Abstract
There are limited data available regarding the effects of age and sex on discrete prefrontal gray and white matter volumes or posterior and anterior hippocampal volumes in healthy humans. Volumes of the superior frontal gyrus, anterior cingulate gyrus, and orbital frontal lobe were computed manually from contiguous magnetic resonance (MR) images in 83 (39M/44F) healthy humans (age range = 16–40) and segmented into gray and white matter. Volumes of the posterior and anterior hippocampal formation were also computed with reliable separation of the anterior hippocampal formation from the amygdala. There were significant age‐by‐tissue type interactions for the superior frontal gyrus and orbital frontal lobe such that gray matter within these regions correlated significantly and inversely with age. In contrast, no significant age effects were evident within regional white matter volumes. Analysis of hippocampal volumes indicated that men had larger volumes of the anterior, but not posterior hippocampal formation compared to women even following correction for total brain size. These data highlight age effects within discrete prefrontal cortical gray matter regions in young and middle aged healthy humans and suggest that the white matter comprising these regions may be more resistant to age effects. Furthermore, understanding the potential role of sex and age in mediating prefrontal cortical and hippocampal volumes may have strong relevance for psychiatric disorders such as schizophrenia that have implicated neurodevelopmental abnormalities within frontotemporal circuits in their pathogenesis. Hum Brain Mapp 34:2129–2140, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: magnetic resonance imaging, healthy volunteer, hippocampus, frontal lobes, aging
INTRODUCTION
There are well established age‐associated changes in gray matter that occur across the lifespan with evidence that older individuals activate different cortical networks compared to younger individuals [Gunning‐Dixon et al., 2000]. Longitudinal MR imaging studies that included individuals at the later stages of the lifespan suggest that age‐associated reductions in gray matter may be evident across the entire cortex [Raz et al., 2004; Resnick et al., 2010], although several studies noted that age effects may be most robust in the prefrontal region [Allen et al., 2005; Raz et al., 2010; Tisserand et al., 2003]. Furthermore, cortical thinning has also been observed in adolescence and young adulthood [Gur et al., 2009; Tamnes et al., 2010] as well as among children and adolescents [Sowell et al., 2009].
The role of sex differences in mediating gray matter changes across the lifespan has produced discrepant findings. Some work suggests that gray matter reductions are more pronounced in men compared to women [e.g., Cowell et al., 2007; Sullivan et al., 2002]. In contrast, however, other investigators reported gray matter reductions across the lifespan that were independent of sex [Allen et al., 2005; Pfefferbaum et al., 2004]. Thus, although findings have been contradictory, significant age‐associated gray matter reductions have been reported most reliably among men. Fewer studies have been directed at understanding the possible effects of age on the brain white matter, although the prefrontal region may be most susceptible [Gunning‐Dixon et al., 2003]. There is some evidence that white matter increases may occur through the fourth decade of life [Bartzokis et al., 2001]. Along these lines Ge et al. 1997 reported that white matter volume loss was delayed until middle adulthood and that effects appeared to be independent of sex.
Some volumetric studies investigating the prefrontal cortex may be limited by the use of gross neuroanatomic regions that may be insensitive to subtle changes in regional volume across the lifespan, although several approaches have distinguished between lateral prefrontal regions, anterior cingulate, and orbital frontal cortex [e.g., Raz et al., 2010, 2004; Salat et al., 2008; Tisserand et al., 2000, 2002]. The examination of neuroanatomically defined volumes of the superior frontal gyrus may be less well studied, however. Perhaps the most general approach for subdividing the brain consists of a dorsal/ventral division [Altmann and Brivanlou, 2001; Campbell et al., 2003], which may have important implications for frontal lobe functioning [Stuss and Levine, 2005]. Furthermore, both animal [Markham et al., 2008] and human [Peiffer et al., 1979] data suggest that aging may differentially impact dorsal versus ventral brain regions and thus, this distinction may be an important consideration in studies examining age effects on brain structure volumes. Similarly, we [Christensen and Bilder, 2000; Szeszko et al., 2005, 1999b) and others [Antonova et al., 2004; King et al., 1994] have used the dual cytoarchitectonic trends theory [Sanides, 1969], which distinguishes between dorsal “archicortical” and ventral “paleocortical” brain regions, as a framework for understanding healthy human frontal lobe functioning and associated cognitive dysfunction in psychiatric disorders such as schizophrenia. Following this dorsal/ventral distinction we investigated prefrontal volumes in this study using an approach for cortical parcellation developed by Rademacher et al. [1992]. These methods have been extended subsequently to reliably and validly subdivide the brain white matter based on neuroanatomical consideration of white matter tracts [Makris et al., 2006; Meyer et al., 1998].
The majority of studies examining age and sex effects on the hippocampus have examined it as a unitary structure. In several studies that examined sex effects robust maturation of the human hippocampus was particularly pronounced among males [Suzuki et al., 2005] with greater hippocampal volume among men [Good et al., 2005; Raz et al., 2010], although findings have not always been consistent [Mu et al., 1993]. Additionally, in cross‐sectional studies Sullivan et al. 2004 reported no significant correlations between age and hippocampal volume among men or women, despite findings of significant age‐associated reductions in temporal volume. In contrast, Pruessner et al. 2002 reported hippocampal volume reductions that were most pronounced among men that began around the third decade of life. Some work suggests, however, that the long axis of the hippocampus is not homogeneous, but may be comprised of functionally distinct subregions with different connections [Fanselow and Dong, 2010; Friedman et al., 2002]. Specifically, the dorsal hippocampus, corresponding to the posterior hippocampus in humans [Sasaki et al., 2001], appears to be connected with sensory cortical areas including the parietal cortex and plays a role in spatial learning [Moser et al., 2006]. In contrast, the ventral hippocampus, which corresponds to the anterior hippocampus in humans [Fanselow and Dong, 2010], has strong connections with the prefrontal cortex [Barbas and Blatt, 1995], may be associated with processing novel information [Strange et al., 1999] and has been implicated in neuropsychiatric disorders such as schizophrenia [Bilder et al., 1995; Szeszko et al., 2000, 2002]. In one study examining age‐associated volumetric changes along the long axis of the hippocampus in healthy humans Malykhin et al. 1999 reported that changes were progressively more severe from head to tail. In contrast, other work has not implicated robust age effects in healthy humans in young and early adulthood [Grieve et al., 2008; Sullivan et al., 2004].
The goal of this study was to examine the differential effects of age and sex on tissue type within prefrontal subregions as well as posterior and anterior hippocampal volumes in healthy young and middle‐aged adults. We investigated gray and white matter volumes of the superior frontal/anterior cingulate gyri and orbital frontal lobe as probes of dorsal and ventral brain integrity, respectively to assess the potential differential effects of age in these regions. We separated the anterior hippocampus from the amygdala using a reliable approach that involved visualizing these structures in the three orthogonal planes. We did not have any a priori hypotheses regarding potential sex effects on the hippocampus, but consistent with prior published work [e.g., Honeycutt and Smith, 2006] we did hypothesize that hippocampal volumes would not be significantly correlated with age in the range examined. We hypothesized that there would be significant sex‐by‐age effects within prefrontal gray matter regions comprising both dorsal and ventral trends suggesting that age‐associated decreases in gray matter would be most robust among males.
MATERIALS AND METHODS
Subjects
Eighty‐three (39M/44F) healthy volunteers were recruited from local newspaper advertisements and through word of mouth in the community. Healthy volunteers denied any history of Axis I psychopathology determined using the SCID nonpatient version and serious medical conditions including Tourette's, Huntington's Disease, Parkinson's Disease, encephalitis, strokes, aneurysms, tumors, CNS infections, degenerative brain diseases, prior psychosurgery and documented learning disabilities. All procedures were approved by the North Shore—Long Island Jewish Health System IRB and written informed consent was obtained from all participants.
Magnetic Resonance (MR) Imaging Procedures
MR imaging exams comprised 124 images in the coronal plane using a high resolution 3D‐spoiled grass sequence on a 1.5 Tesla GE system (General Electric, Milwaukee, WI) yielding in‐plane resolution of 0.86 mm × 0.86 mm in a 256 × 256 matrix. All scans were reviewed clinically by a neuroradiologist and none demonstrated gross pathology (e.g., tumor). Scans were also reviewed by a member of the research team, and any scan with significant artifacts was repeated. All measurements were completed in MEDx. No identifying information was available to the operator from the scan. The images were aligned along the anterior and posterior commissures for standardization across subjects and flipped randomly in the right‐left axis so that the rater was blind to hemisphere.
Total Intracranial Contents
Measurement of total intracranial contents was completed in MEDx by computing the volume of the total cerebrum, cerebrospinal fluid, cerebellum, and brainstem. Interrater reliability between two raters as assessed by intraclass correlations [ICCs]) in nine cases was 0.99.
Frontal Lobe Subregions
Measurement of the frontal lobe subregions was completed using methods described previously [Szeszko et al., 2005] that were adapted from Rademacher et al. [1992] for use in our MR images. This method has been used in our previous work [Szeszko et al., 1999b, 2007, 2008a] and utilizes the cerebral sulci in combination with a set of coronal planes that “close” the borders of selected regions of interest. Intraclass correlations between two or three operators for these brain structures (number of cases ranged from 8 to 10) were (right hemisphere, left hemisphere): anterior cingulate gyrus gray matter (0.90, 0.94), anterior cingulate gyrus white matter (0.94, 0.94), superior frontal gyrus gray matter (0.92, 0.97), superior frontal gyrus white matter (0.95, 0.95), orbital frontal lobe gray matter (0.92, 0.99) and orbital frontal lobe white matter (0.94, 0.90). Volumes obtained in this study compare favorably with prior work by our group investigating frontal and hippocampal regions [e.g., Szeszko et al., 2007, 2008a, 2008b].
The boundaries of the superior frontal gyrus were (anterior, posterior, lateral, and medial): tip of the cingulate sulcus, connection of the superior and precentral sulci, superior frontal sulcus, and cingulate sulcus. The boundaries of the anterior cingulate gyrus were (anterior, posterior, ventral, and dorsal): tip of the cingulate sulcus, connection of the superior and precentral sulci, callosal sulcus and cingulate sulcus. The boundaries of the orbital frontal region were (anterior, posterior, lateral, and medial): last appearance of the anterior horizontal ramus, last appearance of the olfactory sulcus, anterior horizontal ramus/circular sulcus of insula and the olfactory sulcus. One of the sulci required for measurement of the orbital frontal region (i.e., the anterior horizontal ramus) was not present in every hemisphere [Ono et al., 1971; Szeszko et al., 1999a, 2007, 2008b] and thus, orbital frontal volumes could not be computed for seven and six individuals for the right and left hemispheres, respectively. All frontal regions were outlined manually in the coronal plane on a slice by slice basis. A figure illustrating these regions and their corresponding boundaries is provided in Szeszko et al. 2005. After outlining the region an operator segmented it into gray and white matter using a thresholding algorithm [Otsu, 2006], as described previously [Lim and Pfefferbaum, 2007; Szeszko et al., 2007, 2008a, 2008b]. The superior frontal gyrus gray matter corresponded approximately to Brodmann areas 6, 8, and 9 whereas the white matter in this region was comprised mainly of the superior longitudinal fasciculus and occipitofrontal fasciculus. The anterior cingulate gray matter included Brodmann area 24 and the white matter comprised the cingulum bundle. The orbital frontal gray matter corresponded approximately to Brodmann area 47 whereas the white matter was comprised mainly of the uncinate fasciculus and inferior fronto‐occipital fasciculus.
Hippocampus‐Amygdala Complex
Two contiguous portions of the hippocampus were measured in each hemisphere: posterior hippocampus and anterior hippocampus. Neuroanatomical boundaries were based on operationalized criteria from postmortem histological work [Bogerts et al., 1985] and prior published studies [Szeszko et al., 2002]. The anatomic regions are illustrated in Szeszko et al. 2002. The operator used the neuroanatomical information available in each orthogonal plane to facilitate measurement of these regions and to distinguish them from surrounding structures. The posterior boundary of the posterior hippocampus began where an ovoid mass of gray matter appeared inferiomedially to the trigone of the lateral ventricle. Moving anteriorly the operator used the axial and sagittal views to better visualize the first appearance of the pulvinar in the coronal plane to exclude it from measurement. The crus of the fornix was excluded from measurements. Following the interruption of the pulvinar by the crus of the fornix all CA‐segments (CA1, CA2, CA3, CA4), dentate gyrus, alveus, parasubiculum, presubiculum, subiculum proper, and prosubiculum, were included in the measurements. The anterior boundary was the coronal slice posterior to the one where the cistern pontis became clearly visible. The rationale for using the cistern pontis to subdivide the hippocampus into posterior and anterior segments was based on functional magnetic resonance imaging data demonstrating a dissociation between these regions regarding stimulus familiarity [Strange et al., 2001, 1999].
The posterior boundary of the anterior hippocampus was the slice where the cistern pontis became clearly visible, including all segments in the posterior hippocampus as well as uncus. The shape of the anterior hippocampus began to change markedly at the first appearance of the intralimbic gyrus. The distinction between anterior hippocampus and amygdala was sometimes difficult and we utilized several strategies to deal with this problem while visualizing these structures in the three orthogonal planes. The temporal horn was useful to separate these structures. Also, when visible, the alveus provided a good superior boundary for the hippocampus. At times, however, the anterior hippocampus became fused with the amygdala across the ventricular cavity. In this event, a straight horizontal line was drawn from the most superiomedial portion of the temporal horn laterally to the most medial part of the ambient gyrus. The intralimbic gyrus (including dentate gyrus and Ammon's horn) was included in the measurement of the anterior hippocampus. Intraclass correlations between three operators for nine cases were: (right, left): posterior hippocampus (0.87, 0.88), anterior hippocampus (0.94, 0.87).
Handedness
Handedness was assessed by a modified 20‐item Edinburgh Inventory [Oldfield, 2000]. The total number of right‐ and left‐handed items was scored, and the laterality quotient was computed as [(total right ‐ total left)/(total right + total left)] × 100. Subjects with a laterality quotient >0.70 were classified as dextral; the rest were classified as nondextral. Thirteen subjects were classified as right‐ or left‐handed based on preference for handwriting alone. There were missing handedness data for three males and three females.
Statistical Procedures
The mixed models approach (SAS; v8.2) for repeated measures analysis of variance was used to compare brain structure volumes. Analyses were conducted separately for each of the frontal regions because of their neuroanatomical heterogeneity and to specifically test tissue type‐by‐age interactions. In addition, a single analysis for both the posterior and anterior hippocampal regions was performed. In each analysis the statistical model included sex as a between subjects factor. Tissue type (gray versus white) and hemisphere were repeated measures for the frontal lobe subregions. Region (posterior versus anterior) and hemisphere were repeated measures for the hippocampal analysis. To assess the effects of age on the observed findings it was included in the statistical model. Intracranial volume was included as a covariate to control for nonspecific differences in brain size among individuals. Significant tissue type‐by‐age interactions were followed by partial correlation analyses between individual tissue volumes and age while controlling for total intracranial volume. In addition, to disassociate aging effects on regional gray and white matter volumes from global ones we also used partial correlation analysis to investigate correlations of individual brain structure volumes and age using total brain gray or total brain white matter volumes, respectively as statistical covariates to demonstrate that specific tissue type volume loss was independent from global volume loss. Given prior data from both human and animal studies that examined the relationship between handedness and brain volume, we investigated the potential effects of handedness on the observed findings by examining its interaction with significant main effects and interactions [Gut et al., 2002; Hervé et al., 2008; Pujol et al., 2001]. Pearson product moment correlations were used to examine associations of age with brain volumes. Group differences in demographic variables were examined using independent groups t tests. Chi‐square tests were used to examine differences in categorical variables. Alpha was set to 0.05 for all analyses.
RESULTS
Males and females did not differ significantly in distributions of age, handedness, education or wide range achievement test scaled score (see Table 1). Mean volumes for frontal and hippocampal regions are presented separately by sex in Table II.
Table 1.
Sample characteristics
| Value | Males (N = 39) | Females (N = 44) | df | Test statistic | P |
|---|---|---|---|---|---|
| Age | 28.9 (7.3) | 28.0 (7.1) | 81 | t = 0.60 | ns |
| Education (years) | 14.7 (2.2) | 14.9 (2.2) | 67 | t = −0.23 | ns |
| Handedness | 29R/7L | 36R/5L | 1 | χ2 = 0.77 | ns |
| WRAT III Scaled Score | 105 (8.9) | 103 (10.4) | 54 | t = 0.73 | ns |
| Racea | (25,6,5,2,1) | (20,10,4,3,7) | 2 | χ2 = 2.9 | ns |
Notes: Data are presented as mean ± SD in parentheses, unless otherwise indicated. There were data missing for the following variables: education (6 males, 8 females); handedness (3 males and 3 females); WRAT III SS (13 males, 14 females). NS = not significant.
Race coded as Caucasian, African American, Hispanic, Asian and other. Because >20% of the categories for race had expected frequencies of <5 we combined the latter three groups (i.e., Hispanic, Asian and other) into a single group for analysis.
Table 2.
Mean frontal and hippocampal volumes by sex
| All subjects (N = 83) | Male (N = 39) | Female (N = 44) | |
|---|---|---|---|
| Frontal lobe tissue types (cm3) | |||
| Superior frontal gyrus | |||
| Right hemisphere | |||
| Gray matter | 16.2 (3.0) | 16.5 (3.3) | 16.0 (2.6) |
| White matter | 12.8 (2.5) | 13.1 (3.0) | 12.5 (1.8) |
| Left hemisphere | |||
| Gray matter | 16.4 (2.6) | 16.4 (3.0) | 16.4 (2.3) |
| White matter | 12.9 (2.3) | 13.1 (2.8) | 12.7 (1.9) |
| Anterior cingulate gyrus | |||
| Right hemisphere | |||
| Gray matter | 4.3 (1.0) | 4.3 (1.2) | 4.3 (1.0) |
| White matter | 2.3 (0.6) | 2.3 (0.7) | 2.3 (0.5) |
| Left hemisphere | |||
| Gray matter | 3.8 (1.0) | 3.9 (1.0) | 3.7 (0.9) |
| White matter | 2.1 (0.6) | 2.2 (0.6) | 2.0 (0.6) |
| Orbital frontal | |||
| Right hemisphere | |||
| Gray matter | 4.7 (1.2) | 4.9 (1.2) | 4.6 (1.2) |
| White matter | 3.0 (0.8) | 3.2 (0.9) | 2.9 (0.7) |
| Left hemisphere | |||
| Gray matter | 5.1 (1.3) | 5.2 (1.5) | 5.0 (1.3) |
| White matter | 3.3 (0.9) | 3.5 (1.0) | 3.2 (0.8) |
| Hippocampal regions (cm3) | |||
| Posterior hippocampus | |||
| Right hemisphere | 1.4 (0.24) | 1.4 (0.24) | 1.4 (0.24) |
| Left hemisphere | 1.4 (0.26) | 1.5 (0.26) | 1.4 (0.26) |
| Anterior hippocampus | |||
| Right hemisphere | 2.0 (0.44) | 2.2 (0.44) | 1.9 (0.38) |
| Left hemisphere | 1.9 (0.37) | 2.0 (0.38) | 1.8 (0.31) |
Superior Frontal Gyrus
Mixed models analyses revealed a significant (F = 189.01, df = 82, P < 0.001) main effect of tissue type with more gray compared to white matter in the superior frontal gyrus. The main effects of hemisphere and sex were not statistically significant. The age‐by‐tissue type interaction was significant (F = 81.64, df = 246, P < 0.001; Fig. 1) indicating that the rate of change in gray matter was significantly different than the rate of change in white matter. Specifically, age was inversely correlated with total superior frontal gyrus gray matter (r = ‐0.49, df = 80, P < 0.001), but not white matter volume (r = 0.13, df = 80; P = 0.23) while controlling for total intracranial volume. Similar correlations were evident when total gray matter and total white matter volumes were used respectively as covariates. The sex‐by‐age interaction was not statistically significant indicating that age‐associated changes were comparable in males and females.1
Figure 1.
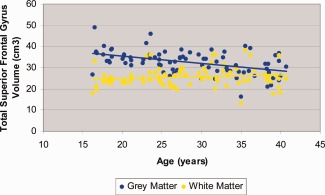
Scatterplot of superior frontal gyrus gray and white matter volume with age. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Anterior Cingulate Gyrus
Mixed models analyses revealed a significant (F = 159.55, df = 1, 82, P < 0.001) main effect of tissue type with more gray compared to white matter in the anterior cingulate gyrus. There was a significant (F = 17.44, df = 1, 82, P < 0.001) main effect of hemisphere with greater right compared to left hemisphere volume. There was a significant (F = 17.44, df = 1, 82, P < 0.001) tissue type‐by‐hemisphere interaction indicating that the magnitude of right greater than left asymmetry was larger for the gray (t = ‐3.98, df = 82, P < 001) compared to the white matter (t = ‐2.58, df = 82, P = 0.012). The age‐by‐tissue type interaction was statistically significant (F = 16.99, df = 1, 245, P < 0.001; Fig. 2) indicating that the rate of change in gray matter was significantly different than the rate of change in white matter. Although the interaction was statistically significant, the correlations of age with total anterior cingulate gray matter volume (r = ‐0.20, df = 80, P = 0.08) and total anterior cingulate white matter volume (r = 0.19, df = 80, P = 0.09) only demonstrated trend level significance. The main effects of sex and sex‐by‐age interactions were not statistically significant.
Figure 2.
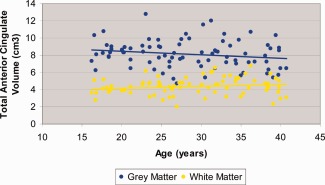
Scatterplot of anterior cingulate gyrus gray and white matter volume with age. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Orbital Frontal Lobe
There were no significant differences in age, sex, or handedness between individuals with or without orbital frontal volumes. The main effects of sex and sex‐by‐age interactions were not statistically significant. Mixed models analyses revealed a significant (F = 162.66, df = 78, P < 0.001) main effect of tissue type with more gray compared to white matter in the orbital frontal lobe. In addition, there was a significant (F = 5.95, df = 73, P < 0.017) main effect of hemisphere with greater left compared to right hemisphere volume. The age‐by‐tissue type interaction was significant (F = 34.43, df = 224, P < 0.001; Fig. 3) indicating that the rate of change in gray matter was significantly different compared to the rate of change in white matter. Age correlated inversely with total orbital frontal gray (r = ‐0.34, df = 71, P = 0.003), but not white matter volume (r = 0.07, df = 71, P = 0.54) while controlling for total intracranial volume. Similar correlations were evident when total gray matter and total white matter volumes were used respectively as covariates.
Figure 3.

Scatterplot of orbital frontal lobe gray and white matter volume with age. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Hippocampus Volumes
A significant (F = 116.89, df = 1, 81, P < 0.001) main effect of region for the hippocampus revealed greater anterior compared to posterior volume. A significant (F = 11.04, df = 1, 82, P = 0.001) main effect of hemisphere was also evident with overall right compared to left hemisphere volume. In addition, the region‐by‐hemisphere interaction was statistically significant (F = 28.37, df = 1, 82, P < 0.001) with follow‐up analyses indicating that there was greater right compared to left anterior hippocampus volume (t = ‐5.40, df = 82, P < 0.001) and greater left compared to right posterior hippocampal volume (t = 3.03, df = 82, P = 0.003). The age‐by‐region interaction was not statistically significant for either the posterior or anterior hippocampus. There was a significant (F = 8.14, df = 1, 81, P < 0.006) region‐by‐sex interaction for the hippocampal volumes indicating that males had larger volumes of the anterior (t = 2.78, df = 81, P = 0.007; Fig. 4), but not posterior (P > 0.05) hippocampal formation compared to females. No significant sex differences were evident for the posterior hippocampal volumes. The sex‐by‐age and sex‐by‐age‐by‐region interactions were not statistically significant (all P values > 0.05) for the hippocampus.
Figure 4.
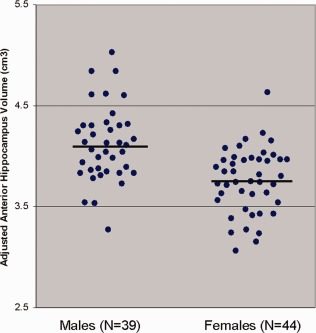
Anterior hippocampal volumes adjusted for total intracranial volume by sex. Note: horizontal lines represent mean values. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Additional Analyses
We also considered the possibility that our study might conceivably be mixing age‐associated effects due to deleterious biological processes with those related to normal late neurodevelopmental processes and thus, we repeated the analyses excluding subjects (n = 16) below the age of 21. We replicated the tissue type‐by‐age interactions for each of the three frontal volumes and the correlations of age with the frontal structure volumes followed the same pattern as the entire sample. In addition, we systematically investigated the potential effects of handedness on the findings described above by repeating each analysis with handedness as a between subjects factor and exploring its interaction with each of the significant main effects or interactions described above. We did not find any evidence, however, for an effect of handedness on the observed findings (Ps > 0.05).
DISCUSSION
Few studies have examined the regional specificity of aging within discrete prefrontal regions in both the gray and white matter or along the long axis of the hippocampus in young and middle‐aged healthy humans. Using a method for subdividing the prefrontal cortex based on the sulcal anatomy our results suggest that age‐associated decreases in gray matter volume were evident in regions comprising dorsal archicortical (superior frontal gyrus) and ventral paleocortical (orbital frontal lobe) brain regions and that these effects were comparable in both men and women. In contrast, no significant effects of aging were observed for anterior cingulate gray matter volume. In addition, we did not find any evidence for the effects of aging on prefrontal white matter volumes, which remained relatively stable across the agespan examined. Investigation of posterior and anterior hippocampal volumes revealed no significant effects of aging, but an overall effect of sex on anterior hippocampal volumes such that men had larger volumes compared to women even after controlling for the effects of total intracranial volume.
Our investigation of age‐by‐tissue type interactions supports the differential impact of aging in the gray, but not the white matter within the superior frontal gyrus and orbital frontal lobe. Our data thus converge with prior work suggesting that cortical thinning occurs in adolescence and young adulthood [Tamnes et al., 2010] as well as studies demonstrating aging effects within the prefrontal gray matter in older cohorts [Allen et al., 2005; Raz et al., 2010; Tisserand et al., 2003]. More specifically, however, evidence for aging effects within dorsal archicortical regions in the current study converges with prior work reporting an association of age with less dorsolateral prefrontal gray matter volume in young adulthood [Gur et al., 2009] and findings of an inverse association between gray matter density reduction and brain growth in the superior frontal region among children, adolescents and young adults [Sowell et al., 2009]. Furthermore, our results converge with Fjell et al. [2009] who similarly reported that age effects were most pronounced in the superior frontal gyri (among other regions) in a sample of 883 subjects. Although our findings are consistent with the hypothesis of neuronal loss, it should also be acknowledged that an additional mechanism relevant to the age effects reported herein might be neuronal shrinkage [Fjell and Walhovd, 2010].
The age‐related changes observed in archicortical regions in our study and others may be related to the development of working memory capacity into young adulthood [Huizinga et al., 1995] and/or changes in executive functions subserved by this region across the agespan [Silver et al., 2006]. Comparable inverse associations of aging with ventral paleocortical (i.e., orbital prefrontal) volumes have also been reported [Convit et al., 2001; Lemaitre et al., 2006; Zimmerman et al., 2006], which may relate to purported deficits on olfactory discrimination [Enwere et al., 2004] and go no/go performance deficits [Lamm et al., 2010] across the agespan. Although we could not identify the precise neurobiological mechanisms by which gray matter changes might become evident in our young and middle‐aged cohort, it is conceivable that oxidative effects in general [Tripathy et al., 2010; Venarucci et al., 2006] and changes in dopamine synthesis [Ota et al., 1990], which have been observed over the age range examined in this study, could contribute to these effects.
In our study the rate of change in the anterior cingulate gray matter differed significantly from the rate of change in the anterior cingulate white matter across the agespan; however, age did not correlate significantly with anterior cingulate gray or white matter volumes. The less robust effects of age‐associated changes in anterior cingulate gyrus gray matter volume observed in our study is consistent with prior work [Fjell et al., 2009; Raz et al., 2010]. Although we could not determine why the anterior cingulate should be less susceptible to the effects of age compared to the other prefrontal brain regions examined in this study, one possible explanation may relate to the fact that this region had the largest ratio of gray to white matter, thus potentially providing additional reserve during the aging process [May, 2007]. It may also be noteworthy that gray matter volume only partially mediates the effects of age on regional cerebral blood flow in the anterior cingulate [Vaidya et al., 2007], suggesting that neurons may just be less active, but not necessarily reduced in number. It should also be acknowledged that further subdivision of the anterior cingulate could conceivably demonstrate aging effects as well as possible sex differences in volume [Chen et al., 2007].
Our data suggest that the white matter in the prefrontal subregions examined remains stable at least until the fourth decade of life, which is consistent with prior work [Ge et al., 1997]. Relative preservation of the brain white matter in the frontal subregions examined may be neurobiologically related to the lack of robust age effects on the myelin sheath, oligodendrocytes and glial cells during young and middle‐adulthood. For example, animal studies suggest age‐associated changes in oligodendrocytes when comparing young versus old monkeys that began only during middle age [Peters and Sethares et al., 2009]. In contrast to our findings Bartzokis et al. [2001, 2003] reported brain white matter increases until the fourth decade of life as assessed using regional morphometry. An important difference between our study and prior investigations, however, is our use of discrete prefrontal regions, thus making it difficult to directly compare our results with larger measures of prefrontal white matter volume.
There was relative preservation of both the posterior and anterior hippocampus in our cohort of young and middle‐aged adults. The lack of significant age‐associated changes in hippocampal volume in the age range of our study converges with prior MR imaging studies in healthy humans [Grieve et al., 2008; Sullivan et al., 2004] and rhesus monkeys [Shamy et al., 2004]. Some data suggests, however, that hippocampal shrinkage may accelerate over time with increasing age [Raz et al., 1997, 2005] and thus, might not be observed in our cohort of young and middle‐aged adults. This possibility would be consistent with animal data suggesting that bioenergetic properties could play a role in functions associated with hippocampal integrity, but only later in life [Kadish et al., 2000]. Other cross‐sectional MR imaging work suggests volume loss progressively from the hippocampal head to its tail [Malykhin et al., 1999]. In addition, Pruessner et al. 2002 reported a significant inverse association between age and volume of the right and left hippocampus among men with effects most pronounced in the head and tail. No significant age‐associated changes were evident in women, however, in the study by Pruessner et al. 2002. Differences between our study and prior studies may relate to differences in morphometric delineation criteria and sampling issues. For example, a prior study [Rajah et al., 1993] reported that in healthy humans older age (mean = 67.7) was associated with less volume in the hippocampal head and body, but not tail.
Anterior hippocampal volume was larger in men compared to women even after controlling for the effects of total intracranial volume. These findings converge with Raz et al. 2010 who reported that the volume of the total hippocampal formation was larger in men compared to women even while controlling for height. Our findings thus extend this prior work by suggesting that sex differences may be most robust in the anterior part of the mesiotemporal lobe and more specifically, the hippocampus. Although the possible functional significance of sex differences in anterior hippocampal volume were not examined in the present investigation, some work suggests that this part of the hippocampus plays an important role in spatial navigation [Hartley et al., 2007; Iaria et al., 2006; Maquire et al., 2009; Moffat et al., 1999] and learning/memory [Gold and Squire, 2007; Kircher et al., 2008] and that sex differences in these functions have been reported [Andreano and Cahill, 2009; Ruggiero et al., 2003]. These findings could also have implications for understanding sex differences contributing to the pathophysiology of neuropsychiatric disorders. For example, prior work demonstrated a significant pattern of structure–function relations localized to the anterior hippocampus in men, but not women with schizophrenia [Szeszko et al., 2000]. Furthermore, it may be noteworthy that there may be sex differences in hormones associated with stress [Solomon and Herman, 2009] and these hormones may moderate morphology differentially within the dorsal versus ventral hippocampus [Maggio and Segal, 1989]. In healthy humans, greater self‐perceived stress predicted smaller hippocampal volume [Gianaros et al., 2002] with effects observed most robustly in the anterior hippocampal formation [Szeszko et al., 2006].
Consistent with the results of several prior neuroimaging studies [e.g., Allen et al., 2005; Raz et al., 2004] we did not observe any differential impact of sex on age within prefrontal or hippocampal volumes. Although prior work [e.g., Cowell et al., 2007] noted such effects, it should be acknowledged that differences among studies may be related to the age range examined [Cowell et al., 1994], methodology [Kennedy et al., 2009], and analytic approaches for modeling tissue changes [Greenberg et al., 2001]. Thus, in our study it is conceivable that such effects would be observed across a broader age range. Moreover, sex differences might be most pronounced within specific prefrontal and hippocampal regions that were not the focus of the current investigation [e.g., Gur et al., 2009; La Joie et al., 2008]. Nevertheless, our data highlight the apparent lack of sex differences on age in young and middle‐aged healthy adults within discrete prefrontal tissue types and along the long axis of the hippocampus.
There were significant asymmetry effects in both prefrontal and hippocampal regions. Specifically, we observed right greater than left asymmetry in the anterior cingulate gyrus replicating our prior findings [Szeszko et al., 1999a] in an independent cohort. In a prior study that noted hemispheric differences in cingulate/paracingulate morphology that could conceivably contribute to the volume differences reported herein Yucel et al. 2001 reported that the paracingulate sulcus was more “commonly prominent” in the left hemisphere, but in contrast was “commonly absent” in the right hemisphere. We also observed significant left greater than right hemispheric asymmetry for the orbital frontal cortex also replicating our prior findings [Szeszko et al., 1999a] in an independent cohort. The finding of larger left compared to right orbital frontal volumes could have implications for language‐associated functions. For example, using fMRI Binder et al. 1997 reported activation of left pars orbitalis during a semantic decision task in healthy humans. In addition, Keller et al. 2009 reported that while considering the cortical sulci in this region there was leftward asymmetry of the pars opercularis region in healthy humans. The finding of right greater than left total hippocampus volume converges with prior studies reporting similar asymmetry in healthy volunteers [Fukuzako et al., 2002; Li et al., 2005; Niemann et al., 1999], although its significance is not known. One possible interpretation of hippocampal asymmetry, however, is that it could have functional implications given prior work demonstrating that left hippocampal integrity mediates spatial memory whereas the right hippocampus is associated with spatial navigation [Burgess, 2002; Burgess et al., 2002].
There were a number of study limitations that should be acknowledged. Investigation of the effects of age on the white matter was limited to regional volumetry and some work suggests diffusion tensor imaging may be a more sensitive technique for assessing white matter integrity compared to morphometry [Fjell et al., 2008]. In this regard although the regions‐of‐interest used in our study were based on a prior published neuroanatomical parcellation scheme, we did not have specific information regarding white matter tracts in these regions‐of‐interest. In addition, although some studies have examined hippocampal subregions in relationship to aging, resolution of individual subfields was not possible from these images. The neuropsychological sequelae of these aging effects were not investigated and thus, require further investigation [e.g., Gunning‐Dixon and Raz, 2005; Jack et al., 2006, 1999; Rajah et al., 1993]. Along these lines, it should be acknowledged that cortical reductions observed at the later stages of the lifespan could have different functional significance compared to volume changes observed during childhood or adolescence. More specifically, volume changes associated with young aging could play a role in the decline of some cognitive skill whereas volume changes during late adolescence might contribute to maturational changes and concomitant refinement of cognitive functions [e.g., Pine et al., 1994]. Also, our study focused on individuals in the age range from 16 to 40 and thus, possible effects of sex and age on brain volumes in older individuals could not be investigated. For example, sexually dimorphic changes have been reported in older compared to younger cohorts in the superior frontal gyrus [Berchtold et al., 2008].
In sum, these findings suggest that significant age‐associated changes in gray, but not white matter volume occur within discrete prefrontal brain regions in young and middle‐adulthood in healthy humans. Moreover, no significant age‐associated changes in hippocampus volume were evident in either its posterior or anterior segments, although men had larger volume of the anterior hippocampal formation compared to women. Our findings could have implications for understanding the potential role of age in mediating prefrontal and hippocampal brain volumes in the pathophysiology of psychiatric disorders such as schizophrenia where neurodevelopmental abnormalities have been implicated in their pathogenesis. For example, a defect in cortical pruning [Keshavan et al., 2008] and/or a dysregulation in brain white matter development [Bartzokis et al., 2003] have been suggested to play a role in schizophrenia.
REFERENCES
- Allen JS, Bruss J, Brown CK, Damasio H (2005): Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol Aging 26:1245–1260. [DOI] [PubMed] [Google Scholar]
- Altmann CR, Brinvanlou AH (2001): Neural patterning in the vertebrate embryo. Int Rev Cytol 203:447–482. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L (2009): Sex influences on the neurobiology of learning and memory. Learn Memory 16:248–266. [DOI] [PubMed] [Google Scholar]
- Antonova E, Sharma T, Morris R, Kumari V (2004): The relationship between brain structure and neurocognition in schizophrenia: A selective review. Schizophrenia Res 70:117–145. [DOI] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ (1995): Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5:511–533. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Mintz J (2001): Age‐related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry 58:461–465. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J (2003): White matter structural integrity in healthy aging adults and patients with alzheimer disease. Arch Neurol 60:393–398. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Cribbs DH, Coleman PD, Rogers J, Head E, Kim R, Beach T, Miller C, Troncoso J, Trojanowski JQ, Zielke HR, Cotman CW (2008): Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci USA 105:15605–15610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder RM, Bogerts B, Ashtari M, Wu H, Alvir JM, Jody D, Reiter G, Bell L, Lieberman JA (1995): Anterior hippocampal volume reductions predict frontal lobe dysfunction in first episode schizophrenia. Schizophrenia Res 17:47–58. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T (1997): Human brain language areas identified by functional magnetic resonance imaging. J Neurosci 17:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerts B, Meertz E, Schönfeldt‐Bausch R (1985): Basal ganglia and limbic system pathology in schizophrenia. A morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 42:784–791. [DOI] [PubMed] [Google Scholar]
- Burgess N (2002): The hippocampus, space, and viewpoints in episodic memory. Quart J Exp Psychol Hum Exp Psychol 55:1057–1080. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maquire EA, O'Keefe J (2002): The human hippocampus and spatial and episodic memory. Neuron 35:625–641. [DOI] [PubMed] [Google Scholar]
- Campbell K (2003): Dorsal‐ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol 13:50–56. [DOI] [PubMed] [Google Scholar]
- Chen X, Sachdev PS, Wen W, Anstey KJ (2007): Sex differences in regional gray matter in healthy individuals aged 44–48 years: A voxel‐based morphometric study. Neuroimage 36:691–699. [DOI] [PubMed] [Google Scholar]
- Christensen BK, Bilder RM (2000): Dual cytoarchitectonic trends: An evolutionary model of frontal lobe functioning and its application to psychopathology. Can J Psychiatry 45:239–240. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, deLeon MJ, Patalinjug M, Kandil E, Caraos C, Scherer A, Saint Louis LA, Cancro R (2001): Volumetric analysis of the pre‐frontal regions: Findings in aging and schizophrenia. Psychiatry Res 107:61–73. [DOI] [PubMed] [Google Scholar]
- Cowell PE, Turetsky BI, Cur RC, Grossman RI, Shtasel DL, Gur RE (1994): Sex differences in aging of the human frontal and temporal lobes. J Neurosci 14:4748–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell PE, Sluming VA, Wilkinson ID, Cezayirli E, Romanowski CA, Webb JA, Keller SS, Mayes A, Roberts N (2007): Effects of sex and age on regional prefrontal brain volume in two human cohorts. Eur J Neurosci 25:307–318. [DOI] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S (2004): Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci 24:8354–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW (2010): Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB (2009): High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex 19:2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB (2010): Structural brain changes in aging: Courses, causes and cognitive consequences. Rev Neurosci 21:187–221. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Greve DN, Fischl B, Benner T, van der Kouwe AJ, Salat D, Bj⊘rnerud A, Due-T⊘nnessen P, Walhovd KB (2008): The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage 42:1654–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC (2002): Comparison of hippocampal amygdala and perirhinal projections to the nucleus accumbens: Combined anterograde and retrograde tracing study in the macaque brain. Journal of Computational Neurology 450:345–365. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Yamada K, Kodama S, Yonezawa T, Fukuzako T, Takenouchi K, Kajiya Y, Nakajo M, Takigawa M (1997): Hippocampal volume asymmetry and age at illness onset in males with schizophrenia. Eur Arch Psychiatry Clin Neurosci 247:248–251. [DOI] [PubMed] [Google Scholar]
- Ge Y (2002): Age‐related total gray and white matter changes in normal adult brain. Part 1: Volumetric MR imaging analysis. Am J Neuroradiol 23:1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews K (2007): Prospective reports of chronic life stress predict decreased gray matter volume in the hippocampus. Neuroimage 35:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Squire LR (2005): Quantifying medial temporal lobe damage in memory‐impaired patients. Hippocampus 15:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJFR (2001): Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel‐based morphometric analysis of 465 normal adult human brains. Neuroimage 14:685–700. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Messer DF, Payne ME, Macfall JR, Provenzale JM, Steffens DC, Krishnan RR (2008): Aging, gender, and the elderly adult brain: An examination of analytical strategies. Neurobiol Aging 29:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E (2005): Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp 25:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Raz N (2000): The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology 14:224–232. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, et al. (2003): Age‐related differences in brain activation during emotional face processing. Neurobiol Aging 24:285–295. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS (2009): Aging of cerebral white matter: A review of MRI findings. Int J Geriatric Psychiatry 24:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gunning‐Dixon FM, Turetsky BI, Bilker WB, Gur RE (2002): Brain region and sex differences in age association with brain volume: A quantitative MRI study of healthy young adults. Am J Geriatric Psychiatry 10:72–80. [PubMed] [Google Scholar]
- Gut M, Urbanik A, Forsberg L, Binder M, Rymarczyk K, Sobiecka B, Kozub J, Grabowska A (2007): Brain correlates of right‐handedness. Acta Neurobiol Exp 67:43–51. [DOI] [PubMed] [Google Scholar]
- Hartley T, Maguire EA, Spiers HJ, Burgess N (2003): The well‐worn route and the path less traveled: Distinct neural bases of route following and wayfinding in humans. Neuron 37:877–888. [DOI] [PubMed] [Google Scholar]
- Head DRK, Kennedy KM, Raz N (2008): Neuroanatomical and cognitive mediators of age‐related differences in episodic memory. Neuropsychology 22:491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé PY, Crivello F, Perchey G, Mazoyer B, Tzourio‐Mazoyer N (2006): Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage 29:1066–1079. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA, Smith CD (1995): Hippocampal volume measurements using magnetic resonance imaging in normal young adults. J Neuroimaging 5:95–100. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW (2006): Age‐related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia 44:2017–2036. [DOI] [PubMed] [Google Scholar]
- Iaria G, Chen J, Guariglia C, Ptito A, Petrides M (2007): Retrosplenial and hippocampal brain regions in human navigation: Complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci 25:890–899. [DOI] [PubMed] [Google Scholar]
- Ito H, Ota M, Ikoma Y, Seki C, Yasuno F, Takano A, Maeda J, Nakao R, Suzuki K, Suhara T (2006): Quantitative analysis of dopamine synthesis in human brain using positron emission tomography with L‐[beta‐11C]DOPA. Nuclear Med Commun 27:723–731. [DOI] [PubMed] [Google Scholar]
- Jack CRJ, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E (1999): Prediction of AD with MRI‐based hippocampal volume in mild cognitive impairment. Neurology 52:1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CRJ, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E (2000): Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 55:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW (2009): Hippocampal and cognitive aging across the lifespan: A bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci 29:1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Shen L, Gomez RG, Garrett A, Slovason HB, Reiss A, Schatzberg AF (2008): Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry 165:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Anderson S, Pettegrew JW (1994): Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The feinberg hypothesis revisited. J Psychiatr Res 28:239–265. [DOI] [PubMed] [Google Scholar]
- King JP, Christiansen BK, Westwood DA (2008): Grasping behavior in schizophrenia suggests selective impairment in the dorsal visual pathway. J Abnorm Psychol 117:799–811. [DOI] [PubMed] [Google Scholar]
- Kircher T, Weis S, Weube D, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Krach S (2008): Anterior hippocampus orchestrates successful encoding and retrieval of non‐relational memory: An event‐related fMRI study. Eur Arch Psychiatry Clin Neurosci 258:363–372. [DOI] [PubMed] [Google Scholar]
- La Joie R, Fouquet M, Mézenge F, Landeau B, Villain N, Mevel K, Pélerin A, Eustache F, Desgranges B, Chételat G (2010): Differential effect of age on hippocampal subfields assessed using a new high‐resolution 3T MR sequence. Neuroimage 53:506–514. [DOI] [PubMed] [Google Scholar]
- Lamm C, Zelazo PD, Lewis MD (2006): Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia 44:2139–2148. [DOI] [PubMed] [Google Scholar]
- Lema?tre H, Crivello F, Grassiot B, Alpérovitch A, Tzourio C, Mazoyer B (2005): Age‐ and sex‐related effects on the neuroanatomy of healthy elderly. Neuroimage 26:900–911. [DOI] [PubMed] [Google Scholar]
- Li YJ, Ga SN, Huo Y, Li SY, Gao XG (2007): Characteristics of hippocampal volumes in healthy chinese from MRI. Neurol Res 29:803–806. [DOI] [PubMed] [Google Scholar]
- Lim KO, Pfefferbaum A (1989): Segmentation of MRbrain images into cerebrospinal fluid spaces, white and gray matter. J Comput Assist Tomogr 13:588–593. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M (2009): Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. J Neurosci 29:2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A, Woollett K, Spiers HJ (2006): London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus 16:1091–1101. [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS (1999): MRI‐based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage 9:18–45. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Bouchard TP, Camicioli R, Coupland NJ (2008): Aging hippocampus and amygdala. Neuroreport 19:543–547. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM (2007): Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience 144:961–968. [DOI] [PubMed] [Google Scholar]
- May A (2011): Experience‐dependent structural plasticity in the adult human brain. Trends Cogn Sci 15:475–482. [DOI] [PubMed] [Google Scholar]
- MEDx (1998): Sensor Systems, Virginia, USA.
- Meyer JW, Makris N, Bates JF, Caviness VS, Kenndy DN (1999): MRI‐based topographic parcellation of human cerebral white matter. Neuroimage 9:1–17. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Elkinsa W, Resnick SM (2006): Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiol Aging 27:965–972. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P (1993): Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 13:3916–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z (1999): A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40–90 years of age. Am J Neuroradiol 20:207–211. [PMC free article] [PubMed] [Google Scholar]
- Niemann K, Hammers A, Coenen VA, Thron A, Klosterkötter J (2000): Evidence of a smaller left hippocampus and left temporal horn in both patients with first episode schizophrenia and normal control subjects. Psychiatry Res 99:93–110. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971)The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologica 9:97–114. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathy CD (1990): Atlas of the Cerebral Sulci, 1st ed Stuttgart: Georg Thieme Verlag. [Google Scholar]
- Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T, Suhara T (2006): Age related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L‐[beta‐11C]DOPA. Life Sci 79:730–736. [DOI] [PubMed] [Google Scholar]
- Otsu NA (1979): Thresholding selection method from gray‐level histogram. Inst Electrical Electron Eng 9:62–66. [Google Scholar]
- Peiffer AM, Hugenschmidt CE, Maldjian JA, Casanova R, Srikanth R, Hayasaka S, Burdette JH, Kraft RA, Laurienti PJ (2009): Aging and the interaction of sensory cortical function and structure. Hum Brain Mapp 30:228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C (2004): Oligodendrocytes, their progenitors and other neuroglial cells in the aging primate cerebral cortex. Cereb Cortex 14:995–1007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO (1994): A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 51:874–887. [DOI] [PubMed] [Google Scholar]
- Pine DS, Grun J, Maguire EA, Burgess N, Zarahn E, Koda V, Fyer A, Szeszko PR, Bilder RM (2002): Neurodevelopmental aspects of spatial navigation: A virtual reality fMRI study. Neuroimage 15:396–406. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Collins DL, Pruessner M, Evans AC (2001): Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J Neurosci 21:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Lopez‐Sala A, Deus J, Cardoner N, Sebastian‐Galles N, Conesa G, Capdevila A (2002): The lateral asymmetry of the human brain studied by volumetric magnetic resonance imaging. Neuroimage 17:670–679. [PubMed] [Google Scholar]
- Rademacher J, Caviness VSJ, Steinmetz H, Galaburda AM (1993): Topographical variation of the human primary cortices: Implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex 3:313–329. [DOI] [PubMed] [Google Scholar]
- Rajah MN, Kromas M, Han JE, Pruessner JC (2010): Group differences in anterior hippocampal volume and in the retrieval of spatial and temporal context memory in healthy young versus older adults. Neuropsychologia 48:4020–4030. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD (1997): Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex 7:268–282. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning‐Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD (2004): Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol Aging 25:377–396. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Denise Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005): Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U (2010): Trajectories of brain aging in middle‐aged and older adults: Regional and individual differences. Neuroimage 51:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C (2003): Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci 23:3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero G, Sergi I, Iachini T (2008): Gender differences in remembering and inferring spatial distances. Memory 16:821–835. [DOI] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS (2001): Selective preservation and degeneration within the prefrontal cortex in aging and alzheimer disease. Arch Neurol 58:1408. [DOI] [PubMed] [Google Scholar]
- Sanides F, Krishnamurti A (1967): Cytoarchitectonic subdivisions of sensorimotor and prefrontal regions and of bordering insular and limbic fields in slow loris (nycticebus coucang coucang). J Hirnforschung 9:225–252. [PubMed] [Google Scholar]
- SAS (2001): Statistical Software, 8,2nd ed Cary, NC: SAS. [Google Scholar]
- Sasaki M, Tohyama K, Matsunaga S, Nakamura M, Tomizawa N, Inoue T, Ogawa H, Ehara S, Ogawa A (2004): MRI identification of dorsal hippocampus homologue in human brain. Neuroreport 15:2173–2176. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Buonocore MH, Makaron LM, Amaral DG, Barnes CA, Rapp PR (2006): Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta). Neurobiol Aging 10:1405–1415. [DOI] [PubMed] [Google Scholar]
- Silver H, Goodman C, Bilker W (2009): Age in high‐functioning healthy men is associated with nonlinear decline in some “executive” functions in late middle age. Dementia Geriatric Cogn Disord 27:292–300. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Herman JP (2009): Sex differences in psychopathology: Of gonads, adrenals and mental illness. Physiol Behav 97:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW (2001): Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci 21:8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ (1999): Segregating the functions of the human hippocampus. Proc Natl Acad Sci USA 96:4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Duggins A, Heinze HJ, Dolan RJ (2005): Dissociating intentional learning from relative novelty responses in the medial temporal lobe. Neuroimage 25:51–62. [DOI] [PubMed] [Google Scholar]
- Stuss D, Levine B (2002): Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annu Rev Psychol 53:401–433. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A (2004): Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging 25:185–192. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A (2005): Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging 26:1093–1098. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hagino H, Nohara S, Zhou SY, Kawasaki Y, Takahashi T, Mie Matsui M, Seto H, Ono T, Kurachi M (2005): Male‐specific volume expansion of the human hippocampus during adolescence. Cereb Cortex 15:187–193. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JM, Ashtari M, Wu H, Lieberman JA (1999a): Investigation of frontal lobe subregions in first‐episode schizophrenia. Psychiatry Res 90:1–15. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robertson D, Alvir JMJ, Bilder RM, Lencz T, Astari M, Wu H, Bogerts B (1999b): Orbital frontal and amygdala volume reductions in obsessive compulsive disorder. Arch Gen Psychiatry 56:913–919. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter G, Wu H, Lieberman JA (2000): Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first‐episode schizophrenia. Schizophrenia Res 43:97–108. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Strous RD, Goldman RS, Ashtari M, Knuth KH, Lieberman JA, Bilder RM (2002): Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. Am J Psychiatry 159:217–226. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Goldberg E, Gunduz‐Bruce H, Ashtari M, Robinson D, Malhotra AK, Lencz T, Bates J, Crandall DT, Kane JM, Bilder RM (2003): Smaller anterior hippocampal formation volume in antipsychotic‐naive patients with first‐episode schizophrenia. Am J Psychiatry 160:2190–2197. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Betensky JD, Mentschel C, Gunduz‐Bruce H, Lencz T, Ashtari M, Malhotra AK, Bilder RM (2006): Increased stress and smaller anterior hippocampal volume. Neuroreport 17:1825–1828. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Sevy S, Kumra S, Rupp CI, Betensky JD, Lencz T, Ashtari M, Kane JM, Malhotra AK, Gunduz-Bruce H, Napolitano B, Bilder RM (2007): Anterior cingulate gray‐matter deficits and cannabis use in first‐episode schizophrenia. Br J Psychiatry 190:230–236. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ashtar M, Vogel J, Betensky J, Sevy S, Ardenkani BA, Lencz T, Malhotra AK, McCormack J, Miller R, Lim KO, Gunduz-Bruce H, Kane JM, Bilder RM (2008a): Clinical and neuropsyological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology 33:976–984. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Christian C, MacMaster F, Lencz T, Mirza Y, Taormina SP, Easter P, Rose M, Michalopoulou GA, Rosenberg DR (2008b): Gray matter structural alterations in psychotropic drug‐naive pediatric obsessive‐compulsive disorder: An optimized voxel‐based morphometry study. Am J Psychiatry 165:1299. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due‐T⊘nnessen P, Walhovd KB (2010): Brain maturation in adolescence and young adulthood: Regional age‐related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex 20:534–548. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J (2003): On the involvement of prefrontal networks in cognitive ageing. Cortex 39:1107–1128. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Visser PJ, van Boxtel MP, Jolles J (2000): The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging 21:569–576. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings H (2002): Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel‐based morphometry. Neuroimage 17:657–669. [PubMed] [Google Scholar]
- Tripathy D, Yin X, Sanchez A, Luo J, Martinez J, Grammas P (2010): Cerebrovascular expression of proteins related to inflammation, oxidative stress and neurotoxicity is altered with aging. J Neuroinflammation 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya JG, Paradiso S, Boles Ponto LL, McCormick LM, Robinson RG (2007): Aging, gray matter, and blood flow in the anterior cingulate cortex. Neuroimage 37:1346–1353. [DOI] [PubMed] [Google Scholar]
- Venarucci D, Venarucci V, Vallese A, Battilà L, Casado A, De la Torre R, Lopez Fernandez ME (1999): Free radicals: Important cause of pathologies refer to ageing. Panminerva Med 41:335–339. [PubMed] [Google Scholar]
- Yücel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, Pantelis C (2001): Hemispheric and gender‐related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: An MRI morphometric study. Cereb Cortex 11:17–25. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E (2006): The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am J Geriatric Psychiatry 14:823–833. [DOI] [PubMed] [Google Scholar]


