Abstract
OBJECTIVE
• To evaluate the role of bladder sensory purinergic P2X3 and P2X2/3 receptors on modulating the activity of lumbosacral neurones and urinary bladder contractions in vivo in normal or spinal cord-injured (SCI) rats with neurogenic bladder overactivity.
MATERIALS AND METHODS
• SCI was induced in female rats by complete transection at T8 – T9 and experiments were performed 4 weeks later, when bladder overactivity developed. Non-transected rats were used as controls (normal rats).
• Neural activity was recorded in the dorsal horn of the spinal cord and field potentials were acquired in response to intravesical pressure steps via a suprapubic catheter. Field potentials were recorded under control conditions, after stimulation of bladder mucosal purinergic receptors with intravesical ATP (1 mm), and after intravenous injection of the P2X3/P2X2/3 antagonist AF-353 (10 mg/kg and 20 mg/kg).
• Cystometry was performed in urethaneanaesthetised rats intravesically infused with saline. AF-353 (10 mg/kg) was systemically applied after baseline recordings; the rats also received a second dose of AF-353 (20 mg/kg). Changes in the frequency of voiding (VC) and non-voiding (NVC) contractions were evaluated.
RESULTS
• SCI rats had significantly higher frequencies for field potentials and NVC than NL rats. Intravesical ATP increased field potential frequency in control but not SCI rats, while systemic AF-353 significantly reduced this parameter in both groups.
• AF-353 also reduced the inter-contractile interval in control but not in SCI rats; however, the frequency of NVC in SCI rats was significantly reduced.
CONCLUSION
• The P2X3/P2X2/3 receptors on bladder afferent nerves positively regulate sensory activity and NVCs in overactive bladders.
Keywords: overactive bladder, spinal cord injury, purinergic receptor, afferents, electrophysiology
INTRODUCTION
More than 10% of the USA population with urinary incontinence have overactive bladder (OAB) symptoms, and < 30% continue antimuscarinic therapy, the most common OAB treatment, after 1 year [1]. OAB symptoms can result from conditions, such as damage to neuronal pathways controlling bladder sensation and filling; myogenic pathologies involving increased detrusor excitability; injury to afferent pathways in the urothelium; or a combination of the above [2,3]. In any case, new pharmacological targets to improve health-related quality of life and effectively treat OAB symptoms are necessary [2,4].
It is now clear that the urothelium is not just a passive barrier but contributes to the activation of the bladder sensory system that transmits, via afferent fibres, the organ status to the brain [3,5]. Urothelial cells are capable of releasing ATP during bladder filling to activate, in paracrine and autocrine means, various ionotropic purinergic (P) receptors of the P2X type that regulate afferent sensory pathways during normal bladder function and perhaps during bladder pain and bladder overactivity [6,7]. It has been shown that in bladder sensory neurones, P2X3 receptors are expressed and function as homomeric or heteromeric (P2X2/3) channels, as indicated by the use of selective antagonists [8,9]. The expression of P2X3 receptors has also been suggested in urothelial cells, where their activation has been implicated in mediating the painful symptoms of interstitial cystitis [10].
Noxious pressure stimuli to the bladder induce urothelial ATP release that activates P2X3/P2X2/3-type receptors located in afferent C-fibres [6,9]. To evaluate whether P2X3/P2X2/3 ionotropic receptors mediate noxious sensory signals in normal rats and in rats with neurogenic induced bladder overactivity, we recorded field potentials in the spinal cord after noxious pressure stimulation and in the presence of high concentrations of intravesical ATP. Previous findings have shown purinergic modulation of noxious bladder afferent signals in normal rat bladders using the non-selective purinergic antagonist suramin [11]. In the present study we investigated whether a specific antagonist for P2X3/P2X2/3 receptors, AF-353 [12], would inhibit the frequency of sensory field potentials evoked by activation of intravesical purinergic receptors or after noxious pressure stimulation. The systemic effects of AF-353 on urodynamic parameters were also evaluated in normal and spinal cord-injured (SCI) rats with neurogenic detrusor overactivity.
MATERIALS AND METHODS
Experimental procedures were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine, and performed in accordance with the guidelines from the National Institutes of Health on the care and use of laboratory animals. Antibiotics were administered to decrease the risk of infection. To minimise pain or discomfort, experiments were performed under general anaesthesia and postoperative pain was mitigated with analgesics.
SCI
Female Sprague-Dawley rats (weighing 250–300 g) were used. SCI rats underwent a complete spinal cord transection at the T8–T9 levels under 2 % isofluorane anaesthesia and were studied 4 weeks later, when hyper-reflexic pathways had fully developed [13]. The rats received buprenorphine (0.5 mg/kg; s.c.) at the time of surgery and every 12 h for a further 24 h to minimise pain. Penicillin (100 mg/kg; i.m.) was administered for 5 days. Bladders from SCI rats were manually expresed twice a day until development of neurogenic bladder overactivity, usually at 10–14 days. Both the SCI and normal control rats underwent the procedures described below.
SURGICAL PREPARATION FOR ELECTROPHYSIOLOGICAL RECORDINGS
SCI or control rats were anaesthetised with 2 % isofluorane. A suprapubic catheter (polyethylene [PE]-90) was implanted at the bladder dome; a second catheter (PE-10) was introduced into the jugular vein for drug infusion, and a tracheostomy was performed to introduce another catheter (PE-120) for artificial ventilation. A laminectomy was done at the L1–L2 spinal level to expose the L6–S1 spinal cord. The cervical spinal cord was transected after local injection with 150 μL of 2% lidocaine and rats were mechanically pithed [11,14]. Rats were transferred to a stereotaxic workstation and immobilised with a spinal cord fixer (Kopf Instruments, Tujunga, CA, USA) on an anti-vibration table. Rats were connected to an artificial ventilator (Harvard Apparatus, Holliston, MA, USA), and the muscle relaxant pancuronium (0.1–0.2 mg/kg; i.v.) was administered.
ELECTROPHYSIOLOGICAL RECORDINGS AND BLADDER PRESSURE
Changes in urinary bladder pressure were monitored through a suprapubic catheter connected to a pressure transducer and a bridge amplifier (both from World Precision Instruments, Sarasota, FL, USA). The same catheter was used to abruptly increase the intravesical pressure by elevating, 60 cm above the bladder level, a container filled with saline solution or containing 1 mm ATP (Sigma, St. Louis, MO, USA). Neural activity was recorded using tungsten electrodes (0.5 MΩ , MicroProbes, Gaithersburg, MD, USA) inserted into the L6–S1 spinal cord. The electrode head-stage was connected to an AC amplifier (both from Grass Telefactor, Warwick, RI, USA). Using a micromanipulator (Kopf, Tujunga, CA, USA), the electrode was placed adjacent to the dorsal-cord artery and lowered for 800–1000 μm towards the dorsal commissure region, where a significant number of bladder afferent neurones project [15]. Bladder sensory responses were identified by suddenly changing the bladder pressure to 60 cmH2O [11]. Once a clear electrophysiological response to increased vesical pressure was obtained, the electrode was kept in that position for the rest of the experiment. Bladder pressure and neural activity were recorded in parallel using the WINDAQ data acquisition software at a sampling rate of 10 kHz (WindaqPro+, DataQ Instruments, Akron, OH, USA).
STIMULATION PROTOCOLS
Spinal cord field potentials were evaluated during intravesical pressure steps from 0 to 60 cmH2O. For stimulation of intravesical purinergic receptors each experiment was divided into three sections. First, bladders were infused with saline solution; second, bladders were filled with a saline solution containing 1 mm ATP; third, bladders were filled with 1 mm ATP during two (10 or 20 mg/kg) consecutive i.v. injections of AF-353 (i.e. 5-[5-iodo-4-methoxy-2-(1-methylethyl)phenoxy]-2,4-pyrimidinediamine hydrochloride, RO-4 hydrochloride). In each case, bladder pressure stimulation was maintained for 1 min followed by 3 min of recovery without pressure. This procedure was repeated twice. The total time of the experiment varied from 4 to 6 h and the interval between AF-353 applications was set at 90 min.
SURGICAL PREPARATION FOR CYSTOMETRY
Control or SCI rats were anaesthetised with 1.0 g/kg urethane (s.c.). A suprapubic catheter was placed through the bladder dome and another catheter was inserted into the jugular vein for drug delivery.
CYSTOMETRIC EVALUATION
The bladder catheter was connected to a syringe pump and saline solution was infused at 0.12 mL/min for 2 h, while the bladder contractions were measured with a pressure transducer (World Precision Instruments) connected to a data acquisition system (DATAQ, DataQ Instruments). Bladder contractions were designated as voiding (VC) or non-voiding (NVC) contractions depending on whether saline was expelled during a bladder contraction [16, 17]. After baseline recordings, AF-353 was administered at a dose of 10 mg/kg, and 2 h later rats received a second application of 20 mg/kg. The frequency of the contractions was calculated during the last 60 min of each condition using the WINDAQ playback program (WindaqEx; DataQ Instruments).
VITAL SIGNS MONITORING
To monitor the vital signs of the rats during electrophysiological recordings, a Mouse-Ox rat tight clip (STARR Life Sciences, Oakmont, PA, USA) was used to non-invasively document changes in arterial oxygen saturation and pulse rate.
DATA ANALYSES AND STATISTICS
Electrophysiological recordings were digitized at a sampling rate of 10 kHz using an AD converter (DI-720, DATAQ). Electrical activity was evaluated over a period of 20 s under each stimulation step from 0 to 60 cmH2O. Non-filtered single ASCCI files were imported into the OriginPro software (Origin Lab Corporation, Northampton, MA, USA) and the peak analyser function was used to determine the frequency of field potentials with a distinguishable magnitude above the background noise. Neural activity was expressed as the number of field potentials per 20 s. For cystometric evaluation, the total number of VC and NVC were calculated during the last hour of the intravesical saline infusion or the AF-353 injections. Bladder pressure changes >5 cmH2O were considered as a contraction event. Field potential panels were generated with OriginPro; other figures were generated with GraphPad Prism software (GraphPad Software, Inc. La Jolla, CA, USA). Data are presented as the mean (sem); statistical comparisons were performed by paired (intragroup) or unpaired (intergroup) t-tests using GraphPad Prism software. A P < 0.05 was considered to indicate statistical significance, and indicated in the corresponding figure legends.
CHEMICALS
Sodium chloride, urethane, ATP. and pancuronium were purchased from Sigma-Aldrich. Isoflurane was purchased from Bulter Animal Health Supply (Dublin, OH, USA) and used combined with oxygen. AF-353 was obtained as a gift from Afferent Pharmaceuticals (San Mateo, CA, USA) and was freshly prepared for each experiment in a solution containing 10% propylene glycol, 10% 0.01 M HCl, and 80% distilled water and sonicated for 5 min before application. Lidocaine (2%) was purchased from APP Pharmaceuticals (Schaumburg, IL, USA).
RESULTS
VITAL PARAMETERS
As previously shown [11], the complete transection of the spinal cord results in some loss of weight in SCI rats after 4 weeks (Table 1). During the electrophysiological procedures the oxygen saturation and heart rate did not change significantly in control or SCI rats (Table 1), suggesting that the systemic application of the P2X3/P2X2/3 receptor antagonist AF-353 did not compromise oxygen levels or cardiac function.
TABLE 1.
Physiological parameters for the SCI and control rats used in the in vivo electrophysiological experiments
| Variable | Control | SCI |
|---|---|---|
| N | 6 | 5 |
| Mean (SEM): | ||
| Body weight, g | 272 (7.5) | 249 (8.7) |
| Oxygen saturation, % | ||
| Initial | 92.8 (4.5) | 97.3 (1.9) |
| End | 90.0 (5.2) | 94.7 (3.2) |
| Heart rate, beats/min | ||
| Initial | 197 (18) | 170 (12) |
| End | 181 (13) | 175 (10) |
BASAL NEURAL ACTIVITY
We measured the basal spinal cord electrical activity at 0 cmH2O bladder pressure in both control and SCI rats. The frequency of field potentials was significantly higher (P < 0.001) in SCI than in control rats (Fig. 1C).
FIG. 1.
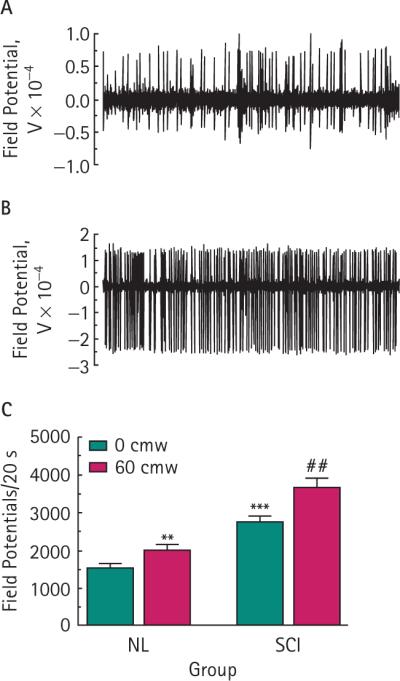
Changes in basal neural activity in normal control (NL) and SCI rats. Representative 5-s traces for field potential activity in NL (A) and SCI (B) rats. SCI rats show a significant baseline activity in comparison with control rats (***P < 0.001; unpaired t-test), and noxious pressure significantly increased neural activity in NL (**P < 0.01) and SCI (##P < 0.01) rats when compared to baseline (C). Bars represent the mean ± sem for six NL and five SCI rats.
PRESSURE AND INTRAVESICAL ATP-INDUCED NEURAL ACTIVITY
A sudden change in bladder pressure from 0 to 60 cmH2O produced a significant increase in the frequency of neural activity in both control (26 %; P < 0.01) and SCI rats (33%; P < 0.01; Fig. 1C). The spinal neural activity at 60 cmH2O was further and significantly enhanced by activation of purinergic receptors by instilling 1 mm ATP into the bladders of control rats (25 %; P < 0.05) but not when instilled into the bladders of SCI rats (10 %; P > 0.05; Fig. 2). For comparison against the effect of saline by itself, data from 60 cmH2O in Fig. 1C was duplicated in Fig. 2.
FIG. 2.
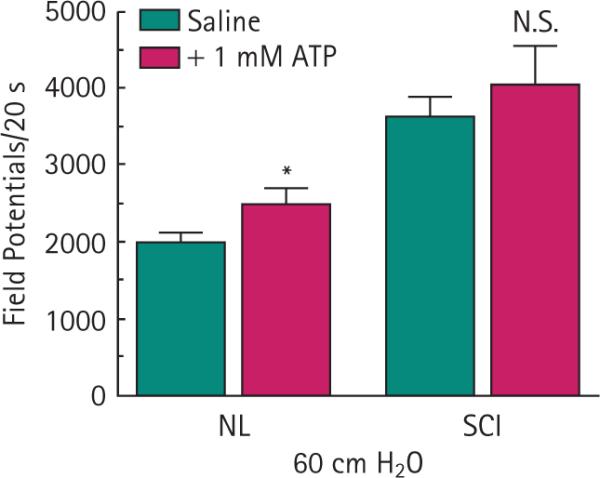
Intravesical ATP effects on afferent neural activity. Noxious bladder pressure combined with 1 mm ATP further stimulated the generation of field potentials in normal control (NL; *P < 0.05) but not SCI rats (not statistically significant.). Data from 60 cm H2O in Fig. 1 were merged for comparison. Bars represent the mean ± sem for six NL and five SCI rats.
INHIBITION OF AFFERENT NEURAL ACTIVITY
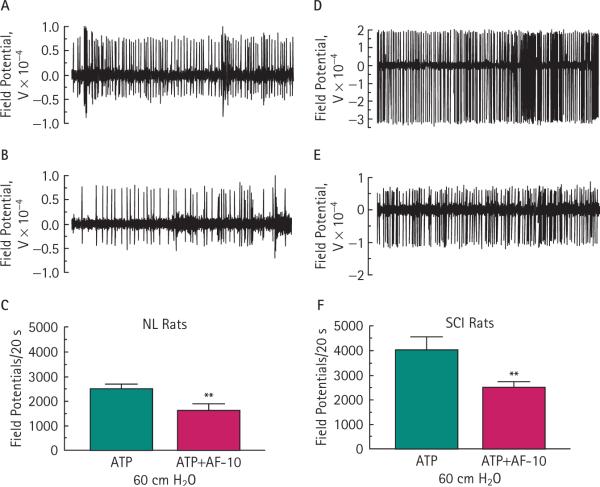
The i.v. injection of the selective P2X3/P2X2/3 antagonist AF-353 applied at a dose of 10 mg/kg (AF-10) decreased the 60 cmH2O bladder pressure and ATP-induced afferent activity by 35 % (P < 0.01) in control and by 38 % (P < 0.01) in SCI rats (Fig. 3C,F, respectively). For comparison and to make the purinergic antagonist effect more evident, data from 60 cmH2O and ATP in Fig. 2 was duplicated in Fig. 3C,F. A second injection of 20 mg/kg AF-353 90 min later did not further inhibit the afferent neuronal response. These results suggest that the purinergic response in both control and SCI rats can be inhibited by the selective P2X3/P2X2/3 antagonist AF-353 (Fig. 3).
FIG. 3.
Inhibition of P2X3/P2X2/3 receptors attenuates the pressure- and ATP-induced increase in afferent neural activity. Representative neuronal activity traces in normal control (NL; A, B) and SCI (D, E) rats before and after i.v. AF-353 (10 mg/kg). Systemic application of AF-353 significantly decreased the generation of field potentials in NL (**P < 0.01; C) and SCI (**P < 0.01; F) rats. Traces represent 5 s of afferent activity. Data from the 60 cmH2O with intravesical ATP from Fig. 2 were merged for comparison. Bars represent the mean ± sem for six NL and five SCI rats.
CYSTOMETRIC EFFECTS OF PURINERGIC INHIBITION
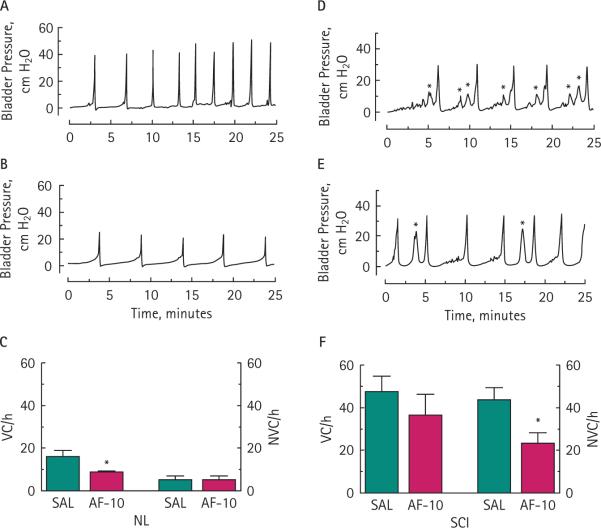
The continuous instillation of saline into the bladders of control rats (six rats) generated well-paced VCs, with a negligible number of NVCs (Fig. 4A,C). In contrast, SCI rats (five rats) exhibited more VCs and NVCs (Fig. 4D,F), perhaps associated with the higher release of ATP as indicated in prior investigations of animal models of neurogenic bladder overactivity [16,18,19]. In control rats the VC but not the NVC frequency was significantly reduced by 43% (P < 0.05) after 10 mg/kg AF-353 i.v. (Fig. 4B,C). In SCI rats, the purinergic antagonist AF-353 did not alter the frequency of VCs but significantly reduced the frequency of NVCs (Fig. 4E,F) by 47 % (P < 0.05). A second injection of 20 mg/kg AF-353 did not further inhibit the bladder contraction frequency in control or SCI rats.
FIG. 4.
Inhibition of P2X3/P2X2/3 receptors decreases VC frequency in normal control (NL) rats and the frequency of NVCs in SCI rats. Representative cystometrograms for NL (A) and SCI (D) rats perfused with a saline solution (NVCs are indicated with asterisks on their top). Systemic application of 10 mg/kg AF-353 decreased the frequency of VCs in NL rats (B) and NVCs in SCI rats (E). The frequency of VCs was statistically significant in NL rats (*P < 0.05; C), whereas the frequency of NVCs was statistically significant in SCI rats (*P < 0.05; F).
DISCUSSION
We used in vivo electrophysiological and cystometric methods to evaluate the effect of the selective P2X3/P2X2/3 antagonist AF-353 on bladder sensory pathways in normal and SCI rats. The present results showed: (i) basal spinal neural activity was amplified in SCI rats compared with normal rats; (ii) in contrast to normal rats, spinal neural activity in SCI rats in response to noxious pressure was not augmented by activation of purinergic receptors with intravesical ATP; (iii) spinal neural activity in response to chemical (ATP) and noxious pressure stimulation was significantly reduced in normal and SCI rats by systemic application of the selective P2X3/P2X2/3 antagonist AF-353; and (iv) in agreement with the electrophysiological results, reflex bladder contractions were markedly decreased in both normal and SCI rats by AF-353.
These results support previous findings that showed purinergic modulation of noxious bladder afferent signals in normal rat bladders using the non-selective purinergic antagonist suramin [11]. The earlier study indicated that spinal neuronal activity is increased in a linear manner with step-wise increases in intravesical pressure and is also enhanced by activation of purinergic receptors with intravesical application of ATP. SCI rats, well-known models of neurogenic bladder overactivity [13,17,20], were used in these experiments to examine changes in lumbosacral spinal activity and the effects of a selective sensory purinergic receptor antagonist compared with changes in neurally intact rats.
Spinal activity under basal conditions was markedly elevated after SCI. These findings were consistent with results of earlier investigations showing enhanced basal urothelial and spinal ATP levels in SCI rats when compared with those in normal rats [18,19]. These results suggest that sensory neural pathways are sensitised after a pathological insult such as SCI, and account not only for the enhanced basal spinal neural activity but also for the prominently increased frequency of field potentials in SCI rats after noxious pressure or chemical stimulation.
ATP is an important signalling transmitter for bladder sensation, acting on purinergic receptors to facilitate the micturition reflex [6]. ATP-activated purinergic receptors in sensory fibres localised in the urothelium mediate physiological and noxious sensation to bladder afferent pathways [21,22]. In experimental rat models of bladder overactivity, the release of ATP is greater in the urothelial layer from diabetic as well as SCI rat bladders when compared with normal rats [16,18,23]. Similarly, the ATP levels in the spinal cord of SCI rats are significantly higher than those found in normal rats [19]. The minimal intravesical effect of ATP in SCI rats might be due to the statistical power in the group size; however, based on previous reports showing higher ATP levels in rats with bladder overactivity [16,18,19], these observations suggest that purinergic terminals are already maximally activated in SCI, such that additional ATP cannot induce further activation (Fig. 2).
Increases in ATP release from the urothelium activate purinergic receptors in nerve terminals of sensory fibres [22,24]. That urothelial ATP levels are elevated in rats with neurogenic bladder overactivity suggests that the higher neural and bladder activity is at least in part a consequence of increased activation of P2X3/P2X2/3 receptors. Indeed, the present electrophysiological recordings from neurones in the spinal cord from SCI rats showed a significantly higher neural activity under baseline conditions.
Although both the efferent and afferent pathways to the bladder can be affected by endogenous or exogenous ATP, under the present experimental conditions (chronic and acute spinal cord transection and mechanical pithing) all efferent neural activity was eliminated. Thus the field potentials we measured in the spinal cord should correspond only to the sensory activity driven by bladder afferent pathways. Consequently, we expected that predominantly signals resulting from activation of sensory purinergic receptors and transmitted by noxious sensing C-fibres would be recorded in the spinal cord [25,26].
In accordance with the increased ‘purinergic tone’, a significant increase in bladder afferent neural activity occurred in SCI rats mediated via P2X3 and P2X2/3 receptors. In contrast, reduced bladder activity was reported in mice lacking P2X3 receptors as a consequence of a decreased purinergic output [27]. Using non-specific [11] or specific antagonists to block purinergic receptors, we can expect the same decrease in purinergic receptor activation and effective inhibition of purinergically driven pathways.
In fact, purinergic inhibition by systemic application of AF-353 is analogous to the cystometric results seen in P2X3 knock-out and P2X3/P2X2/3 double-knockout mice [21,28]. Specific antagonistic effects of the AF-353 compound on the frequency of bladder contractions and spinal neuronal activity in an animal model of neurogenic bladder overactivity strongly suggests that P2X3/P2X2/3 receptors are involved in the pathophysiology of bladder overactivity [7,8]. The present results support further investigations examining the feasibility of using P2X3 antagonists as a therapeutic approach for the treatment of neurogenic bladder overactivity symptoms.
In conclusion, increased levels of ATP in the bladder are associated with neurogenic bladder overactivity and the activation of P2X3 and P2X2/3 receptors. The present observations suggest that high levels of intravesical ATP in a rat model of neurogenic detrusor overactivity positively modulates P2X3 and P2X2/3 receptors to intensify sensory signals and generate NVCs. These data open new avenues for therapeutic approaches to treat neurogenic bladder overactivity, and potentially the OAB syndrome, by focusing on targeting the inhibition of sensory purinergic signals.
What's known on the subject? and What does the study add?
It is well known that urinary bladder sensation requires the activation by ATP of ionotropic purinergic P2X3/P2X2/3 receptors located in bladder afferent C-fibres. Furthermore, in rat models of neurogenic bladder hyperactivity the release of ATP from the bladder urothelium is greater than ATP release in neurally intact rats. Therefore, the activation of purinergic receptors in bladder sensory fibres seems to be a sentinel event for the development of bladder hyperactivity after spinal cord injury.
We found that inhibition of P2X3/P2X2/3 purinergic receptors decreased the frequency of sensory field potentials evoked by activation of bladder noxious pathways. At the same time, the pharmacological blockade of these receptors significantly decreased the frequency of non-voiding contractions in rats with neurogenic bladder hyperactivity. The present study uncovers sensory purinergic receptors as potential therapeutic targets to treat neurogenic bladder hyperactivity, especially when the release of ATP from the urothelium is elevated.
ACKNOWLEDGMENTS
This project was supported by the USA-Department of Veterans Affairs (200600158 to CPS), and the National Institutes of Health (DK-069988 to GTS). We would like to thank the editorial assistance of Carolyn W. Schum from the Scott Department of Urology.
Abbreviations
- OAB
overactive bladder
- P2X
ionotropic purinergic receptor
- SCI
spinal cord-injured
- (N)VC
(non-)voiding contraction
- PE
polyethylene
Footnotes
CONFLICT OF INTEREST
Anthony P. Ford is an employee of Afferent Pharmaceuticals.
REFERENCES
- 1.Levy R, Muller N. Urinary incontinence: economic burden and new choices in pharmaceutical treatment. Adv Ther. 2006;23:556–73. doi: 10.1007/BF02850045. [DOI] [PubMed] [Google Scholar]
- 2.Ashok K, Wang A. Detrusor overactivity: an overview. Arch Gynecol Obstet. 2010;282:33–41. doi: 10.1007/s00404-010-1407-3. [DOI] [PubMed] [Google Scholar]
- 3.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–66. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashim H, Abrams P. Overactive bladder: an update. Curr Opin Urol. 2007;17:231–6. doi: 10.1097/MOU.0b013e32819ed7f9. [DOI] [PubMed] [Google Scholar]
- 5.Birder LA, Wolf-Johnston AS, Chib MK, Buffington CA, Roppolo JR, Hanna-Mitchell AT. Beyond neurons: involvement of urothelial and glial cells in bladder function. Neurourol Urodyn. 2010;29:88–96. doi: 10.1002/nau.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002;59:43–50. doi: 10.1016/s0090-4295(01)01637-5. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura N, Kaiho Y, Miyazato M, et al. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:437–48. doi: 10.1007/s00210-007-0209-z. [DOI] [PubMed] [Google Scholar]
- 8.Kaan TK, Yip PK, Grist J, et al. Endogenous purinergic control of bladder activity via presynaptic P2X3 and P2X2/3 receptors in the spinal cord. J Neurosci. 2010;30:4503–7. doi: 10.1523/JNEUROSCI.6132-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford AP, Cockayne DA. ATP and P2X purinoceptors in urinary tract disorders. Handb Exp Pharmacol. 2011;202:485–526. doi: 10.1007/978-3-642-16499-6_22. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol. 2004;171:448–52. doi: 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- 11.Munoz A, Somogyi GT, Boone TB, Smith CP. Lumbosacral sensory neuronal activity is enhanced by activation of urothelial purinergic receptors. Brain Res Bull. 2011;86:380–4. doi: 10.1016/j.brainresbull.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Gever JR, Soto R, Henningsen RA, et al. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160:1387–98. doi: 10.1111/j.1476-5381.2010.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khera M, Somogyi GT, Salas NA, Kiss S, Boone TB, Smith CP. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord-injured rats. Urology. 2005;66:208–12. doi: 10.1016/j.urology.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 14.Ness TJ, Castroman P. Evidence for two populations of rat spinal dorsal horn neurons excited by urinary bladder distension. Brain Res. 2001;923:147–56. doi: 10.1016/s0006-8993(01)03216-4. [DOI] [PubMed] [Google Scholar]
- 15.Birder LA, de Groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol. 1993;265:R326–33. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- 16.Munoz A, Smith CP, Boone TB, Somogyi GT. Overactive and underactive bladder dysfunction is reflected by alterations in urothelial ATP and NO release. Neurochem Int. 2011;58:295–300. doi: 10.1016/j.neuint.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munoz A, Somogyi GT, Boone TB, Smith CP. Central inhibitory effect of intravesically applied botulinum toxin A in chronic spinal cord injury. Neurourol Urodyn. 2011;30:1376–81. doi: 10.1002/nau.21068. [DOI] [PubMed] [Google Scholar]
- 18.Khera M, Somogyi GT, Kiss S, Boone TB, Smith CP. Botulinum toxin A inhibits ATP release from bladder urothelium after chronic spinal cord injury. Neurochem Int. 2004;45:987–93. doi: 10.1016/j.neuint.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Salas NA, Somogyi GT, Gangitano DA, Boone TB, Smith CP. Receptor activated bladder and spinal ATP release in neurally intact and chronic spinal cord injured rats. Neurochem Int. 2007;50:345–50. doi: 10.1016/j.neuint.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groat WC, Kawatani M, Hisamitsu T, et al. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990;30(Suppl.):S71–7. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- 21.Vlaskovska M, Kasakov L, Rong W, et al. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–7. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birder LA, Kanai AJ, Cruz F, Moore K, Fry CH. Is the urothelium intelligent? Neurourol Urodyn. 2010;29:598–602. doi: 10.1002/nau.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daly DM, Collins VM, Chapple CR, Grundy D. The afferent system and its role in lower urinary tract dysfunction. Curr Opin Urol. 2011;21:268–74. doi: 10.1097/MOU.0b013e3283476ea2. [DOI] [PubMed] [Google Scholar]
- 24.Fry CH, Ikeda Y, Harvey R, Wu C, Sui GP. Control of bladder function by peripheral nerves: avenues for novel drug targets. Urology. 2004;63:24–31. doi: 10.1016/j.urology.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol Urodyn. 2010;29:77–81. doi: 10.1002/nau.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aizawa N, Igawa Y, Andersson KE, Iijima K, Nishizawa O, Wyndaele JJ. Effects of intravesical instillation of ATP on rat bladder primary afferent activity and its relationship with capsaicin-sensitivity. Neurourol Urodyn. 2011;30:163–8. doi: 10.1002/nau.20940. [DOI] [PubMed] [Google Scholar]
- 27.Cockayne DA, Hamilton SG, Zhu QM, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–5. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 28.Cockayne DA, Dunn PM, Zhong Y, et al. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–39. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]