane NAD(P)H oxidase signaling pathways, and the secretion of TGF-β1 in cultured proximal tubular cells.26 In addition, CD36 mediates AOPP–albumin endocytic uptake and subsequent lysosomal degradation in proximal tubular cells. Whether RAGE mediates AOPP–albumin endocytosis remains to be further analyzed.
THERAPEUTIC INTERVENTIONS
AOPP accumulation is associated with renal function decline in CKD patients and therefore may be a target for intervention. A clinical study shows that angiotensin II type 1 receptor blocker (ARB) Candesartan can reduce the plasma AOPP level when administered to non-diabetic patients on peritoneal dialysis.28 In addition, a cross-sectional study reveals that, in elderly people with impaired glucose metabolism, the vitamin D status is inversely associated with plasma levels of AOPPs, especially in subjects with hypovitaminosis D, suggesting a role for vitamin D in preventing AOPP-related pathologies.29 As many effects of AOPPs are mediated by RAGE activation, antagonism of this receptor appears to be an appropriate therapeutic concept. Indeed, direct blockade of RAGE with an antibody or silencing its expression by small interfering RNA prevented AOPP-induced podocyte apoptosis27 and intrarenal RAS activation11 in animal models. Thus, the development of a synthetic RAGE inhibitor could be of interest.
SUMMARY
There is increasing evidence supporting a pathogenic role of AOPPs in the initiation and progression of CKD. Intensive studies have also led to a better understanding of the cellular and molecular mechanisms by which AOPPs exert their pathogenic actions. Figure 2 summarizes the mechanism and effect of AOPPs on renal cells and presents an integrated picture for understanding a pathogenic role of AOPPs in CKD. Clearly, AOPPs are a leading candidate target for future CKD treatment, and more studies are needed to develop new therapeutic strategies for the prevention of AOPP-associated renal injury in CKD patients.
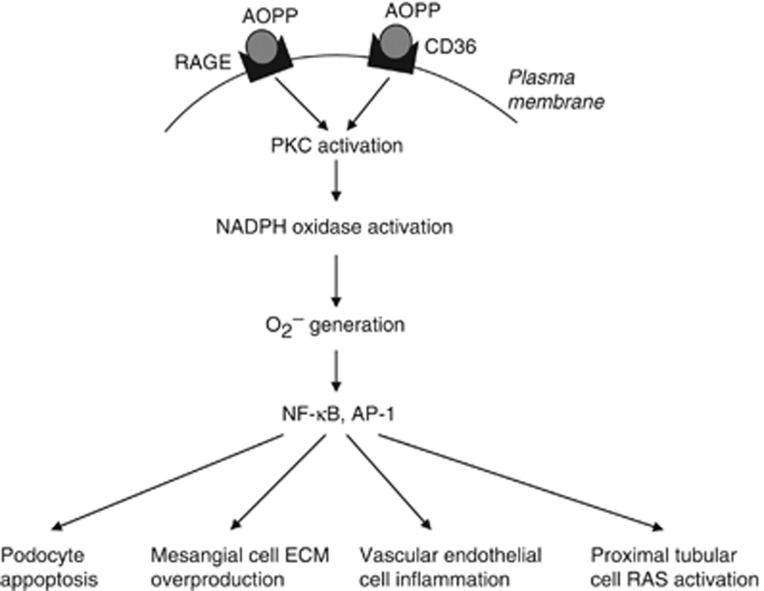
Figure 2.
Schematic diagram summarizing the pathogenic role of advanced oxidation protein products (AOPPs) in the progression of chronic kidney disease (CKD). Chronic accumulation of AOPPs in CKD, through its interaction with RAGE or CD36, triggers a cascade of signaling events that lead to protein kinase C/NAD(P)H oxidase activation, superoxide (O2−) generation, NF-κB, AP-1 activation, and finally podocyte apoptosis, mesangial cell ECM overproduction, vascular endothelial cell inflammation, and tubular RAS activation.
Acknowledgments
This work was supported by the Major State Basic Research Development Program of China (no. 2012CB517700), National Natural Science Foundation for Distinguished Young Scholars of China (no. 81288001), National Nature and Science Young Investigator Grant (no. 81200502), and the Guangdong Medical Association. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
All the authors declared no competing interests.
References
- Tucker PS, Dalbo VJ, Han T, et al. Clinical and research markers of oxidative stress in chronic kidney disease. Biomarkers. 2013;18:103–115. doi: 10.3109/1354750X.2012.749302. [DOI] [PubMed] [Google Scholar]
- Granata S, Zaza G, Simone S, et al. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics. 2009;10:388. doi: 10.1186/1471-2164-10-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Gausson V, Descamps-Latscha B. Are advanced oxidation protein products potenti@@al uremic toxins. Kidney Int Suppl. 2003;84:S11–S14. doi: 10.1046/j.1523-1755.63.s84.47.x. [DOI] [PubMed] [Google Scholar]
- Capeillere-Blandin C, Gausson V, Descamps-Latscha B, et al. Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochim Biophys Acta. 2004;1689:91–102. doi: 10.1016/j.bbadis.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Friedlander M, Nguyen Khoa T, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–2532. [PubMed] [Google Scholar]
- Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–748. doi: 10.1056/NEJMra020109. [DOI] [PubMed] [Google Scholar]
- Floege J, Feehally J. IgA nephropathy: recent developments. J Am Soc Nephrol. 2000;11:2395–2403. doi: 10.1681/ASN.V11122395. [DOI] [PubMed] [Google Scholar]
- Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, et al. Early prediction of IgA nephropathy progression: proteinuria and AOPP are strong prognostic markers. Kidney Int. 2004;66:1606–1612. doi: 10.1111/j.1523-1755.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- Camilla R, Suzuki H, Dapra V, et al. Oxidative stress and galactose-deficient IgA1 as markers of progression in IgA nephropathy. Clin J Am Soc Nephrol. 2011;6:1903–1911. doi: 10.2215/CJN.11571210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Xu J, Zhou ZM, et al. Advanced oxidation protein products activate intrarenal renin-angiotensin system via a CD36-mediated, redox-dependent pathway. Antioxid Redox Signal. 2013;18:19–35. doi: 10.1089/ars.2012.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini P, de Cal M, Cruz D, et al. The role of advanced oxidation protein products in intensive care unit patients with acute kidney injury. J Crit Care. 2010;25:605–609. doi: 10.1016/j.jcrc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Zhou LL, Hou FF, Wang GB, et al. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 2009;76:1148–1160. doi: 10.1038/ki.2009.322. [DOI] [PubMed] [Google Scholar]
- Guo ZJ, Niu HX, Hou FF, et al. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid Redox Signal. 2008;10:1699–1712. doi: 10.1089/ars.2007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XF, Zhou QG, Hou FF, et al. Advanced oxidation protein products induce mesangial cell perturbation through PKC-dependent activation of NADPH oxidase. Am J Physiol Renal Physiol. 2009;296:F427–F437. doi: 10.1152/ajprenal.90536.2008. [DOI] [PubMed] [Google Scholar]
- Li HY, Hou FF, Zhang X, et al. Advanced oxidation protein products accelerate renal fibrosis in a remnant kidney model. J Am Soc Nephrol. 2007;18:528–538. doi: 10.1681/ASN.2006070781. [DOI] [PubMed] [Google Scholar]
- Shi XY, Hou FF, Niu HX, et al. Advanced oxidation protein products promote inflammation in diabetic kidney through activation of renal nicotinamide adenine dinucleotide phosphate oxidase. Endocrinology. 2008;149:1829–1839. doi: 10.1210/en.2007-1544. [DOI] [PubMed] [Google Scholar]
- Coppo R, Camilla R, Alfarano A, et al. Upregulation of the immunoproteasome in peripheral blood mononuclear cells of patients with IgA nephropathy. Kidney Int. 2009;75:536–541. doi: 10.1038/ki.2008.579. [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Gausson V, Nguyen AT, et al. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003;64:82–91. doi: 10.1046/j.1523-1755.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, et al. Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. Am J Kidney Dis. 2005;45:39–47. doi: 10.1053/j.ajkd.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wu S, Jiang J, et al. Accumulation of circulating advanced oxidation protein products is an independent risk factor for ischaemic heart disease in maintenance haemodialysis patients. Nephrology (Carlton) 2012;17:642–649. doi: 10.1111/j.1440-1797.2012.01640.x. [DOI] [PubMed] [Google Scholar]
- Kocak H, Gumuslu S, Sahin E, et al. Advanced oxidative protein products are independently associated with endothelial function in peritoneal dialysis patients. Nephrology (Carlton) 2009;14:273–280. doi: 10.1111/j.1440-1797.2008.01062.x. [DOI] [PubMed] [Google Scholar]
- Marsche G, Frank S, Hrzenjak A, et al. Plasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivo. Circ Res. 2009;104:750–757. doi: 10.1161/CIRCRESAHA.108.193169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SX, Hou FF, Guo ZJ, et al. Advanced oxidation protein products accelerate atherosclerosis through promoting oxidative stress and inflammation. Arterioscler Thromb Vasc Biol. 2006;26:1156–1162. doi: 10.1161/01.ATV.0000214960.85469.68. [DOI] [PubMed] [Google Scholar]
- Marsche G, Semlitsch M, Hammer A, et al. Hypochlorite-modified albumin colocalizes with RAGE in the artery wall and promotes MCP-1 expression via the RAGE-Erk1/2 MAP-kinase pathway. FASEB J. 2007;21:1145–1152. doi: 10.1096/fj.06-7439com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao Y, Nakajou K, Nagai R, et al. CD36 is one of important receptors promoting renal tubular injury by advanced oxidation protein products. Am J Physiol Renal Physiol. 2008;295:F1871–F1880. doi: 10.1152/ajprenal.00013.2008. [DOI] [PubMed] [Google Scholar]
- Zhou LL, Cao W, Xie C, et al. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82:759–770. doi: 10.1038/ki.2012.184. [DOI] [PubMed] [Google Scholar]
- Furuya R, Odamaki M, Kumagai H, et al. Impact of angiotensin II receptor blocker on plasma levels of adiponectin and advanced oxidation protein products in peritoneal dialysis patients. Blood Purif. 2006;24:445–450. doi: 10.1159/000095361. [DOI] [PubMed] [Google Scholar]
- Gradinaru D, Borsa C, Ionescu C, et al. Vitamin D status and oxidative stress markers in the elderly with impaired fasting glucose and type 2 diabetes mellitus. Aging Clin Exp Res. 2012;24:595–602. doi: 10.3275/8591. [DOI] [PubMed] [Google Scholar]