Abstract
Background:
The International Maternal, Pediatric, and Adolescent Clinical Trials P1060 trial demonstrated superior outcomes for HIV-infected children less than 3 years old initiating antiretroviral therapy (ART) with lopinavir/ritonavir compared to nevirapine, but lopinavir/ritonavir is four-fold costlier.
Design/methods:
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)-Pediatric model, with published and P1060 data, to project outcomes under three strategies: no ART; first-line nevirapine (with second-line lopinavir/ritonavir); and first-line lopinavir/ritonavir (second-line nevirapine). The base-case examined South African children initiating ART at age 12 months; sensitivity analyses varied all key model parameters. Outcomes included life expectancy, lifetime costs, and incremental cost-effectiveness ratios [ICERs; dollars/year of life saved ($/YLS)]. We considered interventions with ICERs less than 1× per-capita gross domestic product (South Africa: $7500)/YLS as ‘very cost-effective,’ interventions with ICERs below 3× gross domestic product/YLS as ‘cost-effective,’ and interventions leading to longer life expectancy and lower lifetime costs as ‘cost-saving’.
Results:
Projected life expectancy was 2.8 years with no ART. Both ART regimens markedly improved life expectancy and were very cost-effective, compared to no ART. First-line lopinavir/ritonavir led to longer life expectancy (28.8 years) and lower lifetime costs ($41 350/person, from lower second-line costs) than first-line nevirapine (27.6 years, $44 030). First-line lopinavir/ritonavir remained cost-saving or very cost-effective compared to first-line nevirapine unless: liquid lopinavir/ritonavir led to two-fold higher virologic failure rates or 15-fold greater costs than in the base-case, or second-line ART following first-line lopinavir/ritonavir was very ineffective.
Conclusions:
On the basis of P1060 data, first-line lopinavir/ritonavir leads to longer life expectancy and is cost-saving or very cost-effective compared to first-line nevirapine. This supports WHO guidelines, but increasing access to pediatric ART is critical regardless of the regimen used.
Keywords: Africa, cost-effectiveness, first-line antiretroviral therapy, IMPAACT, P1060 trial, pediatric HIV
Introduction
Approximately 3 million children live with HIV/AIDS worldwide, and nearly 260 000 infants are HIV-infected each year [1]. HIV-infected children below 3 years of age face high risks of AIDS and death without effective antiretroviral therapy (ART) [2]. The majority of HIV-infected children live in sub-Saharan Africa, where fewer than one-third have access to ART and the optimal sequence of available ART medications remains unknown [3–5]. Most African programs offer two sequential ‘lines’ of pediatric ART: a ‘first-line’ regimen including nevirapine [a non-nucleoside reverse transcriptase inhibitor (NNRTI)], followed after virologic failure by a ‘second-line’ regimen containing lopinavir/ritonavir (a protease inhibitor) [5]. Nevirapine is inexpensive, widely available, and formulated in fixed-dose combinations [6,7].
The recent International Maternal, Pediatric, and Adolescent Clinical Trial (IMPAACT) P1060 study, however, demonstrated superior 72-week suppression of HIV when children aged below 3 years initiated first-line lopinavir/ritonavir compared to first-line nevirapine, regardless of prior antiretroviral drug exposure to prevent mother-to-child HIV transmission [8,9]. The WHO now recommends lopinavir/ritonavir-based first-line ART for children below 3 years old [10]. Liquid lopinavir/ritonavir is needed for children too young to swallow pills; it has a high alcohol content and has been reported to have very poor palatability, relies on a cold chain to the point of distribution, and is four-fold costlier than nevirapine [6,7,11]. Incorporating these tradeoffs in efficacy, tolerance, and cost, we projected the long-term clinical outcomes and cost-effectiveness of first-line nevirapine and lopinavir/ritonavir for HIV-infected children below 3 years of age.
Methods
Analytic overview
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)-Pediatric model to evaluate three treatment strategies for HIV-infected children below 3 years of age: no ART (comparator); first-line nevirapine, followed by second-line lopinavir/ritonavir; and first-line lopinavir/ritonavir, followed by second-line nevirapine [12]. In our base-case analysis, we simulated HIV-infected children presenting to care and initiating ART at 12 months of age in South Africa. In sensitivity analyses, we examined the impact of age at presentation to care and ART initiation (6–35 months), lower healthcare costs from Côte d’Ivoire, and variations in all other clinical and economic parameters, on the comparison of the first-line ART regimens. Model outcomes included yearly survival and healthcare costs over short and long-term time horizons, cohort life expectancy, and average lifetime healthcare costs. We calculated an incremental cost-effectiveness ratio (ICER) for each strategy compared to its next less expensive alternative: difference in lifetime costs divided by difference in life-years ($/YLS). Following WHO guidance, we considered interventions with ICERs less than three-times per-capita gross domestic product (GDP; South Africa: $7500, Côte d’Ivoire: $1200)/YLS to be ‘cost-effective,’ those with ICERs below 1× GDP/YLS to be ‘very cost-effective,’ and those leading to longer projected life expectancy and lower lifetime costs to be ‘cost-saving’ [13,14].
CEPAC-Pediatric model
The CEPAC-Pediatric model is a microsimulation model of pediatric HIV disease (Appendix, see also http://web2.research.partners.org/cepac/model.html) [12]. At the start of each simulation, HIV-infected children draw from distributions of HIV RNA and CD4+ levels; the model uses CD4+ percentage (CD4+%) for children below 5 years old and absolute CD4+ cell count thereafter [15]. Current age and CD4+% or CD4+ cell count in each month determine the risks of disease progression, including development of acute opportunistic infections and death. Without effective ART, CD4+% or CD4+ cell count declines monthly. Children can initiate ART based on a combination of observed criteria, including age, CD4+%, and diagnosis of opportunistic infections. Once on ART, children have an initial probability of virologic suppression, with a corresponding increase in CD4+% or CD4+ cell count. Among patients with virologic suppression, there is a small monthly risk of subsequent loss of treatment efficacy (‘late failure’). This leads to virologic rebound, CD4+% or CD4+ decline after a 12-month delay and increased risks of disease progression until the next available, effective ART regimen is initiated.
We previously calibrated the model to match survival data for HIV-infected African children not treated with ART [12,16]. For this analysis, we also fitted the model to rates of opportunistic infection (0.7–9.3/100 person-years) and mortality (3.29/100 person-years) from ART-treated children in the P1060 trial, as well as published probabilities of switching from first-line to second-line ART (9–22% at 36 months; Appendix) [8,9].
Modeled population
In the base-case analysis, we modeled South African children infected during pregnancy/delivery and presenting to care at 12 months of age. Although WHO guidelines combine first-line ART recommendations for all children below 3 years of age, mortality and disease progression differ in infants compared to 2 and 3-year-olds [16,17]. Because HIV-infected children present to ART programs in sub-Saharan Africa at a range of ages, we conducted sensitivity analyses to examine whether age at presentation to care (6, 24, and 35 months) impacted the specific comparison between first-line lopinavir/ritonavir and first-line nevirapine (Table 1) [17,19]. In the absence of data specific to children infected through breastfeeding, who may have slower disease progression than children infected during pregnancy or delivery, we also examined the impact of wide ranges in opportunistic infection and mortality rates [16].
Table 1.
Selected model input parameters (children aged 0–5 years).a
| Clinical Inputs | Value | Sources | |
| CD4+% at presentation to care, by age | [18] | ||
| 6 months (sensitivity analysis) | 25% | ||
| 12 months (base-case) | 22% | ||
| 24 and 35 months (sensitivity analyses) | 19% | ||
| ART efficacy: HIV-RNA <400 copies/ml at 24 weeks on ARTb | P1060 | PENPACT-1 | |
| First-line nevirapine strategy | |||
| Nevirapine (in first-line ART) | 75% | 77% | |
| Lopinavir/ritonavir (in second-line ART) | 75% | 81% | |
| (sensitivity analysis: 10–80%) | Derived from [8,9,28] | ||
| First-line lopinavir/ritonavir strategy | |||
| Lopinavir/ritonavir (in first-line ART) | 91% | 72% | |
| Nevirapine (in second-line ART) | 75% | 74% | |
| (Sensitivity analysis: 10–80%) | |||
| Darunavir-based regimen (sensitivity analysis) | 95% (assumption) | ||
| Risk of virologic failure after suppression (any ART) | 0.91% month (Sensitivity analysis: 0.5–3.6%) | Derived from [8,9,28] | |
| Loss to follow-up after ART initiation (% risk/month) | 0.2 (Sensitivity analysis: 0–0.8) | [31,32] | |
| Cost Inputsc | Cost (2012 USD) | Sources |
| Opportunistic infection care (per event; range by type of OI) | ||
| South Africa | $310–2490 | [24] |
| Côte d’Ivoire | $60–420 | [25] |
| Routine care (per month, range by CD4+%/CD4+)d | ||
| South Africa | $25–205 | [22,23] |
| Côte d’Ivoire | $30–40 | [25,26] |
| Care in the last month of life | ||
| South Africa | $800 | [22,23] |
| Côte d’Ivoire | $65 | [25,26] |
| Antiretroviral regimen costs (per month, range by age/weight)e | ||
| Lopinavir/ritonavir (liquid: age <3 years (base case) or <5 years (sensitivity analyses) | $17–27 (Sensitivity analysis: ↑1–15x) | [6,11] |
| Lopinavir/ritonavir (pediatric or adult tablets) | $13–29 | |
| Nevirapine (pediatric or adult tablets) | $3–8 | |
| Nevirapine/zidovudine/lamivudine (pediatric tablets, age <3 years) | $6–8 | |
| Abacavir/lamivudine (pediatric or adult tablets) | $8–18 | |
| Zidovudine/lamivudine (pediatric or adult tablets) | $4–9 | |
| Efavirenz (pediatric or adult tablets, age ≥3 years) | $3–7 | |
| Darunavir/ritonavir-based third-line ART (in sensitivity analyses only) | $36–92 |
aThis table includes selected inputs for children entering care at ages 0–35 months, which are applied until children reach 59 months of age. At age 60 months and beyond, separate sets of input data are applied. Complete inputs are shown in Appendix Table A for children aged below and at least 60 months.
bART efficacy: probability of suppressing HIV-RNA to below 400 copies/ml by 24 weeks (in base case analysis) or 48 weeks (in sensitivity analysis) after initiation of ART. Results from the PENPACT-1 trial are from a posthoc subgroup of children limited to those enrolling before 3 years of age and treated with nevirapine or lopinavir/ritonavir [8,9,28].
cIn sensitivity analyses, all costs were varied from 0.5–2.0 times the costs shown.
dRoutine clinical care costs include CD4+ and viral load monitoring, according to the modeled scenario.
eMonthly ART drug doses were calculated for children ages 0–13 years old based on the WHO weight-based dosing recommendations. Daily doses were then multiplied by unit drug costs from the May 2012 Clinton Health Access Initiative (CHAI) antiretroviral drug price list to determine monthly ART costs by age and weight. All children were assumed to receive liquid/syrup drug formulations until age 3 years for lopinavir/ritonavir (5 years in sensitivity analyses), and until age 6 months for all other medications, for which dispersible tablets are available. After these ages, children were assumed to transition to pediatric or adult tablet formulations based on weight-based dosing recommendations. Fixed-dose combinations were assumed to be used where available [11]. In the absence of data on darunavir/r costs for children, we assumed third-line ART would have costs equal to twice first-line lopinavir/r-based regimen costs.
Modeled treatment strategies
No ART included routine clinical care, as well as opportunistic infection prophylaxis and treatment. First-line nevirapine additionally included nevirapine with abacavir/lamivudine, followed after observed first-line failure by second-line lopinavir/ritonavir/zidovudine/lamivudine. First-line lopinavir/ritonavir included lopinavir/ritonavir with abacavir/lamivudine, followed by a second-line NNRTI (nevirapine if switching before 3 years of age, or efavirenz if switching after age 3 years) with zidovudine/lamivudine [10]. ART initiation, monitoring, and switching followed WHO 2013 Guidelines, with two additions: ART switching before age 3 years and a 6-month delay between confirmation of first-line failure and initiation of second-line ART (Appendix) [10,15]. Given limited current treatment options, modeled children remained on the last available line of ART lifelong [15,20].
Input data
We used International Epidemiologic Database to Evaluate AIDS (IeDEA) East African data to derive rates of CD4+% or CD4+ decline, opportunistic infections, and death through age 13 years, and data from the Cape Town AIDS Cohort to derive event risks at ages greater than 13 (Appendix) [21,22]. Base-case ART data were from the P1060 trial, including 24 and 48-week rates of RNA suppression, CD4+% gains on suppressive ART, and risk of late failure on each regimen (Table 1; Appendix) [8,9].
We calculated the costs of HIV-related care by multiplying the resources used (e.g. outpatient visits, inpatient days, laboratory testing, and medications) by country-specific unit costs for South Africa and Côte d’Ivoire, using pediatric-specific data when possible (Table 1; Appendix) [23–27]. ART costs were from Clinton Health Access Initiative (CHAI) price lists, calculated for each age from WHO weight-based dosing recommendations (Appendix) [6,11,15]. Costs were from the healthcare system perspective in 2012 USD, discounted at 3% per year.
Sensitivity analyses
We first simulated children presenting to care at age 6, 24, and 35 months. Next, although we lacked clinical and resource use data to directly simulate children in Côte d’Ivoire, we examined the impact of markedly lower healthcare costs by using cost data from this country. We also varied opportunistic infection and mortality rates (reflecting populations with different disease progression rates, such as children with postpartum infection [16]), healthcare costs, ART costs, and the discount rate (Appendix).
Univariate sensitivity analyses: antiretroviral therapy initiation, switching, and sequencing strategies
In sensitivity analyses, we also modeled the still widely used WHO 2010 ART initiation guidelines: initiation for all children below 2 years of age, and initiation based on CD4+%, CD4+ cell count, or opportunistic infections in children aged at least 2 years [15]. Modeled monitoring and switching strategies included various CD4+ and HIV-RNA testing frequencies; delays to confirming first-line ART failure and initiating second-line ART; and clinical, CD4+% or CD4+ cell count, and RNA thresholds for switching to second-line ART (Appendix). Modeled ART sequencing strategies included: one available line of ART, availability of third-line darunavir/ritonavir based ART, and additional strategies requested by the WHO HIV Guidelines Committee (Appendix).
Univariate sensitivity analyses: clinical impact, virologic outcomes, and costs of antiretroviral therapy
We derived separate RNA suppression rates and CD4+% changes on suppressive ART from the PENPACT-1 trial in Europe, and North and South America [28]. In a subgroup of children below 3 years old, PENPACT-1 demonstrated nonsignificantly higher virologic suppression rates with first-line nevirapine than lopinavir/ritonavir, in contrast to P1060 (Table 1). We also varied the efficacy of each second-line regimen (10–80% 24-week RNA <400 copies/ml); time required for initial RNA suppression (24 or 48 weeks, reflecting slower virologic suppression in children than adults); loss to follow-up before and after ART initiation (0–0.8%/month); and late failure rates for each regimen (0.5–3.6%/month, reflecting medication availability, tolerance, and adherence over time) [5,29–32]. Next, we varied opportunistic infection and mortality rates for patients on ART, reflecting data from trials and routine care settings (Appendix) [33,34]. Finally, in the absence of data about cold chain costs, we examined 1–15-fold increases in the cost of liquid lopinavir/ritonavir [35].
Multivariate sensitivity analyses
We next varied the most influential parameters simultaneously in multivariate sensitivity analyses. These included first-line lopinavir/ritonavir efficacy, first-line nevirapine efficacy, and the cost of both regimens.
Results
Base-case results
Impact of any antiretroviral therapy regimen
In the base-case, projected undiscounted life expectancy for no ART was 2.8 years (Table 2). Both first-line ART regimens markedly increased projected life expectancy (27.6–28.8 years) compared to no ART. In lifetime projections for South Africa, total healthcare costs were lower with no ART (average undiscounted cost: $11 450/person) than with either ART regimen. Because ART averts costly opportunistic infections and deaths, care costs for children without ART exceeded the costs of providing both care and ART for 12.4 years (149 months; Fig. 1, open arrow) [36].
Table 2.
Base-case model results.
| ART strategy | Undiscounted LE (years) | Discounted LE (years) | Undiscounted lifetime costs | Discounted lifetime costs | ICER ($/life-year saved) |
| South Africa; children presenting at age 12 months after in-utero/intrapartum infection | |||||
| No ART | 2.83a | 2.52 | 11 450 | 10 290 | |
| First-line LPV/r | 28.79 | 17.11 | 41 350 | 21 950 | 800b |
| First-line NVP | 27.61 | 16.59 | 44 030 | 23 370 | Dominatedc |
Costs are in 2012 USD. Discounting is at 3% per year. ART, antiretroviral therapy; ICER, incremental cost-effectiveness ratio; LE, life expectancy; LPV/r, lopinavir/ritonavir; NVP, nevirapine.
aLife expectancies are mean values projected by the model for a cohort of children presenting to care at 12 months of age. Discounted life expectancies, which value life-years in the future to be worth ‘less’ than life-years in the present, are not directly comparable to clinical experience.
bWHO (WHO-CHOICE) recommendations for country-specific gross domestic product (GDP)-based cost-effectiveness thresholds are based primarily on cost per quality-adjusted life-year saved or cost per disability-adjusted life-year averted. Because of limited health utility weight data in children, we project nonquality-weighted life expectancy, and thus calculate ICERs in dollars per life-year saved.
cDominated: Here, refers to a strategy that is more expensive and less effective than its alternative. This indicates that first-line lopinavir/ritonavir is cost-saving compared to first-line nevirapine in these scenarios. By convention, we do not calculate an ICER comparing these two strategies, and instead calculate the ICER of first-line lopinavir/ritonavir compared to no ART.
Fig. 1.
Projected survival and costs with alternative first-line pediatric antiretroviral therapy regimens.
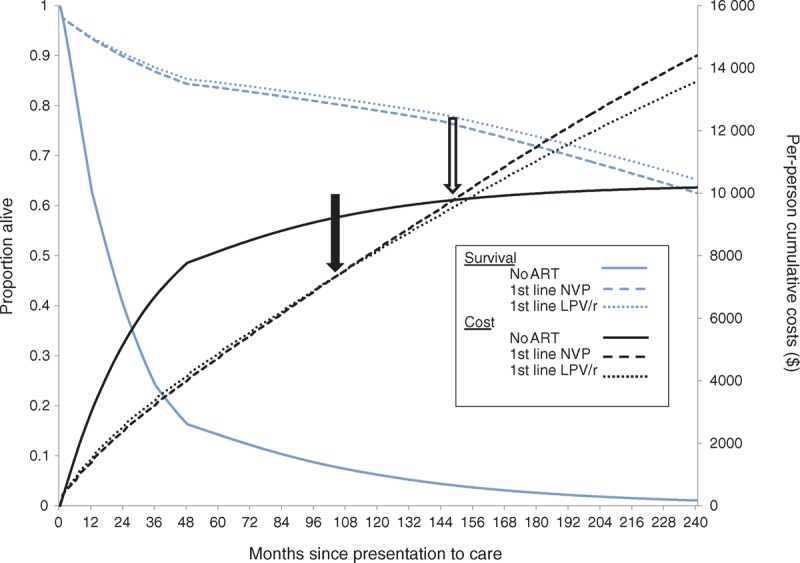
Results are shown for the base case analysis: South African children presenting to care at 12 months of age. The proportion of patients alive is on the left-hand vertical axis (blue lines) and the per-person cumulative costs are on the right-hand vertical axis (black lines). Survival and per-person undiscounted costs are projected over 20 years (240 months) since presentation to care, shown on the horizontal axis. The no ART strategy is represented by solid lines, first-line NVP by dashed lines, and first-line LPV/r by dotted lines. The solid arrow refers to the time after presentation when first-line LPV/r becomes cost-saving compared to first-line NVP: 104 months (8.7 years). Of note, both ART strategies are cost-saving compared to no ART until 149 months (12.4 years) after presentation (open arrow), due to the high costs of care for opportunistic infections and death compared to medication costs. ART, antiretroviral therapy; LPV/r, lopinavir/ritonavir; NVP, nevirapine.
Comparison of first-line antiretroviral therapy regimens
The first-line lopinavir/ritonavir strategy led to a longer projected life expectancy than the first-line nevirapine strategy (28.8 vs. 27.6 years; Table 2). Although first-line lopinavir/ritonavir was the most expensive ART strategy initially ($1400 vs. $1510 at 1 year), it became cost-saving compared to first-line nevirapine by 8.7 years (104 months; Fig. 1, closed arrow). Lifetime costs were $41 350/person with first-line lopinavir/ritonavir and $44 030/person with first-line nevirapine (Table 2). Cost-savings resulted primarily from our assumption of lifelong second-line ART: second-line lopinavir/ritonavir (in the first-line nevirapine strategy) was more expensive than a second-line NNRTI (nevirapine or efavirenz, in the first-line lopinavir/ritonavir strategy), and this difference outweighed the lower costs of nevirapine in first-line ART (Fig. 2). The ICER of first-line lopinavir/ritonavir compared to no ART was $800/YLS (Table 2). Per cost-effectiveness analysis convention, we do not report ICERs for dominated (more expensive and less effective) strategies [37]. However, it may be of policy interest to note that, if first-line lopinavir/ritonavir were not available, first-line nevirapine would be very cost-effective compared to no ART, with an ICER of $930/YLS.
Fig. 2.
Duration on each modeled antiretroviral therapy regimen and impact on total lifetime healthcare costs.
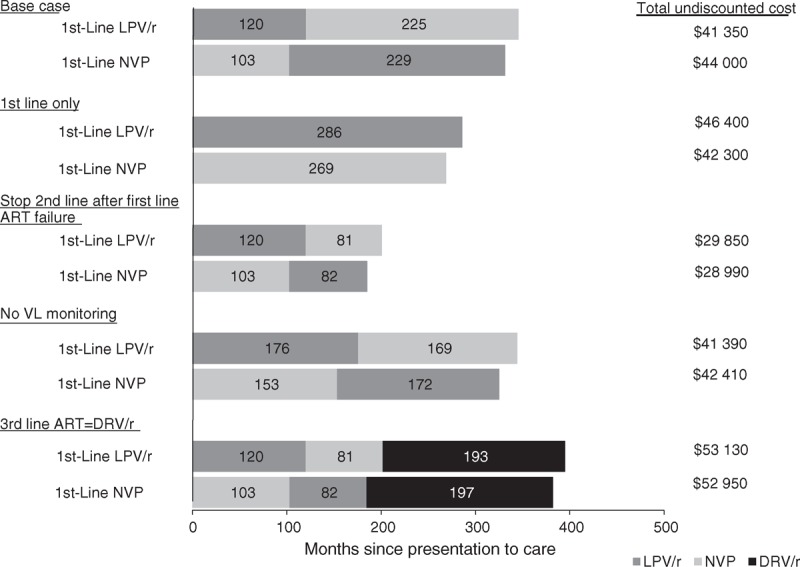
Months spent on NVP-based ART (light gray bars), LPV/r-based ART (dark gray bars), and DRV/r-based ART (black bars) are shown, in undiscounted months, for base case and key sensitivity analyses described in the text. Total per-person, undiscounted lifetime costs are shown at the right of the bars for each treatment strategy. Results shown here are for children presenting to care at 12 months of age in South Africa; findings for children presenting at other ages and using healthcare costs from Côte d’Ivoire followed similar trends. ART, antiretroviral therapy; DRV/r, darunavir boosted with ritonavir; LPV/r, lopinavir/ritonavir; NVP, nevirapine; VL, viral load.
Univariate sensitivity analyses
First-line lopinavir/ritonavir remained cost-saving compared to first-line nevirapine for all ages at presentation (6–35 months) and using Côte d’Ivoire costs (Table 3), although the time required for first-line lopinavir/ritonavir to become cost-saving compared to first-line nevirapine was much longer with Côte d’Ivoire costs than with South Africa costs (26.4 vs. 8.7 years; Appendix). Results were also robust to variation in most clinical and economic parameters (Appendix,). Policy conclusions were, however, sensitive to three key types of parameters (Table 3, section B).
Table 3.
Sensitivity analysis results.
| ART strategy (ordered by costs)a | Undiscounted LE (years) | Discounted LE (years) | Discounted lifetime costs | ICER ($/life-years saved) |
| Alternative patient populations and healthcare costs | ||||
| South Africa: in-utero/intrapartum infection; presenting age 6 months | ||||
| No ART | 2.50 | 2.23 | 8520 | |
| First-line LPV/r | 27.45 | 16.31 | 20 620 | 860 |
| First-line NVP | 26.29 | 15.81 | 21 960 | Dominatedb |
| South Africa: in-utero/intrapartum infection; presenting age 24 monthsc | ||||
| No ART | 3.54 | 3.09 | 12 670 | |
| First-line LPV/r | 28.71 | 17.10 | 27 220 | 710 |
| First-line NVP | 27.52 | 16.59 | 29 600 | Dominated |
| South Africa: in-utero/intrapartum infection; presenting age 35 monthsc | ||||
| No ART | 4.71 | 4.03 | 14 420 | |
| First-line LPV/r | 29.46 | 17.60 | 23 240 | 650 |
| First-line NVP | 28.36 | 17.11 | 24 790 | Dominated |
| Côte d’Ivoire: in-utero/intrapartum infection; presenting age 12 monthsd | ||||
| No ART | 2.83 | 2.52 | 1820 | |
| First-line LPV/r | 28.79 | 17.11 | 15 120 | 910 |
| First-line NVP | 27.62 | 16.58 | 15 480 | Dominated |
| Additional sensitivity analyses (South Africa, in-utero/intrapartum infection; presenting age 12 months) | ||||
| One line of ART available | ||||
| No ART | 2.83 | 2.52 | 10 290 | |
| First-line NVPa | 22.42 | 14.57 | 24 890 | 1210 |
| First-line LPV/r | 23.84 | 15.31 | 26 490 | 2190 |
| Stop second-line ART at failure | ||||
| No ART | 2.83 | 2.52 | 10 290 | |
| First-line NVPa | 23.76 | 15.03 | 17 360 | 565 |
| First-line LPV/r | 25.18 | 15.74 | 17 760 | 570 |
| PENPACT-1 ART efficacies | ||||
| No ART | 2.83 | 2.52 | 10 290 | |
| First-line LPV/r | 29.28 | 16.96 | 22 240 | Weakly dominatede |
| First-line NVP | 30.42 | 17.39 | 22 370 | 810 |
| Second-line NNRTI efficacy (40%) | ||||
| No ART | 2.83 | 2.52 | 10 290 | |
| First-line LPV/r | 26.51 | 16.26 | 23 010 | 930 |
| First-line NVP | 27.61 | 16.59 | 23 370 | 1110 |
| 2.1× late failure for first-line LPV/r (1.9%/month)f | ||||
| No ART | 2.83 | 2.52 | 10 290 | |
| First-line LPV/r | 26.60 | 16.38 | 22 070 | 850 |
| First-line NVP | 27.61 | 16.59 | 23 370 | 6310 |
| 4.5× cost of liquid LPV/r ($80–105 per month for children <3 years of age) | ||||
| No ART | 2.83 | 2.52 | 10 290 | |
| First-line NVPa | 28.77 | 16.59 | 23 480 | Weakly dominated |
| First-line LPV/r | 27.58 | 17.10 | 23 510 | 910 |
| 15.0× cost of liquid LPV/r ($260–330 per month for children <3 years of age) | ||||
| No ART | 2.83 | 2.52 | 10 290 | |
| First-line NVPa | 27.58 | 16.58 | 23 780 | 960 |
| First-line LPV/r | 28.79 | 17.09 | 28 170 | 8640 |
| Liquid LPV/r used until age 5 | ||||
| No ART | 2.83 | 2.52 | 10 290 | |
| First-line LPV/r | 28.77 | 17.09 | 22 730 | 850 |
| First-line NVP | 27.59 | 16.60 | 23 440 | Dominated |
Costs are in 2012 USD. Discounting is at 3% per year (results using alternative discount rates are shown in the Appendix (http://links.lww.com/QAD/A686). ART, antiretroviral therapy; DRV/r, darunavir/ritonavir; ICER, incremental cost-effectiveness ratio; LE, life expectancy; LPV/r, lopinavir/ritonavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; NVP, nevirapine.
aStrategies are listed in order of increasing costs. As a result, the order of the three treatment strategies changes between scenarios. Scenarios in which first-line NVP is less expensive over a lifetime horizon than first-line LPV/r are highlighted with footnote (a).
b‘Dominated’ in this table refers to strong dominance: a strategy is both more expensive and less effective than its next less expensive alternative.
cIn these analyses, the model simulates a cohort of children presenting to care and initiating ART at ages 6, 12, 24, and 35 months. Morbidity and mortality occurring among children before these ages are not included in these analyses. As a result, children presenting to care at older ages have longer projected life expectancies both with and without ART. This occurs because the model incorporates age-stratified mortality risks from HIV and non-HIV causes. High mortality rates among young, untreated children mean that children who survive without treatment to present to care at older ages are generally less sick, reflecting the ‘survivor bias’ seen in most cohorts of HIV-infected children [16–18]. These analyses are intended to evaluate the impact of age at ART initiation on the comparison between the two first-line regimens, and not to compare the outcomes of early versus delayed ART initiation.
dBase-case results using Côte d’Ivoire costs are shown here. Full results for all analyses using Côte d’Ivoire costs are in the Appendix (http://links.lww.com/QAD/A686).
eWeakly dominated. Here, refers to extended dominance: the incremental cost-effectiveness ratio (ICER) of the nondominated strategy compared to the dominated strategy is less than the ICER of the dominated strategy compared to no ART, indicating that the dominated strategy is an inefficient use of healthcare resources.
fIn this scenario, we model a higher risk of late virologic failure (after initial suppression) for lopinavir/ritonavir in first-line ART, but no increase in late failure for nevirapine in first-line ART or for either second-line regimen. Such a scenario might occur if liquid lopinavir/ritonavir (administered to children too young to swallow pills) is much more difficult to tolerate than all other modeled regimens.
Reduced availability or duration of second-line antiretroviral therapy: first-line lopinavir/ritonavir is very cost-effective, but not cost-saving
The assumption of lifelong second-line ART was a key factor in the base case results above. When only one line of ART was available, or when second-line ART was stopped at virologic failure, first-line lopinavir/ritonavir remained more effective than first-line nevirapine, but became more expensive. In these scenarios, first-line lopinavir/ritonavir was very cost-effective compared to first-line nevirapine (ICERs: $2190/YLS and $570/YLS). As in the base-case, however, first-line lopinavir/ritonavir remained cost-saving in South Africa when switching from first-line to second-line ART was delayed (e.g. if HIV-RNA or CD4+ monitoring was unavailable, or if CD4+ confirmation of clinical or virologic failure was required) and when the costs of the last available line of ART were equalized by a darunavir/ritonavir-based third-line regimen in both strategies (Appendix).
Virologic outcomes of first-line and second-line antiretroviral therapy: first-line nevirapine is very cost-effective
When the efficacies of the first-line ART regimens were reversed using PENPACT-1 trial data (Table 1), first-line nevirapine became the more effective and more expensive strategy, and was very cost-effective compared to no ART (ICER: $810/YLS) [28]. If first-line lopinavir/ritonavir and first-line nevirapine efficacies were held constant (using P1060 data, as in the base-case), but the efficacy of a second-line NNRTI following first-line lopinavir/ritonavir was very low (≤40% RNA suppression at 24 weeks), first-line nevirapine was also more effective, more expensive, and very cost-effective (ICER: $1110/YLS at 40% suppression) compared to first-line lopinavir/ritonavir.
Tolerability and cost of liquid lopinavir/ritonavir: first-line nevirapine is very cost-effective
When the risk of late virologic failure for lopinavir/ritonavir in first-line ART was increased 2.1-fold to 1.9%/month (e.g. as might occur with poor tolerability or medication stock-outs of lopinavir/ritonavir syrup), first-line nevirapine was more effective and very cost-effective compared to first-line lopinavir/ritonavir (ICER: $6310/YLS). When the cost of liquid lopinavir/ritonavir was increased 4.5-fold from the base-case (e.g. reflecting costs for establishing and maintaining cold chains), there was no change in projected life expectancies, but first-line lopinavir/ritonavir was no longer cost-saving. At least 15-fold increases in liquid lopinavir/ritonavir costs, first-line lopinavir/ritonavir was no longer very cost-effective.
Multivariate sensitivity analyses
Results of simultaneous variation in first-line ART efficacy and the costs of lopinavir/ritonavir and nevirapine are shown in Fig. 3. When the efficacy (proportion of treated children with HIV-RNA <400 copies/ml at 24 weeks) of one first-line regimen is greater than the efficacy of the other, GDP-based interpretation of cost-effectiveness generally supports the choice of the more effective first-line regimen (panel A). Because lopinavir/ritonavir and nevirapine are used in both strategies (as either first-line or second-line ART), variations in drug costs exert only a modest impact on the economically preferred strategy (panels B and C).
Fig. 3.
Multivariate sensitivity analyses: impact of simultaneous variation in antiretroviral therapy efficacy and costs.
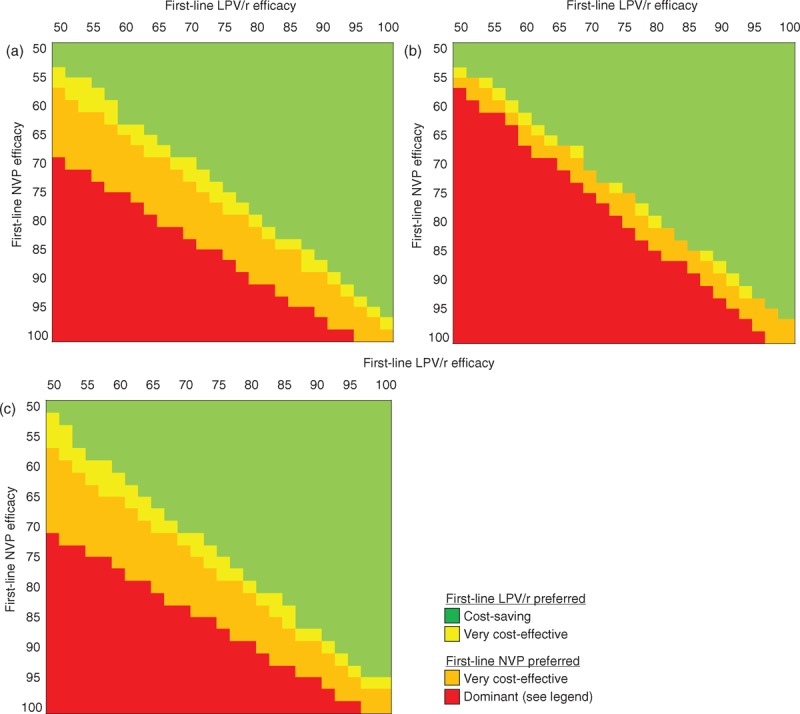
In each panel, the efficacy (proportion of treated children with HIV-RNA <400 copies/ml at 24 weeks) of the first-line LPV/r regimen is shown on the horizontal axis, and the efficacy of the first-line NVP regimen is shown on the vertical axis. Panel a shows results at base-case ART costs. Panel b shows results when the cost of LPV/r is reduced by half (when used in first-line or second-line ART, at any age). Panel c shows results when the cost of NVP is reduced by half (again, when used in first-line or second-line ART, at any age). Costs and life-years are discounted at 3% per year. Green shading indicates scenarios in which the first-line LPV/r strategy is cost-saving (leading to greater life expectancy and lower lifetime costs), compared to the first-line NVP strategy. Yellow shading indicates scenarios in which first-line LPV/r is very cost-effective compared to first-line NVP (ICER of first-line LPV/r compared to first-line NVP is less than South Africa per-capita GDP ($7500)/YLS, or first-line NVP is more effective and more expensive, but its ICER compared to first-line LPV/r is greater than $7500/YLS). Orange shading indicates scenarios in which first-line LPV/r is no longer very cost-effective (first-line LPV/r is more effective and more expensive than first-line NVP, but its ICER relative to first-line NVP is >$7500/YLS, or first-line NVP is more effective and more expensive, and its ICER relative to first-line LPV/r is <$7500). Red shading indicates scenarios in which first-line LPV/r is strongly or weakly dominated by first-line NVP (weak dominance: first-line LPV/r is less effective and less expensive, but represents an inefficient use of resources; strong dominance: first-line LPV/r leads to lower life expectancy and greater lifetime costs). Following WHO GDP-based guidance, cost-effectiveness results support the choice of first-line LPV/r in the green- and yellow-shaded scenarios and the choice of first-line NVP in the orange- and red-shaded scenarios. ART, antiretroviral therapy; GDP: gross domestic product; ICER, incremental cost-effectiveness ratio; LPV/r, lopinavir/ritonavir; NVP, nevirapine; YLS, year of life saved.
Discussion
We used data from the IMPAACT P1060 trial and other sources to estimate the cost-effectiveness of first-line ART for children below 3 years of age in sub-Saharan Africa. There were four key findings from this study. First, treating HIV-infected children with any ART regimen dramatically increased survival; ART also saved money in the short term by averting opportunistic infections, as has previously been reported in South Africa [36]. Compared to no ART, both regimens were very cost-effective in the long term (ICER of first-line lopinavir/ritonavir compared to no ART: $800/YLS; if first-line lopinavir/ritonavir was not available, ICER of first-line nevirapine compared to no ART: $930/YLS). In addition, projected life expectancies were longer for children presenting to care at older compared to younger ages (Table 3). This result reflects very high morbidity and mortality among young infants who do not access care or ART [16,19,38]. Because children who do not survive to access ART were by definition excluded from these analyses, our results are not intended to compare the relative value of ART initiation at older versus younger ages. Increasing access to early pediatric HIV diagnosis and to pediatric ART is therefore critical, regardless of the ART regimen chosen.
Second, first-line lopinavir/ritonavir was cost-saving compared to first-line nevirapine over children's lifetimes. This results both from the greater lopinavir/ritonavir efficacy reported in P1060, averting more care costs for morbidity and mortality in the first few years after ART initiation than nevirapine, and from the assumption of lifelong second-line ART (Fig. 2) [10,15]. Importantly, these cost-savings occurred well beyond the horizons of clinical trials: more than 8 years after ART initiation in South Africa, and more than 26 years after ART initiation with lower healthcare costs from Côte d’Ivoire. In the much nearer future, however, the availability and costs of second-line and third-line pediatric ART will likely change, as will ART monitoring and switching protocols. If the relative duration and cost of first-line compared to second-line ART are altered, first-line lopinavir/ritonavir may no longer be cost-saving. First-line lopinavir/ritonavir remained more effective than first-line nevirapine, and very cost-effective, in many of the scenarios that we evaluated, however, suggesting that it will likely remain of good value as second-line and third-line ART practices evolve.
Third, cost-effectiveness results depended on the relative efficacy of lopinavir/ritonavir and nevirapine in both first-line and second-line ART. The importance of first-line efficacy was demonstrated by PENPACT-1 trial data. If first-line nevirapine has higher rates of virologic suppression than lopinavir/ritonavir, as in the PENPACT-1 subgroup of children below 3 years old, then the first-line nevirapine strategy will be more effective and cost-effective in some settings. The nonsignificant superiority of nevirapine in PENPACT-1 was based on a small post-hoc subgroup analysis; the primary outcome of 4-year change in HIV-RNA was not different between children randomized to protease inhibitor versus NNRTI-based ART [28].
The importance of second-line ART efficacy was highlighted in sensitivity analyses which varied this parameter. The first-line nevirapine/second-line lopinavir/ritonavir sequence is widely used, with excellent second-line lopinavir/ritonavir outcomes in children and adults [29,39,40]. The first-line lopinavir/ritonavir/second-line NNRTI sequence is infrequently described. Sparse observational data suggest 24–48-week suppression rates of 16–45% in children and adults on second-line nevirapine or efavirenz [29,40–42]. These low rates may reflect poor adherence, or may result from accumulation of resistance to nucleoside reverse transcriptase inhibitors (zidovudine, lamivudine, and abacavir) on failing protease inhibitor-based ART, causing the NNRTI used with these drugs in second-line ART to act essentially as monotherapy. This was likely a greater concern with older protease inhibitor regimens (e.g. full-dose ritonavir) [42]. In contrast, PENPACT-1 and the South African Children with HIV Early Antiretroviral (CHER) trial reported few resistance mutations even when children remained on failed protease inhibitor-based ART, and both PENPACT-1 and P1060 observed second-line NNRTI suppression rates greater than 70%, although in small numbers of children [8,9,28,43]. We found that the efficacy of second-line NNRTI-based ART needed to be very low (≤40% RNA suppression at 24 weeks) to render the first-line lopinavir/ritonavir strategy no longer more effective or very cost-effective.
Fourth, lopinavir/ritonavir for young children is formulated as a syrup, which requires refrigeration and, although well tolerated in P1060, may cause adverse gastrointestinal effects [5,8,9]. Although we lacked data on the cost of establishing and maintaining cold chains, we found that these would need to increase the total cost of liquid lopinavir/ritonavir more than 4.5-fold to render the first-line lopinavir/ritonavir strategy no longer cost-saving. Cold chains may, however, be infeasible in some settings with severely constrained human resources and healthcare infrastructure, regardless of cost [35]. We also examined the impact of increased risks of virologic failure after the first 6 months of first-line lopinavir/ritonavir, such as might occur if lopinavir/ritonavir syrup is difficult for young children to tolerate, and thus leads to low adherence over time, or if medication stock-outs lead to development of drug-resistant HIV [7]. We found that this late failure risk would need to increase by only 2.1-fold, from 0.9 to 1.9%/month, to change policy conclusions. Novel formulations, such as the heat-stable lopinavir/ritonavir sprinkles that may soon be US Food and Drug Administration (FDA)-approved [44,45], or a strategy of switching to nevirapine or efavirenz after suppression on lopinavir/ritonavir, may reduce the costs and improve the tolerability of a first-line lopinavir/ritonavir regimen for children too young to swallow pills [30,46].
The present study has several limitations related to model-based cost-effectiveness analyses. Model-based analyses necessarily extrapolate long-term outcomes from short-term data. Because pediatric ART was introduced less than 10 years ago in most African programs, long-term outcomes for children on currently used ART regimens in Africa are not yet available. We therefore calibrated our model to fit short-term opportunistic infection and mortality observations for ART-treated children (Appendix) [8,9]. This led to projected life expectancies (27–28 years) comparable to estimates for young South African adults, but necessarily longer than the current experience of clinicians caring for HIV-infected children [47]. We examined this uncertainty through a range of scenarios leading to shorter and longer life expectancies, and the clinical and economic value of first-line lopinavir/ritonavir remained unchanged. In addition, GDP-based cost-effectiveness thresholds raise important concerns about generalizability and equity across countries, and do not address possible changes in country GDPs over time [48]. Although an alternative approach has not been widely accepted, comparing results to published ICERs for common HIV therapies ($500–5000/YLS), rather than to GDP thresholds, did not change policy conclusions [49]. Finally, by convention, cost-effectiveness analysis considers long-term (lifetime) horizons [37]. Cost-effectiveness is not the same as affordability, however, and is only one of the many critical factors in decision-making. We also present detailed survival and cost estimates for each year of our simulation (Fig. 2; Appendix Fig. A). Given limited current ART availability, providing nevirapine-based ART to greater numbers of children, even if it may be slightly less effective in the short term and more expensive in the long term, may be preferred by some decision-makers.
Additional limitations relate to trial strategies and data availability. We simulated the regimens in the P1060 trial; following the trial protocol, we did not model co-administration with tuberculosis therapy. If efavirenz or other medications that can be co-administered with tuberculosis therapy become widely used for children below 3 years old, the benefit of first-line lopinavir/ritonavir may change [50]. Although we modeled children infected during pregnancy or delivery, sensitivity analyses reflecting lower risks of opportunistic infections and mortality suggest that conclusions would be similar for postnatally HIV-infected children [16]. Finally, we did not have detailed data for children in Côte d’Ivoire; we used clinical data from East and Southern Africa and varied only costs. Although our approach to deriving healthcare costs matched published economic data from South Africa and Zambia (a low-income country with GDP similar to Côte d’Ivoire), such an approach excluded country-specific factors, such as reduced malaria risk with protease inhibitors, which might change the clinical and cost-effectiveness of first-line lopinavir/ritonavir [36,51,52].
In conclusion, we find that treating HIV-infected children with any ART regimen dramatically increases life expectancy, is cost-saving in the short term, and is very cost-effective in the long term, compared to no ART. On the basis of data from the P1060 trial with the assumption of lifelong, currently available second-line ART, a first-line regimen including lopinavir/ritonavir will improve outcomes and save money compared to a first-line regimen including nevirapine. These findings support the adoption of WHO 2013 HIV treatment guidelines in most settings in which lopinavir/ritonavir is feasible. Increasing the availability of pediatric ART is critical and will be of excellent value, regardless of the initial regimen used.
Acknowledgements
The authors are very grateful to the P1060 and PENPACT-1 trial teams, who provided data for primary and sensitivity analyses and assisted in data analysis and interpretation. We thank the CEPAC-Pediatric research team for their role in model development, including Ingrid Bassett, Linda-Gail Bekker, Sophie Desmonde, Jordan Francke, Nancy Kaye Horstmann, Valeriane Leroy, Landon Myer, David Paltiel, Kunjal Patel, George Seage, and Lynn Ramirez-Avila. In particular, we thank Taige Hou for his substantial contributions in coding, debugging, and updating the CEPAC-Pediatrics model. We also appreciate the input of Elaine Abrams and the WHO Maternal-Child Health HIV Guidelines Committee, to whom these analyses were presented in December 2012, to inform development of the WHO 2013 Consolidated HIV Guidelines.
Author contributions: Designed and conducted the analyses (A.L.C., K.D., M.P., K.K.); interpreted model results (A.L.C., K.P., M.P., K.K., R.P.W., S.E., E.L., K.W.K., P.P., K.F.); provided primary data for use in the model-based analyses (J.C.L., L.H., K.W.K., S.A., P.P.); drafted the manuscript (A.L.C., K.D.); critically revised the manuscript and approved its submission (all authors).
Funding sources: Supported by the WHO; the National Institutes of Health through the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT), International Database to Evaluate AIDS (IeDEA), and National Institute of Allergy and Infectious Diseases (K01 AI078754, R01 HD079214, K24 AI062476, R01 AI058736, R01 AI093269, U01 AI069911, U01AI09919, and the Harvard Center for AIDS Research); the March of Dimes Foundation; and the Massachusetts General Hospital Executive Committee on Research. The funders had no role in study design, data analysis, interpretation of results, or decision to publish.
Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9–001/HHSN267200800001C).
Overall support for the IeDEA network is provided by the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Cancer Institute.
Ethics: This study was approved by the Partners Healthcare IRB, Boston, Massachusetts, USA.
This manuscript represents the views of the authors, and the findings and conclusions included here do not necessarily represent the views of the World Health Organization.
Conflicts of interest
All authors report that they have no conflicts of interest to disclose.
Supplementary Material
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS report on the global AIDS epidemic. 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf [Accessed 20 November 2014] [Google Scholar]
- 2.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–1243. [DOI] [PubMed] [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS). Progress report on the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive, 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/20130625_progress_global_plan_en.pdf [Accessed 21 November 2014] [Google Scholar]
- 4.UNICEF, UNAIDS. Towards an AIDS-free generation: children and AIDS sixth stocktaking report. 2013. http://www.childrenandaids.org/ [Accessed 19 November 2014] [Google Scholar]
- 5.Sohn AH, Nuttall JJ, Zhang F. Sequencing of antiretroviral therapy in children in low- and middle-income countries. Curr Opin HIV AIDS 2010; 5:54–60. [DOI] [PubMed] [Google Scholar]
- 6.Clinton Health Access Initiative. Antiretroviral (ARV) Ceiling price list. 2012. http://d2pd3b5abq75bb.cloudfront.net/2012/07/12/15/03/07/163/CHAI_ARV_Ceiling_Price_List_May_2012.pdf [Accessed 18 November 2014] [Google Scholar]
- 7.Prendergast AJ, Penazzato M, Cotton M, Musoke P, Mulenga V, Abrams EJ, Gibb DM. Treatment of young children with HIV infection: using evidence to inform policymakers. PLoS Med 2012; 9:e1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med 2012; 366:2380–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med 2010; 363:1510–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Consolidated guidelines on the use of antiretrovirals for the treatment and prevention of HIV infection. 2013. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html [Accessed 19 November 2014] [Google Scholar]
- 11.Doherty K, Essajee S, Penazzato M, Holmes C, Resch S, Ciaranello A. Estimating age-based antiretroviral therapy costs for HIV-infected children in resource-limited settings based on World Health Organization weight-based dosing recommendations. BMC Health Serv Res 2014; 14:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciaranello AL, Morris BL, Walensky RP, Weinstein MC, Ayaya S, Doherty K, et al. Validation and calibration of a computer simulation model of pediatric HIV infection. PLoS One 2013; 8:e83389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO-CHOICE: cost-effectiveness thresholds. 2010. http://www.who.int/choice/costs/CER_thresholds/en/index.html [Accessed 20 November 2014] [Google Scholar]
- 14.World Bank. GDP per capita (current US$). 2013. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD [Accessed 21 November 2014] [Google Scholar]
- 15.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: Recommendations for a public health approach. 2010. http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html [Accessed 21 November 2014] [PubMed] [Google Scholar]
- 16.Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One 2012; 7:e28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desmonde S, Coffie P, Aka E, Amani-Bosse C, Messou E, Dabis F, et al. Severe morbidity and mortality in untreated HIV-infected children in a paediatric care programme in Abidjan, Cote d’Ivoire, 2004–2009. BMC Infect Dis 2011; 11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Embree J, Bwayo J, Nagelkerke N, Njenga S, Nyange P, Ndinya-Achola J, et al. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. Pediatr Infect Dis J 2001; 20:397–403. [DOI] [PubMed] [Google Scholar]
- 19.Davies MA, Phiri S, Wood R, Wellington M, Cox V, Bolton-Moore C, et al. Temporal trends in the characteristics of children at antiretroviral therapy initiation in southern Africa: the IeDEA-SA Collaboration. PLoS One 2013; 8:e81037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Sources and prices of selected medicines and diagnostics for people living with HIV/AIDS. 2013. http://www.who.int/hiv/pub/prev_care/edm/en/index.html [Accessed 20 November 2014] [Google Scholar]
- 21.Ciaranello AL, Lu Z, Ayaya S, Losina E, Musick B, Vreeman R, et al. Incidence of WHO Stage 3 and 4 events, tuberculosis, and mortality in untreated, HIV-infected children enrolling in care before 1 year of age: an IeDEA (International Epidemiologic Databases to Evaluate AIDS) East Africa regional analysis. Pediatr Infect Dis J 2014; 33:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes CB, Wood R, Badri M, Zilber S, Wang B, Maartens G, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr 2006; 42:464–469. [DOI] [PubMed] [Google Scholar]
- 23.Cleary S, Chitha W, Jikwana S, Okorafor OA, Boulle A. Health systems trust: South African health review. 2005. http://www.healthlink.org.za/publications/682 [Accessed 18 November 2014] [Google Scholar]
- 24.Thomas LS. Costing of HIV/AIDS services at a tertiary level hospital in Gauteng Province. 2006. http://wiredspace.wits.ac.za/handle/10539/2008 [Accessed 19 November 2014] [Google Scholar]
- 25.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet 1999; 353:1463–1468. [DOI] [PubMed] [Google Scholar]
- 26.Yazdanpanah Y, Losina E, Anglaret X, Goldie SJ, Walensky RP, Weinstein MC, et al. Clinical impact and cost-effectiveness of co-trimoxazole prophylaxis in patients with HIV/AIDS in Côte d’Ivoire: a trial-based analysis. AIDS 2005; 19:1299–1308. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization (WHO-CHOICE). Prices for hospitals and health centers. 2014. http://www.who.int/choice/en/ [Accessed 23 November 2014] [Google Scholar]
- 28.Babiker A, Castro nee Green H, Compagnucci A, Fiscus S, Gibb DM, Harper L, et al. First-line antiretroviral therapy with a protease inhibitor versus nonnucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis 2011; 11:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanoni BC, Sunpath H, Feeney ME. Pediatric response to second-line antiretroviral therapy in South Africa. PLoS One 2012; 7:e49591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhn L, Coovadia A, Strehlau R, Martens L, Hu CC, Meyers T, et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis 2012; 12:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis 2008; 8:477–489. [DOI] [PubMed] [Google Scholar]
- 32.Ciaranello A, Chang Y, Margulis A, Bernstein A, Bassett IV, Losina E, Walensky RP. Effectiveness of pediatric ART in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis 2009; 49:1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNairy ML, Lamb MR, Carter RJ, Fayorsey R, Tene G, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda and Tanzania. J Acquir Immune Defic Syndr 2012; 62:e70–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Losina E, Yazdanpanah Y, Deuffic-Burban S, Wang B, Wolf LL, Messou E, et al. The independent effect of highly active antiretroviral therapy on severe opportunistic disease incidence and mortality in HIV-infected adults in Côte d’Ivoire. Antivir Ther 2007; 12:543–551. [PMC free article] [PubMed] [Google Scholar]
- 35.Khan IA, Saha A, Chowdhury F, Khan AI, Uddin MJ, et al. Coverage and cost of a large oral cholera vaccination program in a high-risk cholera endemic urban population in Dhaka. Bangladesh Vaccine 2013; 31:6058–6064. [DOI] [PubMed] [Google Scholar]
- 36.Meyer-Rath G, Brennan A, Long L, Ndibongo B, Technau K, Moultrie H, et al. Cost and outcomes of paediatric antiretroviral treatment in South Africa. AIDS 2013; 27:243–250. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996; 276:1253–1258. [PubMed] [Google Scholar]
- 38.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008; 359:2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies MA, Moultrie H, Eley B, Rabie H, Van Cutsem G, Giddy J, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa: the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr 2011; 56:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy RA, Sunpath H, Lu Z, Chelin N, Losina E, Gordon M, et al. Outcomes after virologic failure of first-line ART in South Africa. AIDS 2010; 24:1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asboe D, Mandalia S, Gazzard BG. Sequencing to NRTI plus NNRTI-only combinations after virological failure of protease inhibitor-based combination HIV-1 therapy. HIV Clin Trials 2003; 4:1–10. [DOI] [PubMed] [Google Scholar]
- 42.Orrell C, Levison J, Ciaranello A, Bekker LG, Kuritzkes DR, Freedberg KA, Wood R, et al. Resistance in pediatric patients experiencing virologic failure with first-line and second-line antiretroviral therapy. Pediatr Infect Dis J 2013; 32:644–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Violari A, Cotton M, Otwombe K, Hunt G, Kalimashe M, et al. Does early initiation of ART in infants affect virological and resistance outcomes? Data from the CHER trial after 6 years of follow-up (oral abstract O224). 11th International Congress on Drug Therapy in HIV Infection, Glasgow 2012 http://dx.doi.org/10.7448/IAS.15.6.18085 [Accessed 21 November 2014] [Google Scholar]
- 44.Chang S. Addressing the drug development needs of infants and young children: DNDi's pediatric HIV program. 19th Interational AIDS Conference, Washington, D.C 2012 http://dndi.org/images/stories/events2012/IAC%20-%20Washington%20-%20July/Chang.pdf [Accessed 21 November 2014] [Google Scholar]
- 45.Musiime V, Fillekes Q, Kekitiinwa A, Kendall L, Keishanyu R, Namuddu R, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr 2014; 66:148–154. [DOI] [PubMed] [Google Scholar]
- 46.French National Institute for Health and Medical Research-French National Agency for Research on AIDS and Viral Hepatitis (Inserm-ANRS). Evaluation of simplified antiretroviral treatment strategies in HIV infected children treated by antiretroviral (ARV) before one year of age. 2012. http://www.clinicaltrials.gov/ct2/show/NCT01127204?term=MONOD&rank=1 [Accessed 18 November 2014] [Google Scholar]
- 47.Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med 2013; 10:e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Revill P, Walker S, Madan J, Ciaranello A, Mwase T, et al. Using cost-effectiveness thresholds to determine value for money in low- and middle-income country healthcare systems: are current international norms fit for purpose? 2014. [[Accessed 17 November 2014]]. https://www.york.ac.uk/media/che/documents/papers/researchpapers/CHERP98_costeffectiveness_thresholds_value_low_middle_income_countries.pdf. [Google Scholar]
- 49.Kahn JG, Marseille EA, Bennett R, Williams BG, Granich R. Cost-effectiveness of antiretroviral therapy for prevention. Curr HIV Res 2011; 9:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institute of Allergy and Infectious Disease (NIAID). Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Efavirenz (EFV) in HIV-infected and HIV/tuberculosis (TB) coinfected children. 2013. http://clinicaltrials.gov/ct2/show/NCT00802802 [Accessed 23 November 2014] [Google Scholar]
- 51.Scott CA, Iyer H, Bwalya DL, McCoy K, Meyer-Rath G, Moyo C, et al. Retention in care and outpatient costs for children receiving antiretroviral therapy in Zambia: a retrospective cohort analysis. PLoS One 2013; 8:e67910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmed BS, Phelps BR, Reuben EB, Ferris RE. Does a significant reduction in malaria risk make lopinavir/ritonavir-based ART cost-effective for children with HIV in co-endemic, low-resource settings?. Trans R Soc Trop Med Hyg 2014; 108:49–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


