Abstract
Current vector control programs are largely dependent on pyrethroids, which are the most commonly used and only insecticides recommended by the World Health Organization for insecticide-treated nets (ITNs). However, the rapid spread of pyrethroid resistance worldwide compromises the effectiveness of control programs and threatens public health. Since few new insecticide classes for vector control are anticipated, limiting the development of resistance is crucial for prolonging efficacy of pyrethroids. In this study, we exposed a field-collected population of Culex pipiens pallens to different insecticide selection intensities to dynamically monitor the development of resistance. Moreover, we detected kdr mutations and three detoxification enzyme activities in order to explore the evolutionary mechanism of pyrethroid resistance. Our results revealed that the level of pyrethroid resistance was proportional to the insecticide selection pressure. The kdr and metabolic resistance both contributed to pyrethroid resistance in the Cx. pipiens pallens populations, but they had different roles under different selection pressures. We have provided important evidence for better understanding of the development and mechanisms of pyrethroid resistance which may guide future insecticide use and vector management in order to avoid or delay resistance.
Author Summary
Successful population control of mosquitoes is key to preventing transmission and epidemics of mosquito-borne diseases. This strategy relies heavily on insecticides, particularly pyrethroids. However, widespread pyrethroid resistance has hindered vector control implementation and sustainability. Generally, pyrethroid resistance in insects is mainly caused by target-site insensitivity and metabolic resistance. Although studies on the two resistance mechanisms have provided insights, how the target-site and metabolic resistance mechanisms jointly confer the resistance phenotype has remained unclear. Understanding the mechanism of resistance to insecticides is essential for vector control measures. Our study investigated the development of resistance to a pyrethroid in three mosquito lines by varying the intensity of selection between lines (intense selection, mild selection and no selection) and then tracking changes in kdr frequency and the activities of three families of metabolic detoxification enzymes over time. These findings may lead to the development of more appropriate use of insecticides and more accurate resistance monitoring systems in the field.
Introduction
Culex mosquitoes are important vectors responsible for transmission of lymphatic filariasis (LF) and several viral pathogens to millions of people worldwide, including St. Louis encephalitis, West Nile encephalitis, eastern equine encephalitis, Venezuelan equine encephalitis and Japanese encephalitis [1]. The World Health Organization estimated over 120 million cases of LF and about 40 million disfigured and incapacitated by the disease [2]. Globally, nearly 1.4 billion people in 73 countries worldwide are currently threatened by LF. Mosquito-borne diseases dramatically affect public health and pose a major burden in terms of economy and development worldwide. Mass drug administration in combination with alternative vector control methods have proven to be more effective and practical in avoiding the re-emergence and re-introduction of LF [3, 4]. Insect control is the primary intervention available for some of the most devastating mosquito-borne diseases, particularly those lacking vaccines such as malaria, dengue and LF [5]. Most vector control programs largely rely on the application of chemical insecticides by the use of outdoor spraying, insecticide-treated nets (ITNs) or indoor residual spraying (IRS) [6]. Because of the relatively low mammalian toxicity and rapid knockdown effect on insects, pyrethroids are the most commonly used insecticides and constitute the only recommended class of insecticides for ITNs. However, insecticide exposure is a potent selective force, presenting a risk of generating resistance that would threaten the efficacy of control programs. Hence, preventing or delaying the emergence and development of resistance to pyrethroids is very important for vector control efforts. Improving vector management involves a better understanding of resistance mechanisms.
Global surveys have indicated that resistance of mosquitoes to pyrethroids mainly occurs through increased detoxification, as well as target site insensitivity [7]. ‘Metabolic resistance’ usually results from enhanced detoxification enzyme activity in resistant organisms [8]. Detoxification enzymes typically linked to insecticide resistance mainly include three major gene families, cytochrome P450 monooxygenases (P450s or CYPs), carboxyl/choline esterases (CCEs) and glutathione-S-transferases (GSTs). Numerous studies have associated these detoxification enzymes with pyrethroid resistance in mosquitoes [9–11]. The primary target sites of pyrethroids, well known as knockdown resistance (kdr), encode voltage-gated sodium channels, and single or multiple substitutions in the sodium channel gene can reduce neuronal sensitivity to pyrethroids [12]. To date, More than 30 unique resistance-associated kdr mutations or combinations of mutations have been detected in several insect species [13]. Among them, the most common kdr mutations are the leucine to phenylalanine (Leu→Phe) substitution and the leucine to serine substitution (Leu→Ser) substitution at codon 1014 in the S6 hydrophobic segment of domain II in the sodium channel gene [14, 15]. These two common mutations have been shown to reduce the pyrethroid sensitivity of sodium channels in Xenopus oocytes, confirming their role in kdr [16, 17]. Many studies have revealed that all these mechanisms can occur simultaneously in resistant populations with cumulative phenotypic effects leading to resistance to a single or multiple insecticides [18, 19]. However, the relative contribution of the metabolic resistance and knockdown resistance in conferring the resistance phenotype has remained elusive.
High levels of resistance to pyrethroids in Culex mosquitoes has been widely reported [20, 21]. Here, we studied Cullex pipiens pallens because it is the most prevalent and important vector in China with high population density. We also chose deltamethrin, a representative pyrethroid insecticide, to explore the evolutionary mechanism of insecticide resistance, as well as the relative contributions of the target-site and metabolic resistance in the development of pyrethroid resistance. We detected kdr and activities of three types of detoxification enzymes (P450s, CCEs, and GSTs) in the same mosquito sample through large-scale population studies. Knowledge of the changing trends and patterns of insecticide resistance may impact future predictions and monitoring of pyrethroid resistance in mosquitoes and other arthropod pests and disease vectors.
Materials and Methods
Set-up of mosquito population
A population of Cx. pipiens pallens was collected from natural habitats (Tangkou, Shandong Province of China) in September 2009. Mosquitoes were reared in standard insectary conditions (28°C, 14 h:10 h light/dark period, 75% relative humidity) with tap water (larvae) and net cages (adults). Adults were supplied with a 10% sucrose solution and blood fed on adult mice. Larvae were fed with fish food.
The population was selected with the pyrethroid insecticide deltamethrin (Jiangsu Yangnong Chemical Group Co., Jiangsu, China) in the laboratory. Selection was performed by exposing each generation of fourth-stage larvae for 24 h to a 50% lethal concentration (LC50) of deltamethrin. The LC50 was determined by a larval bioassay. Initially, the fourth-stage larvae were exposed to a wide range of test concentrations of deltamethrin. After determining the mortality of larvae in this wide range of concentrations, a narrower range (of 5 concentrations, yielding between 5% and 95% mortality in 24 h) was used to determine LC50 values. Five concentrations of deltamethrin and 3 replicates of 20 fourth-instar larvae per concentration were used. A control group was measured using 20 larvae in tap water without any insecticides. Numbers of dead and surviving larvae were recorded after 24 h. All surviving larvae were transferred to tap water, fed with larval food and allowed to emerge. Adults were fed to obtain eggs for the next generation.
After six generations, three strains under different insecticide selection pressures were established. The first strain, designated the ‘intense selection’ (IS) strain, was selected with the LC50 of deltamethrin for each generation causing 50–60% larval mortality. The second selected strain, termed the ‘mild selection’ (MS) strain, was exposed to the constant concentration of 0.05 ppm (LC50 for generation 6) in the subsequent deltamethrin selection. Deltamethrin exposure was withdrawn from the last strain, which was designated the ‘no-selection’ (NS) strain. All three strains were selected for 24 generations with three replicate groups, and each generation of every replicate group was initiated with more than 1000 adult females in order to limit bottleneck effects. The actual doses used for deltamethrin selection per generation were reported in the supporting information (detailed results are shown in S1 Table). A laboratory deltamethrin-susceptible strain of Cx. pipiens pallens (S-LAB) was used as a reference strain.
Sample collection
In every replicate group of each strain, more than 50 female adult mosquitoes, at post-emergence ages of 3–4 d, were collected for determinations of kdr alleles and metabolic enzyme activities. Generations 1, 6, 10, 14, 18, 22, 26 and 30 were analyzed. Two legs of each adult female were removed and preserved individually in 95% alcohol for subsequent DNA analysis. The remaining mosquito body was immediately used for metabolic enzyme activity determination. More than 3,000 female adult mosquitoes were used for analysis in this study.
DNA extraction and kdr allele detection
Genomic DNA was extracted individually from two legs of each adult female by a fast tissue-to-PCR kit (Fermentas, K1091). The region containing kdr mutations within the para sodium channel gene was amplified by PCR with primers Cpp1 (5’-CCTGCCACGGTGGAACTTC-3’) and Cpp-2 (5’-GGACAAAAGCAAGGCTAAGAA-3’). The PCR primers were designed based on the cDNA sequence of Cx. quinquefasciatus para-sodium channel gene alpha subunit (Genbank accession number BN001092) [22]. PCR amplification was carried out in a volume of 20 μl, including 4 μl genomic DNA, 10 μl Tissue Green PCR Master Mix (Fermentas), 10 pmol primers Cpp1 and Cpp2 and 4 μl nuclease-free water. Amplification was performed with the following cycling conditions: initial denaturation at 95°C for 3 min, 35 cycles of 95°C for 30 s, 55°C for 30 s and elongation at 72°C for 40 s, followed by extension at 72°C for 3 min. PCR products were purified using the QIAquick PCR purification kit (Qiagen) and then sent for sequencing (BGI, Shanghai, China). In total, 2,427 female adult samples were successfully sequenced in the study. Genotype frequencies were calculated, and deviation from the Hardy–Weinberg equilibrium was analyzed by the web-based program GENEPOP [23].
Metabolic enzyme activity assays
Metabolic enzyme activity was measured in individual female mosquitoes by using the method described by Daibin et al. [24] with a slight modification. Every individual females was homogenized in a 1.5-ml tube with 300 μl of 0.25 M phosphate buffer (pH 7.2) and diluted by adding 300 μl of phosphate buffer. The tube was mixed and centrifuged, and the same supernatant was used to test the activity of GSTs, P450s and CCEs simultaneously. All assays were carried out in duplicate, and the protein content of the supernatant was measured by the Bradford method [25]. For the GSTs activity assay, a total of 90 μl of reduced glutathione solution (Sigma, G4251) and 90 μl of 1-chloro-2,4'-dinitrobenzene (cDNB) solution was added to 90 μl of mosquito supernatant. The absorbance was measured immediately using a microplate reader at 340 nm and then detected every 2 min for five times, using 0.25 M phosphate buffer as the negative control. For the P450s activity assay, a total of 10 μl of the 60mM 7-ethoxycoumarine (7-EC) solution was added to 100 μl of mosquito supernatant, and samples were incubated at 30°C for 5.5 h. The reaction was stopped by the addition of 150 μl of glycine buffer (pH 10.4, 1 mM), and sample absorbance was measured using a microplate reader at 450 nm with 0.25 M phosphate buffer as a negative control. The OD values were converted into concentrations by using standard regression based on a serial dilution of 7-hydroxycoumarin and its relevant OD values. The content of P450s was calculated for each mosquito. The OD values were converted into concentrations by using standard regression based on OD values of serial dilutions of 7-hydroxycoumarine. For the CCEs activity assay, the 0.1 mM β-nitrophenyl acetate (Sigma, N8130) solution was placed for 5 min in 30°C water bath first, and then a total of 220 μl of the β-nitrophenyl acetate solution was added to 50 μl of mosquito supernatant. The absorbance was measured using a microplate reader at 405 nm every min for five times with 0.25 M phosphate buffer as the negative control. All measurements were performed in duplicate. In total, we successfully detected 1,541 female adult samples for P450s, 1,368 samples for CCEs and 1,665 samples for GSTs.
Statistical analysis
The LC50 was calculated using Probit analysis [26] and Abbott’s correction for the mortality rate in the control group [27]. The resistance ratio (RR) is the ratio of the LC50 of the selected strain to the LC50 of the laboratory deltamethrin-susceptible strain S-LAB. Regression analysis (Curve estimation) was used to determine correlation coefficients between the deltamethrin susceptibility and generations under different selection. The DNA sequences of kdr were assembled and prealigned by BioEdit, aligned in ClustalW implemented in MEGA5 and the alignment was refined manually [28]. Then we used DnaSPv5 to estimate the number of haplotypes (h), the haplotype diversity (Hd) and nucleotide diversity (Pi). The kdr allele frequency in three selected strains was calculated, and statistically significant differences among strains were examined using the t-test. One-way analysis of variance (ANOVA) was used to examine whether metabolic enzyme activity varied in populations with different selection pressures. The generation was treated as a random factor. Metabolic enzyme activity data were square root transformed. The t-test was used for comparison of different generations when appropriate. Linear correlation analysis was used to study the correlation of the frequency of kdr and the metabolic enzyme activity with the degree of resistance.
Accession numbers
Cx. quinquefasciatus para-sodium channel gene alpha subunit: BN001092.
Cx. pipiens pallens kdr haplotypes: GU198936- GU198938, GU339221.
Results
Mosquito populations and deltamethrin susceptibility
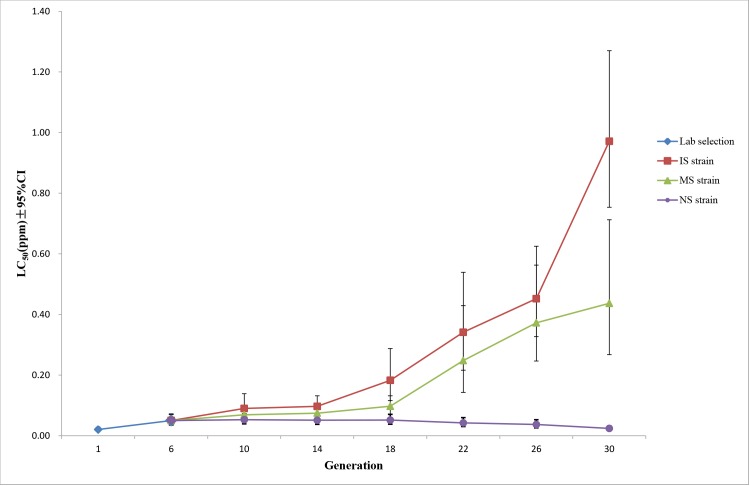
A field population of Cx. pipiens pallens was collected, and three strains were established after being placed for 30 generations (~28 months) under different insecticide selection pressures. After selection for six generations, the RR increased from 1.69 at generation 1 (LC50 = 0.0206 ppm) to 4.11 at generation 6 (LC50 = 0.0501 ppm) (detailed results are shown in S2 Table). The resistance to insecticide was increased from generation to generation with exposure to insecticide, while it was reduced without exposure (Fig 1). Regression analysis showed that the level of resistance grew exponentially in IS and NS strain (Fig 2). The degree of insecticide resistance in the IS strain increased more rapidly than that in the MS strain. At generation 30, the RR of the IS strain had increased significantly to 79.61 (LC50 = 0.9713 ppm) (P < 0.05), and the RR of the MS strain was also increased significantly to 35.80 (LC50 = 0.4367 ppm) (P < 0.05). Notably, the level of insecticide resistance increased faster after generation 14 of the IS strain and generation 18 of the MS strain. By contrast, the level of insecticide resistance in the NS strain was reduced slowly without insecticide selection from generation 6, and significant differences were observed after generation 22. The RR of the NS strain was reduced significantly from 4.11 at generation 6 (LC50 = 0.0501 ppm) to 1.98 at generation 30 (LC50 = 0.0241 ppm) (P < 0.05), and the LC50 of generation 30 was still higher than that of the first generation (P < 0.05) (Fig 1).
Fig 1. Development of larval resistance of populations under different deltamethrin selection pressures.
Levels of deltamethrin resistance were dynamically monitored in three selected strains. Lab selection: the population from 1 to 6 generations were selected with insecticide together and the selection concentration was LC50 of deltamethrin for each generation. IS strain: selection concentration was LC50 of deltamethrin for each generation and resulted in 50–60% larval mortality. MS strain: selection concentration was the LC50 of deltamethrin for generation 6 (0.05 ppm), and the larval mortality was gradually decreased. NS strain: exposure to deltamethrin was withdrawn after generation 6. CI: confidence intervals.
Fig 2. Scatter plot of the level of resistance and generation under different insecticide selection.
In the IS strain, R2 = 0.98, P<0.0001; In the MS strain, R2 = 0.94, P<0.0001; In the NS strain, R2 = 0.98, P<0.0001.
Distribution of kdr allele frequencies in populations with different selection pressures
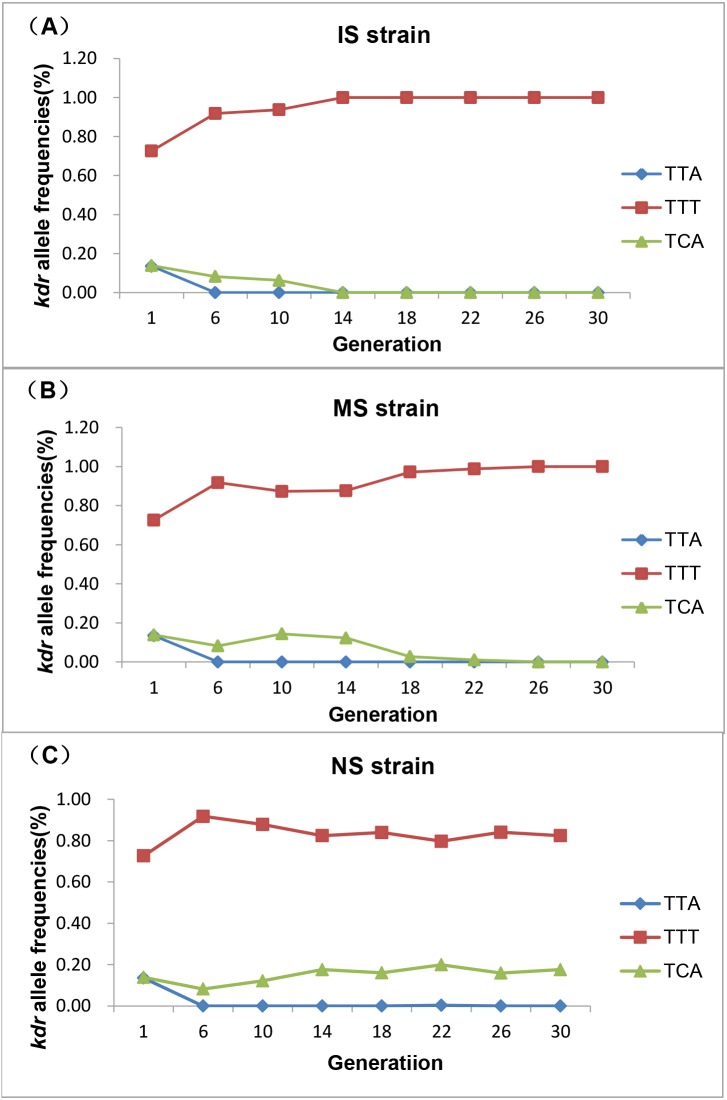
A 480-bp fragment of the para-type sodium channel gene including codon 1014 was sequenced from 2,457 individual Cx. pipiens pallens of the three strains. The wild-type kdr codon sequence spanning position 1014 was TTA (L1014). The two most common types of kdr mutations detected were TTT (L1014F) and TCA (L1014S). A total of six genotypes were identified in all strains: L1014/L1014, L1014F/L1014F, L1014S/L1014S, L1014/L1014F, L1014/L1014S and L1014F/L1014S (Fig 3). The field population of Cx. pipiens pallens carried a high kdr mutation frequency (86.49%) of both the L1014F (72.64%) and L1014S (13.85%) mutations (Table 1). Under insecticide selection, the kdr mutation frequency increased significantly and reached 100.00% at generation 6. But the response of these two mutations to deltamethrin selection were different (Table 1 and Fig 4). Under insecticide selection, the frequency of L1014F was increased and faster in IS strain than MS strain. By contrast, the frequency of L1014S was decreased and slower in MS strain than IS strain. In the IS strain, the frequency of the L1014F mutation became fixed, and the strain became homozygous for kdr (genotype: L1014F/L1014F) at generation 14. In the MS strain, the rate of increase in the allele frequency for this mutation was slower than observed for the IS strain, and the MS strain became homozygous (genotype: L1014F/L1014F) at generation 26. Interestingly, the level of insecticide resistance was increased most significantly after the population became a homozygous population for the kdr resistance gene, suggesting the existence of other mechanism which could induce deltamethrin resistance. Without insecticide selection, the frequency of L1014S increased and L1014F decreased. In the NS strain, the frequency of L1014S increased significantly from 8.21% at generation 6 to 17.73% at generation 30, and the frequency of L1014F declined significantly but was still higher than that of the first generation (72.64%). These results suggest that the L1014F mutation is more closely associated with deltamethrin resistance in the Cx. pipiens pallens population than the L1014S mutation. The wild-type L1014 allele could not be detected from the sixth generation and became extinct with insecticide selection. Because our studied population carried a high kdr mutation frequency and was a closed population, the wild-type sequence was recovered infrequently. The three replicate groups had the same trends, and the detailed results are shown in S1 Fig.
Fig 3. Population kdr genotypes.
A total of six genotypes were identified in this Cx. pipiens pallens population: TTA/TTA, TCA/TCA, TTT/TTT, TTA/TTT, TTT/TCA and TTA/TCA. Red box indicates the 1014 site of the kdr gene.
Table 1. Genotypes and allele frequencies of kdr detected in populations under different insecticide selection pressures.
| kdr genotypes | Kdr alleles frequencies(%) | kdr mutation frequency(%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Generation | N | TTA/TTA | TTT/TTT | TCA/TCA | TTA/TTT | TTA/TCA | TTT/TCA | TTA | TTT | TCA | TTT+TCA |
| Lab | 1 | 148 | 9 | 84 | 6 | 20 | 2 | 27 | 13.51 | 72.64 | 13.85 | 86.49 |
| selection | 6 | 134 | 0 | 120 | 8 | 0 | 0 | 6 | 0.00 | 91.79 | 8.21 | 100.00 |
| IS | 10 | 133 | 0 | 118 | 3 | 0 | 0 | 12 | 0.00 | 93.23 | 6.77 | 100.00 |
| strain | 14 | 102 | 0 | 102 | 0 | 0 | 0 | 0 | 0.00 | 100.00 | 0.00 | 100.00 |
| 18 | 143 | 0 | 143 | 0 | 0 | 0 | 0 | 0.00 | 100.00 | 0.00 | 100.00 | |
| 22 | 97 | 0 | 97 | 0 | 0 | 0 | 0 | 0.00 | 100.00 | 0.00 | 100.00 | |
| 26 | 94 | 0 | 94 | 0 | 0 | 0 | 0 | 0.00 | 100.00 | 0.00 | 100.00 | |
| 30 | 109 | 0 | 109 | 0 | 0 | 0 | 0 | 0.00 | 100.00 | 0.00 | 100.00 | |
| MS | 10 | 109 | 0 | 88 | 8 | 0 | 0 | 15 | 0.00 | 87.61 | 14.22 | 100.00 |
| strain | 14 | 112 | 0 | 89 | 5 | 0 | 0 | 18 | 0.00 | 87.50 | 12.50 | 100.00 |
| 18 | 139 | 0 | 133 | 2 | 0 | 0 | 4 | 0.00 | 97.12 | 2.88 | 100.00 | |
| 22 | 104 | 0 | 103 | 1 | 0 | 0 | 0 | 0.00 | 99.04 | 0.96 | 100.00 | |
| 26 | 127 | 0 | 127 | 0 | 0 | 0 | 0 | 0.00 | 100.00 | 0.00 | 100.00 | |
| 30 | 104 | 0 | 104 | 0 | 0 | 0 | 0 | 0.00 | 100.00 | 0.00 | 100.00 | |
| NS | 10 | 114 | 0 | 95 | 9 | 0 | 0 | 10 | 0.00 | 87.72 | 12.28 | 100.00 |
| strain | 14 | 107 | 0 | 78 | 8 | 0 | 0 | 21 | 0.00 | 82.71 | 17.29 | 100.00 |
| 18 | 133 | 0 | 98 | 8 | 0 | 0 | 27 | 0.00 | 83.83 | 16.17 | 100.00 | |
| 22 | 143 | 0 | 100 | 14 | 1 | 0 | 28 | 0.35 | 80.07 | 19.58 | 99.65 | |
| 26 | 140 | 0 | 106 | 11 | 0 | 0 | 23 | 0.00 | 83.93 | 16.07 | 100.00 | |
| 30 | 141 | 0 | 100 | 9 | 0 | 0 | 32 | 0.00 | 82.27 | 17.73 | 100.00 | |
Fig 4. Development of kdr allele frequencies in populations with different deltamethrin selection pressures.
Dynamic variation of three kdr allele frequencies are shown for (A) IS strain, (B) MS strain, and (C) NS strain.
Patterns of haplotype variation
The haplotype number (K) and haplotype diversity (H) are informative statistics for describing the distribution of haplotypes under an infinite-sites model [29]. During a selective sweep, the reduction in variation around a naturally selected locus will reduce the impact of reshuffling by recombination producing new haplotypes, and recombination is more likely to occur between two copies of the same haplotype [30]. In this study, the intron region downstream of L1014 in the kdr gene was cloned, sequenced then analyzed using DNAspv5 software. The results showed a significant reduction of K and H in the kdr gene under insecticide selection, and the nucleotide diversity (Pi) and haplotype diversity (Hd) decreased with the increase of resistance (Table 2). Pi and Hd were reduced to 0 at generation 26 in the IS strain and at generation 30 in the MS strain, but they were increased after withdrawal of deltamethrin.
Table 2. DNA polymorphisms of kdr gene in populations under different insecticide selection pressures.
| Strain | Generation | Number of sequences | Number of sites | h | Hd | Pi |
|---|---|---|---|---|---|---|
| Lab Selection | 1 | 66 | 480 | 39 | 0.901 | 0.09702 |
| 6 | 81 | 480 | 17 | 0.513 | 0.04526 | |
| IS strain | 10 | 62 | 480 | 13 | 0.45 | 0.02003 |
| 14 | 65 | 480 | 11 | 0.374 | 0.04052 | |
| 18 | 66 | 480 | 9 | 0.281 | 0.00778 | |
| 22 | 57 | 480 | 2 | 0.064 | 0.00014 | |
| 26 | 53 | 480 | 1 | 0 | 0 | |
| 30 | 62 | 480 | 1 | 0 | 0 | |
| MS strain | 10 | 72 | 480 | 15 | 0.438 | 0.01436 |
| 14 | 86 | 480 | 14 | 0.281 | 0.00422 | |
| 18 | 76 | 480 | 13 | 0.356 | 0.01741 | |
| 22 | 63 | 480 | 5 | 0.124 | 0.00104 | |
| 26 | 57 | 480 | 3 | 0.07 | 0.01673 | |
| 30 | 64 | 480 | 1 | 0 | 0 | |
| NS strain | 10 | 65 | 480 | 20 | 0.638 | 0.03574 |
| 14 | 76 | 480 | 24 | 0.752 | 0.04275 | |
| 18 | 71 | 480 | 28 | 0.725 | 0.04657 | |
| 22 | 66 | 480 | 34 | 0.832 | 0.09714 | |
| 26 | 78 | 480 | 32 | 0.876 | 0.08175 | |
| 30 | 68 | 480 | 36 | 0.891 | 0.0398 |
h: Number of Haplotypes, Hd: Haplotype diversity, Pi: Nucleotide diversity.
Metabolic enzyme assay
The field population of Cx. pipiens pallens was selected with deltamethrin in the laboratory due to unknown use of insecticides at the sampling site. Metabolic enzyme activities were detected after deltamethrin selection for six generations. We analyzed in total 1545 samples for P450s, 1371 samples for CCEs and 1653 samples for GSTs (Table 3).
Table 3. Dynamic activities of three detoxification enzymes in populations under different insecticide selection pressures.
| P450s activity | CCEs activity | GSTs activity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Generation | mean(ng/min/mg protein) | SD | N | mean(nmol/min/mg protein) | SD | N | mean(nmol/min/mg protein) | SD | N |
| 6 | 1777.19 | 947.73 | 54 | 42.78 | 23.12 | 47 | 10.86 | 3.52 | 49 | |
| IS | 10 | 2086.80 | 938.54 | 87 | 44.19 | 28.01 | 66 | 9.13 | 4.17 | 98 |
| strain | 14 | 2075.14 | 871.51 | 86 | 46.10 | 28.55 | 72 | 9.88 | 5.11 | 69 |
| 18 | 2354.70 | 1058.67 | 96 | 49.78 | 28.64 | 70 | 11.31 | 5.43 | 98 | |
| 22 | 2727.20 | 1118.18 | 88 | 59.73 | 27.06 | 80 | 9.35 | 4.50 | 97 | |
| 26 | 2768.20 | 1330.72 | 84 | 62.97 | 37.32 | 80 | 9.87 | 3.93 | 97 | |
| 30 | 3941.26 | 1223.07 | 72 | 66.17 | 37.29 | 83 | 12.07 | 7.13 | 96 | |
| MS | 10 | 2184.01 | 693.65 | 89 | 41.07 | 23.08 | 72 | 13.64 | 7.25 | 87 |
| strain | 14 | 2092.73 | 986.13 | 96 | 41.14 | 25.54 | 82 | 16.28 | 9.72 | 88 |
| 18 | 2162.80 | 1057.03 | 86 | 43.12 | 26.16 | 70 | 9.56 | 5.53 | 88 | |
| 22 | 2241.57 | 926.78 | 88 | 41.74 | 29.85 | 71 | 11.48 | 3.90 | 88 | |
| 26 | 2287.26 | 1042.15 | 78 | 45.31 | 29.00 | 85 | 11.19 | 3.77 | 87 | |
| 30 | 2873.73 | 1080.77 | 77 | 49.37 | 34.12 | 83 | 12.92 | 5.02 | 89 | |
| NS | 10 | 1939.10 | 665.47 | 90 | 37.38 | 28.42 | 72 | 8.42 | 3.22 | 78 |
| strain | 14 | 1950.98 | 565.64 | 84 | 36.81 | 24.29 | 63 | 10.92 | 4.85 | 90 |
| 18 | 1692.62 | 564.18 | 69 | 34.81 | 22.30 | 77 | 8.09 | 3.17 | 89 | |
| 22 | 1795.91 | 563.41 | 74 | 31.02 | 20.69 | 70 | 9.02 | 2.99 | 88 | |
| 26 | 1570.71 | 701.16 | 83 | 28.65 | 15.67 | 61 | 9.39 | 3.37 | 88 | |
| 30 | 1438.93 | 531.46 | 64 | 25.16 | 12.51 | 67 | 10.73 | 3.37 | 89 | |
| SUM | 1545 | 1371 | 1653 | |||||||
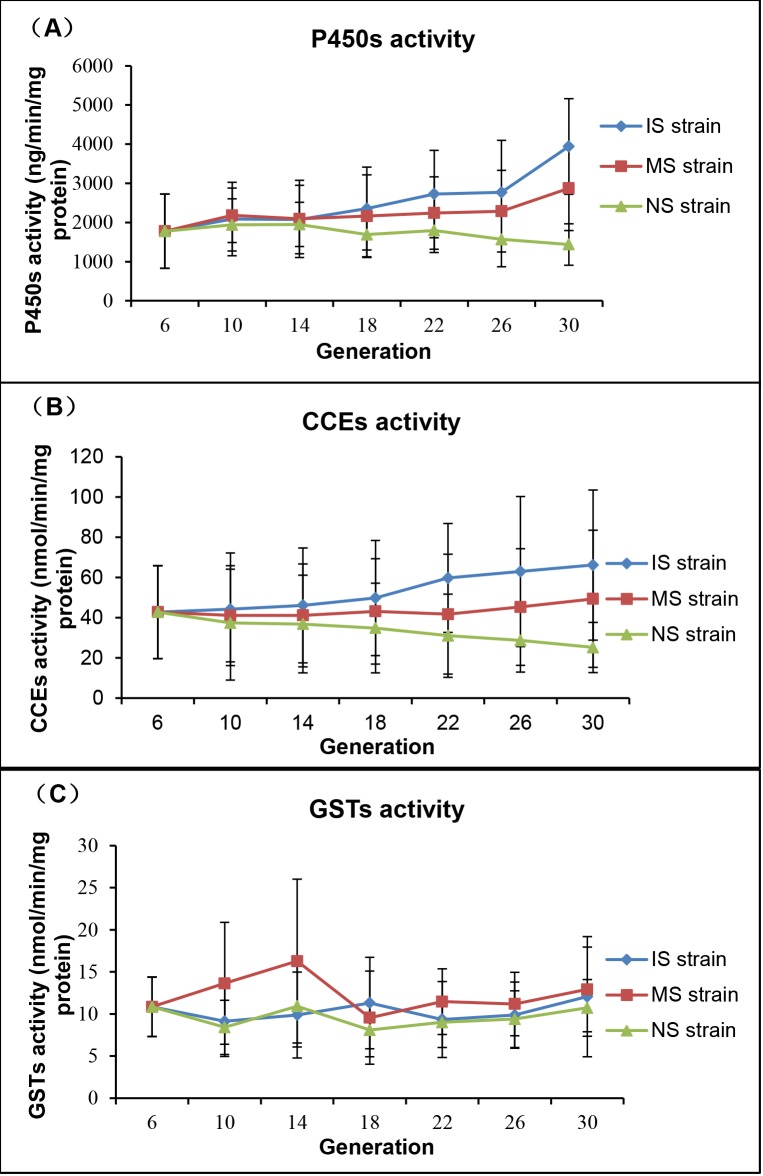
The results showed that the P450s activities of the population changed with different selection conditions (Table 3 and Fig 5). In the IS strain, significant among-population variation in different generations was found (one-way ANOVA, F6, 560 = 31.312, P < 0.0001), and the P450s activities increased significantly with the development of deltamethrin resistance. The P450s activities were increased by 2.21-fold at generation 30, and analysis by t-test showed significant variation after generation 14 when compared with activities in generation 6. The P450s activities increased more rapidly after generation 26. Likewise in the MS strain, significant among-population variation in different generations was observed (one-way ANOVA, F6, 561 = 8.841, P < 0.0001), and P450s activities also increased significantly with the development of deltamethrin resistance, although the increasing trend was slower than that in the IS strain. P450s activities increased by 1.61-fold at generation 30, and analysis by t-test showed significant variation in P450s activities after generation 18. Significant among-population variation was seen in different generations of the NS strain as well (one-way ANOVA, F6, 510 = 7.495, P < 0.0001). Without deltamethrin selection, P450s activities were reduced at each generation, decreasing by 0.81-fold at generation 30, and a significant reduction in those activities after generation 26 was found by t- test analysis.
Fig 5. Development of three enzyme activities in populations under different insecticide selection pressures.
Dynamic variation of three metabolic enzyme activities under different insecticide selection pressures are shown for (A) P450s, (B) CCEs, and (C) GSTs.
Changes of CCEs activities were similar to those of P450s (Table 3 and Fig 5). In the IS strain, significant among-population variation in different generations was observed (one-way ANOVA, F6, 491 = 6.957, P < 0.0001), and the CCEs activities also increased significantly with the development of deltamethrin resistance. At generation 30, the activities increased by 1.55-fold, and activities of CCEs increased significantly as shown by t- test analysis after generation 18 when compared with those in generation 6. In the MS strain, no significant among-population variation in different generations was detected (one-way ANOVA, F6, 503 = 0.710, P = 0. 642 > 0.0001). The CCEs activities were not significantly greater at generation 30 (independent samples test, t = 1.178, P = 0.241 > 0.05). In the NS strain, significant among-population variation in different generations was found (one-way ANOVA, F6, 450 = 4.151, P < 0.0001). Without deltamethrin selection, CCEs activities were also reduced with each generation, decreasing by 0.59-fold at generation 30, and t- test analysis showed significant variations after generation 18.
Activities of P450s and CCEs both increased with the level of deltamethrin resistance, and they decreased after withdrawal of deltamethrin. However, changes of GSTs activities were not associated with deltamethrin resistance (Table 3 and Fig 5). The three replicate groups showed similar trends, and the detailed results are shown in S2 Fig. These results indicated that the enhanced activities of P450s and CCEs led to metabolic resistance in the population, and P450s may hold a prominent role in metabolic resistance.
The correlation of metabolic enzyme activity and the frequency of L1014F with the degree of resistance
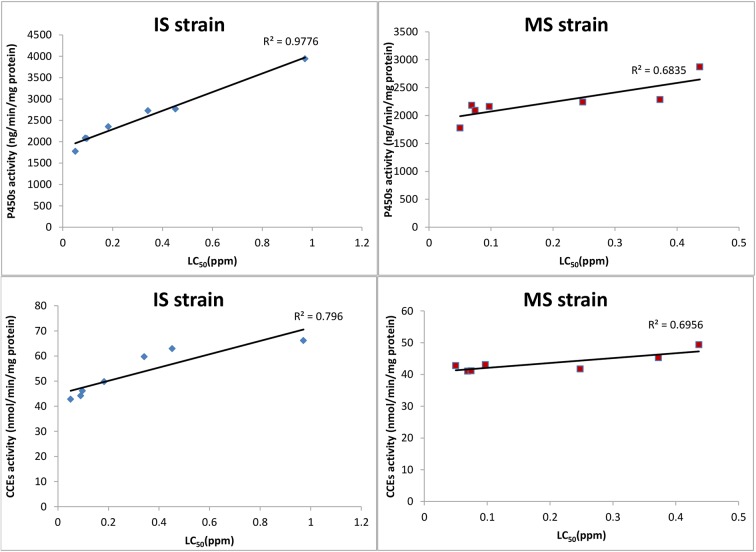
Under insecticide selection, a very strong positive correlation was observed between metabolic enzyme activity and the degree of resistance (Table 4 and Fig 6). The activity of P450s and CCEs were significantly correlated. The correlations between metabolic enzyme activity (P450s and CCEs) and LC50 in the IS strain were high and slightly higher than correlations in MS strain. We also analyzed the relationship between the frequency of L1014F and the degree of resistance (Table 4). It was noted that the significant correlation between the frequency of L1014F and LC50 was only present in MS strain. There were no significant correlations among metabolic enzyme activity, the frequency of L1014F and the degree of resistance in the NS strain.
Table 4. Pearson correlation coefficients between metabolic enzyme activity and the frequency of L1014F with the degree of resistance.
| LC 50 | |||
|---|---|---|---|
| IS | MS | NS | |
| P450s activity | 0.989** | 0.827* | 0.686 |
| CCEs activity | 0.892** | 0.834* | 0.743 |
| GSTs activity | 0.543 | -0.124 | 0.075 |
| The frequency of L1014F | 0.508 | 0.811* | 0.432 |
*The correlation coefficients significant P < 0.05
** The correlation coefficients significant P < 0.01
Fig 6. Relationship between metabolic enzyme activity and the degree of resistance in IS strain and MS strain.
Discussion
Resistance of mosquitoes to pyrethroids appears to rely mainly on target-site and metabolic resistance mechanisms. The two mechanisms can occur singly or simultaneously in resistant populations. A growing number of studies have found both metabolic- and kdr-based resistance mechanisms in most mosquito species [18, 24, 31]. Most researchers found that metabolic detoxification was the most important mechanism for the development of resistance in the mosquito population, whereas the target site played a less important role [32, 33]. Preliminary investigations of underlying resistance mechanisms of the pyrethroid resistance in field populations of Anopheles funestus in southern Africa indicated that a P450-based metabolic resistance was the main mechanism with no kdr mutation identified yet in this species [34]. Ochomo et al. found that phenotypic resistance to permethrin in An. gambiae s.s. was attributed to elevated expression of β-esterase and oxidase enzymes and the presence of kdr alleles at the voltage-gated sodium channel locus, but target-site mechanisms was detected in phenotypic resistance to deltamethrin solely [35]. It was noted that the different mechanisms occurred in the same resistance population. Although studies on the two resistance mechanisms have provided insights, how the target-site and metabolic resistance mechanisms differentially contribute to the resistance phenotype has remained unclear. To tackle these issues, our study detected kdr and three detoxification enzyme activities in the same mosquito sample and dynamically monitored the trends of resistance in populations with different insecticide selection pressures. We found that kdr and metabolic resistance both contributed to deltamethrin resistance in the Cx. pipiens pallens populations, but they had different roles under different selection pressures. The P450s activities increased significantly after generation 14 for IS strain and 18 for MS strain, and only the CCEs increased significantly after generation 18 in IS strain. In both the IS and MS groups, resistance to insecticide and frequencies of the kdr mutation L1014F increased before the detoxification enzyme activities were significantly increased. This phenomenon indicated that the target-site mechanisms was important under a relatively low insecticide selection pressure. After the population became homozygous for the L1014F mutation, the level of resistance grew along with the increase in detoxification enzyme activities. Linear correlation analysis showed that the significant correlation between the frequency of L1014F and LC50 was only present in MS strain, but the correlations between metabolic enzyme activity (P450s and CCEs) and LC50 was significantly high both in IS and MS strain, and slightly higher in IS strain. This finding seemed to indicate that the metabolic resistance increased with the increase of selection pressure, and played a main role in causing a high level of resistance under high insecticide selection pressure. Individual organisms with a low fitness cost will survive under selection. Numerous disruptive mutations can confer resistance (whether through suppression, down-regulation or gene-silencing), and a high dose of a selective agent may overcome fitness costs associated with disruption and thus favor a large pool of normally deleterious mutations [36, 37]. However, fitness costs also select against such alleles in the absence of toxicant selection. Metabolic resistance is mainly caused by the up-regulation of detoxification enzymes with high fitness costs. Some studies suggest that P450s overproduction decreases the fitness of individual organisms that carry them because the overproduced P450s can metabolize hormonal endogenous molecules. However, amino-acid substitutions may possibly involve fewer disturbances to the fitness of the individual [38]. Therefore, the low insecticide selection pressure preferred selection of individuals with kdr. When the selection pressure was increased via a high dose of insecticide, organisms with metabolic resistance were primarily selected.
Metabolic resistance to pyrethroid is known to be associated with three types of detoxification enzymes, P450s, CCEs and GSTs; however, the relative importance and pattern of these enzymes in the development of resistance are still disputed. Pyrethroid resistance is thought to be mediated essentially by P450s. Elevated levels of P450s activity are frequently observed in mosquito species as major malaria vectors in Africa [11, 39, 40]. In whole-genome microarray studies, members of the cytochrome P450 class of metabolic enzymes are frequently up-regulated in pyrethroid-resistant mosquitoes [41]. CCEs are also believed to act as a cause of metabolic resistance in some instances. Recently, the capacity of Aedes aegypti CCEs to metabolize pyrethroids leading to detoxification has been demonstrated in vitro [11]. GSTs are regularly found overexpressed in pyrethroid-resistant mosquitos. The potential role of GSTs in pyrethroid resistance is likely associated with protection against oxidative stress and sequestration of pyrethroids [42, 43]. Detoxification of pyrethroids by P450s either alone or in combination with CCEs and/or GSTs has been suggested to play a role in pyrethroid resistance [8, 44, 45]. In the same population, the metabolic resistance was also different. Ochomo et al. showed the association of elevated activities of β-esterases and P450s with permethrin resistance, but there was no elevated expression of detoxifying enzymes in phenotypic resistance to deltamethrin [35]. The different conclusions among diverse studies confirmed that the development of metabolic resistance is a rather complicated process, which may be affected by the species, strain (field or lab) or insecticides. In our study, the same species and strain were exposed to the identical insecticide, so we could detect the effects of selection pressure on metabolic resistance. Our study found that different selection pressures led to different levels of metabolic resistance with potentially different mechanisms. Metabolic resistance was mainly mediated by P450s under low insecticide selection pressure, but a high level of metabolic resistance was related to P450s and CCEs under high insecticide selection pressure. Results of the metabolic enzyme assay showed that the development of deltamethrin resistance was accompanied by the significant increase in activities of P450s and CCEs in the IS strain, and only P450s were raised in the MS strain. No correlations between GSTs and the level of deltamethrin resistance were found here. The resistance levels and P450s activity were similar at generation 30 for MS strain and generation 26 for IS strain, but the CCEs activity was significantly elevated in IS strain (Table 3 and S2 Table). CCEs activity appeared higher in IS strain, but CCEs activity made substantial jumps from generation 22 to generation 30 in the MS strain. These results suggested that insecticide selection had a finite amount of genetic variation in the base population that could be selected to generate resistant phenotypes. Under mild selection (in MS strain), the P450s activity responded first, then the CCEs activity was necessary and some of the genetic variants responsible for higher CCEs activity were selected from generations 22 to achieve high levels of resistance. Under intense selection pressure (in IS strain), perhaps P450s alleles alone could not segregate fast enough to produce the required resistance level, so CCEs alleles were selected concurrently. As we did not examine the members of the P450s and CCEs families involved in metabolic resistance, further investigations are needed to identify the associated enzymes and their mechanisms in the metabolic-based resistance.
When genomic regions are subjected to strong and recent selection pressure, the adjacent sequences extending outward from the site of selection with reduced diversity provide evidence of a selective sweep [41, 46]. Our study showed the diversity of the kdr gene was reduced with increased resistance under deltamethrin selection by cloning and sequencing the intron region downstream of L1014. These results confirmed that kdr led to the deltamethrin resistance in this population. In our study, a higher association of the L1014F mutation than the L1014S mutation was found with deltamethrin resistance in the Cx. pipiens pallens population. A similar conclusion was made in our previous research [22]. In the current study, we only detected the region around L1014 in the kdr gene, and whether other kdr mutation alleles were related to pyrethroid resistance in this Cx. pipiens pallens population was unclear.
Current chemical control programs are largely dependent on pyrethroids, and their efficacy is now threatened by the rise of resistance in target populations. Therefore, formulating a new insecticide use strategy to delay the development and/or spread of pyrethroid resistance is a priority. Based on results of our study, we propose some considerations for insecticide use. It is a must to avoid long-term exposure of mosquito populations to a constant low concentration of insecticide, because it can lead to significantly increased resistance in populations. In this study, when we selected the population (MS strain) with a constant concentration of deltamethrin (0,05ppm) for 24 generations, the level of resistance grew exponentially and the RR was increased more than eight times. This result suggested that the low concentration of insecticide in environment may affect the vector control, such as insecticides used in agriculture. So reducing pesticide residues and accelerating degradation in the environment may delay the development of resistance. Pesticide use should suit to local conditions, because the kdr and metabolic resistance had different roles under different selection pressures. Our study found that metabolic resistance mainly played a main role under high insecticide selection pressure. So the combination of insecticide and synergists of metabolic enzymes could delay the increase of insecticide in these areas with high resistance. For example the synergist PBO (piperonyl butoxide), a known inhibitor of P450s and esterase activity, has a significant role in vector control and is encouraged by WHO [47]. The NS strain in our study showed a reduction of RR by ~2-fold after 24 generations, but it was still higher than that at the first generation. This indicated that the high frequency of resistance alleles in a population might result in the slow recovery of sensitivity. The kdr alleles were fixed by generation 14 in the NS strain but the RR was still falling. Combined with the results of the steady decline in P450s and CCEs activities (Fig 5), the changes in RR might be caused by reductions in metabolic enzyme activities. However, the RR was still higher than the first generation, it might indicate that fitness costs of metabolic resistance were low. It needs further investigation. Whether in IS or MS, the level of resistance grew exponentially after long term exposure to pesticides. It is a must to rotate the insecticides promptly before the occurrence of high resistance. The insecticides adopted for rotations should have different modes of action to avoid cross-resistance. We demonstrated that the kdr gene and metabolic enzymes played different roles in the development of pyrethroid resistance. Hence, the target sites of insecticides and the resistance induced by metabolic enzymes should both be taken into account in rotations. In addition, as the pattern of decline in resistance can be slow, insecticide withdrawal must be maintained for an extended period of time. A study by Raghavendra et al. showed that persistence of resistance to DDT and malathion was respectively observed 30 yr and 9 yr after withdrawal from IRS, although reversal of deltamethrin resistance was observed relatively rapidly within 2–3 yr after its withdrawal from IRS [48]. Conclusions from our study may provide a reference for future management of insecticide resistance in mosquitoes and other arthropod pests and disease vectors.
Supporting Information
Dynamic changes of kdr allele frequencies are shown for (A) IS strain, (B) MS strain, and (C) NS strain. Ⅰ, Ⅱ and Ⅲ represent the three replicate groups.
(PDF)
(A) Dynamic changes of P450 activities at each generation in IS, MS and NS strains. (B) Dynamic changes of CCE activities at each generation in IS, MS and NS strains. (C) Dynamic changes of GSTs activities at each generation in IS, MS and NS strains. Ⅰ, Ⅱ and Ⅲ represent the three replicate groups.
(PDF)
(DOC)
*Resistance ratio is the ratio of LC50 of the test generation to LC50 of the S-LAB. S-LAB: laboratory deltamethrin-susceptible strain of Cx. pipiens pallens.
(DOC)
Acknowledgments
We thank Zhikuan Jiang, Maoqing Gong, Haifang Wang and Peng Cheng for their assistance with mosquito collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The sources of funding include: the National Institutes of Health of US (NIH)(Grant No. 2R01AI075746), the National Natural Science Foundation of China (Grant No. 81171610, 81101279 and 81301458), the National S & T Major Program (Grant No.2012ZX10004-219 and 2012ZX10004-220), Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No.20113234120007), and Natural Science Foundation of Jiangsu Province (Grant No.81101279). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhang H, Meng F, Qiao C, Cui F. Identification of resistant carboxylesterase alleles in Culex pipiens complex via PCR-RFLP. Parasites & Vectors. 2012;5(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soni N, Prakash S. Green Nanoparticles for Mosquito Control. The Scientific World Journal. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones CM, Machin C, Mohammed K, Majambere S, Ali AS, Khatib BO, et al. Insecticide resistance in Culex quinquefasciatus from Zanzibar: implications for vector control programmes. Parasit Vectors. 2012;5:78 10.1186/1756-3305-5-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keating J, Yukich JO, Mollenkopf S, Tediosi F. Lymphatic filariasis and onchocerciasis prevention, treatment, and control costs across diverse settings: a systematic review. Acta tropica. 2014;135:86–95. 10.1016/j.actatropica.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 5. Lambrechts L, Knox TB, Wong J, Liebman KA, Albright RG, Stoddard ST. Shifting priorities in vector biology to improve control of vector-borne disease. Tropical Medicine & International Health. 2009;14(12):1505–14. [DOI] [PubMed] [Google Scholar]
- 6. Brogdon WG, McAllister JC. Insecticide resistance and vector control. Emerging infectious diseases. 1998;4(4):605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nkya TE, Akhouayri I, Kisinza W, David J-P. Impact of environment on mosquito response to pyrethroid insecticides: Facts, evidences and prospects. Insect biochemistry and molecular biology. 2012;43(4):407–16. 10.1016/j.ibmb.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 8. Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect biochemistry and molecular biology. 2004;34(7):653–65. [DOI] [PubMed] [Google Scholar]
- 9. David J-P, Ismail HM, Chandor-Proust A, Paine MJI. Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecticides on Earth. Philosophical Transactions of the Royal Society B: Biological Sciences. 2013;368(1612):20120429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lumjuan N, Rajatileka S, Changsom D, Wicheer J, Leelapat P, Prapanthadara L-a, et al. The role of the Aedes aegypti Epsilon glutathione transferases in conferring resistance to DDT and pyrethroid insecticides. Insect biochemistry and molecular biology. 2011;41(3):203–9. 10.1016/j.ibmb.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 11. Somwang P, Yanola J, Suwan W, Walton C, Lumjuan N, Prapanthadara L-a, et al. Enzymes-based resistant mechanism in pyrethroid resistant and susceptible Aedes aegypti strains from northern Thailand. Parasitology research. 2011;109(3):531–7. 10.1007/s00436-011-2280-0 [DOI] [PubMed] [Google Scholar]
- 12. Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annual review of entomology. 2000;45:371–91. [DOI] [PubMed] [Google Scholar]
- 13. Rinkevich FD, Du Y, Dong K. Diversity and Convergence of Sodium Channel Mutations Involved in Resistance to Pyrethroids. Pesticide Biochemistry and Physiology. 2013;106(3):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enayati A, Vatandoost H, Ladonni H, Townson H, Hemingway J. Molecular evidence for a kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi . Medical and Veterinary Entomology. 2003;17(2):138–44. [DOI] [PubMed] [Google Scholar]
- 15. Martinez‐Torres D, Chandre F, Williamson M, Darriet F, Berge JB, Devonshire AL, et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect molecular biology. 1998;7(2):179–84. [DOI] [PubMed] [Google Scholar]
- 16. Smith TJ, Hyeock Lee S, Ingles PJ, Knipple DC, M Soderlund D. The L1014F point mutation in the house fly Vssc1 sodium channel confers knockdown resistance to pyrethroids. Insect biochemistry and molecular biology. 1997;27(10):807–12. [DOI] [PubMed] [Google Scholar]
- 17. Burton MJ, Mellor IR, Duce IR, Davies T, Field LM, Williamson MS. Differential resistance of insect sodium channels with kdr mutations to deltamethrin, permethrin and DDT. Insect biochemistry and molecular biology. 2011;41(9):723–32. 10.1016/j.ibmb.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 18. Nwane P, Etang J, Chouaїbou M, Toto JC, Koffi A, Mimpfoundi R, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae s.l. populations from Cameroon, Central Africa. Parasites & Vectors. 2013;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corbel V, N’guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta tropica. 2007;101(3):207–16. [DOI] [PubMed] [Google Scholar]
- 20. Komagata O, Kasai S, Tomita T. Overexpression of cytochrome P450 genes in pyrethroid-resistant Culex quinquefasciatus . Insect biochemistry and molecular biology. 2010;40(2):146–52. 10.1016/j.ibmb.2010.01.006 [DOI] [PubMed] [Google Scholar]
- 21. Cui F, Raymond M, Qiao CL. Insecticide resistance in vector mosquitoes in China. Pest management science. 2006;62(11):1013–22. [DOI] [PubMed] [Google Scholar]
- 22. Chen L, Zhong D, Zhang D, Shi L, Zhou G, Gong M, et al. Molecular ecology of pyrethroid knockdown resistance in Culex pipiens pallens mosquitoes. PloS one. 2010;5(7):e11681 10.1371/journal.pone.0011681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raymond M, Rousset F. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. Journal of heredity. 1995;86(3):248–9. [Google Scholar]
- 24. Zhong D, Chang X, Zhou G, He Z, Fu F, Yan Z, et al. Relationship between Knockdown Resistance, Metabolic Detoxification and Organismal Resistance to Pyrethroids in Anopheles sinensis . PloS one. 2013;8(2):e55475 10.1371/journal.pone.0055475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 26. Finney D. Statisical logic in the monitoring of reactions to therapeutic drugs. Methods Inf Med. 1971;10(4):237–45. [PubMed] [Google Scholar]
- 27. Abbott F. Lights That Are Hidden. Cal West Med. 1925;23(6):758. [PMC free article] [PubMed] [Google Scholar]
- 28. Puizina J, Fredotović Ž, Šamanić I, Šušnjara T, Kekez L, Cukrov D, et al. Phylogeography of the land snail Eobania vermiculata (OF Müller, 1774)(Gastropoda: Pulmonata) along the Croatian coast and islands. Journal of Entomology and Zoology Studies. 2013;1(4):23–31. [Google Scholar]
- 29. Depaulis F, Veuille M. Neutrality tests based on the distribution of haplotypes under an infinite-site model. Molecular biology and evolution. 1998;15(12):1788–90. [DOI] [PubMed] [Google Scholar]
- 30. Rose CJ, Chapman JR, Marshall SD, Lee SF, Batterham P, Ross HA, et al. Selective sweeps at the organophosphorus insecticide resistance locus, Rop-1, have affected variation across and beyond the α-esterase gene cluster in the Australian sheep blowfly, Lucilia cuprina . Molecular biology and evolution. 2011;28(6):1835–46. 10.1093/molbev/msr006 [DOI] [PubMed] [Google Scholar]
- 31. Pocquet N, Milesi P, Makoundou P, Unal S, Zumbo B, Atyame C, et al. Multiple insecticide resistances in the disease vector Culex p. quinquefasciatus from Western Indian Ocean. PloS one. 2013;8(10):e77855 10.1371/journal.pone.0077855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Namountougou M SF, Baldet T, Diabate A, Ouedraogo JB, et al. Multiple Insecticide Resistance in Anopheles gambiae s.l. Populations from Burkina Faso, West Africa. PLoS ONE. 2012;7(11):e48412 10.1371/journal.pone.0048412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xuelian Chang DZ, Qiang Fang, Joshua Hartsel, Guofa Zhou, Linna Shi, Fujin Fang CZ, Guiyun Yan. Multiple Resistances and Complex Mechanisms of Anopheles sinensis Mosquito: A Major Obstacle to Mosquito-Borne Diseases Control and Elimination in China. PLoS Negl Trop Dis. 2014;8(5):e2889 10.1371/journal.pntd.0002889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riveron JM, Irving H, Ndula M, Barnes KG, Ibrahim SS, Paine MJ, et al. Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus . Proceedings of the National Academy of Sciences of the United States of America. 2013;110(1):252–7. 10.1073/pnas.1216705110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ochomo E, Bayoh M, Brogdon W, Gimnig J, Ouma C, Vulule J, et al. Pyrethroid resistance in Anopheles gambiae s.s. and Anopheles arabiensis in western Kenya: phenotypic, metabolic and target site characterizations of three populations. Medical and Veterinary Entomology. 2012;27(2):156–64. 10.1111/j.1365-2915.2012.01039.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taylor M, Feyereisen R. Molecular biology and evolution of resistance of toxicants. Molecular biology and evolution. 1996;13(6):719–34. [DOI] [PubMed] [Google Scholar]
- 37. Labbé P, Sidos N, Raymond M, Lenormand T. Resistance gene replacement in the mosquito Culex pipiens: fitness estimation from long-term cline series. Genetics. 2009;182(1):303–12. 10.1534/genetics.109.101444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bergé J, Feyereisen R, Amichot M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1998;353(1376):1701–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wondji CS, Irving H, Morgan J, Lobo NF, Collins FH, Hunt RH, et al. Two duplicated P450 genes are associated with pyrethroid resistance in Anopheles funestus, a major malaria vector. Genome research. 2009;19(3):452–9. 10.1101/gr.087916.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Djouaka R, Bakare A, Coulibaly O, Akogbeto M, Ranson H, Hemingway J, et al. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC genomics. 2008;9:538 10.1186/1471-2164-9-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilding CS, Smith I, Lynd A, Yawson AE, Weetman D, Paine MJ, et al. A cis-regulatory sequence driving metabolic insecticide resistance in mosquitoes: Functional characterisation and signatures of selection. Insect Biochem Mol Biol. 2012;42(9):699–707. 10.1016/j.ibmb.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 42. Vontas JG, Small GJ, Hemingway J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochemical Journal. 2001;357(Pt 1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kostaropoulos I, Papadopoulos AI, Metaxakis A, Boukouvala E, Papadopoulou-Mourkidou E. Glutathione S–transferase in the defence against pyrethroids in insects. Insect biochemistry and molecular biology. 2001;31(4–5):313–9. [DOI] [PubMed] [Google Scholar]
- 44. Hunt R, Brooke B, Pillay C, Koekemoer L, Coetzee M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus . Med Vet Entomol. 2005;19(3):271–5. [DOI] [PubMed] [Google Scholar]
- 45. Nardini L CR, Coetzer N, Koekemoer LL. DDT and pyrethroid resistance in Anopheles arabiensis from South Africa. Parasit Vectors. 2013;6(1):229 10.1186/1756-3305-6-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saminadin-Peter SS, Kemkemer C, Pavlidis P, Parsch J. Selective sweep of a cis-regulatory sequence in a non-African population of Drosophila melanogaster . Molecular biology and evolution. 2012;29(4):1167–74. 10.1093/molbev/msr284 [DOI] [PubMed] [Google Scholar]
- 47. WHO position statement on integrated vector management to control malaria and lymphatic filariasis. Wkly Epidemiol Rec. 2011;86(13):121–7. http://www.who.int/wer. [PubMed] [Google Scholar]
- 48. Raghavendra K, Verma V, Srivastava H, Gunasekaran K, Sreehari U, Dash A. Persistence of DDT, malathion & deltamethrin resistance in Anopheles culicifacies after their sequential withdrawal from indoor residual spraying in Surat district, India. The Indian journal of medical research. 2010;132:260–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dynamic changes of kdr allele frequencies are shown for (A) IS strain, (B) MS strain, and (C) NS strain. Ⅰ, Ⅱ and Ⅲ represent the three replicate groups.
(PDF)
(A) Dynamic changes of P450 activities at each generation in IS, MS and NS strains. (B) Dynamic changes of CCE activities at each generation in IS, MS and NS strains. (C) Dynamic changes of GSTs activities at each generation in IS, MS and NS strains. Ⅰ, Ⅱ and Ⅲ represent the three replicate groups.
(PDF)
(DOC)
*Resistance ratio is the ratio of LC50 of the test generation to LC50 of the S-LAB. S-LAB: laboratory deltamethrin-susceptible strain of Cx. pipiens pallens.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.