Abstract
Hemolysis can occur as a consequence of extracorporeal membrane oxygenation (ECMO) and is associated with increased mortality and morbidity. Shear stress generated by flow through the circuit and oxygenator is believed to cause ECMO-induced hemolysis. We hypothesize that either a smaller dimension oxygenator or an in-line hemofilter will increase ECMO-associated hemolysis. Circuits were configured with a Quadrox-D Adult oxygenator (surface area 1.8 m2), Quadrox-iD Pediatric oxygenator (surface area 0.8 m2), or Quadrox-D Adult oxygenator with an in-line hemofilter (N=4) and ran for six hours. Samples were collected hourly from the ECMO circuit and a time-based hemolysis control. Plasma hemoglobin levels were assayed. Circuit-induced hemolysis at each time point was defined as the change in plasma hemoglobin standardized to the time-based hemolysis control. Plasma hemoglobin increased with the use of the smaller dimension pediatric oxygenator as compared to the adult oxygenator when controlling for ECMO run time (p=0.02). Further, there was a greater pressure gradient with the smaller dimension pediatric oxygenator (p<0.05). Plasma hemoglobin did not change with the addition of the in-line hemofilter. The use of a smaller dimension pediatric oxygenator resulted in greater hemolysis and a higher pressure gradient. This may indicate that increased shear forces augment ECMO-induced hemolysis.
Keywords: oxygenator, ECMO, hemolysis, pediatric
Introduction
Extracorporeal membrane oxygenation (ECMO) is a life-saving modality for critically ill patients with refractory cardiac and/or respiratory failure. Pediatric patients who utilize ECMO have a greater than 70% survival to decannulation.1 However, the development of acute kidney injury (AKI) on ECMO decreases survival to 40%.1-4 The direct cause of AKI on ECMO outcome is unknown, but hemolysis, a known consequence of ECMO, has been suggested as a possibility.5
Plasma hemoglobin (pHb), a marker of hemolysis has been reported to rise by as much as 10-25 fold during ECMO.6 Free hemoglobin in the plasma has been shown to be cytotoxic leading to tissue hypoxia and, ultimately, cell death.7,8 In addition, free hemoglobin consumes nitric oxide and leads to inappropriate vasoconstriction.8 Factors believed to affect ECMO-induced hemolysis include mechanical shear stress applied to the red blood cells via flow and pressure gradients and red blood cell exposure to the non-endothelialized circuit. 9-12 Hemolysis may be further exacerbated by the use of stored packed red blood cells (pRBCs) required to prime the pump and for transfusions.13,14
A smaller dimension pediatric oxygenator, with a smaller diameter connector, has been introduced to reduce the blood volume typically required to prime the ECMO circuit for younger patients. It is not known if oxygenators with smaller dimensions induce greater hemolysis given the potential increase in mechanical shear force due increased perfusion-pressure and the resulting turbulence. Furthermore, approximately half of the ELSO centers use in-line hemofiltration to augment fluid removal.1 It is unknown whether the addition of a hemofilter to the ECMO circuit, which increases the size of the non-endotheliazed circuit and number of connection points, will further augment hemolysis. We hypothesize that the use of either a smaller dimension oxygenator or an in-line hemofilter will increase ECMO-associated hemolysis.
Methods
Exemption was obtained from the Duke University Health System Institutional Review Board.
Circuit Design
Three ECMO circuit configurations were studied (n=4 for each configuration). Each circuit was built on a Sorin Slll pump console equipped with a Sorin Revolution Centrifugal Pump (SCP) system (Sorin Group, Denver, CO, USA) in a sterile fashion. All circuits were built to replicate typical clinical practice from the institution's standard Extracorporeal Life Support (ECLS) base tubing pack. Components of the circuit pack include 3/8″ SMART coated tubing connections, a PC coated Revolution centrifugal pump, one five-port manifold line, and a CDI 100 Sat/HCT cuvette (Terumo Cardiovascular Systems, Inc., Ann Arbor, MI, USA). The custom base pack was stepped down to accommodate the 1/4″ connections of the pediatric oxygenator. All three circuit configurations were attached to a 500 ml reservoir bag to simulate a patient (Figure 1).
Figure 1. Circuit Design.
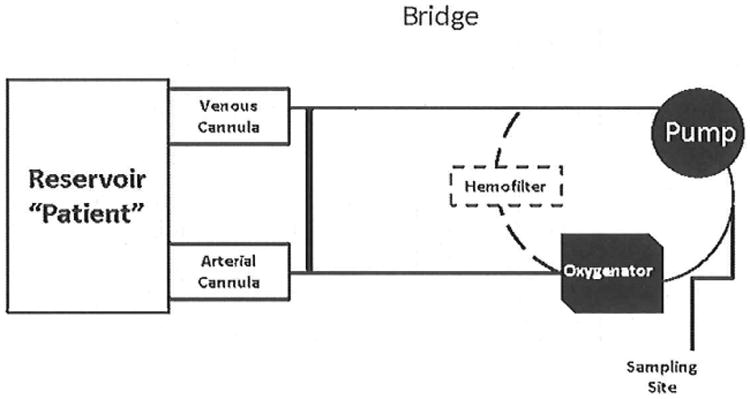
- Quadrox-iD Adult oxygenator: Configured using 3/8″ diameter SMART coated tubing connections. Direct connections were made to the 3/8″ blood inlet and outlet for the adult oxygenator.
- Quadrox-iD Pediatric oxygenator: Configured using 3/8″ diameter SMART coated tubing connections. There was a step down and step up connection made for the 1/4″ blood inlet and outlet for the pediatric oxygenator.
- Quadrox-iD Adult oxygenator with an in-line hemofilter: Configured using 3/8″ diameter SMART coated tubing connections. There was a step down and step up connection made for the 3/16″ blood inlet and outlet for the hemofilter.
A 500mL reservoir bag was used to simulate the patient. Arrows depict direction of flow.
Circuit 1
Maquet Adult Quadrox D Diffusion Membrane Oxygenator (Getinge Group, Rastatt, Germany) with a surface area of 1.8 m2 was used with a standard 3/8″ inch connection. No hemofilter was used in this circuit.
Circuit 2
Maquet Pediatric iD Diffusion Membrane Oxygenator (Getinge Group, Rastatt, Germany) with a surface area of 0.8 m2 was used with a step-down 1/4″ connection. No hemofilter was used in this circuit. The length of this circuit was unchanged from Circuit 1.
Circuit 3
Maquet Adult Quadrox D Diffusion Membrane Oxygenator (Getinge Group, Rastatt, Germany) was used. An in-line hemofilter, with a surface are of 0.25 m2 and length of 145 mm, was added to circuit (DHF0.2 Pediatric Hemoconcentrator; Sorin Group, Denver, CO) with a 3/16″ connection step-down to a 1/8″ manifold connection.
Circuit Prime
The circuits were primed with Plasmalyte A multiple electrolyte solution (Baxter Healthcare Corporation, Deerfield, IL, USA) as well as human donor pRBCs (approximately 450 ml) and fresh frozen plasma (100 ml) that were obtained from the institution's Transfusion Services. In addition, 50 units of heparin, 20 mEq of sodium bicarbonate, and 1 g of calcium gluconate was added according to standard practice. The goal hematocrit was 30% with a total circulating volume of approximately 600 ml. A sample was taken from the circuit prime and maintained in a water bath set at 36°C to provide a time based hemolysis control.
Circuit Calibration
Blood gas monitoring was achieved with the Terumo CDI 500 Blood Parameter Monitoring System (Terumo Cardiovascular Systems, Inc., Ann Arbor, MI, USA). A CDI 500 cell was gas calibrated and placed inline. A venous CDI 100 probe was placed on the circuit. The circuit was warmed to 36°C using the Cincinnati SubZero ECMO-Heater (Cincinnati SubZero Medical, Cincinnati, OH, USA) and circulated through the reservoir at 1 liter/minute. This rate was selected to simulate the flow rate of a 10 kg patient who could use either the pediatric or adult sized oxygenator without exceeding the manufacturer maximum flow rate. The maximum flow rate for adult oxygenator is 7 liter/minute and for the pediatric oxygenator is 2.8 liter/minute. Sweep gas with oxygen, medical air, and carbon dioxide admixtures were used to replicate typical clinical practice. After five minutes of circulation, a sample was drawn from the circuit for blood gas analysis performed by the clinical laboratories of Duke University Hospital. These results were used to calibrate the CDI. The circuit was maintained at a pH of 7.35 - 7.45 via adjustments to the sweep gas and addition of sodium bicarbonate.
Study Design and Sampling
Blood was circulated through each of the three circuit configurations for six hours. For Circuit 3, blood was also circulated continuously through the hemofilter for the duration of the study. At each hour, a 1 ml sample was taken from the circuit and time based hemolysis control (Figure 2). Samples were centrifuged at 1000 × g for 10 min, and the plasma removed. Plasma was stored at -80°C until analyzed. At the time of sampling, all circuit and CDI parameters were recorded. We hemofiltered Circuit 3 at hours 2 and 4 and removed 50 mL of filtrate. Circuit volume was replaced with plasmalyte. Intermittent filtration was limited to two time intervals to minimize fluid shifts to the circuit and hemodilution.
Figure 2. Study Design Sampling.
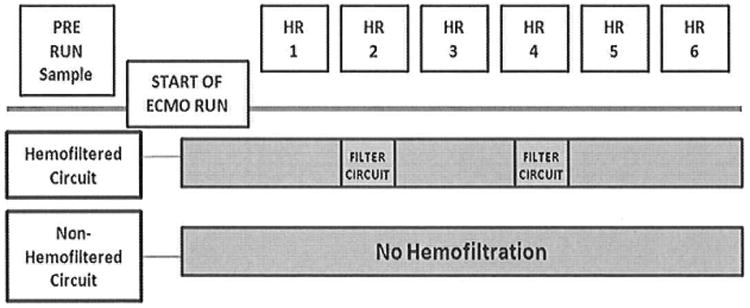
Blood was circulated through the circuit configurations for six hours. Samples were collected hourly from the circuit and time based hemolysis control, which consisted of a sample from the circuit prime maintained in a water bath set at 36°C. 50 ml of filtrate was removed from the hemofiltered circuits at hour 2 and 4.
Hemoglobin Assay
Plasma hemoglobin levels were measured using the QuantiChrom TM hemoglobin assay kit (BioAssay Systems, Hayward, CA) according to the manufacturer's directions. Using a 96-well plate, 50 μl of serum was added in duplicate and mixed with 200 μl of Trition/NaOH reagent. Color intensity, directly proportional to the hemoglobin concentration, was measured at 405 nm by spectrophotometer (Bio-Tek instruments, Winooski, VT).
Statistical Analysis
Hemolysis associated with each ECMO circuit was defined as the hourly change in pHb from baseline in the ECMO circuit standardized to the pHb levels from the time based hemolysis control (TBC) at the same time point (Change in pHb of ECMO Circuit (Time X) - Change in pHb of TBC (Time X)). Multivariable linear regression was used to evaluate the effect of circuit configuration on hemolysis controlling for time. Comparisons between the circuit blood gas, membrane pressures, and revolutions per minute of the pump were made by two-sample Wilcoxon Rank-Sum testing and are expressed as median and interquartile ranges. Comparisons of blood age were made using Kruskal Wallis testing and are also expressed as median and interquartile range. Stata 12.0 (College Station, TX) was used for all analyses, and we considered p < 0.05 statistically significant.
Results
Plasma hemoglobin levels increased to the greatest extent in the pediatric oxygenator circuit group. After controlling for time over the six hour study, the use of the pediatric oxygenator resulted in a mean increase in pHb of 14.5 mg/dL (95% confidence interval 2.3, 26.7) when compared to the adult oxygenator. Median pHb rose in the pediatric oxygenator group 4.6 mg/dL from baseline (interquartile range 2.9, 10.8) in hour 1 to 32.2 mg/dL (16.4, 54.7) in hour 6. Use of the adult oxygenator resulted in an increase in pHb 7.4 mg/dL from baseline (2.7, 11.9) in hour 1 to 8.1 mg/dL (2.5, 18.2) in hour 6 (Figure 3A).
Figure 3A. Use of a smaller dimension pediatric oxygenator was associated with a greater increase in plasma hemoglobin.
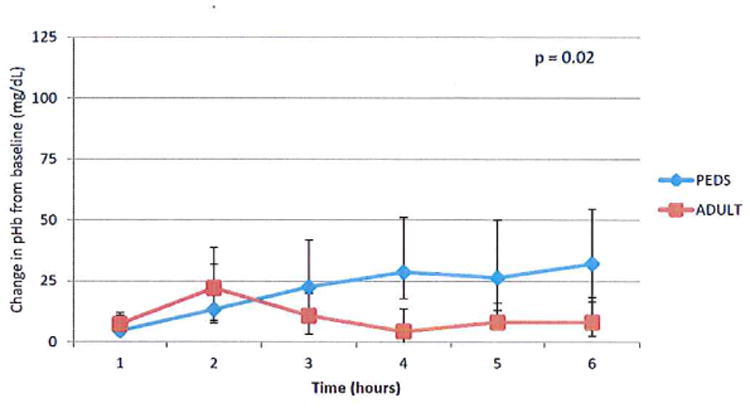
Plasma samples were obtained hourly from both the ECMO circuit and the time based hemolysis control that was maintained at 36°C. Hemoglobin was measured by a Quantichrom assay kit (BioAssay Systems). Hemolysis attributed to the ECMO circuit was defined as the change in pHb at each hour standardizing to pHb measured in time based hemolysis control. Median values (25th, 75th percentile) are displayed. N=4.
After controlling for time, the addition of an in line hemofilter failed to show a statistically significant difference in the degree of hemolysis in comparison to the adult oxygenator circuit (8.5 mg/dl, 95% confidence interval -3.6, 20.7). Median pHb rose in the adult with hemofilter group 6.2 mg/dL from baseline (5.1,31) in hour 1 to 15.4 (-2,30.4) at hour 6 (Figure 3B). At hours 2 and 4, we removed 50 mL of ultrafitrate from the adult with hemofilter circuits. There was no difference in the pHb level in the ultrafiltrate at hour two and hour four (mean 17.9 and 17.6 mg/dL respectively; SD ± 0.5). Furthermore, the difference in the degree of hemolysis did not change between the adult and adult with hemofilter circuits after hemofiltration at hour 3 (15.2 mg/dl, 95% confidence interval -5.6, 28.8) and hour 5 (6.3 mg/dL, 95% confidence interval of- 10.9, 23.5).
Figure 3B. Plasma hemoglobin levels were not significantly increased with the use of the hemofilter when controlled for ECMO run time.
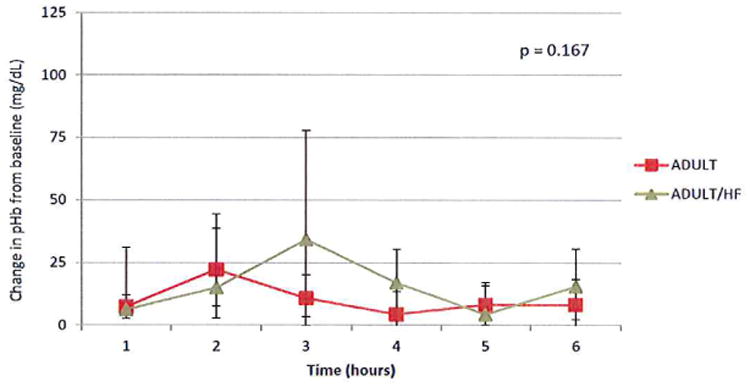
Plasma samples were obtained hourly from the adult circuit in the presence and absence of the hemofilter. Hemoglobin values were measured and standardized to the time based hemolysis control hemoglobin values. Median values (25th, 75th percentile) are displayed. N=4.
Our circuits were built to replicate our clinical practice, and at a flow rate of one liter/minute consistently yielded a premembrane pressure of 120 ± 20 mmHg. At this flow rate there was a significant difference in the postmembrane pressure in comparing the pediatric and adult oxygenator circuit groups resulting in a greater pressure gradient (Table IA). No difference was seen in the postmembrane oxygenator pressures in comparing the adult oxygenator and the adult oxygenator circuits with in line hemofiltration (Table IB). Further, the number of revolutions/minute required to generate this flow rate was not significantly different in any of the circuits tested.
Table I. Use of the smaller dimension pediatric oxygenator resulted in greater negative pressure.
Pressure readings were measured pre and post oxygenator in mmHg. All circuits were circulated at a flow rate of 1 liter/minute. Median values (25th, 75th percentile) are displayed. N=4.
| Table 1A | |||
|---|---|---|---|
| PEDIATRIC | ADULT | p-value | |
| Premembrane | 126 (122,131) | 121 (117, 124) | 0.09 |
| Postmembrane | 110 (104,134) | 118 (113, 120) | 0.05 |
| Pressure Gradient | 16 (15,21) | 3 (3,4.5) | <0.05 |
| Revolutions per minute | 1497 (1468,1521) | 1394 (1379,1420) | 0.18 |
| Table 1B | |||
| ADULT/HF | ADULT | p-value | |
| Premembrane | 115 (107,137) | 121 (117, 124) | 0.42 |
| Postmembrane | 113 (105,134) | 118 (113, 120) | 0.23 |
| Pressure Gradient | 2 (1,4) | 3 (3,4.5) | 0.01 |
| Revolutions per minute | 1366 (1307,1510) | 1394 (1379,1420) | 0.67 |
Given the association between the duration of pRBC storage time and the presence of hemolysis, we assessed the age of the blood used for our study. There was not a statistical difference in the median age in days of the blood used for the pediatric (32; 25,34), adult (20; 12.5, 31), and adult with hemofilter (27; 13,32.5) circuits (p=0.28).
Blood gas and chemistries were analyzed on the prime of each circuit prior to the initiation of circulation. Circuit parameters including pH, PCO2, pO2, pH, hematocrit and potassium values were equivalent at the start of the study (Table II).
Table II. Comparison of initial circuit parameters.
A blood gas was performed by the clinical laboratories of Duke University Medical Center for each circuit configuration after five minutes of circulation. Median values (25th, 75th percentile) are displayed. N=4.
| ADULT | PEDIATRIC | p-value | |
|---|---|---|---|
| PH | 7.41 (7.37,7.43) | 7.41 (7.38,7.43) | 1.00 |
| pCO2 | 39 (36,45) | 37 (35,40) | 0.56 |
| pO2 | 201 (200,206) | 205 (197,210) | 0.56 |
| Bicarbonate | 25 (24,26) | 23.5 (21.5,25.5) | 0.37 |
| Potassium | 7.5 (7.3,7.6) | 7.6 (6.7,8.4) | 0.77 |
| Glucose | 392 (376,446) | 450 (322,522) | 0.77 |
| Lactic Acid | 16.3 (12.5,18) | 9 (7.5,14.2) | 0.25 |
| Hematocrit | 36 (32.5,38) | 31 (29.5,34) | 0.19 |
| Hemoglobin | 11.7 (10.7,12.6) | 9.7 (9.5,10.9) | 0.08 |
| Oxygen Saturation | 100 (99,100) | 100 (99,100) | 0.62 |
| ADULT | ADULT/HF | p-value | |
| PH | 7.41 (7.37,7.43) | 7.39 (7.28,7.42) | 0.55 |
| pCO2 | 39 (36,45) | 38 (30,52) | 0.88 |
| pO2 | 201 (200,206) | 195 (170,208) | 0.66 |
| Bicarbonate | 25 (24,26) | 23.5 (15,33.5) | 0.77 |
| Potassium | 7.5 (7.3,7.6) | 7.4 (7,8) | 0.77 |
| Glucose | 392 (376,446) | 294 (275,328) | 0.02 |
| Lactic Acid | 16.3 (12.5,18) | 9.8 (7.4,10.9) | 0.06 |
| Hematocrit | 36 (32.5,38) | 31 (29.5,31) | 0.14 |
| Hemoglobin | 11.7 (10.7,12.6) | 9.9 (9.4,10.2) | 0.08 |
| Oxygen Saturation | 100 (99,100) | 98 (97,98) | 0.04 |
Discussion
Our results demonstrate that in an ex vivo ECMO model, the use of the smaller dimension pediatric oxygenator compared to the larger dimension adult oxygenator generated a greater increase in pHb (p = 0.02). While the smaller dimension oxygenator was associated with greater hemolysis, an increase in the overall surface area and length of the adult circuit with the inclusion of a hemofilter in the circuit, did not result in a change in pHb over the 6 hour study period (p=0.167).
Hemolysis is of particular concern given its association with AKI following cardiopulmonary bypass in children.15-17 Although a direct clinical comparison cannot be made with our ex vivo study given the duration of time of the study and the lack of inherent scavenging mechanisms present in humans, a recognition of the impact of circuit components on hemolysis suggests a need for further study to more clearly delineate the clinical consequences of this hemolysis.
The increase in hemolysis with the pediatric oxygenator circuit was associated with a greater difference in the pressure gradient to generate equivalent flow rates. In addition to the smaller dimension of the pediatric oxygenator, a smaller connector was required to incorporate it into the circuit (see Figure 1). This increase in pressure may induce hemolysis by the Bernoulli effect via a high pressure jet or by suction.18 However, the relatively low gradient in either circuit suggests that other factors are also likely to play a role.
We did not see an increase in hemolysis with the addition of a hemofilter. These results do not correlate with those reported earlier that demonstrate that prolonged use of continuous renal replacement therapy generates significant hemolysis.15 Such hemolysis has been thought to be due to exposure of blood to additional non-endothelialized surfaces.11,12,15,19 The discrepancy in our findings may be secondary to the small fraction of the total blood flow of the circuit that crossed the hemofilter. In addition, our six hour study duration in comparison to their average run time of 161 ± 68.4 hours may have precluded us from making similar observations.
There are several limitations to our study. First, the six hour study duration is shorter than the average run time (182.4 ± 40.8 hours over the last ten years) for pediatric and neonatal ECMO.1 The duration of our study was limited by hemolysis that occurs in stored blood at physiologic temperature even in the absence of manipulation through an ECMO circuit. We attempted to minimize the impact of basal hemolysis by using a time-based hemolysis control maintained in a 36°C water bath. Second, the sample size for each circuit type was small and may have limited the degree of significance in the hemolysis seen. Third, the study design was an ex vivo model consisting only of stored blood components, which have been shown to be more fragile and prone to hemolysis.13,14 Fourth, while we chose to assess pHb as our measure of hemolysis, the use of a modified index of hemolysis system would provide a more standardized measure of hemolysis that would allow some comparison with previous studies. However, we felt it that this measure didn't accurately account for the baseline degree of hemolysis that occurs over time in the absence of the ECMO circuit. 20-22 Fifth, the degree of turbulence at the connection points was not measured but may be a potential cause for hemolysis. Ongoing investigation to determine the source of hemolysis in the circuit includes the use of flow monitoring at the connection sites of the oxygenators as well as investigation of the individual components. Finally, our investigation focused on the ECMO circuit and pump and did not account for independent patient factors, such as cannula size, flow rate, degree on venous collapse on the cannula, change in illness, and/or development of thrombocytopenia that may affect the degree of hemolysis. Additional investigation will be required to determine the impact of patient interactions on ECMO-induced hemolysis and its ultimate clinical importance.
In summary, greater hemolysis and increased negative pressure were generated with the use of the smaller dimension Quadrox-iD Pediatric Oxygenator when compared to the larger dimension Quadrox-iD Adult oxygenator. We were unable to demonstrate increased hemolysis associated with greater non-endothelialized surface area with the addition of a hemofilter to the larger oxygenator circuit. While we speculate that the difference in the pressure gradient required to maintain equivalent flow rates with the pediatric oxygenator may contribute to hemolysis, the exact mechanism that precipitates the hemolysis, the relative contribution of each component, its impact in vivo, and the clinical impact of this excess level of pHb on critically ill children requiring ECMO warrant further investigation.
Abbreviations
- ECMO
Extracorporeal Membrane Oxygenation
- AKI
Acute Kidney Injury
- pHb
plasma hemoglobin
- pRBCs
packed red blood cells
Footnotes
Conflicts of Interest: The Maquet Getinge Group (Getinge Group, Rastatt, Germany) donated the Quadrox-iD Pediatric and Quadrox-iD Adult oxygenators.
References
- 1.ECLS Registry Report International Summary [database] Ann Arbor, Ml: Extracorporeal Life Support Organization; 2012. Updated January 2012. [Google Scholar]
- 2.Kumar TK, Zurakowski D, Dalton H, et al. Extracorporeal membrane oxygenation in postcardiotomy patients: Factors influencing outcome. J Thorac Cardiovasc Surg. 2010;140:330–336. doi: 10.1016/j.jtcvs.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Smith AH, Hardison DC, Worden CR, Fleming GM, Taylor MB. Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J. 2009;55:412–416. doi: 10.1097/MAT.0b013e31819ca3d0. [DOI] [PubMed] [Google Scholar]
- 4.Swaniker F, Kolla S, Moler F, et al. Extracorporeal Life Support Outcome for 128 Pediatric Patients with Respiratory Failure. Journal of Pediatric Surgery. 2000;35:197–202. doi: 10.1016/s0022-3468(00)90009-5. [DOI] [PubMed] [Google Scholar]
- 5.Steinhorn RH, Isham-Schopf B, Smith C, Green TP. Hemolysis during long-term extracorporeal membrane oxygenation. J Pediatr. 1989;115:625–630. doi: 10.1016/s0022-3476(89)80299-9. [DOI] [PubMed] [Google Scholar]
- 6.Skogby M, Meilgren K, Adrian K, Friberg LG, Chevalier JY, Mellgren G. Induced cell trauma during in vitro perfusion: a comparison between two different perfusion systems. Artif Organs. 1998;22:1045–1051. doi: 10.1046/j.1525-1594.1998.06064.x. [DOI] [PubMed] [Google Scholar]
- 7.Cappellini MD. Coagulation in the pathophysiology of hemolytic anemias. Hematology. 2007;1:74–78. doi: 10.1182/asheducation-2007.1.74. [DOI] [PubMed] [Google Scholar]
- 8.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma haemoglobin. A novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 9.Byrnes J, McKamie W, Swearingen C, et al. Hemolysis during cardiac extracorporeal membrane oxygenation: a case-control comparison of roller pumps and centrifugal pumps in a pediatric population. ASAIO J. 2011;57:456–461. doi: 10.1097/MAT.0b013e31822e2475. [DOI] [PubMed] [Google Scholar]
- 10.Barrett CS, Jaggers JJ, Cook EF, et al. Pediatric ECMO outcomes: comparison of centrifugal versus roller blood pumps using propensity score matching. ASAIO J. 2013;59:145–151. doi: 10.1097/MAT.0b013e31828387cd. [DOI] [PubMed] [Google Scholar]
- 11.Bearss MG. The relationship between membrane oxygenator blood path pressure drop and hemolysis: an in-vitro evaluation. J Extra Corp Technol. 1993;25:87–92. [Google Scholar]
- 12.Williams AR. Shear-induced fragmentation of human erythrocytes. Biorheology. 1973;10:303–311. doi: 10.3233/bir-1973-10303. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama T, Hanaoka K. Hemolysis in stored red blood cell concentrates: modulation by haptoglobin or ulinastatin, a protease inhibitor. Crit Care Med. 2001;29:1979–1982. doi: 10.1097/00003246-200110000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Sawant RB, Jathar SK, Rajadhyaksha SB, Kadam PT. Red cell hemolysis during processing and storage. Asian J Transfus Sci. 2007;1:47–51. doi: 10.4103/0973-6247.33446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betrus C, Remenapp R, Charpie J, et al. Enhanced hemolysis in pediatric patients requiring extracorporeal membrane oxygenation and continuous renal replacement therapy. Ann Thorac Cardiovasc Surg. 2007;13:378–383. [PubMed] [Google Scholar]
- 16.Zager R, Gamelin L. Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol. 1989;256:F446–F455. doi: 10.1152/ajprenal.1989.256.3.F446. [DOI] [PubMed] [Google Scholar]
- 17.Mamikonian L, Mamo L, Smith P, et al. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children. Pediatr Crit Care Med. 2014;15:e111–e119. doi: 10.1097/PCC.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toomasian JM, Bartlett RH. Hemolysis and ECMO pumps in the 21st Century. Perfusion. 2011;26:5–6. doi: 10.1177/0267659110396015. [DOI] [PubMed] [Google Scholar]
- 19.Gbadegesin R, Zhao S, Charpie J, et al. Significance of hemolysis on extracorporeal life support after cardiac surgery in children. Pediatr Nephrol. 2009;24:589–595. doi: 10.1007/s00467-008-1047-z. [DOI] [PubMed] [Google Scholar]
- 20.Thiara A, Hoel T, Kristiansen F, et al. Evaluation of oxygenators and centrifugal pumps for long-term pediatric extracorporeal membrane oxygenation. Perfusion. 2007;5:323–326. doi: 10.1177/0267659107086270. [DOI] [PubMed] [Google Scholar]
- 21.ASTM F1841-97. American Society for Testing and Materials; West Conshohocken, PA: 2004. Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps. [Google Scholar]
- 22.Naito K, Mizuguchi K, Yukihiko N. The need for standardizing the index of hemolysis. Artif Organs. 1994;18(1):7–10. doi: 10.1111/j.1525-1594.1994.tb03292.x. [DOI] [PubMed] [Google Scholar]


