Adolescent idiopathic scoliosis (AIS) is characterized by a lateral curvature of the spine greater than 10 degrees with rotation of the spinal vertebrae. Two to three percent of children younger than 16 years of age will have a curvature of 10 degrees or more, but only 0.3-0.5 percent will require some sort of treatment. 1 Progression of a spinal curve to 50 degrees suggests a high risk for continued curve progression throughout adulthood and usually indicates the need for spinal fusion surgery. Approximately 10 percent of adolescents with AIS end up having curves that progress to this degree; however, these 10 percent impose large costs in the US healthcare system. In 2009 there were over 3600 discharges for idiopathic scoliosis fusion surgery; the total costs of which ranked second only to appendicitis among children aged 10 to 17 (~$514 million total; $137 million to Medicaid alone). 2
Natural History of AIS
Treatment of any condition is an attempt to alleviate current problematic signs and symptoms, and to ultimately alter long-term natural history. The vast majority of AIS patients do not initially present due to symptoms, but due to the finding of truncal asymmetry noted during screening or incidentally during well-child examinations. Few long-term studies exist, but they suggest AIS is primarily a spinal deformity associated with little significant physical or psychological disability, although the population may have a higher prevalence of back pain, and of respiratory compromise if the curve becomes extremely large. 3-8 Therefore, the treatment of AIS during adolescence is mainly an attempt to prevent problems during adulthood by arresting the progression of the curve. Large curves can only be corrected through surgery. Thus, many patients seek and receive essentially prophylactic non-operative treatment for AIS.
Bracing Treatment
Treatment with rigid bracing (thoracolumbosacral and lumbosacral orthoses, TLSO, LSO) is the most common non-operative strategy to prevent curve progression. Many different designs exist, but all attempt to restore the normal contours and alignment of the spine through the use of external forces and, with some designs, the stimulation of active correction as the patient moves the spine away from pressures within the brace. Permanent correction of the curve is typically not expected instead the brace functions as a holding device during the high risk growth phase. Bracing is generally indicated for curves of greater than 20 degrees in adolescents who still have significant skeletal growth remaining. The recommended wear time varies across clinicians, ranging from 12 to 23 hours per day until skeletal maturity is reached (2 – 4 years of treatment). Bracing, however, has many disadvantages for patients including the need for radiographs to monitor brace fit and curve response, out-of-pocket direct and indirect medical expenses, interference with sports and other activities, limited clothing choices, and self-consciousness about the brace. Brace wear for many patients is a constant reminder of their medical condition.
Over the past half century, many investigators have examined the effectiveness of bracing in AIS. 9-69 The majority of studies have been uncontrolled case series or retrospective cohort studies, but conclusions from the few higher-level designs are limited by the lack of an untreated control group, 37,66 randomized assignment, 49 blinded outcome determination, 37,49,66 and a priori determination of the necessary effect size. 37,49,66 Therefore, the results of these studies yield inadequate evidence concerning the effectiveness of bracing.
When designing this trial, we sought to overcome the limitations of previous bracing studies. Therefore, we proposed a multi-center, randomized, controlled trial evaluating the effectiveness of bracing relative to watchful waiting in subjects with (AIS), using blinded, independent outcome measurement. The purpose of this manuscript is to outline the development and initiation of the Bracing in Adolescent Idiopathic Scoliosis Trial. Additional details are provided in the Appendix.
Preliminary Work and Grant Funding
Development of the science and infrastructure of the trial was supported by a Clinical Trial Planning Grant (R21-AR-49587) from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH). The BrAIST planning grant had four general goals: 1) appointing key personnel and creating the structural organization of the trial; 2) developing procedures for data management and safety monitoring; 3) developing materials, methods and the data analysis plan; and 4) recruiting participating institutions. Beyond these goals, most resources during the planning grant phase were directed at demonstrating feasibility: establishing the ethics of randomization; 70 estimating the willingness of adolescents and their parents to enroll into a randomized trial, and their preferences for treatment and their required benefit; 71 and determining the reliability and validity of the brace wear monitoring system. 72 Funding from the NIH/NIAMS for the clinical trial was obtained in September 2006 (RO1-AR-O52113); support for central administration and clinical sites was also obtained through the Canadian Institute of Health Research (CIHR) the Shriners Hospitals for Children.
Trial Organization
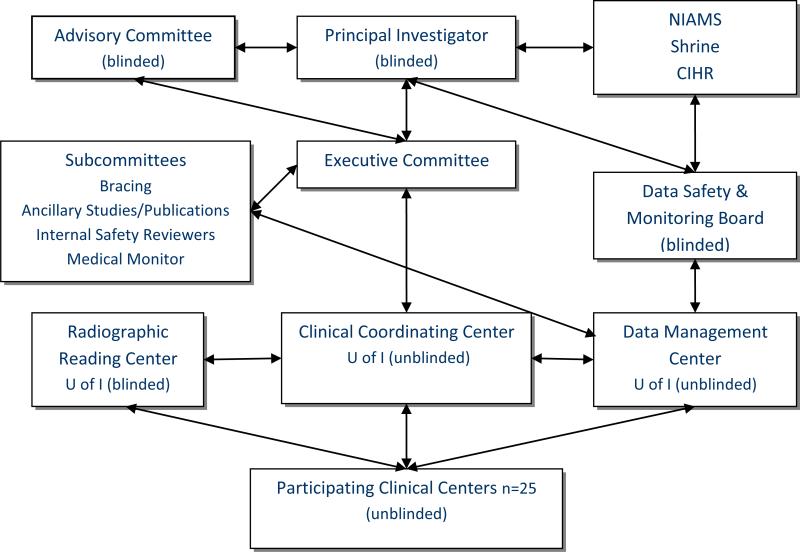
Figure 1 is an overview of the major organizational components of BrAIST. The Executive Committee included the NIH Principal Investigator, the Clinical Trial Director, the PIs of the CIHR and Shrine grants, the director of the Data Management Center (DMC) and a representative from the NIAMS. Three major work groups resided at the University of Iowa. The Trial Director and the staff of the Clinical Coordinating Center (CCC) implemented the policies and procedures set by the PI and the Executive Committee, and coordinated the activities of the DMC, the Radiographic Reading Center (RRC) and the clinical sites.
Figure 1.
Organizational Structure of BrAIST
Study Design
BrAIST is an innovation in AIS research because it combined components never included in a single study to date: simultaneous comparison of untreated and treated subjects; objective brace dose monitoring; comprehensive radiographic, clinical, and psychosocial testing; diversity of participating sites; blinded independent determinations of outcomes; and a priori determination of effect size based on the risk/benefit considerations of potential patients.
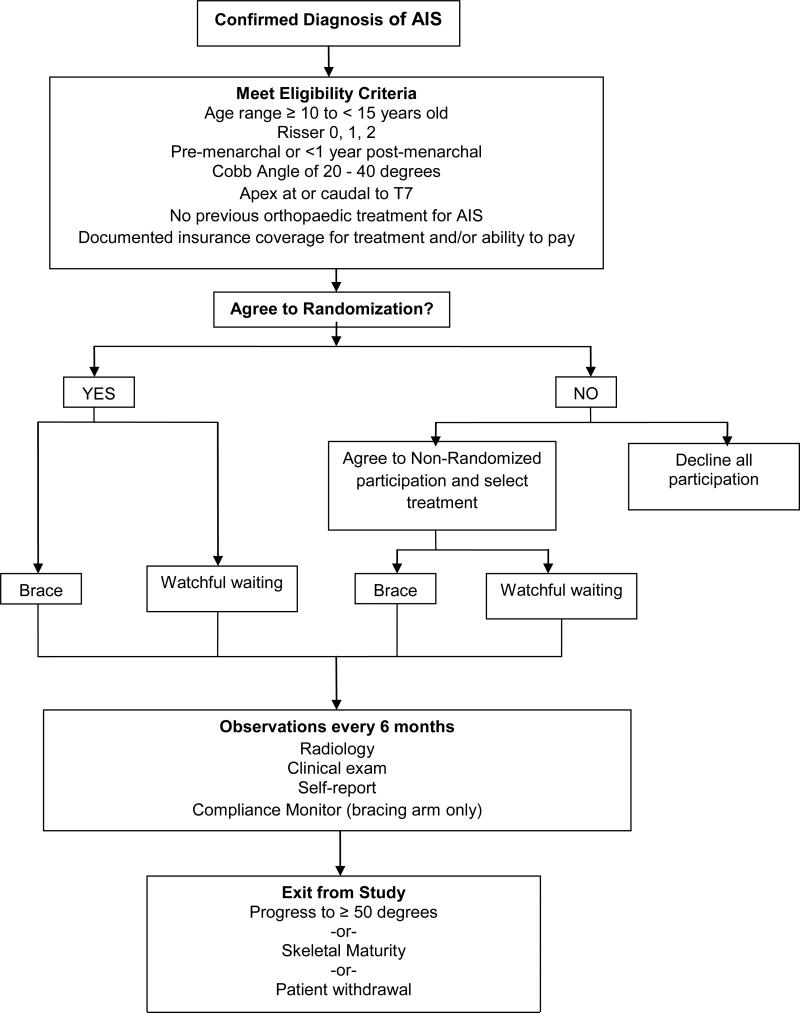
BrAIST was planned and funded as a randomized study. After three years of data collection it became clear enrollment goals would not be reached within a reasonable time frame. Participating sites encountered significantly fewer eligible patients than estimated, and the percentage of patients agreeing to randomization was less than the 25% anticipated based on preliminary work. 71The primary reason reported for declining randomization was holding a strong preference for one treatment over the other. Therefore, in November 2009, BrAIST evolved from a completely randomized study to one including a preference arm. Patients who declined randomization could enter the trial by choosing either bracing or watchful waiting. Figure 2 summarizes the final trial protocol.
Figure 2.
Subject Flow Chart
Study Aims
The primary aim of BrAIST was to compare the risk of curve progression to ≥ 50 degrees (a proxy for surgical indication) in subjects treated by a brace to those treated by watchful waiting. Secondary aims include comparison between the health and functioning, quality of life, and self-image over time in the two treatment groups; determination of the relationship between bracing dose (wear time) and curve response; and development of a predictive model for curve progression based on individual patient characteristics at initial presentation (i.e., sex, skeletal maturity, chronological age), curve characteristics (i.e., curve magnitude, location), and treatment (bracing dose), and based on these characteristics, to estimate degree of risk reduction associated with the use of a brace.
Study Sites
Data was collected at 25 participating sites (Table I) that began recruitment at varying times between March 2007 and January 2010. Four additional sites did not randomize any subjects within their first year of participation and were dismissed from the study.
Table I.
Participating Centers by Funding Source
|
NIH (n=15)
| |
| University of Virginia | University of New Mexico |
| Washington University in St. Louis | Children's Hospital of Boston |
| Children's Hospital of Central California | Cincinnati Children's Hospital Medical Center |
| Rady Children's Hospital | Children's Orthopaedics of Atlanta |
| Nemours/ DuPont Hospital for Children | Seattle Children's Hospital |
| University of Iowa | Children's Hospital of Philadelphia |
| Children's Hospital of Pittsburgh | Hospital for Special Surgery |
| Mercy Children's Hospital Kansas City* | |
|
Shriners Hospitals for Children (n=7)
| |
| Montreal | St. Louis |
| Salt Lake City | Lexington |
| Chicago | Sacramento |
| Twin Cities | |
|
Canadian Institutes of Health Research (n=2) | |
| Hospital for Sick Children, Toronto | University of British Columbia |
|
Internally-Funded (n=1) | |
| University of Rochester | |
Site was original supported by internal funds; currently supported by the NIH
Study Population
The target population for the BrAIST is patients with AIS at high risk for progression due to their age, skeletal immaturity and curve size.73-75 The inclusion criteria are listed in Table II. Patients who were previously braced or who had undergone scoliosis surgery were excluded. Subjects were also required to have insurance coverage or be willing to pay out-of-pocket for their treatment.
Table II.
Inclusion Criteria
| Diagnosis of AIS |
| Presenting without associated musculoskeletal, neurological or other conditions possibly responsible for the curvature |
| Maturity |
| Age between 10 and 15 at consent |
| Risser 0, 1, or 2 |
| Girls: Pre-menarchal OR post-menarchal by less than 1 year |
| Curve Magnitude |
| Largest Cobb angle between 20 and 39 degrees |
| Cobb angle <20 degrees until the age of 10 |
| Treatment |
| No history of previous surgical or orthotic treatment |
| Physical and mental ability to adhere to bracing protocol |
| Ability to read and write English, Spanish or French |
Recruitment and Enrollment
Participating physicians assessed all patients presenting with AIS as part of routine clinical care and introduced the study to those meeting the inclusion criteria. To standardize the information given to patients and to minimize potentially biasing input from their physicians, coordinators, not physicians, were responsible for the education and consent processes. All patients who met the inclusion criteria were asked to read the online BrAIST Education Module (http://www.uiortho.com/braist/index2.htm). The Web-based module introduced the purpose of the trial, as well as the process of random treatment assignment vs. treatment selection, followed by presentation of the natural history of AIS and risk factors for progression, treatment options and a side-by-side comparison of bracing and watchful waiting. Advantages and disadvantages of each treatment were presented.
All eligible patients were registered by the coordinators on the DMC's Web-based enrollment system, which was available 24 hours per day. Those who declined participation were registered as “screened” and the following data were recorded: age, sex, ethnicity, race, SRS curve type, Cobb angle of the largest curve, and reason for declining participation. These data were used to compare participating with non-participating subjects to evaluate selection bias and representativeness of the sample to the population.
Study Interventions
Bracing Protocol
The trial was limited to the use of full-time, rigid TLSO's. Participating physicians and orthotists prescribed and fabricated the type of brace used during normal clinical practice; all were custom-fit using a plaster or fiber cast impression, measurements or via CAD-CAM systems software and completed with pads, slings, relief areas and other modifications. Braces were to be worn at least 18 hours per day. An in-brace standing posteroanterior (PA) spine radiograph was obtained at 4-6 weeks after brace delivery. Stowaway or TidbiT temperature loggers were embedded in the brace (Onset Computer Bourne, Massachusetts), and programmed to log the date, time and temperature every 15 minutes.
Watchful waiting
The subjects in this group underwent clinical and radiographic evaluation every 6 months, but were otherwise untreated.
Change in Treatment Group
Subject-initiated treatment change (crossover) occurred when a subject stated they would not continue in the treatment they were originally assigned to (either by randomization or preference), i.e., when subject in the watchful waiting group requested a brace. This decision was to be made by the subjects and family and never suggested by the physician or coordinator. A subject who wore the brace less than prescribed was considered non-compliant, but still analyzed in the bracing arm.
Blinding
Neither the subject nor the physicians were blinded to treatment. However, the staff at the RRC, who were responsible for evaluating the radiographs and determining endpoints, were blinded to the treatment received.
Data Collection and Follow-up Periods
BrAIST collected radiographic, clinical, orthotic and self-report data at 6-month intervals. A complete list of these data is given in Table III.
Table III.
Data Sources and Collection Schedule
| Enrollment | In-Brace film | Q 6 mo | Yearly | |
|---|---|---|---|---|
| Radiographic Data | PA, lateral, bending, hand | PA | PA, hand | PA, lateral, hand |
| Cobb angle of structural and compensatory curves110 | P, R | R | P, R | P, R |
| Apex and end vertebrae of curves110 | P, R | R | P, R | P, R |
| Risser grade110 | P, R | P, R | P, R | |
| Nash Moe rotation111 | R | R | R | |
| Percent curve correction | R | R | ||
| Sagittal balance110 | R | R | ||
| Coronal balance110 | R | R | R | R |
| Kyphosis110 | ||||
| Lordosis110 | ||||
| Spinal length T1-L457 | R | R | R | |
| Concave-to-convex vertebral height ratio17 | R | R | R | |
| Digital maturity stage81 | R | R | R | |
| Clinical Data | ||||
| Weight, sitting and standing height | P | P | P | |
| Ortho/neuro examination | P | P | P | |
| Orthotic Data (brace arm only) | ||||
| Dose logger data | P | P | ||
| Brace wear diary | S | S | ||
| Skin condition under brace | P | P | ||
| Orthotist clinical notes | P | P | P | P |
| Brace Quality Evaluation | BEC | |||
| Self-Report Data | ||||
| Child Health Questionnaire83 | S | S | S | |
| Self-Image Questionnaire for Young | S | S | S | |
| Adolescents85 | ||||
| PedsQL84 | S | S | S | |
| Spinal Appearance Questionnaire112 | ||||
| Demographic information | S | S | S | |
| Menarchal status | S | S | S | |
Key: P=Participating Clinical Centers; R = Radiographic Reading Center; S = Self Report; BEC=Bracing Evaluation Committee
Radiographic Data
Standing full spine PA and lateral films, supine side-bending (to assess curve flexibility) and left hand films for bone age were obtained at the initial visit. PA spine and hand films were obtained at each 6-month follow-up, with a lateral spine film taken yearly.
All films were uploaded to the RRC file transfer site and underwent extensive evaluation by a research associate and the study radiologist. Critical evaluations, such as the Cobb angle(s), Risser sign, apical vertebral rotation, kyphosis, lordosis and digital maturity stage, were arrived at via consensus between the two readers. Disagreements between the readers that exceeded certain pre-determined tolerance limits (Table IV) were resolved and the consensus measurement/classification was entered into the database.
Table IV.
Tolerance Limits for Radiographic Evaluations
| Evaluation | Tolerance Limit | |
|---|---|---|
| Cobb angle | 0-5 degrees | |
| Risser grade | Within range of 0, 1, 2, or 3, 4, 5 | |
| Rotation (Nash Moe) | Within range of 0, 1, 2, or 3, 4 | |
| Kyphosis | Normal | 10-40 degrees |
| Hypokyphotic | <10 degrees | |
| Hyperkyphotic | >40 degrees | |
| Lordosis | Normal | 40-60 degrees |
| Hypokyphotic | <40 degrees | |
| Hyperkyphotic | >60 degrees | |
| Digital Maturity Stage | Agree on stages 2 and 3; in cases other than the endpoint | |
Clinical Data
Subjects were seen by the clinician at each visit and underwent examination to rule out any neurological or other potential reason for the curvature. Chest, shoulder and back asymmetries were evaluated. The subjects’ height was measured three times, and the average recorded.
Self-Report
All subjects completed the Child Health Questionnaire (CHQ), 76 the PedsQL, 77 the Self-Image Questionnaire for Young Adolescents (SIQYA),78 and the Spinal Appearance Questionnaire 79 at each visit. Subjects in the bracing arm completed a 2-week brace wear diary between each follow-up visit.
Orthotic Evaluation (bracing arm only)
At the initial visit the orthotist recorded evaluations of the curve, coronal decompensation, shoulder and pelvis asymmetry, and described the type of brace to be fabricated (e.g., Boston, Wilmington) along with the specific customizations. At brace delivery and subsequent visits, the orthotist observed the subjects both in, and out, of the brace. Brace fit, curve correction and the condition of skin and bony prominences under the brace were recorded. Any additional modifications were recorded. Temperature monitor data (date, time stamps and temperature) were downloaded at least every 6 months by the research coordinator. Temperatures ≥ 82.4° 72 indicated that the brace was being worn.
Endpoints and Outcomes
Subjects exited the study, and their outcome determined, when the first of two conditions was met: curve progression to ≥50 degrees (treatment failure), or skeletal maturity (treatment success) as determined by consensus evaluation by the RRC. Thus, the length of time in the study differed across subjects. In the case when the two primary readers did not agree whether an endpoint had been met or not, a third, blinded reader was used to break the tie.
Treatment Failure: Curve Progression to ≥50 Degrees
Cobb angle progression to 50 degrees or more has two major implications which make its use appropriate for this study. First, natural history studies have shown that curves greater than 50 degrees at skeletal maturity continue to progress throughout adulthood. Researchers have found greater continued progression in curves between 50 and 75 degrees at skeletal maturity relative to those of smaller magnitude. 73 Secondly, curve progression beyond 50 degrees is commonly used as the threshold for considering surgical correction. This curve threshold indicates treatment failure and therefore has significant clinical implications as well as implications for the patient and family.
Treatment Success: Skeletal Maturity
The continued risk of curve progression is directly tied to amount of skeletal growth remaining. Therefore, reaching skeletal maturity with a Cobb angle less than 50 degrees indicated a treatment success. The original BrAIST maturity endpoint was based on change in vertical height adjusted for change in the Cobb angle. 80 An adjusted change of < 1cm over a 12 month period was defined as end of growth. In the course of the study, we found several instances where determining this endpoint was problematic (i.e. if study visits were missed or different stadiometers were used). Therefore, in 2011 with approval of the DSMB, the maturity endpoint was redefined as a Risser sign of 4 and a Sanders digital maturity stage (DMS) of 7 (Risser 5, DMS 7 for boys). 81,82
Statistical Analysis Plan
Primary Aim
The primary analysis will estimate the adjusted odds ratio (OR) and 95% confidence intervals of the association between bracing and a successful outcome (reaching skeletal maturity with a curve < 50 degrees). Subjects from the randomized and preference arms will be combined and analyzed according to treatment received.
Propensity scores will be used to reduce the effect of treatment-selection bias (due to non-randomized treatment assignment and/or cross-over) in the estimation of the treatment effect. 83,84 Treatment success will be modeled as a function of treatment received, length of follow-up, and the propensity score. A minimally clinically significant difference in treatments will claimed if the relative risk reduction due to bracing is ≥ 50 percent.
Interim analyses will be performed as requested by the DSMB. The cumulative Type I error rate will be maintained at the planned 0.05 by using the Lan and Demets 85 spending function approach with the O'Brien-Fleming86 spending function. These methods adjust the threshold for statistical significance based on information time (the proportion of subjects with an endpoint relative to the number of subjects in the study population).
Sample Size and Power
The initial sample size calculations for BrAIST assumed randomization and equal number of subjects in each arm. The bracing failure rate was set at 15% based on the literature and the consensus of the protocol development committee. A survey of potential patients indicated that the minimally significant difference should be a 50% reduction in risk of curve progression to surgical indications, so the failure rate in the watchful waiting group was set at 30%. 71 For simplicity, it was assumed that a continuity corrected chi-square test would be used to compare the proportions. Setting alpha at 0.05 and power at 90%, a sample of 348 subjects (384 after allowing for 10% loss-to-followup) was required.
Addition of the preference arm resulted in unequal numbers of subjects in each treatment group. The final proportions will not be known until the end of data collection and all cross-overs between arms are accounted for. Assuming the proportion of subjects analyzed in the bracing arm ranges between 25 and 75% of the sample, the power of the test will be at least 80 percent. Table V provides a summary of these calculations.
Table V.
Sample Size and Power Calculations
| Control Failure Rate | 30% |
| Bracing Failure Rate | 15% |
| Significance Level | 0.05 |
| Sample Size | 348 |
| Sample Size Adjusted for 10% drop-out | 384 |
| Percent of subjects in braced group | Power |
| 25% | 0.82 |
| 40% | 0.91 |
| 50% | 0.92 |
| 60% | 0.91 |
| 75% | 0.85 |
Secondary Aims
Health and Functioning
The first secondary aim is to compare the CHQ, PedsQL, SIQYA and SAQ scores over time in the two treatment groups. Analysis will focus on comparing change in variables from baseline to study completion attributable to treatment using a linear mixed model approach.
Dose-Response Relationship
Our efforts will be directed to determining the smallest dose significantly associated with curve stabilization (<10 degrees progression), as well as the maximum dose beyond which no further benefits are seen. We will first establish unadjusted dose response curves, then proceed to address the possibility of different curve response dependent upon patient and curve characteristics.
Prediction of Curve Progression
Curve progression will be defined in two ways: beyond 50 degrees (binary, yes or no) and as the difference between baseline and final follow-up (interval). Logistic and linear mixed models of curve progression will take into account patient and curve characteristics measured at initial presentation such as chronological age, Risser sign, menarche, sex, body-mass index, curve classification, Cobb angle, and other radiographic measures. The composite of brace dose, curve correction in brace, and change in vertebra wedging will then be added into the equations to model the risk of progression with treatment. This will allow estimation of risk for different clinical presentations, and to estimate how bracing may differentially alter the course of progression dependent on presentation.
Training and Ongoing Quality Control
All procedures, measurements and data collection procedures were standardized and described in detail in the MOOP. In order to ensure quality data, the DMC and CCC developed valid and logically consistent case report forms, carefully trained those who were collecting and entering data, pilot-tested all forms and systems, carefully edited all data when it was entered, and monitored onsite to ensure that the data in the database was accurate.
Participating Sites
Training sessions were held for the investigators, site coordinators, site orthotists and funding organization representatives prior to trial initiation. At these meetings details of the trial were discussed, data collection questions clarified, and radiographic and orthotic standards reinforced. Prior to any enrollments, all coordinators were given several vignettes and required to go through the process of registering and enrolling simulated patients. Physicians were given a set of x-rays which had been evaluated by the RRC staff and were required to complete their own evaluations and submit the case report forms. CCC staff held monthly phone calls with coordinators at each site to discuss each subject, including follow-up scheduling, data collection and submission and any issues concerning compliance, adverse events or disease progress or response. Yearly site monitoring visits were undertaken for the purpose of auditing records and making important site-specific recommendations to improve the overall trial quality. Coordinators attended yearly meetings organized by the CCC.
Radiographic Reading Center
The RRC consisted of a research assistant and a musculoskeletal radiologist, both of whom were trained specifically to perform the measurements and evaluations for the study using the standard definitions established by the Spinal Deformity Study Group and published in their manual. 87 An important consideration is that the endpoints (Cobb angle and skeletal maturity), were defined consistently, and measured with equal accuracy, across the treatment groups. This was ensured to a reasonable degree by the standards for x-ray acquisition, blinding of the raters in the RRC, the software used in the RRC, and the ongoing quality assurance program built into the trial oversight.
Data Management Center
Once case report forms were received at the DMC, they were logged into a database, and queries emailed to the coordinators in the case of missing fields or other issues. Once resolved, the DMC staff double-data entered each form.
During the active enrollment period, the DMC produced monthly progress summarizing the recruitment and data entry to date. Similar progress reports were presented to the DSMB. These reports summarized current enrollment, follow-up data acquisition and database entry status, protocol deviations, and baseline characteristics of subjects in both treatment arms.
Data Safety and Monitoring Board (DSMB)
BrAIST was a Phase III trial and oversight by a DSMB was required per NIH policy. The DSMB was composed of five members appointed by the NIAMS, all of whom were completely independent of the investigator and without conflicts of interest with the trial. Prior to the first enrollment, the DSMB reviewed and approved the protocol, informed consent documents and the safety monitoring and statistical analysis plan. During the course of data collection, the Board met approximately every 6 months to evaluate the progress of the trial, including recruitment, data quality and completeness, protocol violations and adverse events. All changes to the protocol, including the maturity endpoint and change from a completely randomized design were presented and approved by the Board.
Conclusion
BrAIST is an innovative effort which has enrolled the largest sample to date to study the effectiveness of bracing and key methodological improvements over past efforts have been incorporated. Clinical decision making will be improved by translation of the BrAIST results into evidence-based prognosis and estimates of how the prognosis, specifically the risk of progressing to surgery, may be altered by the use of bracing.
Supplementary Material
Acknowledgement
The authors would like to acknowledge the contributions of the BrAIST Protocol Development Committee: Keith Bridwell, MD, Robert Dickson, MD, Caroline Goldberg, MD, Jose Morcuende, MD, John Lonstein, MD, Raymond Morrissey, MD, Alf Nachemson, MD, Peter Newton, MD, and Dennis Wenger, MD; and the BrAIST Study Group: Mark Abel, MD, Oheneba Boachie-Adjei, MD, Jacques d'Astous, MD, John Emans, MD, John (Jack) Flynn, MD, Joseph Gerardi, DO, Daniel Green, MD, Kenneth Guidera, MD, Munish Gupta, MD, Kim Hammerberg, MD, M. Timothy Hresko, MD , Henry Iwinski, MD, Antony Kallur, MD, Walter Krengel, MD, Charles Mehlman, MD, Peter Newton, MD, Jean Ouellet, MD, Nigel Price, MD, Chris Reilly, MD, James O. Sanders, MD, Michael Schmitz, MD, Suken Shah, MD, and W. Timothy Ward, MD as well as previous members, Patrick Bosch, MD, Matthew Halsey, MD, Richard Schwend, MD, Kit Song, MD, Peter Sturm, MD and Elizabeth Szalay, MD.
The device(s)/drug(s) that is/are the subject of this manuscript is/are exempt from FDA or corresponding national regulations. The project described was supported by Award Numbers R21AR049587 and R01AR052113 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Weinstein SL, PI); the Shriners Hospitals for Children (#79125, Dobbs, MB, PI); the Canadian Institutes of Health Research (FRN-81050, Wright JG, PI); the University of Rochester (Sanders JO, PI);the Children's Mercy Hospital and Clinics (Price NJ, PI); and the Children's Miracle Network (Weinstein SL, PI). Relevant financial activities outside the submitted work: grants, board membership, royalties, travel/accommodations/meeting expenses, patents, consultancy.
Contributor Information
Stuart L. Weinstein, Department of Orthopaedics and Rehabilitation University of Iowa.
Lori A. Dolan, Department of Orthopaedics and Rehabilitation University of Iowa.
James G. Wright, Department of Surgery - The Hospital for Sick Children (Toronto, Ontario, Canada); the Division of Orthopedic Surgery - University of Toronto (Toronto, Ontario, Canada).
Matthew B. Dobbs, Department of Orthopaedic Surgery Washington University School of Medicine and Saint Louis Shriners Hospital for Children.
References
- 1.Nachemson AL, Lonstein JE, Weinstein SL. Scoliosis Research Society. Denver, Colorado: 1982. Report of the Prevalence and Natural History Committee of the Scoliosis Research Society. [Google Scholar]
- 2.HCUP Kids’ Inpatient Database (KID) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2009. www.hcup-us.ahrq.gov/kidoverview.jsp. [Google Scholar]
- 3.Pehrsson K, Larsson S, Oden A, et al. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine. 1992;17:1091–6. doi: 10.1097/00007632-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein SL, Dolan LA, Spratt KF, et al. Health and function of patients with untreated idiopathic scoliosis: a 50-year natural history study. JAMA. 2003;289:559–67. doi: 10.1001/jama.289.5.559. [DOI] [PubMed] [Google Scholar]
- 5.Ascani E, Bartolozzi P, Logroscino CA, et al. Natural history of untreated idiopathic scoliosis after skeletal maturity. Symposium on Epidemiology, Natural History and Non-operative Treatment of Idiopathic Scoliosis. Spine. 1986;11:784–9. doi: 10.1097/00007632-198610000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg MS, Mayo NE, Poitras B, et al. The Ste-Justine Adolescent Idiopathic Scoliosis Cohort Study. Part II: Perception of health, self and body image, and participation in physical activities. Spine. 1994;19:1562–72. [PubMed] [Google Scholar]
- 7.Mayo NE, Goldberg MS, Poitras B, et al. The Ste-Justine Adolescent Idiopathic Scoliosis Cohort Study. Part III: Back pain. Spine. 1994;19:1573–81. doi: 10.1097/00007632-199407001-00005. [DOI] [PubMed] [Google Scholar]
- 8.Poitras B, Mayo NE, Goldberg MS, et al. The Ste-Justine Adolescent Idiopathic Scoliosis Cohort Study. Part IV: Surgical correction and back pain. Spine. 1994;19:1582–8. doi: 10.1097/00007632-199407001-00006. [DOI] [PubMed] [Google Scholar]
- 9.Allington NJ, Bowen JR. Adolescent idiopathic scoliosis: treatment with the Wilmington brace. A comparison of full-time and part-time use. J Bone and Joint Surg. 1996;78:1056–62. [PubMed] [Google Scholar]
- 10.Andriacchi T, Schultz A, Belytschko T, et al. Milwaukee brace correction of idiopathic scoliosis. J Bone and Joint Surg. 1976;58A:806–15. [PubMed] [Google Scholar]
- 11.Aulisa AG, Guzzanti V, Galli M, et al. Treatment of thoraco-lumbar curves in adolescent females affected by idiopathic scoliosis with a progressive action short brace (PASB): assessment of results according to the SRS committee on bracing and nonoperative management standardization criteria. Scoliosis. 2009;4:21. doi: 10.1186/1748-7161-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassett G, Bunnell W, MacEwen G. Treatment of idiopathic scoliosis with the Wilmington brace. Results in patients with a twenty to thirty-nine degree curve. J Bone and Joint Surg. 1986;68A:602–5. [PubMed] [Google Scholar]
- 13.Bassett GS, Bunnell WP. Influence of the Wilmington brace on spinal decompensation in adolescent idiopathic scoliosis. Clinical Orthopaedics & Related Research. 1987:164–9. [PubMed] [Google Scholar]
- 14.Brox JI, Lange JE, Gunderson RB, et al. Good brace compliance reduced curve progression and surgical rates in patients with idiopathic scoliosis. Eur Spine J. 2012;21:1957–63. doi: 10.1007/s00586-012-2386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bunnell WP. Nonoperative treatment of spinal deformity: the case for observation. Instructional Course Lectures. 1985;34:106–9. [PubMed] [Google Scholar]
- 16.Bunnell WP, MacEwen GD, Jayakumar S. The use of plastic jackets in the non-operative treatment of idiopathic scoliosis. J Bone and Joint Surg. 1980;62A:31–8. [PubMed] [Google Scholar]
- 17.Carr W, Moe J, Winter R, et al. Treatment of idiopathic scoliosis in the Milwaukee brace. J Bone and Joint Surg. 1980;62A:599–612. [PubMed] [Google Scholar]
- 18.Castro FP., Jr. Adolescent idiopathic scoliosis, bracing, and the Hueter-Volkmann principle. Spine Journal: Official Journal of the North American Spine Society. 2003;3:180–5. doi: 10.1016/s1529-9430(02)00557-0. [DOI] [PubMed] [Google Scholar]
- 19.Chase AP, Bader DL, Houghton GR. The biomechanical effectiveness of the Boston brace in the management of adolescent idiopathic scoliosis. Spine. 1989;14:636–42. doi: 10.1097/00007632-198906000-00018. [DOI] [PubMed] [Google Scholar]
- 20.D'Amato CR, Griggs S, McCoy B. Nighttime bracing with the Providence brace in adolescent girls with idiopathic scoliosis. Spine. 2001;26:2006–12. doi: 10.1097/00007632-200109150-00014. [DOI] [PubMed] [Google Scholar]
- 21.Danielsson AJ, Hasserius R, Ohlin A, et al. A prospective study of brace treatment versus observation alone in adolescent idiopathic scoliosis: a follow-up mean of 16 years after maturity. Spine. 2007;32:2198–207. doi: 10.1097/BRS.0b013e31814b851f. [DOI] [PubMed] [Google Scholar]
- 22.Edmonsson AS, Morris JT. Follow-up study of Milwaukee brace treatment in patients with idiopathic scoliosis. Clinical Orthopaedics & Related Research. 1977:58–61. [PubMed] [Google Scholar]
- 23.Emans J, Kaelin A, Bancel P, et al. The Boston bracing system for idiopathic scoliosis. Follow-up results in 295 patients. Spine. 1986;11:792–801. doi: 10.1097/00007632-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Federico DJ, Renshaw TS. Results of treatment of idiopathic scoliosis with the Charleston bending orthosis. Spine. 1990;15:886–7. doi: 10.1097/00007632-199009000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Feliberti R, Flynn J, Ramirez N, et al. Effectiveness of TLSO bracing in the conservative treatment of idiopathic scoliosis. Journal of Pediatric Orthopedics. 1995;15:176–81. [PubMed] [Google Scholar]
- 26.Focarile FA, Bonaldi A, Giarolo MA, et al. Effectiveness of nonsurgical treatment for idiopathic scoliosis. Overview of available evidence. Spine. 1991;16:395–401. doi: 10.1097/00007632-199104000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Gammon SR, Mehlman CT, Chan W, et al. A comparison of thoracolumbosacral orthoses and SpineCor treatment of adolescent idiopathic scoliosis patients using the Scoliosis Research Society standardized criteria. J Pediatr Orthop. 2010;30:531–8. doi: 10.1097/BPO.0b013e3181e4f761. [DOI] [PubMed] [Google Scholar]
- 28.Gardner ADH, Burwell RG, Wozniak AP, et al. Some beneficial effects of bracing and a search for prognostic indicators in idiopathic scoliosis. Spine. 1986;11:779. [Google Scholar]
- 29.Goldberg CJ, Dowling FE, Hall JE, et al. A statistical comparison between natural history of idiopathic scoliosis and brace treatment in skeletally immature adolescent girls. Spine. 1993;18:902–8. doi: 10.1097/00007632-199306000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg CJ, Moore DP, Fogarty EE, et al. Adolescent idiopathic scoliosis: the effect of brace treatment on the incidence of surgery. Spine. 2001;26:42–7. doi: 10.1097/00007632-200101010-00009. [DOI] [PubMed] [Google Scholar]
- 31.Green NE. Part-time bracing of adolescent idiopathic scoliosis. J Bone and Joint Surg. 1986;68:738–42. [PubMed] [Google Scholar]
- 32.Hanks GA, Zimmer B, Nogi J. TLSO treatment of idiopathic scoliosis. An analysis of the Wilmington jacket. Spine. 1988;13:626–9. [PubMed] [Google Scholar]
- 33.Howard A, Wright JG, Hedden D. A comparative study of TLSO, Charleston, and Milwaukee braces for idiopathic scoliosis. Spine. 1998;23:2404–11. doi: 10.1097/00007632-199811150-00009. [DOI] [PubMed] [Google Scholar]
- 34.Janicki JA, Poe-Kochert C, Armstrong DG, et al. A comparison of the thoracolumbosacral orthoses and providence orthosis in the treatment of adolescent idiopathic scoliosis: results using the new SRS inclusion and assessment criteria for bracing studies. J Pediatr Orthop. 2007;27:369–74. doi: 10.1097/01.bpb.0000271331.71857.9a. [DOI] [PubMed] [Google Scholar]
- 35.Jonasson-Rajala E, Josefsson E, Lundberg B, et al. Boston thoracic brace in the treatment of idiopathic scoliosis. Initial correction. Clinical Orthopaedics & Related Research. 1984:37–41. [PubMed] [Google Scholar]
- 36.Karol LA. Effectiveness of bracing in male patients with idiopathic scoliosis. Spine. 2001;26:2001–5. doi: 10.1097/00007632-200109150-00013. [DOI] [PubMed] [Google Scholar]
- 37.Katz DE, Browne RH. The influence of how much a scoliosis orthosis is worn and its ability to prevent curve progression in adolescent idiopathic scoliosis.. Scoliosis Research Society Annual Meeting.; Quebec City, Quebec. 2003. [Google Scholar]
- 38.Katz DE, Durrani AA. Factors that influence outcome in bracing large curves in patients with adolescent idiopathic scoliosis. Spine. 2001;26:2354–61. doi: 10.1097/00007632-200111010-00012. [DOI] [PubMed] [Google Scholar]
- 39.Katz DE, Herring JA, Browne RH, et al. Brace wear control of curve progression in adolescent idiopathic scoliosis. J Bone and Joint Surg. 2010;92:1343–52. doi: 10.2106/JBJS.I.01142. [DOI] [PubMed] [Google Scholar]
- 40.Katz DE, Richards BS, Browne RH, et al. A comparison between the Boston brace and the Charleston bending brace in adolescent idiopathic scoliosis. Spine. 1997;22:1302–12. doi: 10.1097/00007632-199706150-00005. [DOI] [PubMed] [Google Scholar]
- 41.Korovessis P, Kyrkos C, Piperos G, et al. Effects of thoracolumbosacral orthosis on spinal deformities, trunk asymmetry, and frontal lower rib cage in adolescent idiopathic scoliosis. Spine. 2000;25:2064–71. doi: 10.1097/00007632-200008150-00010. [DOI] [PubMed] [Google Scholar]
- 42.Laurnen EL, Tupper JW, Mullen MP. The Boston brace in thoracic scoliosis. A preliminary report. Spine. 1983;8:388–95. doi: 10.1097/00007632-198305000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Lee CS, Hwang CJ, Kim DJ, et al. Effectiveness of the Charleston night-time bending brace in the treatment of adolescent idiopathic scoliosis. J Pediatr Orthop. 2012;32:368–72. doi: 10.1097/BPO.0b013e3182561193. [DOI] [PubMed] [Google Scholar]
- 44.Lonstein JE, Winter RB. The Milwaukee brace for the treatment of adolescent idiopathic scoliosis. A review of one thousand and twenty patients. Journal of Bone & Joint Surgery - American Volume. 1994;76:1207–21. doi: 10.2106/00004623-199408000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Mellencamp DD, Blount WP, Anderson AJ. Milwaukee brace treatment of idiopathic scoliosis: late results. Clinical Orthopaedics & Related Research. 1977:47–57. [PubMed] [Google Scholar]
- 46.Miller JA, Nachemson AL, Schultz AB. Effectiveness of braces in mild idiopathic scoliosis. Spine. 1984;9:632–5. doi: 10.1097/00007632-198409000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Moe JH, Kettleson DN. Idiopathic scoliosis. Analysis of curve patterns and the preliminary results of Milwaukee-brace treatment in one hundred sixty-nine patients. J Bone and Joint Surg. 1970;52:1509–33. [PubMed] [Google Scholar]
- 48.Montgomery F, Willner S. Prognosis of brace-treated scoliosis. Comparison of the Boston and Milwaukee methods in 244 girls. Acta Orthopaedica Scandinavica. 1989;60:383–5. doi: 10.3109/17453678909149302. [DOI] [PubMed] [Google Scholar]
- 49.Nachemson AL, Peterson LE. Effectiveness of treatment with a brace in girls who have adolescent idiopathic scoliosis. A prospective, controlled study based on data from the Brace Study of the Scoliosis Research Society. J Bone and Joint Surg. 1995;77:815–22. doi: 10.2106/00004623-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Negrini S, Atanasio S, Fusco C, et al. Effectiveness of complete conservative treatment for adolescent idiopathic scoliosis (bracing and exercises) based on SOSORT management criteria: results according to the SRS criteria for bracing studies - SOSORT Award 2009 Winner. Scoliosis. 2009;4:19. doi: 10.1186/1748-7161-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Negrini S, Minozzi S, Bettany-Saltikov J, et al. Braces for idiopathic scoliosis in adolescents. Spine (Phila Pa 1976) 2010;35:1285–93. doi: 10.1097/BRS.0b013e3181dc48f4. [DOI] [PubMed] [Google Scholar]
- 52.Noonan KJ, Weinstein SL, Jacobson WC, et al. Use of the Milwaukee brace for progressive idiopathic scoliosis. J Bone and Joint Surg. 1996;78:557–67. doi: 10.2106/00004623-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Olafsson Y, Saraste H, Soderlund V, et al. Boston brace in the treatment of idiopathic scoliosis. Journal of Pediatric Orthopedics. 1995;15:524–7. doi: 10.1097/01241398-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 54.Peltonen J, Poussa M, Ylikoski M. Three-year results of bracing in scoliosis. Acta Orthopaedica Scandinavica. 1988;59:487–90. doi: 10.3109/17453678809148769. [DOI] [PubMed] [Google Scholar]
- 55.Piazza MR, Bassett GS. Curve progression after treatment with the Wilmington brace for idiopathic scoliosis. Journal of Pediatric Orthopedics. 1990;10:39–43. [PubMed] [Google Scholar]
- 56.Price CT, Scott DS, Reed FE, Jr., et al. Nighttime bracing for adolescent idiopathic scoliosis with the Charleston bending brace. Preliminary report. Spine. 1990;15:1294–9. doi: 10.1097/00007632-199012000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Price CT, Scott DS, Reed FR, Jr., et al. Nighttime bracing for adolescent idiopathic scoliosis with the Charleston Bending Brace: long-term follow-up. Journal of Pediatric Orthopedics. 1997;17:703–7. [PubMed] [Google Scholar]
- 58.Rowe DE, Bernstein SM, Riddick MF, et al. A meta-analysis of the efficacy of non-operative treatments for idiopathic scoliosis. J Bone and Joint Surg. 1997;79:664–74. doi: 10.2106/00004623-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Trivedi JM, Thomson JD. Results of Charleston bracing in skeletally immature patients with idiopathic scoliosis. Journal of Pediatric Orthopedics. 2001;21:277–80. [PubMed] [Google Scholar]
- 60.Upadhyay SS, Nelson IW, Ho EK, et al. New prognostic factors to predict the final outcome of brace treatment in adolescent idiopathic scoliosis. Spine. 1995;20:537–45. doi: 10.1097/00007632-199503010-00006. [DOI] [PubMed] [Google Scholar]
- 61.Watts HG, Hall JE, Stanish W. The Boston brace system for the treatment of low thoracic and lumbar scoliosis by the use of a girdle without superstructure. Clinical Orthopaedics & Related Research. 1977:87–92. [PubMed] [Google Scholar]
- 62.Wever DJ, Tonseth KA, Veldhuizen AG, et al. Curve progression and spinal growth in brace treated idiopathic scoliosis. Clinical Orthopaedics & Related Research. 2000:169–79. doi: 10.1097/00003086-200008000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Wiley JW, Thomson JD, Mitchell TM, et al. Effectiveness of the Boston brace in treatment of large curves in adolescent idiopathic scoliosis. Spine. 2000;25:2326–32. doi: 10.1097/00007632-200009150-00010. [DOI] [PubMed] [Google Scholar]
- 64.Willers U, Normelli H, Aaro S, et al. Long-term results of Boston brace treatment on vertebral rotation in idiopathic scoliosis. Spine. 1993;18:432–5. [PubMed] [Google Scholar]
- 65.Willner S. Effect of the Boston thoracic brace on the frontal and sagittal curves of the spine. Acta Orthopaedica Scandinavica. 1984;55:457–60. doi: 10.3109/17453678408992394. [DOI] [PubMed] [Google Scholar]
- 66.Wong MS, Cheng JC, Lam TP, et al. The effect of rigid versus flexible spinal orthosis on the clinical efficacy and acceptance of the patients with adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2008;33:1360–5. doi: 10.1097/BRS.0b013e31817329d9. [DOI] [PubMed] [Google Scholar]
- 67.Ylikoski M, Peltonen J, Poussa M. Biological factors and predictability of bracing in adolescent idiopathic scoliosis. Journal of Pediatric Orthopedics. 1989;9:680–3. doi: 10.1097/01241398-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Yrjonen T, Ylikoski M, Schlenzka D, et al. Effectiveness of the Providence nighttime bracing in adolescent idiopathic scoliosis: a comparative study of 36 female patients. Eur Spine J. 2006;15:1139–43. doi: 10.1007/s00586-005-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaborowska-Sapeta K, Kowalski IM, Kotwicki T, et al. Effectiveness of Cheneau brace treatment for idiopathic scoliosis: prospective study in 79 patients followed to skeletal maturity. Scoliosis. 2011;6:2. doi: 10.1186/1748-7161-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dolan LA, Donnelly MJ, Spratt KF, et al. Professional opinion concerning the effectiveness of bracing relative to observation in adolescent idiopathic scoliosis. J Pediatr Orthop. 2007;27:270–6. doi: 10.1097/01.bpb.0000248579.11864.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dolan LA, Sabesan V, Weinstein SL, et al. Preference assessment of recruitment into a randomized trial for adolescent idiopathic scoliosis. J Bone and Joint Surg. 2008;90:2594–605. doi: 10.2106/JBJS.G.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dolan LA, Weinstein SL, Adams BS. Temperature as a diagnostic test for compliance with a thoracolumbosacral orthosis.. Annual Meeting of the Pediatric Orthopaedic Society of North America; 2010. [Google Scholar]
- 73.Weinstein SL, Ponseti IV. Curve progression in idiopathic scoliosis. J. Bone and Joint. Surg. 1983;65-A:447–55. [PubMed] [Google Scholar]
- 74.Peterson LE, Nachemson AL. Prediction of progression of the curve in girls who have adolescent idiopathic scoliosis of moderate severity. Logistic regression analysis based on data from The Brace Study of the Scoliosis Research Society. J Bone and Joint Surg. 1995;77:823–7. doi: 10.2106/00004623-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone and Joint Surg. 1984;66:1061–71. [PubMed] [Google Scholar]
- 76.Landgraf J, Abetz L, Ware J. The CHQ User's Manual. The Health Institute, New England Medical Center; Boston, MA: 1996. [Google Scholar]
- 77.Varni J, Seid M, Rode C. The PedsQL: Measurement model for the pediatric quality of life inventory. Medical Care. 1999;37:126–39. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Petersen A, Schulenberg J, Abramowitz R, et al. A self-image questionnaire for young adolescents (SIQYA): Reliability and validity. J Youth Adol. 1984;13:93–111. doi: 10.1007/BF02089104. [DOI] [PubMed] [Google Scholar]
- 79.Sanders JO, Harrast JJ, Kuklo TR, et al. The Spinal Appearance Questionnaire: results of reliability, validity, and responsiveness testing in patients with idiopathic scoliosis. Spine. 2007;32:2719–22. doi: 10.1097/BRS.0b013e31815a5959. [DOI] [PubMed] [Google Scholar]
- 80.Ylikoski M. Growth and progression of adolescent idiopathic scoliosis in girls. J Pediatr Orthop B. 2005;14:320–4. doi: 10.1097/01202412-200509000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Sanders JO, Browne RH, McConnell SJ, et al. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone and Joint Surg. 2007;89:64–73. doi: 10.2106/JBJS.F.00067. [DOI] [PubMed] [Google Scholar]
- 82.Sanders JO, Khoury JG, Kishan S, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008;90:540–53. doi: 10.2106/JBJS.G.00004. [DOI] [PubMed] [Google Scholar]
- 83.Rubin DB. For objective causal inference, design trumps analysis. Annals of Applied Statistics. 2008;2:808–40. [Google Scholar]
- 84.D'Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 85.Lan KKG, DeMets DL. Changing frequency of interim analysis in sequential monitoring. Biometrics. 1989;45:1017–20. [PubMed] [Google Scholar]
- 86.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979 [PubMed] [Google Scholar]
- 87.O'Brien MF, Kuklo TR, Blanke KM, et al. In: Radiographic Measurement Manual. Spinal Study Deformity Group, editor. Medtronic Sofamor Danek USA, Inc.; 2004. [Google Scholar]
- 88.Nash CI, Moe JH. A study of vertebral rotation. J Bone Joint Surg AM. 1969;2:223–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.