SUMMARY
Streptococcus pneumoniae and Haemophilus influenzae are members of the normal human nasal microbiota with the ability to cause invasive infections. Bacterial invasion requires translocation across the epithelium; however, mechanistic understanding of this process is limited. Examining the epithelial response to murine colonization by S. pneumoniae and H. influenzae, we observed the TLR-dependent downregulation of claudins 7 and 10, tight junction components key to the maintenance of epithelial barrier integrity. When modeled in vitro, claudin downregulation was preceded by upregulation of SNAIL1, a transcriptional repressor of tight junction components, and these phenomena required p38 MAPK and TGF-β signaling. Consequently, downregulation of SNAIL1 expression inhibited bacterial translocation across the epithelium. Furthermore, disruption of epithelial barrier integrity by claudin 7 inhibition in vitro or TLR stimulation in vivo promoted bacterial translocation. These data support a general mechanism for epithelial opening exploited by invasive pathogens to facilitate movement across the epithelium to initiate disease.
INTRODUCTION
Humans are colonized by approximately 1 × 1013–14 bacteria, with the vast majority residing on mucosal surfaces (Mazmanian and Kasper, 2006); however, very few bacterial species cause invasive infection from this location. This is thought to be due, in part, to tissue barriers preventing extensive bacterial penetration and rapid killing once exposed to bloodstream defenses. However, in the upper respiratory tract several species of encapsulated bacteria, including Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis, are able to cause invasive disease in a fraction of their hosts following the establishment of colonization (Bogaert et al., 2004; Kuppermann, 1999; Moxon, 2009). To cause invasive disease, bacteria must attach and translocate across the epithelium, pass through the extracellular matrix, and then enter the bloodstream by crossing the endothelium. Both innate and adaptive immunity are required to monitor and control the levels of colonizing bacteria, and as the first line of defense, the epithelium plays a central role in orchestrating these responses, in addition to functioning as a physical barrier to bacterial entry (Schleimer et al., 2007). To perform these tasks, the epithelium must be both a responsive and a dynamic structure, capable of recognizing bacteria, communicating with, and recruiting immune cells, and allowing the egress of these cells where required. Studies have addressed many of the steps in the process of invasive disease, including adhesion to the epithelium (Adamou et al., 1998), movement through the extracellular matrix (Attali et al., 2008), and movement across endothelial cells (Ring et al., 1998). Mechanistic understanding of bacterial translocation across the epithelium is currently incomplete.
Initial sensing of bacteria by the epithelium occurs primarily by pattern recognition receptors, such as the Toll-like receptors (TLRs), which recognize highly conserved bacterial structures (Medzhitov, 2007). We have previously shown that TLR2 signaling is required for the recruitment of monocytes/macrophages into the nasal lumen during colonization with S. pneumoniae (Zhang et al., 2009) and that TLR signaling in the upper respiratory tract during colonization with either H. influenzae or S. pneumoniae activates both the p38 MAPK and TGF-β signaling pathways (Beisswenger et al., 2007, 2009). Furthermore, we have shown that p38 MAPK and TGF-β signaling is required for bacterial translocation across the epithelium in vitro and that inhibition of these pathways prevents bacterial movement across epithelial monolayers (Beisswenger et al., 2007). How activation of these pathways facilitates bacterial crossing of the epithelial barrier is, however, currently unknown.
In this study, using a combination of in vivo and in vitro models, we found TLR-mediated activation of p38 MAPK and TGF-β signaling by H. influenzae and S. pneumoniae caused downregulation of claudins. Claudins are key components of tight junctions, structures formed between epithelial cells required for the formation of the epithelial barrier, which control movement through the paracellular spaces of the epithelium (Steed et al., 2010). Furthermore, downregulation of claudin expression was preceded by an upregulation of SNAIL1, a transcriptional repressor of tight junction components. We demonstrate the importance of this upregulation, by showing that inhibition of SNAIL1 inhibits bacterial translocation across the epithelium. We also show that inhibition of claudin 7 expression increased bacterial translocation across the epithelium. Furthermore, we show that stimulation of TLRs facilitates the movement of bacteria across the epithelium in vivo.
RESULTS
In Vivo Analysis of Epithelial Responses to Colonization by H. influenzae and S. pneumoniae
We sought to determine the epithelial responses to colonization with either the Gram-negative H. influenzae or Gram-positive S. pneumoniae, by performing microarrays using RNA isolated from the upper respiratory tract. Mice were intranasally inoculated with either PBS, H. influenzae, or S. pneumoniae, and 24 hr postinoculation the mice were sacrificed and RNA was isolated by lavage of the upper respiratory tract. We choose a 24 hr time point, as previous data showed that bacteria can breach the epithelial barrier, and cause invasive infection, during early colonization (Moxon and Murphy, 1978). Additionally, we have shown that this was a time point prior to any significant influx of other cell types into the nasal lumen and would therefore allow us to look at the initial responses of the epithelium only (summarized in Tables S1 and S2, available online) (Beisswenger et al., 2009). Each of the genes of interest identified by the micro-array as differentially expressed showed significant change in expression by qRT-PCR, validating our microarray data (examples shown in Figure S1). S. pneumoniae induced significant upregulation of many of the same genes as H. influenzae (Figures S1A–S1D), suggesting there is an overlapping response to colonization by these bacteria. By both microarray analysis and qRT-PCR, we have previously shown upregulation of Lipocalin 2 during colonization by both S. pneumoniae and H. influenzae (at day 3 postinoculation) (Nelson et al., 2005), and similar upregulation was found in the current study (Tables S1 and S2), further corroborating our current data and providing additional evidence for common host responses to these different bacterial species.
Colonization of the Upper Respiratory Tract by H. influenzae and S. pneumoniae Causes Downregulation of Genes Involved in the Formation of Tight Junctions
H. influenzae and S. pneumoniae both cause invasive disease originating from the nasal mucosa, and a prerequisite for this is the translocation of bacteria across the epithelial barrier. We were therefore interested in understanding if colonization causes modulation of components of the epithelium involved in maintaining barrier integrity. Our microarray analysis identified significant downregulation of Cldn10, encoding the tight junction component claudin 10 (Table S1 and Figure 1). Tight junctions are structures between epithelial cells that control movement through the paracellular space and are therefore crucial for the functional epithelial barrier (Steed et al., 2010). The microarray also showed there was significant downregulation of the gene encoding zinc-α2-glycoprotein (Table S1), and silencing of this gene has recently been shown to induce the epithelial-to-mesenchymal transition with the concomitant loss of epithelial integrity and downregulation of cell adhesion molecules (Kong et al., 2010). This suggests that colonization results in the down-regulation of genes that could directly, or indirectly, result in the opening of the epithelial barrier. We analyzed the expression of other genes involved in the formation of junctional structures between epithelial cells and found significant downregulation of another claudin family member, claudin 7 (Figure 1A). We found no change in the expression of the genes encoding P-cadherin, connexin31, or ZO-1 (data not shown). These data show that bacterial colonization causes changes in the expression of key genes involved in the formation of tight junctions and the maintenance of the epithelial barrier.
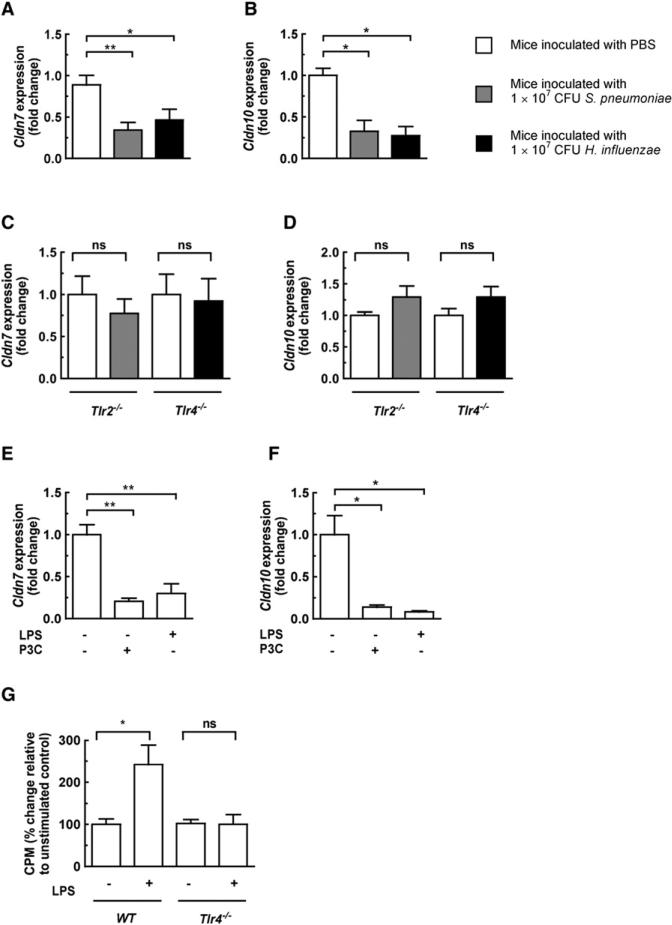
Figure 1. Colonization with H. influenzae or S. pneumoniae Causes a TLR-Dependent Downregulation of Claudins, and TLR Stimulation Facilitates Bacterial Translocation across the Epithelium In Vivo.
(A and B) Wild-type mice were intranasally inoculated with either PBS, S. pneumoniae, or H. influenzae (n = 6, per group) in PBS, and 24 hr postinoculation the mice were sacrificed and RNA was isolated by lavage of the upper respiratory tract.
(C and D) Tlr4−/− mice were intranasally inoculated with either PBS or H. influenzae and Tlr2−/− mice with either PBS or S. pneumoniae (n = 6, per group).
(E and F) Wild-type mice were intranasally inoculated with either LPS (50 μg/naris), P3C (50 μg/naris), or vehicle control (n = 6, per group); 6 hr post-inoculation, mice were sacrificed and RNA was isolated by lavage of the upper respiratory tract.
(G) Wild-type or Tlr4−/− mice (n = 4–6, per group) were pretreated intranasally with LPS (50 μg/naris) or vehicle control and 6 hr later inoculated with [14C]choline-labeled S. pneumoniae; mice were sacrificed 24 postinoculation and counts/g liver tissue compared. Error bars represent ± SEM, *p < 0.05, **p < 0.01; and ns, not significant.
In Vivo Downregulation of Claudin Genes Is Dependent on Toll-like Receptor 2 and 4 Signaling
We next wanted to ascertain how colonization causes downregulation of tight junction-associated genes. Colonization by either species caused a similar downregulation of the same tight junction genes, suggesting that the host response was not tailored to a specific pathogen or due to a certain virulence factor but maybe a generalized response to bacteria. We have previously shown that in our model of nasopharyngeal colonization, Tlr2, but not Tlr4, senses S. pneumoniae (Beisswenger et al., 2009). In contrast, Tlr4 is the predominant receptor for the recognition of H. influenzae (Zola et al., 2008). To investigate the possibility that Toll-like dependent signaling was required for barrier opening, we colonized Tlr4−/− mice with either PBS or H. influenzae, and Tlr2−/− mice with either PBS or S. pneumoniae. In the absence of Tlr4, downregulation of Cldn10 and Cldn7 during colonization with H. influenzae was abrogated; analogously, in the absence of Tlr2, colonization with S. pneumoniae had no effect on Cldn10 or Cldn7 expression (Figures 1C and 1D). To confirm the importance of TLR signaling in Cldn7 and Cldn10 downregulation, we intranasally inoculated mice with either LPS (TLR4 ligand), P3C (Pam3CSK4, a synthetic TLR2 ligand), or vehicle control. These ligands alone were sufficient to cause Cldn7 and Cldn10 downregulation in vivo (Figures 1E and 1F). These observations suggest a general mechanism for barrier opening by bacteria via downregulation of tight junction-associated genes using TLR signaling.
Stimulation of TLR Signaling Promotes Bacterial Translocation across the Epithelium In Vivo
Our data show that activation of TLRs in vivo downregulates tight junction-associated genes, suggesting that TLR stimulation causes disruption of the epithelial barrier. We therefore sought to determine whether this had any influence on bacterial translocation in vivo by using a model of invasive pneumococcal infection (Figure 1G). Culturing blood from animals with invasive disease has demonstrated that bacteremia is often the result of the expansion of a single surviving organism of multiple bacteria that have translocated across the epithelium (Moxon and Murphy, 1978; Rubin, 1987). This implies that culturing blood is an insensitive method for detecting the initial translocation of bacteria. To quantify the population of translocating S. pneumoniae, we grew it in [14C]choline, which specifically labels its C-polysaccharide, and exploited the in vivo stability of C-polysaccharide (Boulware et al., 2007; Faden et al., 2002) and measured radioactivity deposited in the reticuloendothelial system as a means of assessing bacterial invasion. This provides information on all bacteria that translocate irrespective of their survival once transited. We pretreated mice intranasally with either LPS or vehicle control and 6 hr later intranasally inoculated both groups with S. pneumoniae with [14C]choline-labeled cell walls. Stimulation with LPS caused a significant increase in counts in the liver after 24 hr colonization, and this increase was dependent on TLR4 signaling (Figure 1G). These data confirm that TLR stimulation in vivo is sufficient to promote translocation of bacteria across the epithelium.
H. influenzae and S. pneumoniae Cause Downregulation of Genes Involved in the Formation of Tight Junctions in an In Vitro Model of Polarized Airway Epithelial Cells
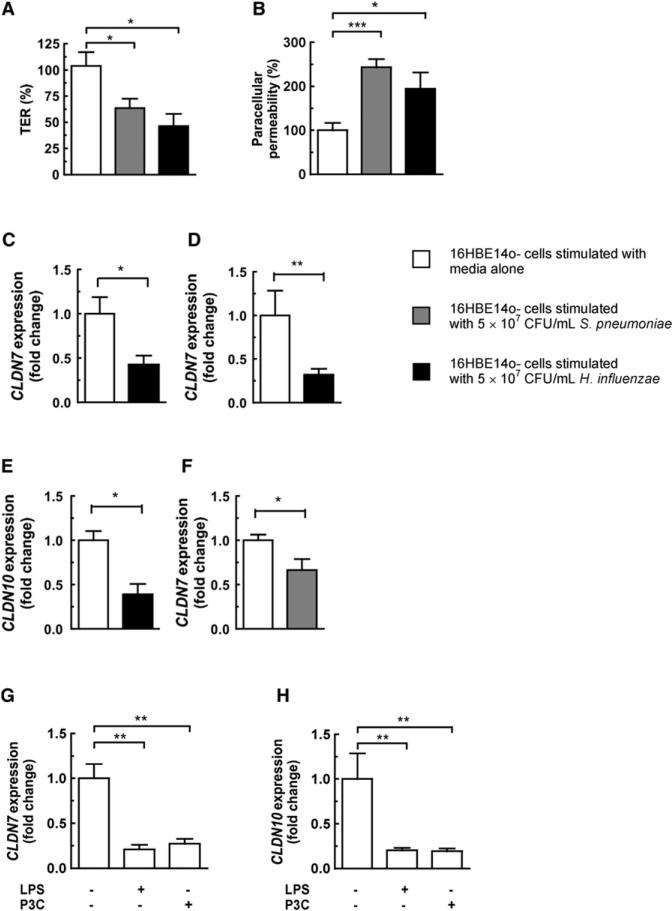
We wanted to gain further mechanistic insight into the causes and consequences of the claudin downregulation we had observed in vivo. We used 16HBE14o- cells grown on transwell inserts as an in vitro model of polarized human bronchial epithelial cells (Beisswenger et al., 2007). We assessed the effect of H. influenzae and S. pneumoniae on epithelial barrier function by measuring the transepithelial resistance (TER) and paracellular permeability of differentiated 16HBE14o- monolayers stimulated with bacteria. After 20 hr of stimulation with H. influenzae, the TER had fallen by 57.6% ± 17.6%, and by 40.3% ± 15.8% after stimulation with S. pneumoniae, relative to uninfected control monolayers (Figure 2A). Paracellular permeability increased 143% ± 24.8% after stimulation with S. pneumoniae, and by 94.6% ± 42.8% after stimulation with H. influenzae (Figure 2B). There was no significant differ ence in lactate dehydrogenase release between epithelial monolayers stimulated with PBS or by either bacteria, confirming that disruption of epithelial barrier integrity was not due to cytotoxicity (data not shown). To relate these data to our in vivo observations, we measured the expression of both CLDN7 and CLDN10 after bacterial stimulation of monolayers. After 3 hr of stimulation with H. influenzae there was significant downregulation of CLDN7 (Figure 2C), and by 20 hr there was downregulation of both CLDN7 and CLDN10 (Figures 2D and 2E). Stimulation with S. pneumoniae caused significant downregulation of CLDN7 after 20 hr stimulation (Figure 2F); although there was a trend toward downregulation of CLDN10, this was not statistically significant (data not shown). These data provided further evidence that H. influenzae and S. pneumoniae may disrupt epithelial barrier integrity by downregulation of tight junction-associated genes.
Figure 2. Stimulation of Epithelial Monolayers with Bacteria or TLR Ligands Causes Downregulation of Claudin Expression in Epithelial Monolayers, and This Correlates with Bacterial Disruption of Barrier Integrity.
(A and B) 16HBE14o- monolayers were stimulated with either bacteria, and the transepithelial resistance (TER) and paracellular permeability were measured after 20 hr. TER is shown relative to the TER at 0 hr, and paracellular permeability is shown relative to permeability of untreated control monolayers.
(C–F) Expression of tight junction-associated genes after bacterial stimulation for 3 hr (C) or 20 hr (D–F).
(G and H) 16HBE14o- monolayers were stimulated for 20 hr with either LPS (2μg/mL) or P3C (150μg/mL). Values represent seven to nine independent determinations ± SEM, *p < 0.05, **p < 0.01.
Stimulation by Toll-like Receptor Ligands Is Sufficient to Cause Downregulation of Genes Involved in the Formation of the Tight Junctions of Polarized Bronchial Epithelial Cells
In vivo downregulation of tight junction-associated genes required TLR signaling (Figures 1C and 1D). We (Beisswenger et al., 2007) and others (He et al., 2009; Pawar et al., 2009) have previously shown that stimulation of epithelial cells with TLR2 and TLR4 ligands disrupts epithelial barrier integrity in vitro. We wanted to establish whether TLR signaling was also required for claudin downregulation in our in vitro model. Stimulation for 20 hr with LPS caused a significant downregulation of both CLDN7 and CLDN10, and stimulation with P3C also caused a significant downregulation of CLDN7 and CLDN10 (Figures 2G and 2H). These data show that activation of TLR signaling is sufficient to downregulate tight junction-associated genes and confirms that our in vitro model recapitulates our in vivo observations.
Activation of Both p38 MAPK and TGF-β Pathways Is Required for Downregulation of Genes Involved in the Formation of Tight Junctions and Disruption of Epithelial Barrier Integrity
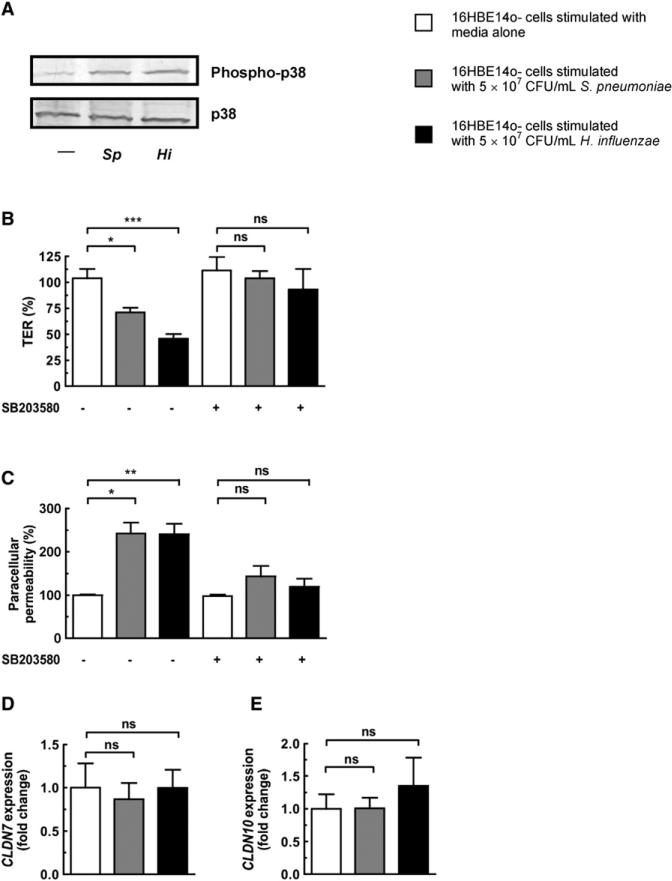
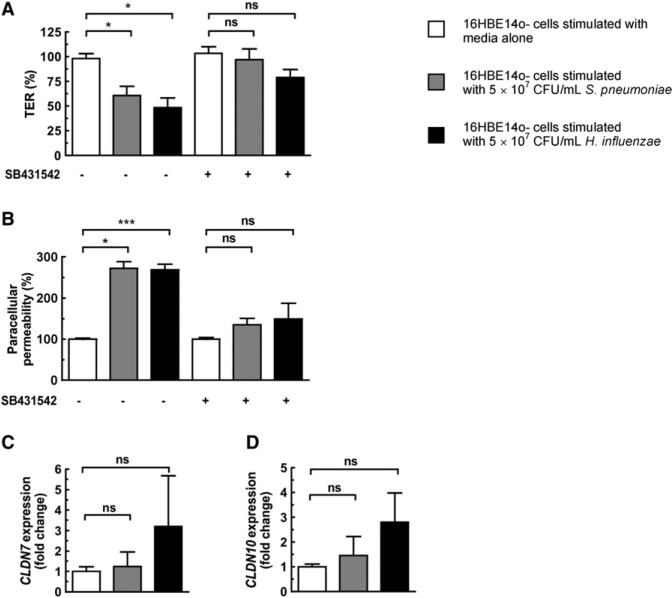
We wanted to define what signaling pathways were being activated downstream of TLR ligation and whether they were mediating claudin downregulation and loss of barrier integrity. We have previously shown in vivo that during colonization there is increased phosphorylation of p38, SMAD2, and SMAD3 in the respiratory epithelium (Beisswenger et al., 2009), demonstrating that H. influenzae and S. pneumoniae are able to activate both p38 MAPK and TGF-β signaling pathways. To investigate the importance of these pathways in mediating the opening of the epithelium and downregulation of claudin genes, we treated monolayers with either SB203580 (p38 MAPK inhibitor) or SB431542 (TGF-β signaling inhibitor) prior to stimulation with bacteria. We detected p38 phosphorylation in epithelial monolayers after bacterial stimulation (Figure 3A), and found that the decreased TER and increased paracellular permeability caused by bacterial stimulation are abrogated after inhibition of p38 MAPK signaling (Figures 3B and 3C). Inhibition of TGF-β signaling also prevented the decrease in TER and increase in paracellular permeability after bacterial stimulation of epithelial monolayers (Figures 4A and 4B). Importantly, the downregulation of CLDN7 and CLDN10 is lost after inhibition of either pathway (Figures 3D and 3E and Figures 4C and 4D), providing further evidence that regulating claudin levels is key to the maintenance of epithelial barrier integrity.
Figure 3. Downregulation of Tight Junction-Associated Genes and Concomitant Disruption of Epithelial Barrier Integrity Requires p38 MAPK Signaling.
(A) Western blot showing that bacterial stimulation of 16HBE14o- monolayers for 1 hr activates the p38 MAPK signaling pathway.
(B–E) 16HBE14o- monolayers were treated with the p38 MAPK inhibitor SB203580 at a final concentration of 10 μM for 0.5 hr prior to stimulation of monolayers with either bacteria for 20 hr. (B and C) TER and paracellular permeability were measured after 20 hr. TER is shown relative to the TER at initial time point (0 hr), and paracellular permeability is shown relative to permeability of untreated control monolayers. (D and E) Expression of tight junction-associated genes after bacterial stimulation for 20 hr. Values represent four to eight independent determinations ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant.
Figure 4. Downregulation of Tight Junction-Associated Genes and Concomitant Disruption of Epithelial Barrier Integrity Requires TGF-β Signaling.
(A–D) 16HBE14o- monolayers were treated with the TGF-β inhibitor SB431542 at a final concentration of 10 μM for 0.5 hr prior to stimulation of monolayers with either bacteria for 20 hr. (A and B) TER and paracellular permeability were measured after 20 hr. TER is shown relative to the TER at initial time point (0 hr), and paracellular permeability is shown relative to permeability of untreated control monolayers. (C and D) Expression of tight junction-associated genes after bacterial stimulation for 20 hr. Values represent four to eight independent determinations ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant.
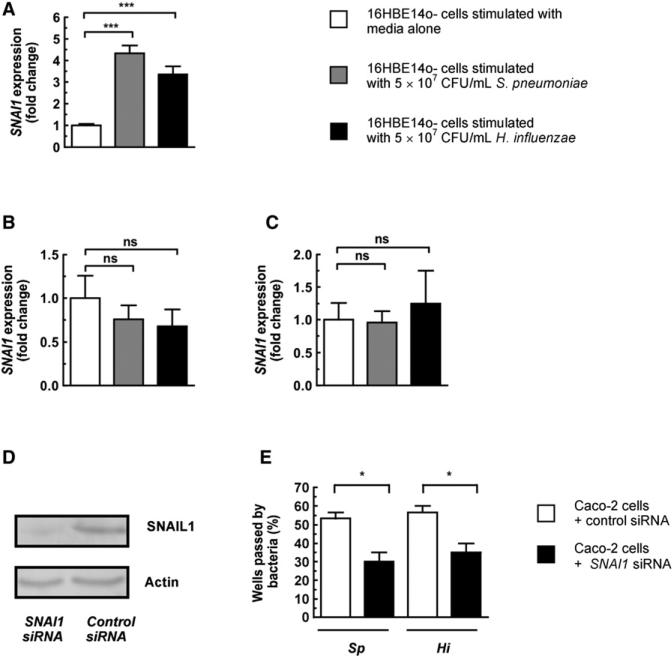
SNAIL1 Is Upregulated in Epithelial Monolayers by H. influenzae and S. pneumoniae in a p38 MAPK- and TGF-β-Dependent Manner, and Inhibition of SNAIL1 Expression Delays Bacterial Movement across Epithelial Monolayers
Having established that bacteria activate both p38 MAPK and TGF-β pathways in vivo (Beisswenger et al., 2009) and that they are required for downregulation of tight junction-associated genes in vitro, we wanted to gain further mechanistic insight into how activation of these signaling pathways could affect tight junction-associated genes. We analyzed the expression of SNAIL1, a transcription factor responsible for transcriptional repression of numerous genes involved in junction formation between epithelial cells (Batlle et al., 2000; Carrozzino et al., 2005; Ikenouchi et al., 2003; Vincent et al., 2009). It has been shown that inducible expression of SNAIL1 disrupts barrier integrity (Carrozzino et al., 2005) and that during the epithelial-to-mesenchymal transition induced by TGF-β, SNAIL1 is recruited to the promoter of tight junction-associated genes and acts in concert with SMAD3 and SMAD4 to represses their expression (Vincent et al., 2009). Furthermore, SNAIL1 has been shown to bind to the E box consensus sequence found in the promoter region of Cldn7 repressing its transcription (Ikenouchi et al., 2003), and the promoter of Cldn10 also contains the E box consensus sequence. After 2 hr stimulation with either H. influenzae or S. pneumoniae, prior to any changes in tight junction gene expression, there was a significant upregulation of SNAI1 expression in epithelial monolayers (Figure 5A). To provide further detail linking SNAIL1 expression and claudin downregulation, we treated monolayers with either SB203580 or SB431542 prior to stimulation with bacteria. Inhibition of either the p38 MAPK or TGF-β pathways prevented the upregulation of SNAI1 by stimulation with either bacteria (Figures 5B and 5C). To determine whether the upregulation of SNAIL1 was involved in epithelial barrier disruption, siRNA was used to inhibit SNAI1 expression in epithelial monolayers. Due to the difficulty of using siRNA with 16HBE14o- cells, we used polarized Caco-2 cells, which are more amenable to transfection with siRNA. Inhibition of SNAIL1 expression was confirmed by western blotting (Figure 5D). We found that inhibition of SNAIL1 expression caused inhibition of S. pneumoniae and H. influenzae translocation across epithelial monolayers (Figure 5E). These data show that activation of p38 MAPK and TGF-β signaling upregulates SNAI1 expression and that inhibition of SNAIL1 inhibits bacterial translocation across the epithelium, thus providing direct evidence that SNAIL1 activity is required for barrier disruption.
Figure 5. H. influenzae and S. pneumoniae Upregulate SNAI1 Expression, and This Is Dependent on TGF-β and p38 MAPK Signaling, and Inhibition of SNAIL1 Expression Delays Bacterial Translocation across Epithelial Monolayers.
(A) 16HBE14o- monolayers were stimulated with either bacteria for 2 hr, and RNA was then harvested.
(B and C) 16HBE14o- monolayers were treated with the p38 MAPK inhibitor SB203580 (B) or TGF-β inhibitor SB431542 (C) at a final concentration of 10 μM for 0.5 hr prior to stimulation of monolayers with either bacteria for 20 hr.
(D) Caco-2 epithelial monolayers were transfected with either an siRNA targeting SNAI1 or control siRNA, and the level of SNAIL1 protein was compared by western blot.
(E) Bacterial translocation across Caco-2 monolayers transfected with either SNAI1 siRNA or control siRNA. The basolateral compartment was sampled every 0.5 hr for the presence of bacteria; data are based on the time point after addition of bacteria when ≥50% of transwells were passed in control wells for the species of translocated bacteria indicated below the graph. Bacteria were at a density of 5 × 106 CFU/mL to the apical compartment. Values represent five to six independent determinations ± SEM, ***p < 0.001, *p < 0.05; ns, not significant.
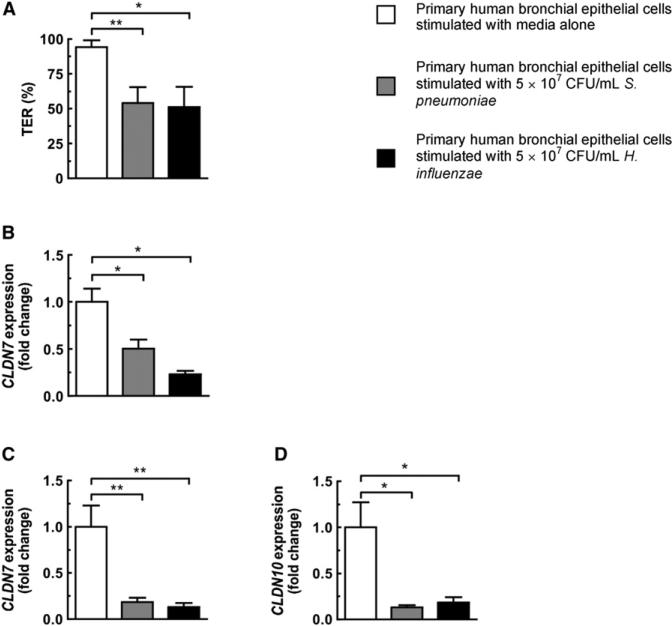
Stimulation of Primary Human Respiratory Epithelial Cells with Either H. influenzae or S. pneumoniae Causes Loss of Barrier Integrity Accompanied by Downregulation of Tight Junction-Associated Genes
The in vitro epithelial model using 16HBE14o- cells displays many characteristics of differentiated human bronchial epithelial cells while allowing easy experimental manipulation. However, 16HBE14o- cells are a transformed cell line and thus less physiologically relevant than primary cells. To address this concern, we grew normal human bronchial epithelial cells on transwell inserts and allowed them to differentiate as air-liquid cultures. Stimulation of these monolayers with bacteria resulted in disruption of the epithelial barrier, with S. pneumoniae and H. influenzae causing a drop in TER of 40.3% ± 12.6% and 43.3% ± 15.5%, respectively (Figure 6A), analogous to stimulation of 16HBE14o- cells. Concurrent with this drop in TER, there was downregulation of CLDN7 after stimulation with either bacteria for 3 hr (Figure 6B), and by 20 hr both CLDN10 and CLDN7 were downregulated (Figures 6C and 6D). Data from primary human epithelial cells further confirmed mouse colonization and 16HBE14o- cell culture findings that S. pneumoniae and H. influenzae cause downregulation of tight junction-associated genes and disruption of the epithelial barrier.
Figure 6. Stimulation of Primary Human Bronchial Epithelial Cells by H. influenzae or S. pneumoniae Causes Disruption of Barrier Integrity and Downregulation of Tight Junction-Associated Genes.
(A) Normal human bronchial epithelial monolayers were stimulated with bacteria for 20 hr. TER was measured after 20 hr and is shown relative to the TER at initial time point (0 hr).
(B–D) Expression of tight junction-associated genes after stimulation with either H. influenzae or S. pneumoniae for 3 hr (B) or 20 hr (C and D). Values represent six to 11 independent determinations ± SEM, *p < 0.05 and **p < 0.01.
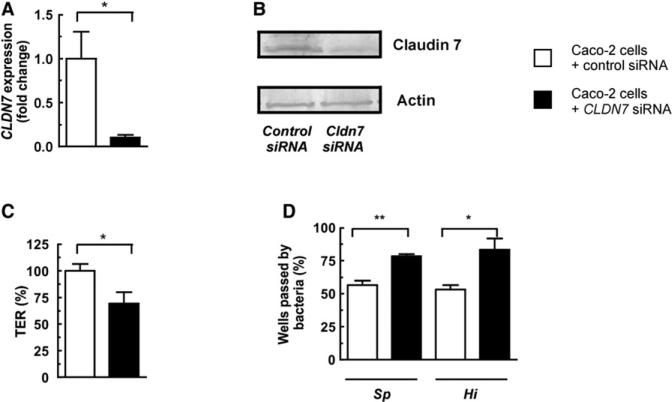
Inhibition of CLDN7 Expression Causes Loss of Barrier Integrity, Allowing More Rapid Translocation of Bacteria across Epithelial Monolayers
To understand the consequences of tight junction gene downregulation on bacterial translocation across the epithelial barrier, we used siRNA to inhibit claudin expression in epithelial monolayers. Due to the low level of CLDN10 expression in these cells, we focused on CLDN7. Successful inhibition of CLDN7 expression was confirmed by both qRT-PCR and western blotting (Figures 7A and 7B). Inhibition of CLDN7 expression was sufficient to cause a significant drop in TER (Figure 7C) and, importantly, facilitated increased bacterial translocation across epithelial monolayers (Figure 7D). This is in agreement with previous data showing that knockdown of CLDN7 in MKN28 cells (a gut adenocarcinoma cell line) resulted in an increase in the paracellular permeability of epithelial monolayers (Oshima et al., 2008).
Figure 7. Loss of Claudin 7 Causes Barrier Disruption and Increased Bacterial Translocation across Epithelial Monolayers.
(A and B) Caco-2 epithelial monolayers were transfected with either siRNA targeting CLDN7 or control siRNA, and the levels of CLDN7 transcript and protein were measured.
(C and D) TER and bacterial translocation across Caco-2 monolayers transfected with either CLDN7 siRNA or control siRNA. The basolateral compartment was sampled every 0.5 hr for the presence of bacteria, and transmigration data are based on the time point after addition of bacteria when ≥50% of transwells were passed in control wells for the species of translocated bacteria indicated below the graph. Bacteria were added at a density of 5 × 106 CFU/mL to the apical compartment. Values represent six to 14 independent determinations ± SEM, *p < 0.05 and **p < 0.01.
DISCUSSION
Diverse bacterial species, including S. pneumoniae and H. influenzae, that colonize the mucosal surfaces are on occasion able to breach the epithelial barrier and cause invasive infection and systemic disease. In this study we used a mouse model of nasopharyngeal colonization, in combination with in vitro cell culture models, to decipher the mechanism of how these bacteria cross the epithelium. Our findings suggest that the epithelial barrier is compromised as a programmed response to conserved microbial signals.
We have previously shown in vivo that colonization by S. pneumoniae and H. influenzae activates both p38 MAPK and TGF-β signaling cascades in the respiratory epithelia (Beisswenger et al., 2009) and that in vitro activation of these pathways increases translocation of bacteria across epithelial monolayers (Beisswenger et al., 2007). Here we show that S. pneumoniae and H. influenzae activate TLR signaling in vivo, resulting in the downregulation of claudins, tight junction components required for maintenance of epithelial integrity. Using in vitro models, we showed that this was dependent on both TGF-β and p38 MAPK signaling cascades and, importantly, demonstrated that down-regulation of these components increases the rate of bacterial translocation across the epithelia. We provide further mechanistic detail by showing that the decreased levels of claudins inversely correlated with the expression of SNAI1, a transcriptional repressor of claudins, and increased expression of SNAI1 also required both p38 MAPK and TGF-β signaling. Stimulation of TLRs in vivo facilitated the movement of bacteria across the epithelium, confirming the in vivo role TLR-mediated barrier disruption plays in bacterial translocation. We therefore posit a model whereby colonizing bacteria activate p38 MAPK and TGF-β signaling pathways using TLRs to cause a transient increase in SNAIL1, with a subsequent downregulation of tight junction components, and that invasive pathogens can take advantage of this to increase their passage between cells. The paracellular route for S. pneumoniae and H. influenzae translocation is supported by previous data from us, and others, showing that S. pneumoniae colocalizes with the cell junction marker E-cadherin (Beisswenger et al., 2007), and by electron micrographs showing that H. influenzae move through the paracellular space between epithelial cells (van Schilfgaarde et al., 1995). Our data demonstrate links between the activation of TLRs via p38 MAPK and TGF-β, the expression of SNAIL1, and the subsequent downregulation of claudin genes. Prior to this study, the role of SNAIL1 has only been documented in the epithelialmesenchymal transition and a variety of developmental processes (Batlle et al., 2000; Morales et al., 2007).
Our data show that there is a strict requirement for the activation of both p38 MAPK and TGF-β signaling cascades for the upregulation of SNAIL1 and downregulation of claudins. It is known that the transcription factor ATF-2 is activated synergistically by both the SMADs and p38 MAPK (Sano et al., 1999), so it is therefore possible that these pathways converge at this point in our system, as supported by our previous work showing that ATF-2 is phosphorylated in the upper respiratory tract in vivo (Beisswenger et al., 2009). A further question is how bacteria activate the TGF-β signaling cascade. We have shown previously that stimulation of epithelial cells with either S. pneumoniae or H. influenzae does not result in a detectable increase in the levels of TGF-β1 (Beisswenger et al., 2007), and this is in agreement with data from Jono et al. (2002), who could not detect increased production of TGF-β1, -2, or -3 after stimulation of epithelial cells with nontypeable H. influenzae. TGF-β production is regulated at the transcriptional, posttranscriptional, and posttranslational levels (Moustakas and Heldin, 2009), with all TGF-β ligands synthesized as precursor proteins with an N-terminal propeptide. It is therefore possible that there is release of the active TGF-β ligands from stores of this latent form present in the extracellular matrix after stimulation by either S. pneumoniae or H influenzae, as suggested by Jono et al. (2002) for nontypeable H. influenzae. Alternatively, other TGF-β ligands, such as activins and BMPs, may be activating the TGF-β signaling cascade. There is also precedent for direct crosstalk between TLRs and TGF-β signaling at multiple levels (Lu et al., 2007; Xiao et al., 2003). The TGF-β inhibitor used in our study inhibits the kinase activity of the TGF-β type I receptor, suggesting the mechanism of crosstalk must occur at, or before, activation of the TGF-β receptor. A possible mechanism that could account for crosstalk between TGF-β and TLR signaling pathways at the receptor level has been postulated (Lu et al., 2007). They show that there is interaction between the IL-1 receptor and TGF-β receptor, and that at high concentrations of TGF-β1, NF-kB is activated via IL-1R, and vice versa, at high concentrations of IL-1β there is activation of SMADs via the TGF-β type I and II receptors. Due to the shared signaling components between the IL-1R and TLRs, cross-activation via TLRs was also found, thus raising the possibility of TLR activation of TGF-β signaling in our model by receptor-receptor interactions between TLRs and the TGF-βR, possibly independent of any TGF-β ligands.
In contrast to mechanisms described for other invasive bacterial pathogens, barrier opening exploited by S. pneumoniae and H. influenzae described here is not a due to a certain virulence factor or specific pathogen feature but occurs by conserved pathways of the innate immune system and consequently could be a route of translocation used by many bacterial species. This raises the question of why the host would want to open the epithelial barrier in response to bacterial challenge. Given the importance of TLRs in mucosal defense, it is likely that the TLR-mediated opening of the epithelial barrier is part of this conserved host response, allowing for the egress of immune cells and antimicrobial factors in to the lumen. Colonization with either H. influenzae or S. pneumoniae causes a robust influx of neutrophils into the nasal lumen (Zhang et al., 2009; Zola et al., 2008), and to enter the lumen the neutrophils must cross the epithelial barrier. It has been shown that as the number of neutrophils crossing the epithelial barrier increases there is a concomitant decrease in epithelial barrier integrity due to increased opening of the paracellular spaces (Nash et al., 1987), showing that in inflammatory conditions the epithelial barrier can become “leaky,” allowing for greater influx of immune cells. In contrast, other studies have shown that activation of TLR2 facilitates neutrophil transmigration while the epithelial barrier integrity is maintained (Chun and Prince, 2009), and in vitro studies show that TLR2 activation transiently strengthens the epithelial barrier (Cario et al., 2004). In the current and previous studies, we (Beisswenger et al., 2007) and others (Pawar et al., 2009) found that TLR2 activation does disrupt barrier integrity; however, we used high concentrations of TLR2 ligands. It is therefore likely that when bacterial numbers are low the epithelium is sufficiently porous to allow for the egress of the required number of neutrophils, but with increasing bacterial load the paracellular spaces in the epithelium open to facilitate the egress of a greater number of antimicrobial factors such as neutrophils, to control bacterial numbers. This also seems to be true for TLR4, as previous work has shown that stimulation of bronchial cells with low concentrations of LPS does not disrupt barrier integrity; at high concentrations, however, there is reduced barrier function (He et al., 2009). Strengthening and weakening of the epithelial barrier through activation of a single receptor, the 5-HT7 receptor, by serotonin has been documented (Pai and Horseman, 2008). This study showed a transient strengthening of the barrier following a short stimulation of epithelial monolayers at low ligand concentrations. Conversely, at high ligand concentrations and at later time points, the barrier was weakened, and analogously to our study, barrier disruption was dependent on p38 MAPK activation. Another example of cellular egress through the paracellular spaces of the epithelium is provided by dendritic cells. These cells have been shown to penetrate the epithelium of both the upper respiratory tract (Takano et al., 2005) and the gut (Rescigno et al., 2001) to allow for direct sampling of bacteria by extending processes that express tight junction components on their surface between epithelial cells. The mechanism of how the existing tight junction components are remodeled to accommodate this is currently unknown. Furthermore, in the upper respiratory tract the processes were only seen beyond the apical tight junctions during inflammation, and the number of intraepithelial processes in the gut is increased after TLR stimulation of the epithelium (Chieppa et al., 2006). Again, this suggests that increased bacterial stimulation of the epithelium results in opening of the paracellular spaces to accommodate greater numbers of immune cells, and this could be due to the TLR-dependent modulation of tight junction components described in this study. In these systems, coculture models were used with dendritic and epithelial cells, and the expression of tight junction components on the dendritic cells maintained epithelial barrier integrity during sampling, in contrast to data present in this study showing disruption of the barrier using epithelial monolayers. However, previous work has documented the differences in behavior between monolayers and cocultures (Lehmann et al., 2009), and the drop in TER due to paracellular opening in the monolayers is not found in cocultures, probably because the resident immune cells rapidly occupy the available paracellular space. The work from these studies and ours shows that the epithelium is constantly being remodeled due to bacterial stimulation and that this is key for the immune system to monitor and control bacterial numbers.
As previously stated, the progression from colonization to invasive H. influenzae or S. pneumoniae disease is a complex multistep process. Previous work has shown that H. influenzae cultured from the blood of bacteremic animals are derived from a small number of parent organisms, often a single organism (Moxon and Murphy, 1978), suggesting that there is a “bottleneck” in invasion and that this could be crossing the epithelium. Moxon and Murphy showed that after intranasal inoculation with a mixture of two genetically marked strains of H. influenzae, both strains were present in the blood within 6 hr; however, after 12 hr almost all cultures were of either single strain only. These data suggest that crossing of the epithelial barrier can be an early event during colonization and is not the major bottleneck in the progression to invasive disease, which must be downstream of translocation across the epithelium. It has been shown that the sera of healthy individuals may contain pneumococcal DNA, with the highest rate in children under 2 years of age (Dagan et al., 1998). Therefore, crossing the epithelial barrier is likely to be a common event during colonization, which is not restricted to a subpopulation of more invasive variants of H. influenzae or S. pneumoniae. This fits with our model of a general mechanism for epithelial barrier opening via TLR stimulation, raising the possibility that commensal bacteria, not just opportunistic pathogens, could cross the epithelium if present in sufficient numbers. This is supported by recent data showing that Lactobacillus murinus, a gut commensal, can translocate across the epithelium of the upper respiratory tract and can be detected in the nasal-associated lymphoid tissue (Costalonga et al., 2009).
The events described in this study link to a wider phenomenon of bacterial translocation and its importance in the development and modulation of mucosal and systemic immunity. Animals raised in a germ-free environment show numerous defects including, but not limited to, problems with the digestive, respiratory, and immune systems (Macpherson and Harris, 2004). We (Clarke et al., 2010) and others (Mazmanian et al., 2005) have described the importance of bacteria and bacterial products in the functional maturation of these systems. Previous work has shown that bacterial products are present systemically in the host and under normal circumstances are important for the proper functioning of the immune system (Clarke et al., 2010), but in pathologic situations increased levels of circulating bacterial products cause deleterious chronic immune activation (Brenchley et al., 2006). One important unresolved question is how these bacterial products gain access to the systemic circulation, and our model of TLR-mediated opening of paracellular spaces provides one potential mechanism for bacteria to reach distal sites. It is probable that the vast majority of translocated bacteria are rapidly killed by the innate immune system, leaving only bacterial fragments, such as peptidoglycan, that are recalcitrant to degradation. It is the ability of pathogens such as S. pneumoniae and H. influenzae to evade these defenses, rather than a unique ability to transit tissue barriers, that allows them to cause invasive disease. It will be important to determine whether it is translocation of whole bacteria in this manner or sampling of bacterial products directly from mucosal surfaces that is the primary source of systemic bacterial products in the circulation.
EXPERIMENTAL PROCEDURES
Bacterial Strains
H. influenzae 636 (a type b clinical isolate) and S. pneumoniae P1121 (a type 23F clinical isolate) were grown as described previously (Beisswenger et al., 2009; Clarke et al., 2010). S. pneumoniae P1547 (a type 6A strain, an invasive clinical isolate) was grown in [14C]choline, as described previously (Kim and Weiser, 1998).
Murine Model of Nasopharyngeal Colonization and Invasive Disease
Six- to eight-week-old C57BL/6J mice were obtained from Taconic Laboratories and housed in accordance with Institutional Animal Care and Use Committee protocols. TLR4-deficient animals (C57BL10ScNJ) were obtained from Jackson Laboratories, and TLR2-deficient animals were described previously (Beisswenger et al., 2009; Zhang et al., 2009). Mice were intranasally inoculated with 107 CFU of either H. influenzae or S. pneumoniae in PBS. TLR ligands were intranasally inoculated at 0 and 3 hr, and mice were sacrificed after 6 hr. For microarray and qRT-PCR analysis, mice were sacrificed 24 hr postinoculation. Tissue samples of the reticuloendothelial system (liver or spleen) were taken to assay for radioactivity (counts/g tissue) by liquid scintillation counting 24 hr postinoculation.
RNA Extraction and Microarray Analysis
RNA was isolated by lavage of the upper respiratory tract as described previously (Beisswenger et al., 2009); RNA quality and integrity was verified using an Agilent Bioanalyzer. Samples (n = 5 per group) were hybridized to Affymetrix GeneChip Mouse Gene 1.0 ST chips, and microarray processing was performed by the University of Pennsylvania Microarray Core Facility as described in the Affymetrix GeneChip Expression Analysis Technical Manual. RNA (5 μg) was used per chip with no amplification beyond RNA transcription. .CEL files were imported into the Partek Genomic Suite for data analysis, and data were normalized using the RMA (Robust Multichip Averaging) algorithm.
Cell Culture
16HBE14o- and Caco-2 cells were grown and maintained as described previously (Beisswenger et al., 2007). Primary human bronchial epithelial cells were isolated and maintained as described in Kreindler et al. (2009). TER was measured with an ohmmeter (EVOM, World Precision Instruments), and transmigration assays were performed as described previously (Beisswenger et al., 2007). P3C was from InvivoGen, and LPS was purified from H. influenzae H636 as described in Masoud et al. (1997). SB203580 (BIOSOURCE) and SB431542 (Sigma) were resuspended in DMSO. Paracellular permeability was measured using the nonionic macromolecular tracer FITC-dextran 4000 (Sigma). Epithelial monolayers were stimulated with bacteria for 20 hr, and then basolateral and apical media was removed; the basolateral media was replaced with HBSS and the apical compartment with HBSS + FITC-dextran 4000 (10 mg/mL). After 1 hr, the basolateral compartment was sampled and absorbance at 490 nm measured. RNA was isolated from cultured cells using an RNeasy kit according to the manufacturer's instructions (QIAGEN). Cytotoxicity was measured by lactic dehydrogenase release (Roche).
Quantitative RT-PCR
cDNA was synthesized using a high-capacity cDNA reverse transcription kit according to the manufacturer's instructions (Applied Biosystems), and qRT-PCR reactions were carried out as described before (Beisswenger et al., 2009) using primers listed in the Supplemental Information and SYBR Green PCR master mix (Applied Biosystems) according to the manufacturer's instructions. Differential expression was calculated using the ΔΔCT method and shown relative to Gapdh ± SEM.
Western Blotting
Cells were lysed in cell lysis buffer (Cell Signaling Technology), and equivalent total protein concentrations were separated on a 4%–15% polyacrylamide gel (BIORAD). Proteins were transferred to a PVDF membrane (Millipore) and probed with either anti-phospho-p38 MAPK (Cell Signaling), anti-p38 MAPK (Cell Signaling), anti-claudin 7 (Santa Cruz), anti-SNAIL1 (Abcam), or anti-actin (Santa Cruz) antibodies. All secondary antibodies were alkaline phosphatase conjugated, and membranes were developed with 4-Nitro blue tetrazolium chloride/5-Bromo-4-Chloro-3-indolyl-phosphate.
Inhibition of SNAIL1 and Claudin 7 Expression by siRNA
siRNA oligonucleotides against CLDN7 (Ambion), SNAI1 SMARTpool (Dharmacon), and control siRNA were used to transfect Caco-2 cells using Dharma-FECT1 transfection reagent according to the manufacturer's instructions. Cells were used 72 hr posttransfection.
Statistical Analysis
Differences between groups were compared using the unpaired Student's t test (GraphPad Prism 4).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. James L. Kreindler for primary human bronchial cells and the University of Pennsylvania School of Medicine Microarray and Bioinformatics core for performing microarrays and assisting with data analysis. This work was supported by grants AI038446 (J.N.W.), AI044231 (J.N.W.), and AI078538 (J.N.W.) from the U.S. Public Health Service.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes one figure, two tables, and primer sequences and can be found with this article online at doi:10.1016/j.chom.2011.04.012.
REFERENCES
- Adamou JE, Wizemann TM, Barren P, Langermann S. Adherence of Streptococcus pneumoniae to human bronchial epithelial cells (BEAS-2B). Infect. Immun. 1998;66:820–822. doi: 10.1128/iai.66.2.820-822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali C, Frolet C, Durmort C, Offant J, Vernet T, Di Guilmi AM. Streptococcus pneumoniae choline-binding protein E interaction with plasminogen/plasmin stimulates migration across the extracellular matrix. Infect. Immun. 2008;76:466–476. doi: 10.1128/IAI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Beisswenger C, Coyne CB, Shchepetov M, Weiser JN. Role of p38 MAP kinase and transforming growth factor-beta signaling in transepithelial migration of invasive bacterial pathogens. J. Biol. Chem. 2007;282:28700–28708. doi: 10.1074/jbc.M703576200. [DOI] [PubMed] [Google Scholar]
- Beisswenger C, Lysenko ES, Weiser JN. Early bacterial colonization induces toll-like receptor-dependent transforming growth factor beta signaling in the epithelium. Infect. Immun. 2009;77:2212–2220. doi: 10.1128/IAI.01224-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- Boulware DR, Daley CL, Merrifield C, Hopewell PC, Janoff EN. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J. Infect. 2007;55:300–309. doi: 10.1016/j.jinf.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Carrozzino F, Soulie P, Huber D, Mensi N, Orci L, Cano A, Feraille E, Montesano R. Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins. Am. J. Physiol. Cell Physiol. 2005;289:C1002–C1014. doi: 10.1152/ajpcell.00175.2005. [DOI] [PubMed] [Google Scholar]
- Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Prince A. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe. 2009;5:47–58. doi: 10.1016/j.chom.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costalonga M, Cleary PP, Fischer LA, Zhao Z. Intranasal bacteria induce Th1 but not Treg or Th2. Mucosal Immunol. 2009;2:85–95. doi: 10.1038/mi.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan R, Shriker O, Hazan I, Leibovitz E, Greenberg D, Schlaeffer F, Levy R. Prospective study to determine clinical relevance of detection of pneumococcal DNA in sera of children by PCR. J. Clin. Microbiol. 1998;36:669–673. doi: 10.1128/jcm.36.3.669-673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden H, Heimerl M, Varma C, Goodman G, Winkelstein P. Urinary excretion of pneumococcal cell wall polysaccharide in children. Pediatr. Infect. Dis. J. 2002;21:791–793. doi: 10.1097/00006454-200208000-00020. [DOI] [PubMed] [Google Scholar]
- He D, Su Y, Usatyuk PV, Spannhake EW, Kogut P, Solway J, Natarajan V, Zhao Y. Lysophosphatidic acid enhances pulmonary epithelial barrier integrity and protects endotoxin-induced epithelial barrier disruption and lung injury. J. Biol. Chem. 2009;284:24123–24132. doi: 10.1074/jbc.M109.007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J. Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- Jono H, Shuto T, Xu H, Kai H, Lim DJ, Gum JR, Jr., Kim YS, Yamaoka S, Feng XH, Li JD. Transforming growth factor-beta -Smad signaling pathway cooperates with NF-kappa B to mediate nontypeable Haemophilus influenzae-induced MUC2 mucin transcription. J. Biol. Chem. 2002;277:45547–45557. doi: 10.1074/jbc.M206883200. [DOI] [PubMed] [Google Scholar]
- Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- Kong B, Michalski CW, Hong X, Valkovskaya N, Rieder S, Abiatari I, Streit S, Erkan M, Esposito I, Friess H, et al. AZGP1 is a tumor suppressor in pancreatic cancer inducing mesenchymal-to-epithelial transdifferentiation by inhibiting TGF-beta-mediated ERK signaling. Oncogene. 2010;29:5146–5158. doi: 10.1038/onc.2010.258. [DOI] [PubMed] [Google Scholar]
- Kreindler JL, Bertrand CA, Lee RJ, Karasic T, Aujla S, Pilewski JM, Frizzell RA, Kolls JK. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L257–L266. doi: 10.1152/ajplung.00344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppermann N. Occult bacteremia in young febrile children. Pediatr. Clin. North Am. 1999;46:1073–1109. doi: 10.1016/s0031-3955(05)70176-0. [DOI] [PubMed] [Google Scholar]
- Lehmann AD, Blank F, Baum O, Gehr P, Rothen-Rutishauser BM. Diesel exhaust particles modulate the tight junction protein occludin in lung cells in vitro. Part. Fibre Toxicol. 2009;6:26. doi: 10.1186/1743-8977-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Tian L, Han Y, Vogelbaum M, Stark GR. Dose-dependent cross-talk between the transforming growth factor-beta and interleukin-1 signaling pathways. Proc. Natl. Acad. Sci. USA. 2007;104:4365–4370. doi: 10.1073/pnas.0700118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Masoud H, Moxon ER, Martin A, Krajcarski D, Richards JC. Structure of the variable and conserved lipopolysaccharide oligosaccharide epitopes expressed by Haemophilus influenzae serotype b strain Eagan. Biochemistry. 1997;36:2091–2103. doi: 10.1021/bi961989y. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat. Rev. Immunol. 2006;6:849–858. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Morales AV, Acloque H, Ocana OH, de Frutos CA, Gold V, Nieto MA. Snail genes at the crossroads of symmetric and asymmetric processes in the developing mesoderm. EMBO Rep. 2007;8:104–109. doi: 10.1038/sj.embor.7400867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- Moxon ER. Bacterial variation, virulence and vaccines. Microbiology. 2009;155:997–1003. doi: 10.1099/mic.0.024877-0. [DOI] [PubMed] [Google Scholar]
- Moxon ER, Murphy PA. Haemophilus influenzae bacteremia and meningitis resulting from survival of a single organism. Proc. Natl. Acad. Sci. USA. 1978;75:1534–1536. doi: 10.1073/pnas.75.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S, Stafford J, Madara JL. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J. Clin. Invest. 1987;80:1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AL, Barasch JM, Bunte RM, Weiser JN. Bacterial colonization of nasal mucosa induces expression of siderocalin, an iron-sequestering component of innate immunity. Cell. Microbiol. 2005;7:1404–1417. doi: 10.1111/j.1462-5822.2005.00566.x. [DOI] [PubMed] [Google Scholar]
- Oshima T, Miwa H, Joh T. Aspirin induces gastric epithelial barrier dysfunction by activating p38 MAPK via claudin-7. Am. J. Physiol. Cell Physiol. 2008;295:C800–C806. doi: 10.1152/ajpcell.00157.2008. [DOI] [PubMed] [Google Scholar]
- Pai VP, Horseman ND. Biphasic regulation of mammary epithelial resistance by serotonin through activation of multiple pathways. J. Biol. Chem. 2008;283:30901–30910. doi: 10.1074/jbc.M802476200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar RD, Castrezana-Lopez L, Allam R, Kulkarni OP, Segerer S, Radomska E, Meyer TN, Schwesinger CM, Akis N, Grone HJ, et al. Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology. 2009;128:e206–e221. doi: 10.1111/j.1365-2567.2008.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- Ring A, Weiser JN, Tuomanen EI. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J. Clin. Invest. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LG. Bacterial colonization and infection resulting from multiplication of a single organism. Rev. Infect. Dis. 1987;9:488–493. doi: 10.1093/clinids/9.3.488. [DOI] [PubMed] [Google Scholar]
- Sano Y, Harada J, Tashiro S, Gotoh-Mandeville R, Maekawa T, Ishii S. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-beta signaling. J. Biol. Chem. 1999;274:8949–8957. doi: 10.1074/jbc.274.13.8949. [DOI] [PubMed] [Google Scholar]
- Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J. Allergy Clin. Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Takano K, Kojima T, Go M, Murata M, Ichimiya S, Himi T, Sawada N. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J. Histochem. Cytochem. 2005;53:611–619. doi: 10.1369/jhc.4A6539.2005. [DOI] [PubMed] [Google Scholar]
- van Schilfgaarde M, van Alphen L, Eijk P, Everts V, Dankert J. Paracytosis of Haemophilus influenzae through cell layers of NCI-H292 lung epithelial cells. Infect. Immun. 1995;63:4729–4737. doi: 10.1128/iai.63.12.4729-4737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat. Cell Biol. 2009;11:943–950. doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Shim JH, Kluppel M, Zhang SS, Dong C, Flavell RA, Fu XY, Wrana JL, Hogan BL, Ghosh S. Ecsit is required for Bmp signaling and mesoderm formation during mouse embryogenesis. Genes Dev. 2003;17:2933–2949. doi: 10.1101/gad.1145603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola TA, Lysenko ES, Weiser JN. Mucosal clearance of capsule-expressing bacteria requires both TLR and nucleotide-binding oligomerization domain 1 signaling. J. Immunol. 2008;181:7909–7916. doi: 10.4049/jimmunol.181.11.7909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.