Abstract
Objective
To determine the effects of high-dose vitamin D on insulin sensitivity in Polycystic Ovary Syndrome (PCOS).
Design
Randomized placebo-controlled trial.
Setting
Academic medical center.
Patients
28 PCOS women.
Interventions
Vitamin D3 12,000 International Units or placebo daily for 12 weeks.
Main Outcome Measures
The primary outcome was quantitative insulin sensitivity check index (QUICKI). Secondary outcomes included glucose and insulin levels during a 75-gram oral glucose tolerance test and blood pressure.
Results
Twenty-two women completed the study. Compared to placebo, vitamin D significantly increased 25-hydroxyvitamin D (mean (95% confidence interval) in vitamin D group 20.1 (15.7 to 24.5) ng/ml at baseline and 65.7 (52.3 to 79.2) ng/ml at 12 weeks; placebo 22.5 (18.1 to 26.8) ng/ml at baseline and 23.8 (10.4 to 37.2) ng/ml at 12 weeks). There were no significant differences in QUICKI and other measures of insulin sensitivity, however we observed trends towards lower 2-hour insulin and lower 2-hour glucose. We also observed a protective effect of vitamin D on blood pressure.
Conclusions
In women with PCOS, insulin sensitivity was unchanged with high-dose vitamin D but there was a trend towards decreased 2-hour insulin and a protective effect on blood pressure.
Clinical Trial registration number
ClinicalTrials.gov Identifier: NCT00907153
Keywords: Polycystic Ovary Syndrome, vitamin D, insulin resistance, blood pressure
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder among reproductive-aged women (1). Women with PCOS are at increased risk for insulin resistance, inflammation, obesity, type 2 diabetes (T2DM) and cardiovascular disease. Interestingly, all of these disease states have been linked with vitamin D insufficiency (2).
Vitamin D insufficiency may contribute to the pathogenesis of PCOS by promoting insulin resistance, which increases the risk of T2DM and cardiovascular disease. Several PCOS studies demonstrate that serum 25-hydroxyvitamin D (25OHD) concentrations are negatively correlated with body mass index (BMI), body fat, and insulin resistance (3–5). Additionally, vitamin D insufficiency-induced alterations in intracellular calcium may contribute to ovulatory dysfunction and reproductive abnormalities in PCOS. Animal studies demonstrate that calcium is important in ovarian follicular development and oocyte maturation (6, 7). In an uncontrolled study of 13 PCOS women with vitamin D insufficiency, calcium and vitamin D normalized menstrual irregularities, and resulted in two pregnancies (7).
Evidence that vitamin D may be important in diabetes is further suggested by the link between variations in vitamin D-binding protein and insulin resistance and T2DM (8). Several cross-sectional studies have described an inverse relationship between serum 25OHD and insulin resistance or T2DM (2, 9–11). An inverse relationship also has been demonstrated between the intake of vitamin D supplements and the risk of T2DM in the Nurses’ Health Study (12).
The primary aim of this study was to determine the effects of vitamin D on insulin sensitivity in PCOS women. To achieve this aim, we conducted a randomized controlled trial of vitamin D in PCOS women living in the United States.
Materials and Methods
Participants
Participants were recruited through Medicine and Obstetrics and Gynecology clinics at Penn State Hershey Medical Center from July 2009 to November 2010 (ClinicalTrials.gov Identifier: NCT00907153). Women, ages 18 to 45 years, diagnosed with PCOS were eligible. PCOS was defined using the 1990 National Institutes of Health criteria, chronic hyperandrogenic anovulation (13). Women were excluded if they were pregnant, nursing, or taking vitamin D or calcium supplements in excess of a regular multivitamin, or if they had diabetes, uncontrolled hypertension, untreated hypothyroidism or hyperthyroidism, liver disease, osteopenia, osteoporosis, or secondary causes of hyperandrogenism such as Cushing’s syndrome, androgen secreting tumor, or hyperprolactinemia. Women using oral contraceptive pills (OCP) were allowed to participate if they planned to continue it during the trial; Randomization was stratified by OCP use to provide balanced assignment to the treatment arms of this potential confounder. Women on metformin or thiazolidinediones required a 3-month wash out period prior to randomization. During the study, women were recommended to avoid tanning salons and traveling to latitudes < 35 degrees north. Participants were advised to maintain their usual diet and lifestyle habits including sun exposure, physical activity and dietary intake of Vitamin D and calcium. The Institutional Review Board of the Pennsylvania State University College of Medicine approved the study. Written informed consent was obtained from all participants.
Study design
This was a 12-week randomized, double-blind, placebo-controlled trial. Participants were randomized in a double-blind fashion to receive either Vitamin D3 (cholecalciferol) 12,000 International Units (IU) or placebo daily for 12 weeks. The primary outcome was quantitative insulin sensitivity check index (QUICKI), a validated measure of insulin sensitivity based on fasting insulin and glucose (14). Major secondary outcomes included glucose and insulin levels during a 75-gram oral glucose tolerance test, blood pressure, lipids and androgens.
The randomization scheme used permuted blocks, having fixed blocks of size 2, and was stratified by current OCP use with equal allocation between the two groups. The biostatistician generated the randomization scheme and provided it to the pharmacist who distributed the blinded active and placebo gel caps so that the participants, research coordinator who administered the intervention, and investigators who assessed the outcomes were blinded.
BTR Group, Inc. (Pittsfield, IL, USA) provided active drug and identical placebo gel caps to the pharmacist. The active drug is the same as their nutritional supplement Maximum D3®, but without imprinting on the gel cap. The active gel caps contain 12,000 IU of Vitamin D3 in soy lecithin oil. The placebo is identical but without vitamin D.
Assays
Blood samples were analyzed in the General Clinical Research Center or Core Endocrine Laboratory at Hershey Medical Center using validated assays. Total 25OHD was assayed by the Immunodiagnostic Systems radioimmunoassay (IDS Inc., Scottsdale, AZ). The coefficients of variation were 6, 10, and 12% for low, medium and high levels of 25OHD concentrations, with an overall average of 9.3%.
Procedures
Eligible participants presented in a 12-hour fasting state for a baseline visit. Blood pressure, pulse, anthropometrics and hirsutism scores were recorded. Urine pregnancy test excluded pregnancy. Fasting blood was drawn to measure total and free testosterone, insulin, glucose, 25OHD, intact parathyroid hormone (i-PTH), high sensitive C-reactive protein (hsCRP), vitamin D binding protein and lipid panel. Participants underwent a 75-gram oral glucose tolerance test, where blood samples for glucose and insulin were obtained at 0 and 2-hours and used to calculate the insulin sensitivity index (ISI0,120) (15). Plasma and sera were stored at −80 C until analysis.
Following the baseline evaluation, participants were randomized in a double-blind fashion to receive either vitamin D3 12,000 IU or placebo daily for twelve weeks. At 4, 8 and 12 weeks, serum calcium was checked to exclude hypercalcemia. At the end of 12 weeks, baseline measurements were repeated to assess change from baseline.
For sample size determination, we used data from a pilot study showing the mean (standard deviation (SD)) of QUICKI to be 0.32 (0.04) in PCOS women (16). Anticipating 15% dropout, we calculated that a sample size of 24 subjects would provide 92% statistical power to detect an absolute mean difference in QUICKI of 0.064 between the two treatment arms using a two-sided test having a significance level of 0.05. We randomized 28 subjects as we allowed additional subjects who had been consented and were undergoing screening to continue in the study.
Linear mixed-effects models, extensions of regression that account for within-subject correlation inherent in longitudinal designs, were fit to continuous outcomes to assess the change from baseline to 12 weeks within and between treatment groups. Results were analyzed by intention-to-treat and adjusted for randomization strata of current OCP use as well as for the season the participant was actively participating in the study. Although the intention-to-treat principle was adhered to for this study, all subjects randomized received and ingested the treatment that they were randomly assigned to. Further, a sensitivity analysis was done examining only the women who completed all phases of the trial. No corrections were made for multiple comparisons testing because the analyses were exploratory in nature. All hypotheses tests were two-sided, and a p-value of less than 0.05 for between-group comparisons was considered to indicate statistical significance, with no adjustment for multiple testing of secondary outcomes. All analyses were performed using SAS software, version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
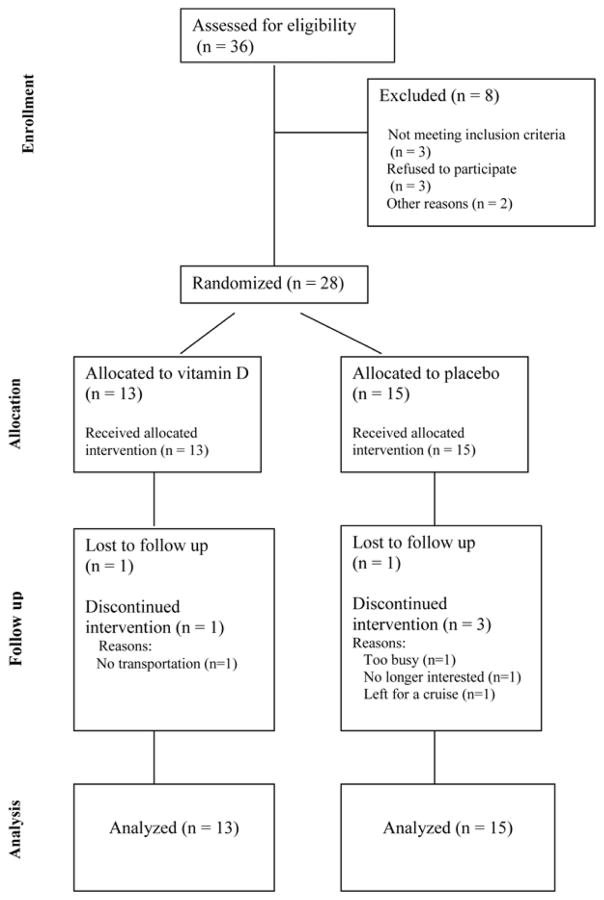
Twenty-eight women were randomized to vitamin D or placebo once daily for twelve weeks. There were 5 current OCP users in each group. Twenty-two women completed the study, eleven in each group. The flow of participants through each stage of the study is shown in Figure 1.
Figure 1.
CONSORT flowchart showing the progress of participants through each stage of this randomized controlled trial.
Both groups were similar in age, BMI and other baseline characteristics (Table 1). The mean age was 28.2 ± 5.2 and 28.7 ± 5.6 years in the vitamin D and placebo groups respectively. The mean BMI was 37.20 ± 4.53 and 35.09 ± 9.81 kg/m2 in the vitamin D and placebo groups respectively. The majority of our subjects were obese. In fact, only 2/15 (13%) of the placebo group were classified as having a normal BMI at baseline, and 0/13 (0%) of the vitamin D group was classified as having a normal BMI at baseline.
Table 1.
Clinical and metabolic profiles of PCOS women before and after treatment.
| Vitamin D group (n=13)
|
Placebo group (n=15)
|
Vitamin D vs. Placebo
|
|||||
|---|---|---|---|---|---|---|---|
| Before Mean (SD) | After Mean (SD) | Within-group Mean Differencea (95% CI) [p-value] | Before Mean (SD) | After Mean (SD) | Within-group Mean Difference a (95% CI) [p-value] | Between-group Mean Difference a (95% CI) [p-value] | |
| Clinical profile | |||||||
| BMI (kg/m2) | 37.20 (4.53) | 37.85 (4.50) | 0.36 (−0.11,0.83) [0.13] | 35.09 (9.81) | 37.62 (10.00) | −0.13 (−0.62,0.35) [0.56] | 0.49 (−0.17.1.15) [0.14] |
| Systolic blood pressure (mm Hg) | 117.46 (10.00) | 118.53 (6.97) | 0.64 (−7.13,8.42) [0.86] | 113.91 (10.21) | 118.41 (10.53) | 4.29 (−3.15,11.74) [0.24] | −3.65 (−14.32,7.02) [0.48] |
| Diastolic blood pressure (mm Hg) | 79.08 (8.28) | 78.97 (5.27) | −0.91 (−4.96,3.14) [0.64] | 74.88 (7.72) | 80.00 (8.31) | 5.60 (1.69,9.52) [0.007] | −6.51 (−12.07, −0.96) [0.02] |
| Vitamin D and PTH profile | |||||||
| 25-hydroxy vitamin D (ng/mL) | 19.95 (9.47) | 67.36 (28.62) | 45.63 (33.50,57.76) [<0.001] | 22.20 (6.86) | 22.45 (7.02) | 1.32 (−10.93,13.58) [0.82] | 44.31 (27.13,61.48) [<0.001] |
| Vitamin D binding protein (mg/dl) | 30.46 (8.53) | 32.53 (6.44) | 1.01 (−1.60,3.62) [0.43] | 31.95 (7.54) | 31.56 (4.99) | −0.55 (−3.14,2.04) [0.66] | 1.56 (−2.08,5.20) [0.38] |
| i-PTH (pg/mL) | 40.81 (27.34) | 15.82 (12.27) | −24.28 (−39.80, −8.76) [0.004] | 33.17 (17.73) | 16.83 (13.20) | −16.45 (−31.40, −1.49) [0.03] | −7.84 (−29.34,13.67) [0.46] |
| Insulin and glucose profile | |||||||
| Fasting Glucose (mg/dl) | 84.92 (9.46) | 83.82 (8.02) | −0.70 (−7.96,6.56) [0.84] | 83.73 (9.33) | 77.64 (14.66) | −6.98 (−14.31,0.34) [0.06] | 6.28 (−3.97,16.54) [0.22] |
| Fasting Insulin (μU/ml) | 26.31 (9.60) | 38.09 (37.60) | 13.04 (−3.96,30.05) [0.12] | 27.13 (15.79) | 28.73 (14.64) | −0.80 (−18.03,16.43) [0.92] | 13.84 (−210.29,37.98) [0.33] |
| 2 hour Glucose (mg/dl) | 122.08 (36.29) | 110.73 (24.84) | −11.66 (−33.22,9.89) [0.27] | 110.07 (23.68) | 111.36 (23.85) | 1.14 (−20.10,22.38) [0.91] | −12.80 (−42.94,17.34) [0.39] |
| 2 hour Insulin (μU/ml) | 214.69 (146.41) | 146.27 (82.83) | −62.02 (−124.5,0.49) [0.05] | 107.07 (55.20) | 122.09 (59.20) | 13.06 (−47.94,74.06) [0.66] | −75.08 (−161.9,11.78) [0.09] |
| Insulin sensitivity index0,120 | 55.55 (23.26) | 62.00 (24.16) | 6.67 (−16.87,30.21) [0.56] | 63.56 (16.37) | 68.05 (41.99) | 5.94 (−17.77,29.65) [0.61] | 0.73 (−32.59,34.06) [0.96] |
| QUICKI | 0.302 (0.014) | 0.296 (0.022) | −0.008 (−0.020,0.004) [0.17] | 0.307 (0.029) | 0.309 (0.039) | 0.009 (−0.003,0.021) [0.14] | −0.017 (−0.034, −0.000) [0.05] |
| HOMA-IR | 5.47 (1.82) | 7.79 (7.37) | 2.57 (−0.90,6.03) [0.14] | 5.80 (3.90) | 5.69 (2.97) | −0.51 (−4.01,2.99) [0.76] | 3.08 (−1.84,7.99) [0.21] |
| Lipid profile and CRP | |||||||
| Total Cholesterol (mg/dl) | 172.00 (42.70) | 177.18 (37.17) | −1.69 (−19.04,15.66) [0.84] | 184.27 (32.52) | 181.09 (40.10) | −1.80 (−19.55,15.95) [0.83] | 0.11 (−24.43,24.64) [0.99] |
| HDL-Cholesterol (mg/dl) | 45.54 (17.60) | 45.73 (18.40) | −0.70 (−6.52,5.12) [0.80] | 37.00 (10.83) | 34.27 (10.73) | 1.23 (−4.68,7.14) [0.67] | −1.93 (−10.12,6.26) [0.62] |
| LDL-Cholesterol (mg/dl) | 98.62 (36.96) | 105.91 (27.69) | −0.12 (−14.70,14.46) [0.99] | 117.33 (28.56) | 116.55 (32.69) | −0.40 (−15.24,14.45) [0.96] | 0.28 (−20.29,20.85) [0.98] |
| Triglycerides (mg/dl) | 139.08 (71.61) | 127.73 (58.09) | −2.21 (−34.11,29.69) [0.89] | 149.47 (85.46) | 150.73 (76.95) | −12.44 (−44.68,19.80) [0.43] | 10.24 (−34.61,55.08) [0.64] |
| hsCRP (mg/L) | 7.95 (5.24) | 9.13 (5.13) | 0.90 (−2.46,4.27) [0.58] | 4.42 (4.34) | 6.33 (7.30) | 2.04 (−1.37,5.45) [0.23] | −1.14 (−5.89,3.62) [0.62] |
| Androgen profile | |||||||
| Total Testosterone (ng/dl) | 54.23 (28.16) | 55.36 (37.28) | 3.01 (−8.05,14.06) [0.57] | 41.27 (17.29) | 50.64 (29.16) | 10.16 (−1.16,21.47) [0.08] | −7.15 (−22.81,8.51) [0.88] |
| Free Testosterone (ng/dl) | 19.08 (15.79) | 19.36 (15.67) | 2.67 (−1.29,6.63) [0.17] | 14.00 (10.88) | 19.45 (14.84) | 5.82 (1.82,9.82) [0.007] | −3.15 (−8.70,2.41) [0.25] |
Model-based differences adjusted for OCP use and season
BMI, body mass index; i-PTH, intact parathyroid hormone; QUICKI, quantitative insulin sensitivity check index; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high sensitive C-reactive protein.
The majority of the subjects were vitamin D deficient at baseline. In fact, only 6 subjects (3 in the placebo group and 3 in the vitamin D group) had normal baseline 25OHD levels ≥ 30 ng/mL. Of the 12 vitamin D deficient subjects in the placebo group, 7 were severely deficient with baseline 25OHD < 20 ng/mL. Of the 10 vitamin D deficient subjects in the vitamin D group, 8 were severely deficient with baseline 25OHD < 20 ng/mL.
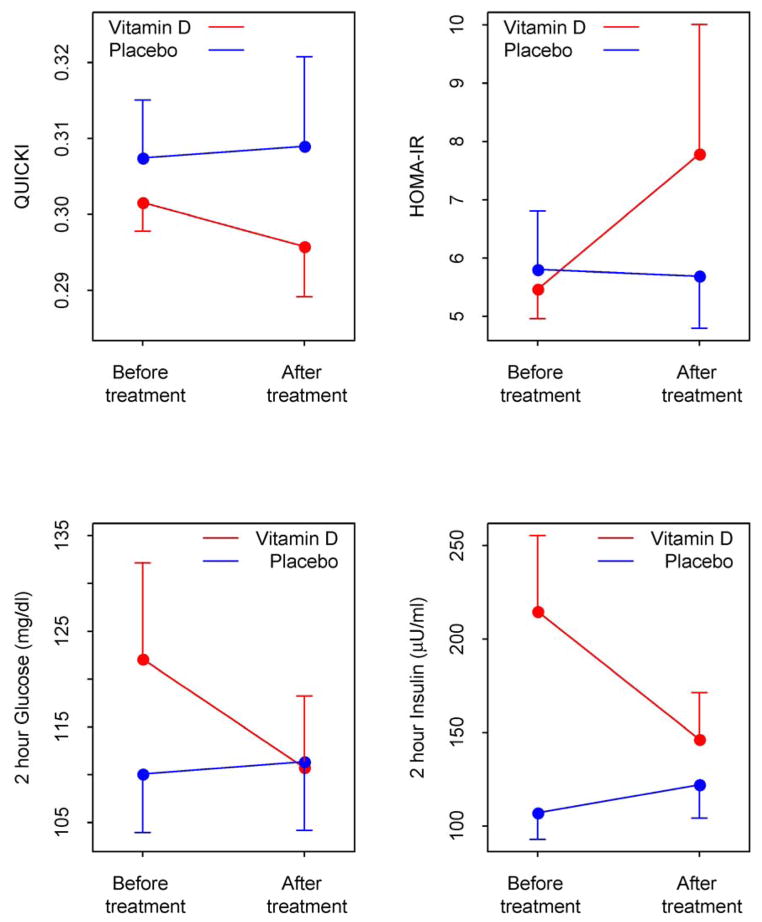
Within the vitamin D group there was a significant increase in mean 25OHD from baseline to 12 weeks (P < 0.001), which confirms compliance with the study medication. Changes in the primary outcome QUICKI, and other measures of glucose and insulin metabolism, homeostatic model assessment of insulin resistance (HOMA-IR) and 2-hour glucose and insulin, are shown in Figure 2. We found no significant difference in QUICKI between the two groups. However, within the vitamin D group there was a reduction in the 2-hour insulin level at 12 weeks, which approached statistical significance (P= 0.05) and a trend towards a reduction in the 2-hour glucose (Table 1). There was no change in the 2-hour insulin or glucose within the placebo group. The between group comparison for the 2-hour insulin approached statistical significance (P= 0.09). There were no significant differences between groups in fasting insulin, fasting glucose, and other measures of insulin sensitivity including HOMA-IR and ISI0,120.
Figure 2.
Changes in QUICKI, HOMA-IR and 2-hour glucose and insulin levels in PCOS women treated with Vitamin D (red ●) or placebo (blue ●). QUICKI, quantitative insulin sensitivity check index; HOMA-IR, homeostatic model assessment of insulin resistance.
We observed a protective effect of vitamin D on diastolic blood pressure in women with PCOS. There were no significant differences in vitamin D binding protein, lipids or androgens. The vitamin D was well tolerated. There were no adverse events.
Recognizing that the study was not powered for subgroup analyses, there does not appear to be any evidence of a strong correlation of BMI with the main outcome of QUICKI. The partial correlation between BMI and the absolute change in QUICKI from baseline to the end of the study, adjusting for OCP usage and change in vitamin D levels was for all subjects combined: correlation coefficient = −0.33 [95% CI: (−0.67, 0.13)]; for the Vitamin D group only: correlation coefficient = −0.22 [95% CI: (−0.77, 0.52)]; and for the Placebo group only: correlation coefficient = −0.19 [95% CI: (−0.76, 0.54)].
Further, we re-analyzed the data using the subset of patients having baseline 25OHD levels < 30 ng/mL. The most notable difference between this “subgroup” analysis and the findings reported in Table 1 is that the change from baseline for QUICKI compared between the two treatment groups approached statistical significance (p=0.05) in Table 1 but was not significant in this subset of vitamin D deficient subjects (p=0.19).
A sensitivity analysis was also conducted examining only the women that completed all phases of the trial (85% of women in the vitamin D group and 73% of women in the placebo group). This sensitivity analysis yielded very similar results (data not shown).
Discussion
In the present study we demonstrate that 12 weeks of high-dose vitamin D significantly increases 25OHD levels in PCOS women. Insulin sensitivity, both with homeostatic and integrated measures, was unchanged despite the trend towards decreased 2-hour insulin. Although it has been hypothesized that vitamin D insufficiency-induced alterations in the calcium flux of pancreatic β cells reduces insulin secretion (17), we found that vitamin D may actually reduce insulin secretion, based on the reduction in 2-hour insulin.
Vitamin D insufficiency induces elevations in PTH, which can adversely affect glucose metabolism (2). As expected i-PTH levels decreased within the vitamin D group. Unexpectedly, i-PTH levels also decreased within the placebo group, possibly due to unintentional increased consumption of dietary calcium in the placebo group as subjects are known to behave differently while participating in a trial. This could explain why there were no significant differences in insulin sensitivity or glucose levels between groups. An interesting observation is the significant increase in diastolic blood pressure in the placebo group, not seen in the vitamin D group which suggests a protective effect of vitamin D on blood pressure.
To our knowledge, there has only been one other randomized, placebo-controlled trial to study the effects of high-dose vitamin D in women with PCOS. The previous study included 50 women with PCOS and used doses of vitamin D3 equivalent to 2,500 IU per day (50,000 IU every 20 days for a total of 3 doses) (18). Their results were similar to ours, showing no significant effect of vitamin D on insulin sensitivity in PCOS despite statistically significant reduction in PTH and increase in 25OHD (18). Another recent uncontrolled study also found no improvement in insulin sensitivity after 3 months of vitamin D in women with PCOS (19). The relatively short duration of vitamin D treatment could account for the lack of effect on insulin and glucose metabolism. In fact, significant reductions in fasting and stimulated glucose and C-peptide levels have been demonstrated in PCOS women after 6 months of vitamin D3, however this was an uncontrolled study (20).
A major strength of our study is its randomized double-blind, placebo-controlled design. According to a recent systematic review there has only been one randomized controlled trial of vitamin D and insulin sensitivity in women with PCOS (21). Therefore, our study, although a relatively small pilot study, adds significantly to the field. An additional strength is that we utilized high doses of vitamin D compared to previous studies. As a result of this pilot study, we now have a more precise estimate of effect size and variability for future trials. A future randomized controlled trial having at least 81% statistical power would require a sample size of 140 subjects to detect a difference in 2-hour insulin of 450 pmol/L (SD=930 pmol/L) between vitamin D and placebo treated participants using a two-sided test having a significance level of 0.05. A future randomized controlled trial having at least 85% statistical power would require a sample size of 100 subjects to detect a difference in diastolic blood pressure of 6.5 mmHg (SD=10.8 mmHg) between vitamin D and placebo treated participants using a two-sided test having a significance level of 0.05. We chose to power the future study using 2-hour insulin and diastolic blood pressure as the primary outcomes as these were the most clinically relevant effects we observed in our pilot study.
In conclusion, high-dose vitamin D significantly increased 25OHD levels and had a protective effect on blood pressure in women with PCOS. Insulin sensitivity, both with homeostatic and integrated measures, was unchanged despite the trend towards decreased 2-hour insulin. It is important to note that the dose of vitamin D used in our study is significantly above the current vitamin D guidelines. There is the potential for serious adverse effects from using such high doses. Until further research becomes available, we do not recommend routine use of such high doses in clinical settings.
Acknowledgments
Funding: This work was supported by Pennsylvania State College of Medicine Dean’s Feasibility Grant. The project described was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000127.
We acknowledge Barb Scheetz, the research coordinator, for her outstanding conduct of the study.
Footnotes
DISCLOSURE STATEMENT: ARK reports ownership of < $5,000 in stock from Merck. The remaining authors have none to declare.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. BTR Group, Inc. provided the study drug and placebo.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Tai K, Need AG, Horowitz M, Chapman IM. Vitamin D, glucose, insulin, and insulin sensitivity. Nutrition. 2008;24:279–85. doi: 10.1016/j.nut.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, et al. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol. 2009;161:575–82. doi: 10.1530/EJE-09-0432. [DOI] [PubMed] [Google Scholar]
- 4.Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, et al. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2006;114:577–83. doi: 10.1055/s-2006-948308. [DOI] [PubMed] [Google Scholar]
- 5.Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280:559–63. doi: 10.1007/s00404-009-0958-7. [DOI] [PubMed] [Google Scholar]
- 6.De Felici M, Dolci S, Siracusa G. An increase of intracellular free Ca2+ is essential for spontaneous meiotic resumption by mouse oocytes. J Exp Zool. 1991;260:401–5. doi: 10.1002/jez.1402600314. [DOI] [PubMed] [Google Scholar]
- 7.Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids. 1999;64:430–5. doi: 10.1016/s0039-128x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 8.Hirai M, Suzuki S, Hinokio Y, Hirai A, Chiba M, Akai H, et al. Variations in vitamin D-binding protein (group-specific component protein) are associated with fasting plasma insulin levels in Japanese with normal glucose tolerance. J Clin Endocrinol Metab. 2000;85:1951–3. doi: 10.1210/jcem.85.5.6569. [DOI] [PubMed] [Google Scholar]
- 9.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–8. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 10.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattila C, Knekt P, Mannisto S, Rissanen H, Laaksonen MA, Montonen J, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–70. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 12.Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–6. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 13.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic ovary syndrome. Boston: Blackwell Scientific; 1992. pp. 377–84. [Google Scholar]
- 14.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 15.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–84. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 16.Raja-Khan N, Shuja SA, Kunselman AR, Hogeman CS, Demers LM, Gnatuk CL, et al. Brachial artery conductance during reactive hyperemia is increased in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;155:49–53. doi: 10.1016/j.ejogrb.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–5. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 18.Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and vitamin D deficiency. Nutr Res. 2012;32:195–201. doi: 10.1016/j.nutres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Pal L, Berry A, Coraluzzi L, Kustan E, Danton C, Shaw J, et al. Therapeutic implications of vitamin D and calcium in overweight women with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28:965–8. doi: 10.3109/09513590.2012.696753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wehr E, Pieber TR, Obermayer-Pietsch B. Effect of vitamin D3 treatment on glucose metabolism and menstrual frequency in polycystic ovary syndrome women: a pilot study. J Endocrinol Invest. 2011;34:757–63. doi: 10.3275/7748. [DOI] [PubMed] [Google Scholar]
- 21.Krul-Poel YH, Snackey C, Louwers YV, Lips P, Lambalk CB, Laven J, et al. The role of vitamin D in metabolic disturbances in polycystic ovary syndrome (PCOS): a systematic review. Eur J Endocrinol. 2013 doi: 10.1530/EJE-13-0617. [DOI] [PubMed] [Google Scholar]