Abstract
Pathogenic members of the genus Leptospira are the causative agents of leptospirosis, a neglected disease of public and veterinary health concern. Leptospirosis is a systemic disease that in its severest forms leads to renal insufficiency, hepatic dysfunction, and pulmonary failure. Many strains of Leptospira produce hemolytic and sphingomyelinase activities, and a number of candidate leptospiral hemolysins have been identified based on sequence similarity to well-characterized bacterial hemolysins. Five of the putative hemolysins are sphingomyelinase paralogs. Although recombinant forms of the sphingomyelinase Sph2 and other hemolysins lyse erythrocytes, none have been demonstrated to contribute to the hemolytic activity secreted by leptospiral cells. In this study, we examined the regulation of sph2 and its relationship to hemolytic and sphingomyelinase activities produced by several L. interrogans strains cultivated under the osmotic conditions found in the mammalian host. The sph2 gene was poorly expressed when the Fiocruz L1-130 (serovar Copenhageni), 56601 (sv. Lai), and L495 (sv. Manilae) strains were cultivated in the standard culture medium EMJH. Raising EMJH osmolarity to physiological levels with sodium chloride enhanced Sph2 production in all three strains. In addition, the Pomona subtype kennewicki strain LC82-25 produced substantially greater amounts of Sph2 during standard EMJH growth than the other strains, and sph2 expression increased further by addition of salt. When 10% rat serum was present in EMJH along with the sodium chloride supplement, Sph2 production increased further in all strains. Osmotic regulation and differences in basal Sph2 production in the Manilae L495 and Pomona strains correlated with the levels of secreted hemolysin and sphingomyelinase activities. Finally, a transposon insertion in sph2 dramatically reduced hemolytic and sphingomyelinase activities during incubation of L. interrogans at physiologic osmolarity. Complementation of the mutation with the sph2 gene partially restored production of hemolytic and sphingomyelinase activities. These results indicate that the sph2 gene product contributes to the hemolytic and sphingomyelinase activities secreted by L. interrogans and most likely dominates those functions under the culture condition tested.
Author Summary
The spirochete Leptospira causes leptospirosis, a potentially deadly disease of humans and animals. Candidate factors that promote infection include hemolysins encoded by several leptospiral genes. Hemolysins rupture red blood cells in vitro. Some hemolysins are sphingomyelinases, which target sphingomyelin in the host cell membrane. Hemolysins have the potential to disrupt organ function during infection. It is not known which hemolysins and sphingomyelinases are responsible for the hemolytic and sphingomyelinase activities secreted by L. interrogans. We found that the production of hemolytic activity is regulated and is tied to expression of sph2, which encodes a hemolysin with sphingomyelinase, cytotoxic, and fibronectin-binding activities. Hemolytic and sphingomyelinase activities and sph2 expression were higher when the osmolarity of the culture medium was raised to the level found in the mammalian host. Similarly, sph2 expression was substantially higher in an L. interrogans strain that secreted large amounts of hemolytic and sphingomyelinase activities than in a strain that generated negligible amounts. Most importantly, disruption of the sph2 gene eliminated hemolysin production and yielded substantially less sphingomyelinase than the wild-type strain. Our findings indicate that sph2 is a major contributor to the hemolytic and sphingomyelinase activities secreted by L. interrogans and that the hemolytic and sphingomyelinase activities measured in standard L. interrogans cultures may underestimate the levels produced during infection.
Introduction
Leptospirosis is a neglected zoonotic disease that afflicts humans and animals [1–3]. Although the disease occurs worldwide, it is observed most frequently in tropical countries, where conditions for environmental survival and transmission are most favorable [4]. The causative organisms are spirochetes belonging to the genus Leptospira, which comprise pathogenic and nonpathogenic species. The bacteria are able to enter the mammalian host through skin abrasions and mucous membranes. Following entry, the spirochetes disseminate via the bloodstream to many organs [2]. Patients exhibit a wide range of signs and symptoms from undifferentiated fever to liver dysfunction, renal insufficiency, hemolytic anemia, bleeding, and respiratory failure [5]. Proposed mechanisms of pathogenesis include vascular damage, which is frequently manifested as hemorrhage in the lungs and other organs on post-mortem examination [6]. Additionally, reproductive failure is observed in livestock animals with leptospirosis [7]. Leptospira bacteria are spread and maintained in the environment by rats and other reservoir hosts, whose renal tubules are persistently colonized by the spirochete. Humans and animals can be infected by direct or indirect contact with contaminated urine or other tissue.
Many Leptospira strains secrete hemolysins and sphingomyelinases during in vitro growth [8–11]. Strains of serovar Pomona secrete the highest levels of hemolytic activity [9] and cause hemolytic anemia in ruminants [12–14]. The single peak of hemolytic activity detected by isoelectric focusing of the culture supernatant fluid of one Pomona strain co-purified with sphingomyelinase C activity, suggesting that hemolysis is due to sphingomyelinase [15]. It is not known which gene or genes encode the secreted hemolytic and sphingomyelinase activities.
Hemolytic activity has been reported for purified recombinant forms of the L. interrogans protein HlyX, HlpA, TlyA, and the sphingomyelinase-like paralogs Sph1, Sph2, Sph3, and SphH [16–19]. Among the sphingomyelinase paralogs, sphingomyelinase C activity has been demonstrated only for Sph2, which cleaves sphingomyelin to ceramide and phosphocholine [20]. Sphingomyelinase activity has also been reported for Sph1, Sph3, and Sph4 [21], although these proteins lack some of the catalytic amino acid residues required for enzymatic activity [22]. The genes encoding the sphingomyelinase-like proteins are missing from the nonpathogen Leptospira biflexa. Similarly, sphingomyelinase activity has been detected only in pathogenic strains of Leptospira [10]. These observations suggest that sphingomyelinase-like proteins function during infection [23].
Hemolysins are assumed to assist microbial pathogens in acquiring iron by lysing host erythrocytes during infection [24]. Heme can be used by L. interrogans as an iron source for growth [25]. They express the hemin-binding protein HbpA, which may participate in transporting heme into the cell, and a heme oxygenase, which is required for heme utilization and for virulence in the hamster model of leptospirosis [26–28]. Iron depletion resulted in increased levels of a sphingomyelinase-like protein, which is consistent with the notion that hemolysins are involved in iron acquisition by L. interrogans [29]. Nevertheless, experimental evidence indicates that leptospiral hemolysins are capable of pathogenic processes that do not involve red blood cells. For example, recombinant Sph2 protein is cytotoxic towards equine endothelial cells, mouse lymphocytes and macrophages, and a human liver cell line [18, 30]. Additionally, L. biflexa ectopically expressing sph2 captures plasma fibronectin in vitro [31].
Sph2 has not been detected in appreciable amounts in cell lysates of L. interrogans grown in the standard culture medium EMJH except in strains of serovar Pomona [32]. However, sph2 expression in the Fiocruz L1-130 strain is dramatically upregulated by environmental conditions that occur during infection. In our previous whole-genome microarray study examining the changes in transcript levels to an increase in osmolarity from low osmolarity found in EMJH medium to physiologic levels found in the mammalian host, sph2 was the second most strongly upregulated gene in the Fiocruz L1-130 strain [33]. Levels of cellular and extracellular Sph2 in the Fiocruz L1-130 strain were also upregulated upon addition of sodium chloride or sucrose to physiological levels of osmolarity [34]. Anti-Sph2 antibody is detected in patients with leptospirosis, suggesting that sph2 is expressed during infection [32]. In a recent RNA-seq experiment, sph2 transcript levels were higher in L. interrogans bacteria growing in dialysis membrane chambers in rats than in those growing in in vitro culture [35]. These observations suggest that upregulation of sph2 is involved in L. interrogans infection.
The enzymatic domain of Sph2 is flanked by unusual sequences missing from bacterial sphingomyelinases whose crystal structures have been determined [20]. Three or four ~20 residue imperfect tandem repeats are present to the amino terminus of Sph2, and the carboxy terminus comprises a unique segment of ~186 amino acid residues [18, 22]. The enzymatic domain may not be sufficient for hemolytic activity of Sph2. A sphingomyelinase with a similar C-terminal extension is found in the sphingomyelinase protein of a fish isolate of Pseudomonas species [36]. Removal of the C-terminal domain eliminates its hemolytic activity despite its enzymatic domain being left intact [36]. L. interrogans releases Sph2 in smaller forms whose apparent molecular masses are ~13 and ~21 kDa less than that of the cell-associated form of Sph2 [34]. These observations raise the question of whether L. interrogans secretes Sph2 in an active form because the hemolytic activity of Sph2 was demonstrated with full-length recombinant protein instead of the shortened forms detected in culture supernatant fluids [16, 18, 34]. One approach to establish that Sph2 contributes to the secreted hemolytic activity is to demonstrate inhibition of hemolysis when Sph2 antibody is added to the spent culture medium. However, both anti-Sph2 antiserum and normal serum inhibited the hemolytic activity secreted by a Pomona strain of L. interrogans [32].
In this study, we extend our earlier findings of osmotic induction of sph2 expression with the Fiocruz L1-130 strain to show that the sph2 genes of four additional strains of L. interrogans are also regulated by sodium chloride, including a Pomona strain that displays high levels of basal sph2 expression. In addition, we demonstrate that the levels of secreted hemolytic and sphingomyelinase activities are regulated by osmolarity. In our attempts to maximize sph2 expression, we also found that rat serum increased Sph2 production further at physiologic osmolarity, although the presence of serum inhibited hemolytic activity in liquid-phase assays. Finally, we present genetic evidence that sph2 contributes to the hemolytic and sphingomyelinase activities secreted from L. interrogans.
Materials and Methods
Bacterial strains and culture conditions
The leptospiral strains Leptospira interrogans serovars Manilae (strain L495), Pomona subtype kennewicki (strain LC82-25), and Lai (strains 56601 and L391) were grown at 30°C in EMJH medium assembled with Probumin BSA (Vaccine Grade, lot 103) as described previously [37] or purchased as Probumin Vaccine Grade Solution (lot 103) from EMD Millipore (Temecula, California, USA). The Pomona LC82-25 and Lai 56601 strains were obtained from Rich Zuerner (National Animal Disease Center, Ames, Iowa, USA) and Mathieu Picardeau (Institut Pasteur, Paris, France), respectively. Leptospira interrogans serovar Copenhageni strain Fiocruz L1-130 was grown at 30°C in EMJH medium supplemented with 1% heat-inactivated rabbit serum (Rockland; Gilbertsville, Pennsylvania, USA). The L391 strain and an L391 mutant with a kan r-marked Himar1 transposon inserted in the sph2 gene were provided by Gerald Murray [38]. The sph2 mutant was maintained in EMJH containing 40 μg/mL kanamycin. Culture densities were determined by directly counting the number of Leptospira using an AxioLab A1 microscope with a darkfield condenser (Zeiss Microscopy Division; Pleasanton, California, USA). To examine the influence of environmental conditions on sph2 expression, L. interrogans cells were grown to a cell density of 0.6–1 x 108 cells/mL and then supplemented with 120 mM NaCl, 10% rat serum (Rockland Immunochemicals, Limerick, Pennsylvania, USA), or a mixture of nine parts of EMJH supplemented with 120 mM NaCl and one part of rat serum and allowed to incubate for 4 h. Cultures were harvested by centrifugation at 9,200 x g for 20 min at 4°C in a Sorvall high-speed centrifuge (Thermo Scientific; Marietta, Ohio, USA), and the spent medium was collected for storage. Cells were washed once with cold PBS-5 mM MgCl2. Sodium chloride was added to the spent medium obtained from the EMJH cultures to equalize the osmolarity across all samples. Cell pellets and spent growth medium were stored in -80°C.
Immunoblot analysis
For Western blots, the proteins were fractionated on 10% PAGEr Gold precast Tris-Glycine gels (Lonza; USA). Dual Color Precision Plus Protein Standards from Bio-Rad (catalog # 161–0374) (Hercules, California, USA) were included in adjacent lanes. Proteins were transferred from gels on to PVDF Immobilon-P transfer membrane (EMD Millipore; USA) using Trans-blot SD Semi-Dry transfer Cell system (Bio-Rad) (10V for 45 min). The membranes were incubated in blocking solution (5% skim milk in PBS-0.05% Tween 20 [PBS-T]) for 30 min and then incubated with rabbit anti-Sph2 and anti-LipL41 antisera [34, 39] at dilutions of 1:1,000 and 1:10,000, respectively, for 30 min and washed three times (5 min each) with PBS-T. The membrane was then incubated with donkey anti-rabbit antibody (1:5,000) (Amersham Biosciences; Piscataway, New Jersey, USA) or protein A-horseradish peroxidase conjugate (1:3,000) (Amersham Biosciences) in blocking solution for 30 min and again washed three times with PBS-T. The membranes were developed with the ECL Western blot detection system (Thermo Scientific), and the bands were visualized with Hyperfilm (Amersham Biosciences).
Immunoprecipitation of Sph2 from the spent culture medium was performed as described [37], except the culture supernatant fluid was preadsorbed with 25 μL of EZview Red Protein A Affinity gel (Sigma-Aldrich; St. Louis, Missouri, USA) for 60 min at 4°C prior to immunoprecipitation with anti-Sph2 antiserum [32]. The resuspension volumes of the immunoprecipitates with Laemmli sample buffer were adjusted according to the cell densities of the cultures.
Plasmid DNA
Restriction enzymes and T4 DNA ligase were obtained from New England Biolabs (Beverly, Massachusetts, USA). PCR amplifications were conducted with Phusion DNA polymerase (Thermo Scientific). L. interrogans sequences cloned into plasmid vectors were verified by Sanger sequencing (Laragen; Culver City, California, USA).
The sph1, sph3, and sph4 sequences were amplified by PCR with the primer pairs lic12632(Nd)-3F and lic12632(Xh)-4R, lic13198(Nd)-3F and lic13198(Xh)-4R, and lic11040(Nd)-3F and lic11040(Xh)-4R, respectively (Table 1). Forward and reverse primers included NdeI and XhoI restriction sites near the 5' ends. Genomic DNA from L. interrogans Fiocruz L1-130 served as template. Each amplicon was digested with NdeI and XhoI and ligated to pET20b+ (EMD Biosciences; La Jolla, California USA) that had been digested with the same enzymes. The sph1, sph3, and sph4 expression plasmids were named pRAT547, pRAT548, and pRAT549, respectively. The sph2 expression plasmid pTOPO-Sph2(27–623) was described previously [34].
Table 1. List of oligonucleotides for PCR.
| Gene | Primer name* | Sequence (5’to 3’) # | Role |
|---|---|---|---|
| sph1 | lic12632(Nd)-3F | CTTACAcatatgAAACCTGGCAAACAAAATTCCATAAATC | P |
| lic12632(Xh)-4R | GCActcgagATGATGATAGATTAAATCTTTACTCCA | ||
| sph2 | lic12631(Kp)-17F | GACCATggtaccAGCGAGACGCTGAGTCTGA | P |
| lic12631(Xh)-18R | GTCCATctcgagGGTATTTTATTGAATAAGATTAGGAAAGT | ||
| sph3 | lic13198(Nd)-3F | AATTGTcatatgAAAGATTCATTAGTATACAATAAATTTTTA | P |
| lic13198(Xh)-4R | GCActcgagACGATAAATTAGATCCTTGCTCCAATCT | ||
| sph4 | lic11040(Nd)-3F | CTATCTcatatgGATTGTTTACCTGATTCACAGTCTTCTG | P |
| lic11040(Xh)-4R | CCTctcgagTTCCTCAGGGCCTTCATTCAATTGAACA | ||
| sph1 | lic12632-7F | TGAAACCGTTTTGATTACGGGTGA | Q |
| lic12632-8R | CGGCAAGTGCGTTTGTTTTTGTAT | ||
| sph2 | lic12631-11F | ATGTTGCCCACAAATCTTCCACG | Q |
| lic12631-12R | CCACATCTGTTTGATACGGATATTCTTCT | ||
| lipL41 | lipL41-7F | TCGGAAATCTGATTGGAGCGGAAGCA | Q |
| lipL41-8R | AGAAGCGGCGAAACCTGCCACT |
* F, forward primer; R, reverse primer
# Restriction sites in lower case
P, plasmid construction; Q, quantitative RT-PCR
To construct the sph2 complementation plasmid, the oligonucleotides lic12631(Kp)-17F and lic12631(Xh)-18R (Table 1) were used to PCR amplify sph2 along with its flanking regions using Fiocruz L1-130 genomic DNA as template. The amplicon included sequences 327 bp upstream and 130 bp downstream of the start and stop codons, respectively. Restriction sites for KpnI and XhoI were included near the 5' ends of the oligonucleotides. Following digestion of the amplicon with KpnI and XhoI, the sph2-containing fragment was inserted into the similarly-digested plasmid pRAT575 [40] to create pRAT613 for the current and future studies. The KpnI-XhoI fragment containing sph2 was subsequently transferred to the mobilizable plasmid pAL614, which contains KpnI and XhoI sites between the ends of a Himar1 element that also harbored a gene encoding resistance to spectinomycin [41]. The resulting sph2 plasmid was designated pRAT708.
Recombinant protein expression and purification
Frozen competent E. coli BLR(DE3)/pLysS (EMD Biosciences) was transformed with the plasmids pRAT547 (sph1), pRAT548 (sph3), pRAT549 (sph4), and pTOPO-Sph2(27–623) (sph2), and transformants were selected on LB plates containing 100 μg/mL carbenicillin. Colonies were inoculated into 10 mL LB containing carbenicillin for overnight growth at 37°C. 5 mL of each overnight culture was then placed into 200 mL LB with carbenicillin and incubated at 37°C. When the OD at 600 nm reached 0.6, 1 M IPTG was added to a final concentration of 0.5 mM. For expression of recombinant Sph1, Sph3, and Sph4, the cultures were incubated for 2 hrs at 37°C. The bacteria were collected by centrifugation, and the cell pellets were stored at -80°C. For expression of recombinant Sph2, the culture was incubated overnight at 16°C and harvested by centrifugation. To lyse the bacteria, cell pellets were suspended in 5 mL (for Sph1, Sph3, and Sph4) or 10 mL (Sph2) of BugBuster (EMD Biosciences) containing 20 unit/mL DNase I (Thermo Scientific) and 0.25 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich), and the suspension was swirled for 20 min at room temperature. The lysates were poured into Corex tubes and subjected to centrifugation at 15,000 x g for 20 min at 4°C in a Sorvall RC-5B Superspeed centrifuge.
To wash the Sph1, Sph3, and Sph4 inclusion bodies, the pellets were suspended in 10 mL of a 10-fold dilution of BugBuster. Following centrifugation at 15,000 x g for 20 min at 4°C, the pellets were suspended in 5 mL of 100 mM NaH2PO4, 10 mM Tris-HCl, and 8M urea, pH 8.0 (Buffer B) and swirled for 15 min at room temperature to solubilize the inclusion bodies. The material was centrifuged at 10,000 x g at room temperature to pellet the cell debris. 1 mL of 50% Ni2+-NTA slurry (Qiagen) was added to the solubilized inclusion bodies, and the suspension was mixed for 60 min at room temperature. The material was then poured into a 5-mL polypropylene column (Qiagen) for purification of the recombinant protein. The column was washed with 4 mL Buffer B that was adjusted to pH 6.3 and then with 2 mL Buffer B at pH 5.9. The recombinant protein was eluted with Buffer B adjusted to pH 4.5, and 0.5 mL fractions were collected. The fractions were neutralized by adding 55.5 μl of 1 M Tris-HCl, pH 8.0, to each.
To purify soluble Sph2, 250 μl of 0.5 M imidazole and 2 mL of the 50% Ni2+-NTA slurry (Qiagen) were added to 10 mL of lysate. The mixture was mixed for 60 min at 4°C and then poured into a 5 mL polypropylene column. After collecting the flow through, the column was washed at 4°C with a total of 30 mL of 20 mM imidazole in Wash Buffer (50 mM NaH2PO4, 0.5 M NaCl, pH 8.0). The protein was eluted at 4°C with 0.5 M imidazole in Wash Buffer, and 1 mL fractions were collected. Protein concentrations of all purified Sph proteins were determined using the Pierce BCA Protein Assay kit (Thermo Scientific) with BSA standards.
RNA extraction and DNase treatment
For preparation of RNA, 25 mL of culture was transferred into an Erlenmeyer flask, quickly chilled by swirling for 7 s in a dry ice-ethanol bath, and centrifuged at 9,200 x g for 20 min at 4°C. RNA was isolated from leptospires as follows: 1 mL Trizol (Life Technologies; Grand Island, New York, USA) was added to the cell pellet, resuspended thoroughly by pipetting followed by incubation at room temperature for 5 min. 200 μL of chloroform (Sigma-Aldrich) was added, mixed vigorously for 15 s followed by incubation at room temperature for 3 min. The tubes were centrifuged at 12,000 x g for 15 min at 4°C, and the upper aqueous layer was pipetted into fresh tubes. RNA was precipitated by the addition of equal volumes of isopropanol, mixed and incubated for 10 min and subjected to centrifugation at 16,000 x g for 15 min at 4°C. The pellet was washed with 1 mL of 75% ethanol, air dried and dissolved in 84 μL of RNase free water. The dissolved RNA was protected from degradation by addition of 1μL of 20 U/μL SUPERase In RNase inhibitor (Life Technologies). DNA was digested by addition of 10 μL of 10X Turbo DNase buffer and 5 μL of Turbo DNase followed by incubation in 37°C water bath for 2 h. The RNA samples were subjected to clean-up using the RNeasy Mini kit (Qiagen; USA) as described in the manufacturer’s instructions. The concentration of the RNA was determined using the NanoVue spectrophotometer (GE Healthcare; Piscataway, New Jersey, USA) and the quality of RNA was assessed by examining the A260/A280 and A260/A230 ratios and by electrophoresis of 200 ng of RNA onto a 1.2% agarose gel.
Quantitative RT-PCR
cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad) following the manufacturer’s instructions. The reaction was set up in a microcentrifuge tube in a reaction volume of 20 μL containing 4 μL of 5X iScript Reverse Transcription Supermix, 1 μg RNA, and nuclease-free water. cDNA synthesis was carried out in a Master Cycler gradient thermal cycler (Eppendorf; Hauppauge, New York, USA) with priming at 25°C for 5 min followed by reverse transcription at 42°C for 30 min and enzyme inactivation at 85°C for 5 min. A separate reaction without reverse transcriptase was included as a negative control. The synthesized cDNA was diluted with RNase free water (Ambion) to obtain a working concentration of 10 ng/μL.
The quantification of cDNA was done using real-time PCR using iQ5 SYBR Green Supermix (Bio-Rad) and the iQ5 Multicolor Real-time PCR Detection System (Bio-Rad). Each reaction contained cDNA derived from 50 ng of RNA, 10 pmol of each forward and reverse primers, and 12.5 μL of 2X iQ5 SYBR Green supermix in a total volume of 25 μL. The sph1-, sph2- and lipL41-specific primers (Table 1) were designed using Primer Premier (Premier Biosoft; Palo Alto, California, USA). The amplification protocol consisted of an initial denaturation for 15 min (95°C) followed by 40 cycles of amplification (15 s at 95°C, 30 s at 58°C, 30 s at 72°C) and a final extension of 2 min at 72°C. Standard curves were constructed by 5-fold serial dilutions (50 ng to 0.08 ng) of cDNA as template in triplicate. Amplification efficiency was evaluated from the standard curves by determining the E value; results in the range of 90% to 110% were considered to be acceptable. The CT values were normalized to that of lipL41, and expression levels were calculated using 2ΔΔC T method [42]. The relative fold change was calculated by comparison with the EMJH control. The assay was performed using three biological replicates.
Hemolytic activity
To assess hemolytic activity qualitatively, 5 μL of culture supernatant fluid was spotted onto BBL Trypticase Soy Agar with 5% sheep red blood cells (TSA II) (Becton Dickinson; Sparks, Maryland, USA). Because magnesium is necessary for sphingomyelinase activity, 100 mM MgCl2 was added to the culture supernatant to a final concentration of 10 mM prior to spotting the plates. 0.05 units of Bacillus cereus sphingomyelinase (Sigma-Aldrich) was spotted as a positive control. The plates were incubated for 20 hours at 37°C and then at 4°C for at least three days.
The liquid-phase hemolysis assay was set up in a 96 well round-bottomed microtiter plate as reported earlier [20] with some modifications. Briefly, sheep erythrocytes were procured commercially from Quad Five (Ryegate, Montana, USA) as a 50% (v/v) suspension in Alsever's's solution. Erythrocytes were collected by centrifugation at 400 x g for 10 min at 8°C, washed three times with cold PBS (pH 7.4), and resuspended in cold PBS to a final concentration of 10%. Each reaction mixture (200 μL) contained 10 mM MgCl2 in PBS, with 40 μL 10% washed sheep erythrocytes and 100 μL of culture supernatant fluid. For background measurements, EMJH with 120 mM sodium chloride replaced the spent medium. Three biological replicates were examined. The hemolysis reaction proceeded at 37°C for 90 min followed by incubation at 4°C for 30 min. The plate was centrifuged at 800 x g in an Eppendorf 5430 centrifuge to pellet intact erythrocytes, and the supernatant fluid from each well was transferred to a flat-bottom 96-well ELISA plate. The plate was read in an iMark Microplate Absorbance Reader (Bio-Rad) at 415 nm. Percent hemolysis was calculated by multiplying the PBS background-subtracted absorbance of the sample by 100 and dividing by the absorbance of the osmotically-lysed erythrocytes.
Sphingomyelinase assay
Sphingomyelinase activity was measured by a coupled assay using the Amplex Red Sphingomyelinase assay kit (Molecular Probes, Invitrogen, USA) as described in the manufacturer’s instructions. The reactions were set up in 96-well special optics flat clear bottom black polystyrene Microplate (Corning, product # 3720). The reaction mixture (200 μL) contained 100 μL test sample and 100 μL of 100 μM Amplex red reagent (containing 2 U/mL horseradish peroxidase, 0.2 U/mL choline oxidase, 8 U/mL alkaline phosphatase, and 0.5 mM sphingomyelin) (Life Technologies). The reaction proceeded for 90 min at 37°C. The fluorescence was measured at excitation and emission wavelengths of 530 nm and 590 nm respectively using the Synergy2 Multi-Mode Microplate Reader (BioTek; Winooski, Vermont, USA). The background fluorescence was corrected by subtracting the negative control, which contained the reaction buffer without sphingomyelinase. Standard curves were generated with B. cereus sphingomyelinase, and the measurements were fit by nonlinear regression to a hyperbola (one-site binding) model with GraphPad Prism, version 5.04 (GraphPad Software; La Jolla, California, USA). Experiments were performed with three biological replicates.
Complementation
For complementation of the sph2 mutant, the mobilizable sph2 plasmid pRAT708 was transformed into the diaminopimelic acid auxotroph E. coli β2163, which expresses the RP4 conjugation machinery [43]. The plasmid was transferred into the L. interrogans sph2 mutant by conjugation as described [44, 45]. Transconjugants were selected on EMJH agar plates containing 40 μg/mL kanamycin and 40 μg/mL spectinomycin. After two weeks of incubation at 30°C, colonies were inoculated into EMJH liquid medium. The insertion site of the sph2-containing transposon was identified by nested PCR using primers TnK1 and Deg1 for the first PCR reaction and primers TnkN1 and Tag for the second [45].
Statistical analysis
All values for hemolytic and sphingomyelinase activities were log transformed prior to statistical analysis to achieve similar variances across all groups. One-way ANOVA was conducted with R version 3.0.3 [46]. The Tukey post test was used for group comparisons.
Results
Regulation of sph2 expression in different strains of L. interrogans
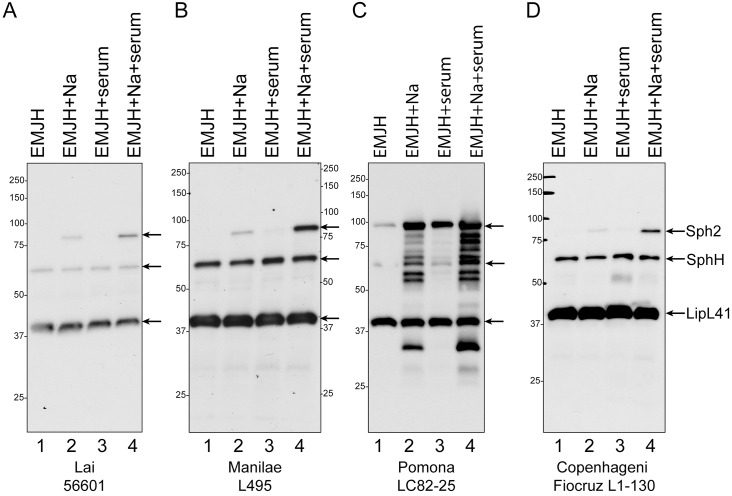
Our earlier work demonstrated that sph2 gene expression in L. interrogans strain Fiocruz L1-130 (serovar Copenhageni) increases substantially when incubated in EMJH supplemented with sodium chloride to attain physiological osmolarity [33, 34]. In the current study, we examined sph2 regulation in three additional strains of L. interrogans, L495 (serovar Manilae), 56601 (serovar Lai), and LC82-25 (serovar Pomona subtype kennewicki). The Pomona strain was included because members of the serovar produce larger amounts of hemolysin than other serovars [9]. Immunoblots were performed to determine the effects of NaCl and serum on Sph2 protein levels. As shown in Fig 1 (lane 1), Sph2 was not detected in the Fiocruz L1-130 [34], Manilae L495, or Lai 56601 strain when grown in EMJH. The addition of 120 mM sodium chloride to EMJH increased Sph2 production to detectable levels in these three strains (lane 2). On the other hand, Sph2 was detected easily in the Pomona LC82-25 strain growing in EMJH, and Sph2 levels increased further when the medium was supplemented with 120 mM sodium chloride (Fig 1C, lanes 1 and 2). The presence of rat serum to the culture medium had an effect on Sph2 expression independent of osmolarity; Sph2 levels were noticeably higher than that when only sodium chloride was present, despite the osmolarity of the culture medium being similar (Fig 1, lanes 2 and 4). Relative to LipL41 levels, Sph2 levels were substantially higher in the Pomona LC82-25 strain than in the Manilae L495 strain under all conditions (Fig 1). The apparent molecular masses of Sph2 was higher than the calculated masses of 71.0, 71.0, 70.4, and 73.5 kDa in the Copenhageni, Lai, Manilae, and Pomona strains, respectively. The apparent molecular mass of Sph2 in the LC82-25 strain was ~13 kDa higher than those of the other strains (Fig 1). As noted in our earlier study and by others, anti-Sph2 antibodies also recognized SphH in these samples (Fig 1) [32, 34].
Fig 1. Effect of salt and rat serum on cellular Sph2 production in different strains of L. interrogans.
L. interrogans serovar Lai strain 56601 (A), serovar Manilae strain L495 (B), serovar Pomona strain LC82-25 (C), and serovar Copenhageni Fiocruz L1-130 (D) were grown in EMJH (lane 1), EMJH supplemented with 120 mM sodium chloride (lane 2) or 10% rat serum (lane 3) or both sodium chloride and rat serum (lane 4) for 4 hours at 30°C. Whole-cell lysates were analyzed by immunoblotting with anti-Sph2 and anti-LipL41 antisera. Arrows identify Sph2, SphH, and LipL41. The anti-Sph2 antiserum cross-reacts with SphH. Positions of molecular mass standards are shown in kilodaltons.
Sph2 breakdown products were visible by immunoblot after growth of the serovar Pomona strain in 120 mM NaCl with and without serum (Fig 1C). To exclude the possibility that some of the bands originated from the other sphingomyelinase-like proteins, we examined the cross-reactivity of our anti-Sph2 antiserum with purified recombinant forms of Sph1, Sph3, and Sph4. Equal masses of Sph1, Sph2, Sph3, and Sph4 were subjected to SDS-polyacrylamide gel electrophoresis (Fig 2A) and immunoblot analysis with the anti-Sph2 antiserum (Fig 2B). As observed for the native form of Sph2, the recombinant form ran more slowly than expected from its calculated molecular mass, whereas Sph1, Sph3, and Sph4 migrated as expected (Fig 2A). The immunoblot shows that the anti-Sph2 antibody does not cross-react with Sph3 or Sph4 and reacts poorly with Sph1 (Fig 2B). These results indicate that the bands observed below the Sph2 species in the Pomona LC82-25 lysate are Sph2 breakdown products, although we cannot rule out the possibility that the bands running below SphH arose from SphH degradation.
Fig 2. Cross-reaction of anti-Sph2 antibody with other sphingomyelinase-like proteins.
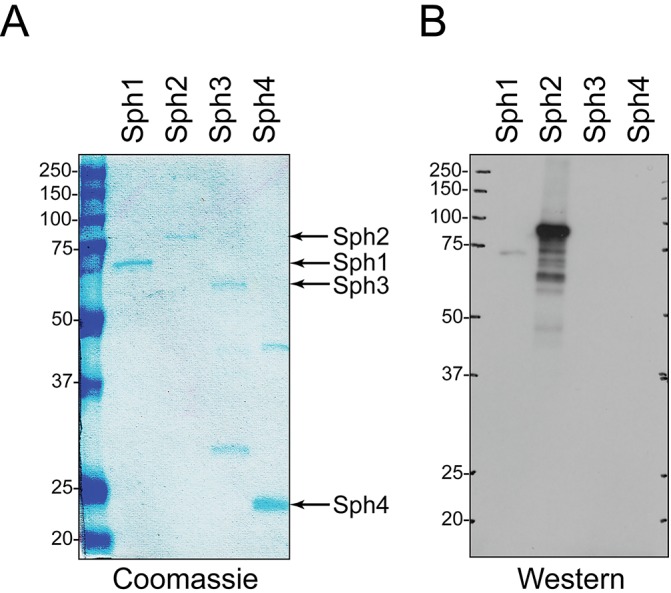
50 ng of recombinant Sph1, Sph2, Sph3, and Sph4 were subjected to SDS-polyacryamide gel electrophoresis in duplicate gels. One gel was stained with Coomassie blue (A), and the second was analyzed by Western blot with anti-Sph2 antiserum (B). Positions of molecular mass standards are shown in kilodaltons.
Immunoprecipitation of Sph2 was performed with the spent growth medium from cultures of the Manilae L495 and Pomona LC82-25 strains to assess the effects of salt and serum on the levels of extracellular Sph2. We did not detect Sph2 with the L495 strain grown in EMJH. When EMJH was supplemented with sodium chloride, with or without rat serum, Sph2 released from the L495 strain was detected in two smaller forms (Fig 3, lanes 3 and 4), as shown in our earlier study with Fiocruz L1-130 [30]. The Pomona LC82-25 strain exhibited similar regulation of extracellular Sph2 levels by sodium chloride and rat serum, except the amount of Sph2 detected was much greater than that in the L495 strain (Fig 3, lanes 2–4 vs. 5–7). The two Sph2 bands in the serovar Pomona immunoprecipitates were smaller than the species detected in the corresponding cell lysate (Fig 3, lanes 5–8). The apparent molecular masses of the extracellular forms of Sph2 were greater in the LC82-25 strain than those of the L495 strain, as was the case for the cellular form.
Fig 3. Effect of salt and serum on the amount of Sph2 released by L. interrogans.
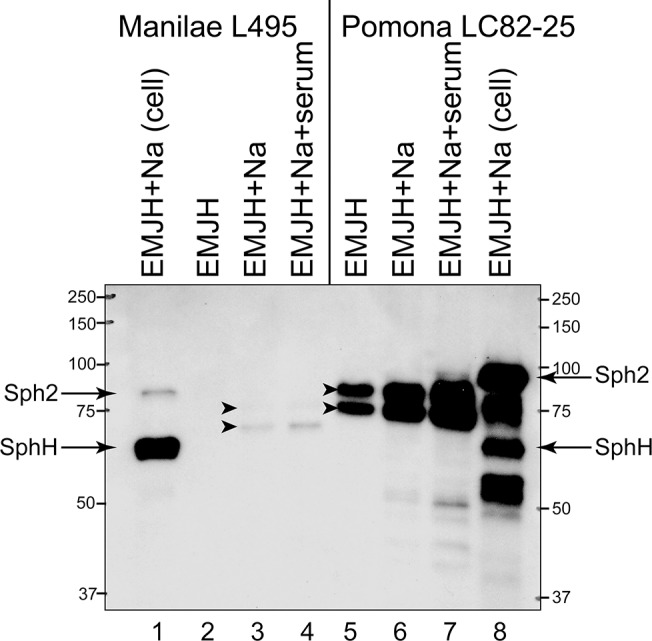
Sph2 was recovered by immunoprecipitation from spent growth medium of L. interrogans Manilae L495 (lanes 2–4) and Pomona LC82-25 (5–7) strains grown under different conditions for 4 hours at 30°C in EMJH (lanes 2 and 5), EMJH with 120 mM sodium chloride (lanes 3 and 6), and EMJH with sodium chloride and 10% rat serum (lanes 4 and 7). Spent medium was collected, and Sph2 was detected by immunoprecipitation (lanes 2–7). Cell lysates are shown for comparison (lanes 1 and 8). The Pomona cell lysate was diluted four fold before loading. Arrows indicate Sph2 and SphH observed in cell lysates. The anti-Sph2 antiserum cross-reacts with SphH. Arrowheads mark Sph2 detected in the spent medium. Positions of molecular mass standards are shown in kilodaltons.
We compared sph2 transcript levels in the serovar Pomona strain LC82-85 with those of the serovar Manilae strain L495 in response to environmental conditions. Quantitative RT-PCR measurements revealed that when grown in standard EMJH medium sph2 transcript levels were 21 fold higher in the Pomona strain than in the Manilae strain (Fig 4). In both strains, the addition of 120 mM sodium chloride to the cultures increased sph2 transcript levels by over 100 fold (Fig 4). At the higher osmolarity, levels of sph2 transcript remained nearly 20 fold higher in the Pomona strain than in the Manilae strain. Addition of 10% rat serum increased sph2 transcript levels by four fold. The ~20-fold difference in sph2 transcript levels between the two strains was maintained when both 10% rat serum and the additional 120 mM sodium chloride were present in the culture medium.
Fig 4. Effect of salt and serum on sph2 transcript levels in L. interrogans.
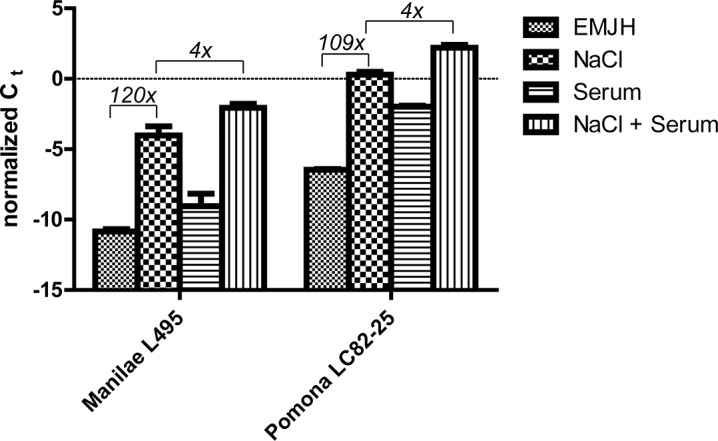
Transcript levels were determined by quantitative RT-PCR. Ct values for sph2 transcript were normalized to those for lipL41 transcript. Fold differences between growth conditions are shown in italics. Mean and standard deviation from three biological replicates are shown; p < 0.001 for all group comparisons (one-way ANOVA, Tukey multiple comparisons post-test).
Regulation of hemolysin and sphingomyelinase production by L. interrogans
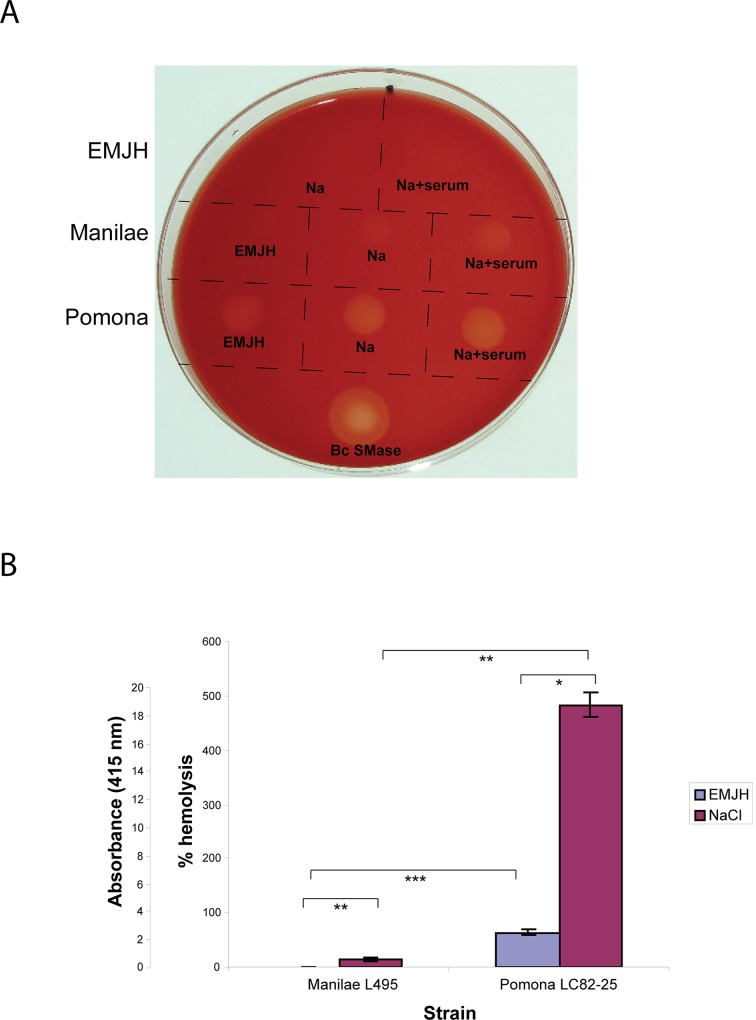
The levels of hemolytic and sphingomyelinase activities in the spent growth medium of the Pomona LC82-25 and Manilae L495 strains grown in EMJH and in EMJH supplemented with 120 mM sodium chloride were determined. To assess hemolytic activity qualitatively, the culture supernatant fluid was spotted onto sheep erythrocyte agar plates. The culture medium, which was adjusted to physiological osmolarity with sodium chloride, did not cause detectable lysis of the erythrocytes (Fig 5A). On the other hand, the spent growth medium from both strains, including those obtained from cultures containing rat serum, caused partial clearance of erythrocytes on the plate (Fig 5A).
Fig 5. Hemolytic activity in spent growth medium of L. interrogans.
A. Hemolytic activity associated with spent growth medium from L. interrogans Manilae L495 and Pomona LC82-25 strains grown in EMJH, EMJH supplemented with 120 mM sodium chloride (Na) and EMJH supplemented with 120 mM sodium chloride and 10% rat serum (Na+serum) was examined on blood agar plates. 50 mU of Bacillus cereus sphingomyelinase (Bc SMase) was spotted onto the agar as a positive control. B. Hemolytic activities of spent growth medium from Manilae L495 and Pomona LC82-25 cultures incubated in EMJH and EMJH supplemented with 120 mM sodium chloride were measured by a quantitative hemolytic assay. The spent medium from the Pomona LC82-25 cultures were diluted 10 fold into EMJH plus 120 mM sodium chloride prior to conducting the assay. Measurements obtained with the diluted Pomona samples were multiplied by ten and plotted on the graph. Data are presented as mean ± standard deviation of triplicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (one-way ANOVA, Tukey multiple comparisons post-test).
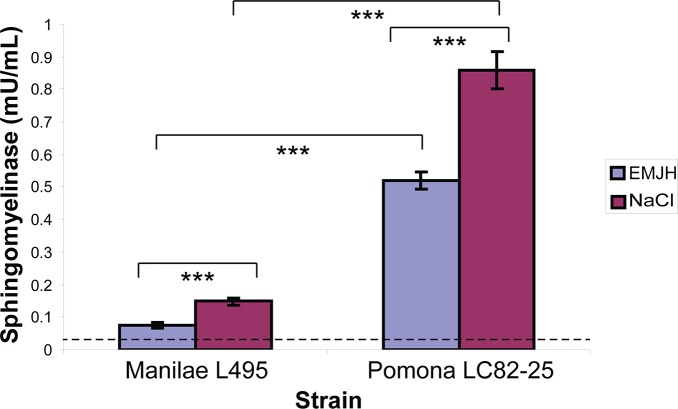
The amount of hemolytic activity in the spent growth medium was quantified with a liquid-phase assay. In EMJH, the Pomona strain showed greater hemolytic activity for sheep erythrocytes than the Manilae strain (Fig 5B). Both strains released higher levels of hemolytic activity when EMJH was supplemented with 120 mM sodium chloride. In the process of developing the hemolysis assay, we discovered that hemolytic activity in spent medium from the Pomona strain incubated in EMJH with 120 mM sodium chloride was at least 92% lower when 10% rat serum was also present, even though it was detected in sheep erythrocyte plates (Fig 5A). The hemolysis results with the strains grown in EMJH and EMJH with 120 mM sodium chloride were reflected in an assay for sphingomyelin hydrolase activity (Fig 6). The results from the Western blots, hemolysis assay, and enzymatic assay reveal a correlation between sph2 expression and extracellular hemolysis and sphingomyelinase activities of L. interrogans.
Fig 6. Sphingomyelinase activity in spent growth medium of L. interrogans.
A sphingomyelinase assay was conducted with the spent growth medium of L. interrogans Manilae L495 and Pomona LC82-25 strains grown in EMJH (blue) and in EMJH supplemented with 120 mM NaCl (purple). The dashed line indicates the background enzymatic activity of EMJH with 120 mM NaCl alone. Data are presented as mean ± standard deviation of three biological replicates; ***, p < 0.001 (one-way ANOVA, Tukey multiple comparisons post-test).
Reduced hemolytic and sphingomyelinase activity secreted by an L. interrogans sph2 mutant
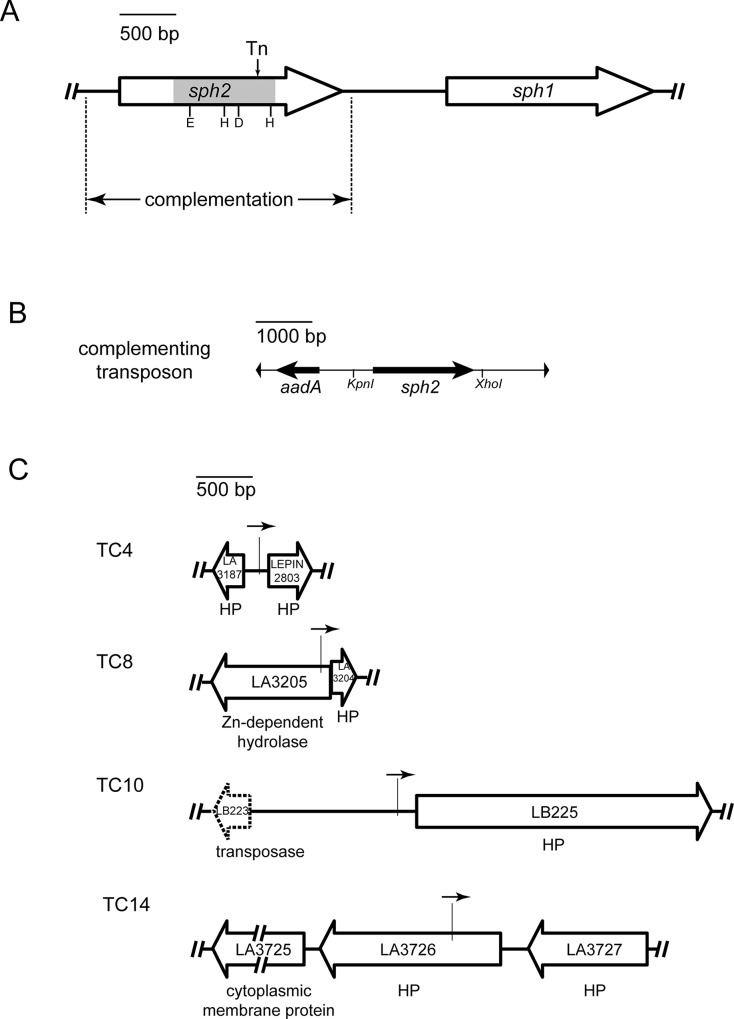
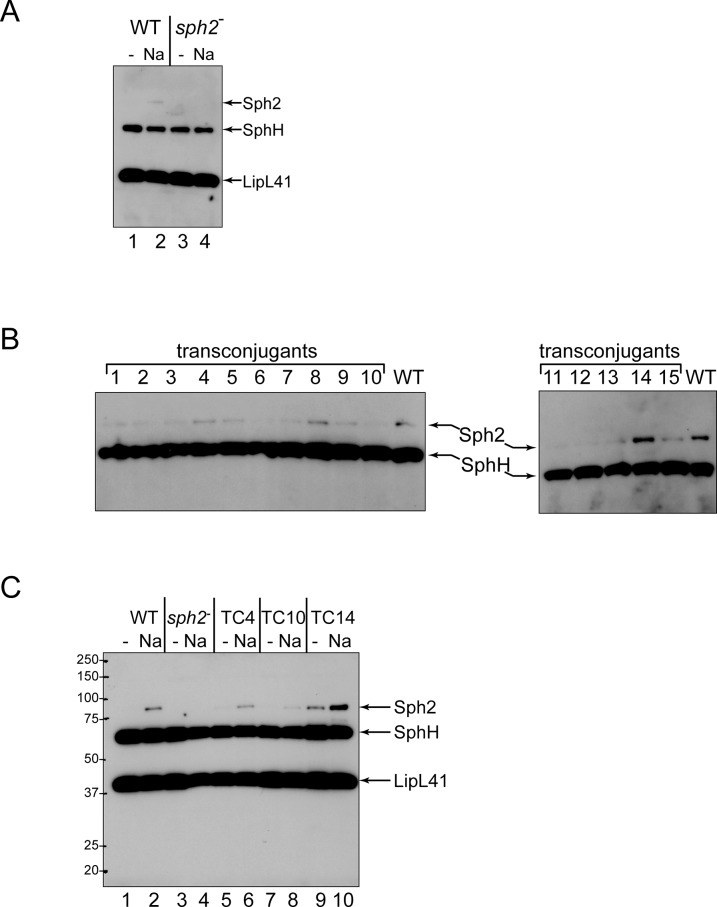
To examine the contribution of sph2 to secreted hemolytic and sphingomyelinase activities by a genetic approach, we were provided with the highly-passaged L. interrogans strain L391 (serovar Lai) and an isogenic sph2 mutant, which was generated by transposon insertional mutagenesis [38]. The transposon is located within sph2 near the end of the segment encoding the enzymatic domain of the protein (Fig 7A). The truncated form predicted to be generated by the mutant allele lacks the second catalytic histidine residue in the enzymatic domain. Substitution of this histidine with alanine in Bacillus cereus sphingomyelinase completely abolishes its enzymatic activity [47]. Therefore, the truncated form expressed from the mutant sph2 allele is unlikely to possess residual enzymatic function. Immunoblot analysis with the sph2 mutant and its wild-type parent confirmed that the mutant was unable to produce the full-length form of Sph2 following incubation at physiological osmolarity for six hours (Fig 8A, lane 4). The sph1 coding region lies 915 bp downstream of sph2 (Fig 7A). Quantitative RT-PCR analysis indicates that sph1 transcript levels were 34% lower in the sph2 mutant compared with that in the wild-type strain.
Fig 7. Location of tranposons in an L. interrogans sph2 mutant and in sph2 complemented isolates.
A. Genetic organization of L. interrogans sph1-sph2 locus. The location of the transposon insertion in the sph2 mutant is designated with "Tn." The ends of the sequence cloned for complementation studies are marked with dashed lines. The segment of sph2 encoding the enzymatic domain is shaded gray, and the catalytic residues demonstrated experimentally to be necessary for Bacillus subtilis sphingomyelinase activity are abbreviated by their single-letter amino acid codes. B. Genetic structure of the complementing transposon. The sph2 sequences were cloned between the KpnI and XhoI restriction sites in the transposon. The aadA gene encodes resistance to spectinomycin. C. Insertion sites of the complementing transposon. The location of the complementing transposon in four transconjugants (TC) is indicated by the vertical line. The arrow above the vertical line depicts the orientation of the sph2 gene in the transposon. The dashed open arrow indicates a pseudogene. HP, hypothetical protein.
Fig 8. Immunoblot analysis of the L. interrogans sph2 mutant and its complemented isolates.
A. Immunoblot analysis of the sph2 mutant. The wild-type (WT) and mutant (sph2 -) strains were incubated in EMJH (-) or EMJH with 120 mM sodium chloride (Na) for six hours, and cell lysates were examined by immunoblot analysis with anti-Sph2 and anti-LipL41 antisera. The anti-Sph2 antiserum cross-reacts with SphH. B. Screen for complemented isolates producing Sph2. The sph2 gene was introduced into the sph2 mutant by conjugation. 15 transconjugants were examined for production of Sph2 following incubation in EMJH with 120 mM sodium chloride for six hours. The immunoblot was probed with anti-Sph2 antiserum. C. Regulation of Sph2 production in three transconjugants. The transconjugants TC4, TC10, and TC14 were examined for Sph2 production following incubation in EMJH (odd-numbered lanes) and EMJH with 120 mM sodium chloride (even-number lanes). The immunoblot was probed with anti-Sph2 and anti-LipL41 antisera.
We introduced an intact copy of sph2 into the mutant for complementation studies. We selected the sph2 gene from the Fiocruz L1-130 strain because of the possibility that the Sph2 protein synthesized by the highly-passaged L391 strain was not fully active. The Sph2 protein sequences of the Fiocruz L1-130 and Lai 56601 strains are 99.5% identical (621/624), with none of the three differences occurring in predicted catalytic residues [22]. The sph2 gene was introduced by conjugation on a suicide plasmid carrying sph2 within a Himar1 transposon carrying a gene encoding resistance to spectinomycin (Fig 7B). Following mating and incubation on selective plates, 15 transconjugants were screened for Sph2 production. The transconjugants exhibited various levels of Sph2 following incubation at physiologic osmolarity (Fig 8B), as would be expected from random insertion of the complementing transposon. Transconjugants that produced high (TC14), medium (TC4), and low (TC10) levels of Sph2 were further examined for control of Sph2 production (Fig 7C). TC14 showed higher basal levels of Sph2 than the wild-type strain (Fig 8C, lane 9 vs 1), and Sph2 levels increased further upon incubation at physiologic osmolarity (Fig 8C, lane 10). The complementing transposon in TC14 was located in the hypothetical gene LA3726, with sph2 in the transposon oriented opposite of the disrupted gene (Fig 7C). In TC10, Sph2 levels were regulated by sodium chloride, but the amount produced at physiologic osmolarity was low relative to the levels produced in the wild-type strain (Fig 8C, lane 8 vs 2). The sph2 gene in the complementing transposon was positioned near the promoter region of the hypothetical gene LB225 with the two genes directed in the same orientation (Fig 7C). TC4 generated similar levels of Sph2 as the wild-type strain (Fig 8C, lanes 5 and 6 vs. 1 and 2). The complementing transposon in TC4 was inserted in the promoter region of the hypothetical gene LEPIN2803 with sph2 oriented in the same direction (Fig 7C).
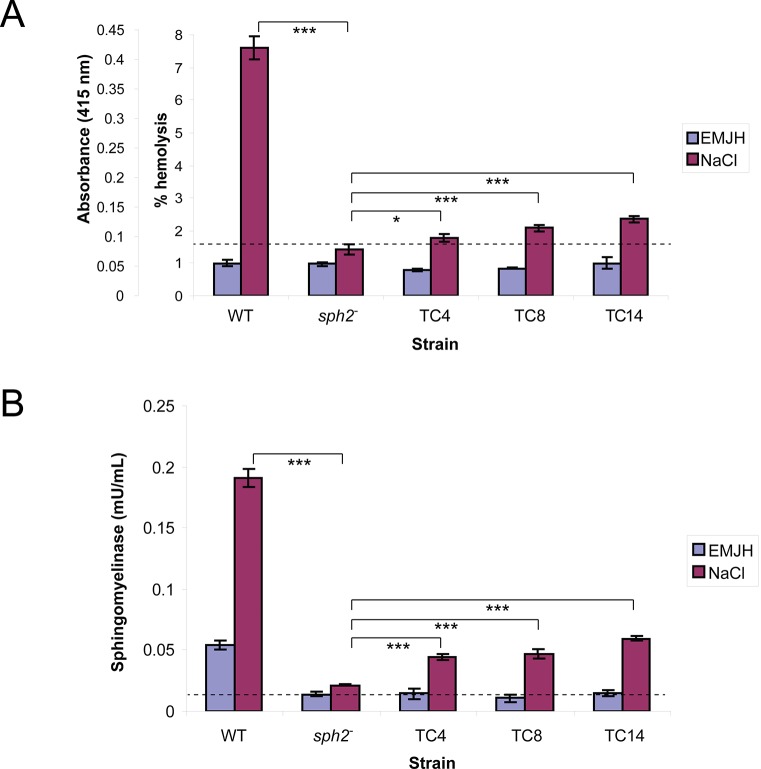
For the hemolysis assay, cultures were incubated for 24 hours to permit accumulation of Sph2. Hemolytic activity was higher in spent medium collected from cultures of the wild-type strain incubated at physiological osmolarity compared to incubation in EMJH alone (Fig 9A). However, hemolytic activity remained at background levels in spent medium collected from cultures of the sph2 mutant (Fig 9A), indicating that sph2 is required for hemolytic activity. For complementation analysis, three transconjugants were selected for their high levels of Sph2 production at physiologic osmolarity: TC4, TC8, and TC14 (Fig 8B). The complementing transposon in TC8 was inserted in a gene encoding a zinc-dependent hydrolase (LA3205), with sph2 oriented opposite of the disrupted gene (Fig 7C). The three complemented strains produced significantly more hemolytic activity than the sph2 mutant (Fig 9A). However, the hemolytic activity secreted by the complemented mutants was less than that generated by the wild-type strain.
Fig 9. Hemolytic and sphingomyelinase activities secreted from L. interrogans sph2 mutant and complemented isolates.
Culture supernatant fluid from wild-type strain Lai L391, sph2 mutant, and sph2 mutant complemented with wild-type sph2 (TC4, TC8, and TC14) incubated in EMJH and EMJH with 120 mM sodium chloride for 24 hours was assayed for hemolytic activity (A) and sphingomyelinase activity (B). The dashed line indicates the background activity in culture medium (EMJH plus 120 mM NaCl) alone. Mean and standard deviation from three biological replicates are shown; *, p < 0.05; ***, p < 0.001 (one-way ANOVA, Tukey multiple comparisons post-test).
The sphingomyelinase activity present in the samples was also quantified. The sph2 mutation resulted in a substantial reduction of sphingomyelinase activity, although activity above background levels was detected following incubation at physiologic osmolarity (Fig 9B). Similar to what we observed with the hemolysis assay, sphingomyelinase activity was partially restored with the three complemented strains (Fig 9B).
Discussion
In this study we provide the first experimental evidence that demonstrates that Sph2 contributes to the hemolytic and sphingomyelinase activities secreted by L. interrogans. The amounts of transcript and protein generated from sph2 correlated with the levels of hemolysis and sphingomyelinase activities observed in spent culture medium when examining the Manilae L495 and Pomona LC82-25 strains incubated in standard culture medium and in medium supplemented with additional sodium chloride. More importantly, hemolysis associated with the spent growth medium from an sph2 mutant failed to rise above background levels. Additionally, the sph2 mutation eliminated most of the sphingomyelinase activity. These results suggest that Sph2 is the dominant hemolysin secreted by L. interrogans during cultivation in EMJH adjusted to physiologic osmolarity with sodium chloride.
Complementation of the mutant with the sph2 gene partially restored hemolytic and sphingomyelinase activities in three transconjugants producing similar or higher levels of Sph2 as the wild-type strain. These results confirm that sph2 contributes to the secreted hemolytic and sphingomyelinase activities, most likely by encoding a single protein that possesses both activities. However, wild-type levels of hemolytic and sphingomyelinase activities were not recovered in the complemented strains. It is unlikely that all three insertions of the complementing transposon disrupted Sph2 secretion or another process necessary for wild-type expression of the two activities. Partial complementation of the hemolytic activity may be explained in part by the mild reduction of sph1 transcript levels observed in the sph2 mutant. Another possibility for the incomplete dominance of the wild-type sph2 gene is that the truncated form of Sph2 produced by the mutant allele interferes with the function of the wild-type protein. We did not detect a truncated Sph2 protein in our immunoblots with the expected molecular mass of 46 kDa. Nevertheless, the same property of Sph2 that causes it to migrate slower than its calculated mass during electrophoresis could also cause the truncated form to run slowly, causing the protein to be obscured by the intense SphH band in the blot.
The sph2 mutation left behind a trace amount of sphingomyelinase activity above background levels. Our previous sequence analysis of the sphingomyelinase-like paralogs revealed that Sph1 and Sph3 lacked a few of the catalytic residues necessary for sphingomyelin hydrolysis activity [22]. Nevertheless, Sph1 and Sph3 may retain a limited capacity to hydrolyze sphingomyelin that is detected only when the dominant sphingomyelinase, Sph2, is removed genetically or when large amounts of recombinant Sph1 and Sph3 are analyzed [21].
Additional hemolysins may also contribute to hemolytic activity secreted by L. interrogans, depending on growth conditions. The hemolysins Sph1, Sph2, Sph3, HlpA, and TlyA were detected extracellularly in co-cultures with macrophage-like cell lines in tissue culture medium supplemented with 10% fetal calf serum [16]. Despite the secretion of several hemolysins, our findings predict that Sph2 would be a major hemolysin secreted by L. interrogans during colonization of the rat host since rat serum increased Sph2 production beyond what was observed without serum at physiologic osmolarity. This finding indicates that undiscovered factors in rat serum are responsible for enhancing sph2 expression.
As demonstrated in another study [32], we found that a Pomona strain incubated in standard culture medium produced large amounts of Sph2, in contrast to the negligible levels produced by the other four strains tested (Figs 1 and 2 and 6A). For the Pomona and Manilae strains, these results correlated with the secreted hemolytic activity (Fig 4B). The large amount of Sph2 and hemolytic activity observed in vitro with a Pomona strain in our study may be implicated in the hemolytic anemia and hemoglobinuria observed in ruminants infected with Pomona strains [12–14]. High basal sph2 expression may be a consequence of a insertion sequence-like element present near the promoter region of sph2 or the extra 75 nucleotides located within the 5' end of the coding region in the Pomona strain [18, 22, 48]. The latter sequences, which correspond to one of four tandem 25 amino acid residue repeats lying amino-terminal to the enzymatic domain, are likely to account for the higher molecular masses of the cellular and released forms of Pomona Sph2 relative to that of the other strains, which possess only three repeats. Moreover, the difference in the apparent molecular mass of Sph2 from the Pomona strain versus the other L. interrogans strains examined in this study is much greater than the calculated difference of 3 kDa. This observation indicates that the unexpectedly slow migration of Sph2 in acrylamide gels observed in this and in our earlier study is related to the repeats, which cause an increase of the apparent molecular mass of Sph2 relative to its calculated mass of up to 18 kDa [34]. The repeats are rich in proline, which could place conformational constraints on the protein to retard its migration through the acrylamide matrix.
Hemolytic activity was detected easily when spent medium from cultures containing rat serum was spotted onto agar plates containing sheep erythrocytes. However, despite the large amount of Sph2 released by the Pomona strain, hemolytic activity measured with the liquid phase assay was reduced considerably when rat serum was present. The inhibitory effect of rabbit and bovine serum on leptospiral hemolytic activity has been noted by others [8, 32, 49]. The ability to detect hemolytic activity in the presence of rat serum on erythrocyte agar plates suggests that the inhibitor is labile or highly diffusible in agar. The hemolysin inhibitor in bovine serum was shown to be phosphatidylethanolamine, phosphotidylcholine, and sphingomyelin [50]. In addition, because rats are a reservoir host for Leptospira, rat serum may contain anti-Sph2 antibodies that inhibit the function of Sph2. Our results raise the question of whether Sph2 can function as a hemolysin during hematogenous dissemination. It is possible that uncharacterized host or bacterial processes minimize the inhibitory action of phospholipids during leptospiral infection. Alternatively, the critical function of Sph2 during infection may not involve hemolysis. For example, Sph2 may function as an adhesin or trigger host signaling pathways via its sphingomyelinase when expressed by leptospires that colonize the renal tubule, where plasma proteins that carry phospholipids are likely to be excluded by the glomerulus [22, 31].
Several studies have demonstrated that most of the hemolytic activity detected in Leptospira cultures is present in the culture supernatant fluid [8, 9, 11, 32]. Similarly, almost all of the sphingomyelinase activity in another strain of Pomona is extracellular [51]. Although we did not measure the hemolytic and sphingomyelinase activities that remained associated with L. interrogans cells, the earlier studies suggest that the full-length Sph2 protein that is associated with the bacterial cell remains inactive until secretion, despite the full-length recombinant protein being active in vitro [16, 20]. These observations suggest that Sph2 and other hemolysins such as the pore-former SphH [17] are inaccessible to the surface, tied up in the secretion apparatus, or bound by an inhibitor until released from the cell, perhaps to prevent injury to the bacterium. Our findings presented in Fig 3 suggest that processing of Sph2 occurs during its release from the cell and that at least one of the two truncated forms of Sph2 detected extracellularly in L. interrogans cultures are functional rather than degradation products. However, we cannot rule out the possibility that the true active secreted product is the full-length species that degrades to inactive truncated forms during the lengthy immunoprecipitation procedure. Definitive proof will require demonstration of hemolytic and sphingomyelinase activities of purified forms of the truncated Sph2 species.
The pathway leading to secretion and processing of Sph2 remains to be identified. The processing sites leading to the released forms of Sph2 are predicted to lie within its N- or C-terminal domains, outside of the enzymatic domain. Sph2 appears to lack a classic amino-terminal signal peptide. Given the presence of genes encoding TolC, HlyB, and HlyD orthologs in the L. interrogans genome [1], secretion of Sph2 and the other sphingomyelinase-like proteins may involve a type I secretion pathway. In one study, a TolC homolog was co-immunoprecipitated using anti-Sph3 immunoglobulin, providing some experimental support for a TolC-based secretion system for the leptospiral sphingomyelinase-like proteins [29].
In conclusion, we report that sph2 expression and Sph2 secretion are regulated by salt and rat serum. Salt also regulated the levels of hemolytic and sphingomyelinase activities, potentially pathogenic functions of L. interrogans. These findings indicate that the quantities of hemolytic and sphingomyelinase activities measured in standard cultures of L. interrogans may underestimate the levels produced during infection, even for strains that produce large amounts in culture. Partial restoration of hemolytic and sphingomyelinase activities of an sph2 mutant by introduction of an intact sph2 gene strongly suggests that Sph2 is secreted as an active hemolysin and sphingomyelinase. Given the profound response of sph2 to host tissue-like conditions and the potential importance of Sph2 in leptospiral pathogenesis, further studies are needed to better understand the molecular mechanisms of sph2 gene regulation and secretion of its product.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a Veterans Affairs Merit Award to JM, a Fulbright-Nehru Doctoral and Professional Research Fellowship (Grantee ID: 15111348) from the United States India Educational Foundation (USIEF) to SAN, and National Institutes of Health grant R01 AI 034431 to DAH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sritharan M. Insights into Leptospirosis, a Neglected Disease In: Lorenzo-Morales J, editor. Zoonosis. InTech; 2012. pp. 167–92. http://www.intechopen.com/books/zoonosis/leptospirosis-a-neglected-zoonotic-disease. [Google Scholar]
- 2. Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009;7(10):736–47. Epub 2009/09/17. 10.1038/nrmicro2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2(6):e256 Epub 2008/06/26. 10.1371/journal.pntd.0000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3(12):757–71. Epub 2003/12/04. [DOI] [PubMed] [Google Scholar]
- 5. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. Epub 2014/11/13. 10.1007/978-3-662-45059-8_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arean VM. The pathologic anatomy and pathogenesis of fatal human leptospirosis (Weil's disease). Am J Pathol. 1962;40:393–423. Epub 1962/04/01. [PMC free article] [PubMed] [Google Scholar]
- 7. Grooms DL. Reproductive losses caused by bovine viral diarrhea virus and leptospirosis. Theriogenology. 2006;66(3):624–8. Epub 2006/05/24. [DOI] [PubMed] [Google Scholar]
- 8. Alexander AD, Smith OH, Hiatt CW, Gleiser CA. Presence of hemolysin in cultures of pathogenic leptospires. Proc Soc Exp Biol Med. 1956;91(2):205–11. Epub 1956/02/01. [DOI] [PubMed] [Google Scholar]
- 9. Russell CM. A hemolysin associated with leptospirae. J Immunol. 1956;77(6):405–9. Epub 1956/12/01. [PubMed] [Google Scholar]
- 10. Kasarov LB. Degradation of the erythrocyte phospholipids and haemolysis of the erythrocytes of different animal species by leptospirae. J Med Microbiol. 1970;3(1):29–37. Epub 1970/02/01. [DOI] [PubMed] [Google Scholar]
- 11. Imamura S, Kuribayashi K, Kameta M. Studies on toxins of pathogenic Leptospira . Jpn J Microbiol. 1957;1(1):43–7. Epub 1957/01/01. [DOI] [PubMed] [Google Scholar]
- 12. Smith BP, Armstrong JM. Fatal hemolytic anemia attributed to leptospirosis in lambs. J Am Vet Med Assoc. 1975;167(8):739–41. Epub 1975/10/15. [PubMed] [Google Scholar]
- 13. Hanson LE. Bovine leptospirosis. J Dairy Sci. 1976;59(6):1166–70. Epub 1976/06/01. 10.3168/jds.S0022-0302(76)84339-1 [DOI] [PubMed] [Google Scholar]
- 14. Ayanegui-Alcerreca MA, Wilson PR, Mackintosh CG, Collins-Emerson JM, Heuer C, Midwinter AC, et al. Leptospirosis in farmed deer in New Zealand: a review. N Z Vet J. 2007;55(3):102–8. Epub 2007/05/31. [DOI] [PubMed] [Google Scholar]
- 15. Bernheimer AW, Bey RF. Copurification of Leptospira interrogans serovar pomona hemolysin and sphingomyelinase C. Infect Immun. 1986;54(1):262–4. Epub 1986/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H, Wu Y, Ojcius DM, Yang XF, Zhang C, Ding S, et al. Leptospiral hemolysins induce proinflammatory cytokines through Toll-like receptor 2-and 4-mediated JNK and NF-kappaB signaling pathways. PloS One. 2012;7(8):e42266 Epub 2012/08/08. 10.1371/journal.pone.0042266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SH, Kim S, Park SC, Kim MJ. Cytotoxic activities of Leptospira interrogans hemolysin SphH as a pore-forming protein on mammalian cells. Infect Immun. 2002;70(1):315–22. Epub 2001/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Artiushin S, Timoney JF, Nally J, Verma A. Host-inducible immunogenic sphingomyelinase-like protein, Lk73.5, of Leptospira interrogans . Infect Immun. 2004;72(2):742–9. Epub 2004/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hauk P, Negrotto S, Romero EC, Vasconcellos SA, Genovez ME, Ward RJ, et al. Expression and characterization of HlyX hemolysin from Leptospira interrogans serovar Copenhageni: potentiation of hemolytic activity by LipL32. Biochem Biophys Res Commun. 2005;333(4):1341–7. Epub 2005/07/02. [DOI] [PubMed] [Google Scholar]
- 20. Narayanavari SA, Kishore NM, Sritharan M. Structural Analysis of the Leptospiral sphingomyelinases: in silico and experimental evaluation of Sph2 as an Mg++-dependent sphingomyelinase. J Mol Microbiol Biotechnol. 2012;22(1):24–34. 10.1159/000337013 [DOI] [PubMed] [Google Scholar]
- 21. Zhang YX, Geng Y, Bi B, He JY, Wu CF, Guo XK, et al. Identification and classification of all potential hemolysin encoding genes and their products from Leptospira interrogans serogroup Icterohaemorrhagiae serovar Lai. Acta Pharmacol Sin. 2005;26(4):453–61. Epub 2005/03/23. [DOI] [PubMed] [Google Scholar]
- 22. Narayanavari SA, Sritharan M, Haake DA, Matsunaga J. Multiple leptospiral sphingomyelinases (or are there?). Microbiology. 2012;158(Pt 5):1137–46. Epub 2012/03/17. 10.1099/mic.0.057737-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Picardeau M, Bulach DM, Bouchier C, Zuerner RL, Zidane N, Wilson PJ, et al. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PloS One. 2008;3(2):e1607 Epub 2008/02/14. 10.1371/journal.pone.0001607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006;188(24):8421–9. Epub 2006/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guegan R, Camadro JM, Saint Girons I, Picardeau M. Leptospira spp. possess a complete haem biosynthetic pathway and are able to use exogenous haem sources. Mol Microbiol. 2003;49(3):745–54. Epub 2003/07/17. [DOI] [PubMed] [Google Scholar]
- 26. Asuthkar S, Velineni S, Stadlmann J, Altmann F, Sritharan M. Expression and characterization of an iron-regulated hemin-binding protein, HbpA, from Leptospira interrogans serovar Lai. Infect Immun. 2007;75(9):4582–91. Epub 2007/06/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murray GL, Ellis KM, Lo M, Adler B. Leptospira interrogans requires a functional heme oxygenase to scavenge iron from hemoglobin. Microbes Infect. 2008;10(7):791–7. Epub 2008/06/06. 10.1016/j.micinf.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 28. Murray GL, Srikram A, Henry R, Puapairoj A, Sermswan RW, Adler B. Leptospira interrogans requires heme oxygenase for disease pathogenesis. Microbes Infect. 2009;11(2):311–4. Epub 2008/12/31. 10.1016/j.micinf.2008.11.014 [DOI] [PubMed] [Google Scholar]
- 29. Velineni S, Ramadevi S, Asuthkar S, Sritharan M. Effect of iron deprivation on expression of sphingomyelinase in pathogenic serovar Lai. Online J Bioinform. 2009;10(2):241–58. [Google Scholar]
- 30. Zhang YX, Geng Y, Yang JW, Guo XK, Zhao GP. Cytotoxic activity and probable apoptotic effect of Sph2, a sphigomyelinase hemolysin from Leptospira interrogans strain Lai. BMB Rep. 2008;41(2):119–25. Epub 2008/03/05. [DOI] [PubMed] [Google Scholar]
- 31. Pinne M, Matsunaga J, Haake DA. Leptospiral outer membrane protein microarray, a novel approach to identification of host ligand-binding proteins. J Bacteriol. 2012;194(22):6074–87. Epub 2012/09/11. 10.1128/JB.01119-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carvalho E, Barbosa AS, Gomez RM, Oliveira ML, Romero EC, Goncales AP, et al. Evaluation of the expression and protective potential of leptospiral sphingomyelinases. Curr Microbiol. 2010;60(2):134–42. Epub 2009/10/15. 10.1007/s00284-009-9519-3 [DOI] [PubMed] [Google Scholar]
- 33. Matsunaga J, Lo M, Bulach DM, Zuerner RL, Adler B, Haake DA. Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect Immun. 2007;75(6):2864–74. Epub 2007/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsunaga J, Medeiros MA, Sanchez Y, Werneid KF, Ko AI. Osmotic regulation of expression of two extracellular matrix-binding proteins and a haemolysin of Leptospira interrogans: differential effects on LigA and Sph2 extracellular release. Microbiology. 2007;153(Pt 10):3390–8. Epub 2007/10/02. [DOI] [PubMed] [Google Scholar]
- 35. Caimano MJ, Sivasankaran SK, Allard A, Hurley D, Hokamp K, Grassmann AA, et al. A model system for studying the transcriptomic and physiological changes associated with mammalian host-adaptation by Leptospira interrogans serovar Copenhageni. PLoS Pathog. 2014;10(3):e1004004 Epub 2014/03/15. 10.1371/journal.ppat.1004004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sueyoshi N, Kita K, Okino N, Sakaguchi K, Nakamura T, Ito M. Molecular cloning and expression of Mn2+-dependent sphingomyelinase/hemolysin of an aquatic bacterium, Pseudomonas sp. strain TK4. J Bacteriol. 2002;184(2):540–6. Epub 2001/12/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsunaga J, Sanchez Y, Xu X, Haake DA. Osmolarity, a key environmental signal controlling expression of leptospiral proteins LigA and LigB and the extracellular release of LigA. Infect Immun. 2005;73(1):70–8. Epub 2004/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murray GL, Morel V, Cerqueira GM, Croda J, Srikram A, Henry R, et al. Genome-wide transposon mutagenesis in pathogenic Leptospira species. Infect Immun. 2009;77(2):810–6. Epub 2008/12/03. 10.1128/IAI.01293-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shang ES, Summers TA, Haake DA. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect Immun. 1996;64(6):2322–30. Epub 1996/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsunaga J, Coutinho ML. Positive regulation of Leptospira interrogans kdp expression by KdpE as demonstrated with a novel β-galactosidase reporter in Leptospira biflexa . Appl Environ Microbiol. 2012;78(16):5699–707. Epub 2012/06/12. 10.1128/AEM.00713-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eshghi A, Becam J, Lambert A, Sismeiro O, Dillies MA, Jagla B, et al. A putative regulatory genetic locus modulates virulence in the pathogen Leptospira interrogans . Infect Immun. 2014;82(6):2542–52. Epub 2014/04/02. 10.1128/IAI.01803-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC(T) method. Methods. 2001;25(4):402–8. Epub 2002/02/16. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 43. Demarre G, Guerout AM, Matsumoto-Mashimo C, Rowe-Magnus DA, Marliere P, Mazel D. A new family of mobilizable suicide plasmids based on broad host range R388 plasmid (IncW) and RP4 plasmid (IncPα) conjugative machineries and their cognate Escherichia coli host strains. Res Microbiol. 2005;156(2):245–55. Epub 2005/03/08. [DOI] [PubMed] [Google Scholar]
- 44. Picardeau M. Conjugative transfer between Escherichia coli and Leptospira spp. as a new genetic tool. Appl Environ Microbiol. 2008;74(1):319–22. Epub 2007/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Slamti L, Picardeau M. Construction of a library of random mutants in the spirochete Leptospira biflexa using a mariner transposon. Methods Mol Biol. 2012;859:169–76. Epub 2012/03/01. 10.1007/978-1-61779-603-6_9 [DOI] [PubMed] [Google Scholar]
- 46. R Core Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 47. Matsuo Y, Yamada A, Tsukamoto K, Tamura H, Ikezawa H, Nakamura H, et al. A distant evolutionary relationship between bacterial sphingomyelinase and mammalian DNase I. Protein Sci. 1996;5(12):2459–67. Epub 1996/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Timoney JF, Kalimuthusamy N, Velineni S, Donahue JM, Artiushin SC, Fettinger M. A unique genotype of Leptospira interrogans serovar Pomona type kennewicki is associated with equine abortion. Vet Microbiol. 2011;150(3–4):349–53. Epub 2011/04/01. 10.1016/j.vetmic.2011.02.049 [DOI] [PubMed] [Google Scholar]
- 49. Yanagihara Y, Kojima T, Mifuchi I. Hemolytic activity of Leptospira interrogans serovar canicola cultured in protein-free medium. Microbiol Immunol. 1982;26(7):547–56. Epub 1982/01/01. [PubMed] [Google Scholar]
- 50. Kojima T, Yanagihara Y, Mifuchi I. Characterization of inhibitor to leptospiral hemolysin present in bovine serum. Microbiol Immunol. 1984;28(3):291–302. Epub 1984/01/01. [DOI] [PubMed] [Google Scholar]
- 51. Segers RP, van der Drift A, de Nijs A, Corcione P, van der Zeijst BA, Gaastra W. Molecular analysis of a sphingomyelinase C gene from Leptospira interrogans serovar hardjo. Infect Immun. 1990;58(7):2177–85. Epub 1990/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.