Abstract
Background
Postoperative pancreatic fistulas (POPFs) are potentially morbid complications that often require therapeutic interventions. Distal pancreatectomy performed during cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemoperfusion (HIPEC) puts patients at risk for POPF. The authors hypothesized that POPFs are more severe after CRS/HIPEC than after pancreatectomy alone.
Methods
Clinicopathologic and perioperative details, including POPF by International Study Group of Pancreatic Fistula criteria (ISGPF), and oncologic outcomes for patients undergoing distal pancreatectomy during CRS/HIPEC for peritoneal carcinomatosis of appendiceal (n = 31) or colorectal (n = 23) origin (HIPEC group) were compared with those for patients undergoing minimally invasive or open distal pancreatectomy without HIPEC (n = 66) for locally resectable pancreatic adenocarcinoma (non-HIPEC group).
Results
The incidence of POPF was similar between the HIPEC and non-HIPEC groups (26 %). The severity of POPF according to the ISGPF criteria was significantly worse in the HIPEC group. The HIPEC patients had 13 grade B fistulas and 1 grade C fistula compared with 12 grade A fistulas and 4 grade B fistulas in the non-HIPEC group. The HIPEC patients with POPF did not differ in the extent of their CRS, peritoneal cancer index, length of hospital stay, or other postoperative complications from the the HIPEC patients without POPF. The HIPEC patients with colorectal carcinomatosis who experienced POPF had higher disease recurrence in the first year after CRS/HIPEC than those without POPF.
Conclusion
The findings showed that POPFs are more severe when distal pancreatectomy is combined with CRS/HIPEC. Moreover, selective use of distal pancreatectomy is important during CRS/HIPEC because POPFs may increase early disease recurrence for patients with colorectal carcinomatosis.
Postoperative pancreatic fistulas (POPFs) have traditionally been associated with significant morbidity and mortality.1,2 They can require prolonged hospitalization and therapeutic interventions and may lead to adverse short- and long-term consequences. Usually, POPFs are encountered after formal pancreatic resection as a result of pancreatic duct disruption. However, they also may occur from pancreatic parenchymal violation during peripancreatic surgical procedures (e.g., splenectomy or peritoneal stripping) commonly performed in the setting of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemoperfusion (HIPEC) for the management of disseminated intraabdominal malignancies.
Among clinicians, CRS/HIPEC has gained acceptance for the locoregional management of peritoneal surface malignancies including pseudomyxoma peritonei, malignant peritoneal mesothelioma, and ovarian and colorectal carcinomatosis confined to the peritoneal cavity.3-6 Frequently, CRS requires multivisceral resection and extensive peritoneal stripping to clear disseminated macroscopic disease within the peritoneal cavity. The common operative procedures required for optimal CRS that may predispose to POPF include splenectomy with or without distal pancreatectomy and peripancreatic peritoneal stripping. Several series have reported incidences of POPF after CRS/HIPEC ranging from 4.8 % for all patients to 29 % for patients undergoing splenectomy.7-9
The inherent complexity and aggressive nature of CRS could conceivably affect the clinical course, management, and outcome of POPF, especially considering that these procedures are associated with relatively high overall morbidity (range, 24–74 %)9-15 and mortality rates (3.8 % in the largest reported series to date).16 Moreover, the additional impact of HIPEC on the development and clinical course of POPF is unknown, and this issue has not been addressed in the published literature.
We hypothesized that HIPEC may increase the frequency or severity of POPF after distal pancreatectomy performed during CRS. Furthermore, POPF after HIPEC may lead to increased overall morbidity and mortality for patients and potentially affect oncologic outcomes adversely. We addressed this hypothesis by comparing POPF among patients undergoing distal pancreatectomy as a component of CRS/HIPEC (HIPEC group) for peritoneal carcinomatosis of appendiceal or colorectal origin against POPF among patients undergoing distal pancreatectomy alone (non-HIPEC group) for pancreatic adenocarcinoma.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University of Pittsburgh. We retrospectively reviewed a prospectively maintained database of patients with peritoneal carcinomatosis from colorectal cancer (CRC) (n = 23) or mucinous appendiceal cancer (MAN) (n = 31) who underwent CRS/HIPEC with a distal pancreatectomy between 2001 and 2012 (HIPEC group). We compared these patients and a cohort of 66 patients with pancreatic adenocarcinoma who underwent laparoscopic or open distal pancreatectomy from a retrospective database created during this same period (non-HIPEC group).
The exclusion criteria for patients in the non-HIPEC group specified those with borderline resectable or locally advanced unresectable disease according to the National Comprehensive Cancer Network Guidelines, those receiving preoperative chemotherapy or radiotherapy, and those with incomplete follow-up data.17 The CRS was performed in accordance with techniques described by Bao and Bartlett.18 A standard institutional protocol for HIPEC was initiated after CRS. Using the closed technique, a roller-pump heat exchanger perfusion machine (ThermoChem HT-100; ThermaSolutions, Melbourne, FL, USA) allowed adequate saline flow (> 800 ml/min) and a target intraperitoneal tissue temperature of 42 °C. Mitomycin C 30 mg (5-fluorouracil (5-FU) and oxaliplatin in one patient) was added to the perfusate initially for 60 min followed by an additional 10 mg for a further 40 min.
Our primary end points were the rate and severity of POPF for the HIPEC and non-HIPEC groups. We collected demographic information including age at surgery, American Society of Anesthesiology (ASA) grade, body mass index (BMI), gender, and race. The intraoperative data recorded included the extent of CRS as defined by individual organs resected, peritoneal cancer index (PCI), number of anastomoses and need for colostomy, blood loss, and requirement for transfusion. Frequency and grade (Clavien-Dindo grade 3 or 4) of individual complications,19 length of hospital stay, intensive care unit (ICU) admission, 30- and 90-day readmission, and mortality were noted. Specific attention was paid to the development of POPFs, and if present, they were scored based on the International Study Group of Pancreatic Fistula criteria (ISGPF).20 In this scoring system, biochemical fistulas without clinical sequelae are graded as A. Those requiring any therapeutic intervention (including drainage for more than 3 weeks) are graded as B, and fistulas with severe clinical sequelae are graded as C.
Statistical analysis was performed using R 3.0, and p values lower 0.05 were considered significant. Continuous variables were compared using the two-sample t test, and categorical variables were compared with Fisher’s exact test. Overall survival was calculated from the date of surgery to the date of death. Patients who did not die were censored at their last follow-up visit. Time to recurrence was calculated from the date of surgery to the date of first recurrence. Patients who did not experience recurrence were censored on the date of their last follow-up visit or at the date of death when the cause of death was clearly not related to their cancer. Patients with missing recurrence records or cancer that could not be ruled out as a cause for death were treated as having disease progression, and the time was calculated from the date of surgery to the date of death. The rates of POPF were compared using Fisher’s exact test. Survival times were estimated using the Kaplan–Meier method and compared using the log-rank test.
RESULTS
The HIPEC group comprised 54 patients with a diagnosis of MAN (n = 31) or CRC (n = 23) who underwent CRS/HIPEC and had a distal pancreatectomy as a component of their CRS. Of the 31 MAN patients, 4 (13 %) did not undergo formal pancreatectomy but were found to have a pancreas in the final pathology after splenectomy. This was true for 6 (26 %) of 23 patients with CRC. Mitomycin C was used for chemoperfusion for all the patients except one who was treated with a combination of 5-fluorouracil (5-FU) and oxaliplatin in the perfusate.
The non-HIPEC group included 62 patients who underwent laparoscopic (n = 23) or open (n = 39) distal pancreatectomy for pancreatic ductal adenocarcinoma. The patients in the HIPEC group were younger than those in the non-HIPEC group (median age, 54 vs. 62 years; p < 0.0001). The remaining demographic characteristics were similar between the two groups (Table 1).
TABLE 1.
Baseline characteristics of the HIPEC and non-HIPEC groups
| HIPEC group (n = 54) n (%) |
Non-HIPEC group (n = 62) n (%) |
|
|---|---|---|
| Mean age (years)a | 54 | 62 |
| Gender | ||
| Female | 27 (50) | 40 (65) |
| Mean BMI (kg/m2) | 26.3 | 26.5 |
| Median ASA | 2.8 | 2.7 |
| Diagnosis | ||
| CRC | 23 (43) | |
| MAN | 31 (57) | |
| Pancreatic adenocarcinoma | 62 (100) | |
| Surgical approach | ||
| Open | 54 (100) | 39 (63) |
| Minimally invasive | 0(0) | 23 (37) |
HIPEC hyperthermic intraperitoneal chemoperfusion, BMI body mass index, ASA American Society of Anesthesiology physical status classification CRC colorectal cancer, MAN mucinous appendiceal neoplasm
p Value <0.05
The incidence of POPF was similar between the HIPEC and non-HIPEC groups (26 %). The severity of POPF in the HIPEC group was significantly worse than in non-HIPEC group, as characterized by the ISGPF criteria. The HIPEC patients had 13 grade B fistulas and 1 grade C fistula compared with 12 grade A fistulas and 4 grade B fistulas in the non-HIPEC group (Table 2). Most of the patients in both groups underwent stapled transection of the pancreas (93.5 % in the HIPEC group and 75 % in the non-HIPEC group). However, the method of transection differed significantly between the two groups, with more patients in the non-HIPEC group undergoing pancreatic transection by electrocautery (20 vs. 4.3 %) or by sharp dissection (5 vs 2.2 %; p = 0.036).
TABLE 2.
Rate and severity of postoperative pancreatic fistulas in the HIPEC and non-HIPEC groups
| ISGPF grade A fistula n (%) |
ISGPF grade B fistula n (%) |
ISGPF grade C fistula n (%) |
|
|---|---|---|---|
| HIPEC group–CRC (n = 23) |
0(0) | 6 (5.2) | 1 (0.9) |
| HIPEC group–MAN (n = 31) |
0 (0) | 7 (6) | 0(0) |
| Non-HIPEC group (n = 66)a |
12 (10.3) | 4 (3.4) | 0 (0) |
| Non-HIPEC group (open distal pancreatectomy only) (n = 39)b |
7 (17.9) | 4 (4.3) | 0 (0) |
HIPEC hyperthermic intraperitoneal chemoperfusion, ISGPF international study group of pancreatic fistula criteria, CRC colorectal cancer, MAN mucinous appendiceal neoplasm
Fisher’s exact test p value compared with the HIPEC group–CRC and the HIPEC group–MAN: 0.0006
Fisher’s exact test p value compared with the HIPEC group–CRC and the HIPEC group–MAN: 0.014
The patients who underwent stapled pancreatic transection showed no difference in the rate of staple line reinforcement using either a technique of oversewing or the Seamguard insert (p = 0.2). However, only one patient in the HIPEC group had explicit control of the pancreatic duct compared with 17.8 % of the patients in the non-HIPEC group (p = 0.039), wherein the duct was identified and closed separately from division of the pancreatic parenchyma or reinforcement of the staple line.
Using the PCI 21 as a surrogate for disease burden, we noted no significant difference in the intraoperative PCI in the HIPEC group between the patients who did and those who did not experience POPF (Table 3). When the patients were grouped by low PCI (<15) versus high PCI (>15), there was no difference in the occurrence of POPF. The rate of fistula among the patients with a low PCI did not differ from that of those who did not undergo CRS/HIPEC (p = 0.46) and this was also true for the group of patients with a high PCI (p = 0.4). In the HIPEC group, age, gender, ASA, BMI, length of operation, estimated blood loss, and intraoperative transfusion requirements did not differ significantly between those who experienced POPF and those who did not. The extent of CRS did not differ between those who had POPF and those who did not. The patients who had POPF did not have a higher likelihood of other postoperative morbidities, a longer hospital stay, or higher rates of ICU admission than those who did not experience POPF. The occurrence of Clavien-Dindo grades 3 and 4 complications did not differ between the groups. Finally, the occurrence of POPF was not influenced by tumor grade in the patients undergoing CRS/HIPEC (Table 3).
TABLE 3.
Perioperative characteristics of the HIPEC group of patients with and without POPF (n = 54)
| No POPF n (%) |
POPF n (%) |
p Value | |
|---|---|---|---|
| Omentectomy | 36 (92.3) | 13 (92.9) | 1 |
| Total abdominal colectomy | 4(10) | 1 (7.1) | 1 |
| Small bowel resection | 9 (22.5) | 2 (14.3) | 0.71 |
| Partial gastrectomy | 10 (25) | 3 (21.4) | 1 |
| Stoma | 19 (48.7) | 7(50) | 1 |
| Mean no. of anastamoses (standard deviation) |
0.85 ± 0.83 | 0.71 ± 0.91 | 0.63 |
| Sepsis | 6 (15.4) | 4 (28.6) | 0.43 |
| Postoperative bleed | 2 (5.1) | 1 (7.1) | 1 |
| Delayed gastric emptying | 6(17.1) | 3 (21.4) | 0.7 |
| Ileus >3 weeks | 2 (5.9) | 2 (14.3) | 0.57 |
| Cardiac complication | 3 (7.7) | 4 (28.6) | 0.07 |
| Pulmonary complication | 11 (28.21) | 7(50) | 0.19 |
| Mean ICU days (standard deviation) |
3.6 ± 2.7 | 3.3 ± 2.21 | 0.76 |
| Mean hospital stay (standard deviation) |
18 ± 15.5 | 19.4 ± 8.2 | 0.69 |
| High-grade tumor | 15 (40.5) | 4 (30.8) | 0.74 |
| Mean intraoperative PCI score |
15.2 | 12.9 | 0.16 |
HIPEC hyperthermic intraperitoneal chemoperfusion, POPF postoperative pancreatic fistula, ICU intensive care unit, PCI peritoneal cancer index
For 10 HIPEC patients with POPF (71 %), a peripancreatic drain was placed intraoperatively, whereas the remaining patients required postoperative drain placement. A POPF was suspected in the four patients without operatively placed drains when they experienced decreased appetite, ileus, ascites, and shortness of breath associated with acidosis in the postoperative setting. These patients underwent operative or image-guided placement of drains for the management of their fistulas. The drains were left in place for a median of 39 days (range 31–106 days). Five patients were treated with octreotide to help manage their POPF. Four patients required drainage of associated pleural effusions. Notably, 80 % of the patients with POPF had peritoneal stripping of the left hemidiaphragm, and two had partial left diaphragm resections, which can be associated with pleural effusion. However, the POPF in these patients met the criteria for grade B fistulas, even when their pleural effusions were not considered. Four patients required readmission due to their POPF. One patient experienced fever and leukocytosis from a malfunctioning drain. A second patient had a recurrent collection after inadvertent removal of an operatively placed drain and required image-guided drainage. A third patient experienced pancreatitis, and a fourth patient was readmitted with bleeding from a branch of the superior pancreatic artery in the setting of a grade C POPF that required reoperation.
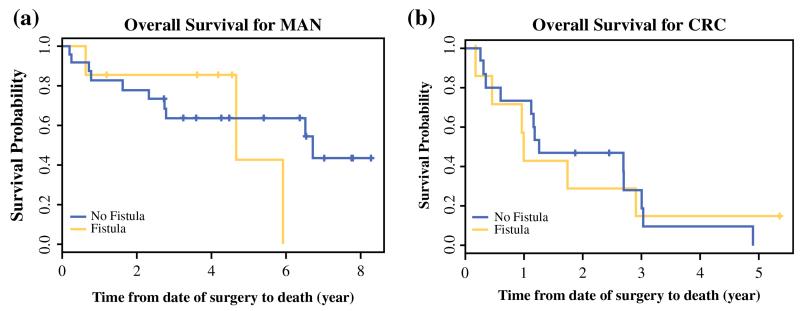
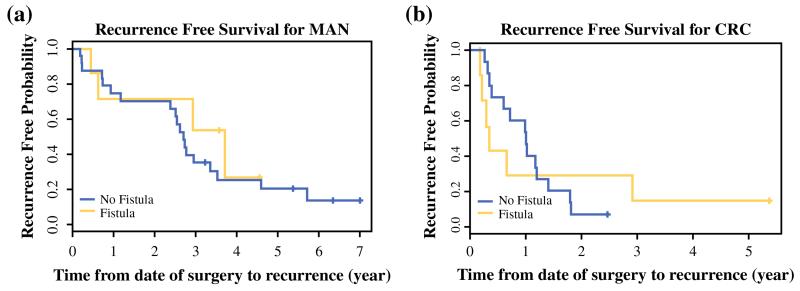
The median follow-up time was 4.2 years. Death occurred for 32 patients, and tumor recurrence was experienced by 43 patients in the HIPEC group overall. The median overall survival (OS) was 2.9 years for the patients who experienced POPF in this group and 3 years for the patients who did not. Similarly, the median recurrence-free survival (RFS) was 1.78 years after CRS/HIPEC for the patients who experienced POPF and 1.79 years for those who did not. Stratification by diagnosis showed that the patients with MAN had an OS of 6.5 years compared with 1.3 years for the patients with CRC. The RFS also differed significantly between the two groups (2.7 years for MAN vs. 1 year for CRC). For the MAN patients with POPF, the median OS was 4.7 years compared with 6.7 years for those without POPF, although this difference was not significant (Fig. 1). The patients with CRC and POPF had a median OS similar to the OS of those without POPF. Interestingly, for the patients with CRC and POPF, recurrence appeared to be more frequent in the first year than for those without POPF, although the difference was not significant. This difference was not apparent among the MAN patients with POPF (Fig. 2).
FIG. 1.
Kaplan–Meier OS curves of patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion who did and did not experience postoperative pancreatic fistulas, stratified by diagnosis. a Of the patients with mucinous appendiceal cancer (MAN), the median OS was 6.7 years for the patients without postoperative pancreatic fistulas (POPFs) (n = 24) and 4.7 years for the patients with POPFs (n = 7). b Of the patients with colorectal cancer (CRC), the median OS was 1.3 years for the patients without POPF (n = 16) and 1 year for the patients with POPF (n = 7)
FIG. 2.
Kaplan-Meier curves of recurrence-free survival for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion who did and did not experience postoperative pancreatic fistulas, stratified by diagnosis. a Of the patients with mucinous appendiceal cancer (MAN), the median progression free survival was 2.7 years for the patients without postoperative pancreatic fistulas (POPFs) (n = 24) and 3.7 years for the patients with POPFs (n = 7). b Of the patients with colorectal cancer (CRC), recurrence-free survival was 1 year for the patients without POPF (n = 16) and 4.1 months for the patients with POPF (n = 7)
DISCUSSION
Cytoreductive surgery is an aggressive locoregional approach that frequently requires multivisceral resections, including pancreatectomy and splenectomy as well as peritonectomy procedures that predispose to POPF. Moreover, the addition of HIPEC to CRS may increase the potential morbidity associated with these complex surgical procedures. Our study is unique in that it compared the characteristics of POPF in patients undergoing distal pancreatectomy as a component of CRS/HIPEC (HIPEC group) for colorectal and appendiceal malignancies and those in patients undergoing distal pancreatectomy without HIPEC (non-HIPEC group) for pancreatic adenocarcinoma. Our data demonstrated that the HIPEC group experienced more severe pancreatic fistulas than the non-HIPEC group, whereas the rate of POPF remained equivalent.
The higher severity of POPF seen in the HIPEC group, as demonstrated by our study, could have been due to several factors. First, patients undergoing CRS/HIPEC frequently require more extensive procedures than those undergoing pancreatectomy alone, perhaps accounting for an inherent risk of increased morbidity. Diffuse peritoneal dissemination of tumors and associated fibrosis often obscure normal tissue planes, leading to challenging dis- section and resection of organ structures such as the pancreas and spleen. Moreover, aggressive stripping of the visceral peritoneum and routine omentectomy may have a negative impact on the ability of tissues to heal well, especially those of the soft pancreatic parenchyma. However, we did not see a difference in the intraoperative PCI or the extent of surgery in the HIPEC patients who did or did not experience POPF.
Second, HIPEC is known to cause physiologic and metabolic derangements, in addition to those caused by CRS alone, which theoretically could contribute to poor healing and ultimately more severe fistulas.22-26 The higher severity of POPF experienced by the HIPEC patients in our study would support this hypothesis.
Third, a high incidence of overall postoperative morbidity among patients undergoing CRS/HIPEC may engender a tendency to leave postoperative drains in place for a longer period, which may inadvertently predispose to a higher rate of grade B or C POPF. Additionally, it is possible that surgeons have a lower threshold for placement of intraoperative drains at the time of CRS/HIPEC given the potential morbidity of undrained pancreatic fistulas in these fragile patients, leading to higher recognition of POPF that otherwise would not have been clinically relevant or detected.
A few studies have reported the incidence of POPF in the setting of CRS/HIPEC. To our knowledge, however, a comparison of fistula severity between patients managed with HIPEC and those not treated with HIPEC has not been reported. The incidence of POPF in our study is in line with what has been reported in the literature after CRS/HIPEC, which ranges from 4.8 % for all patients to as high as 29 % for patients undergoing splenectomy.7-9,27 In one study of 17 patients who underwent distal pancreatectomy and splenectomy for ovarian, peritoneal, or fallopian tube cancers, the patients who experienced POPF (n = 4) had a longer hospital stay than those who did not, but this did not have an impact on the time to chemotherapy.10 In a series of 270 cases by Kusamura et al.,7 the POPF rate was 4.8 % among all the patients undergoing CRS, not only those undergoing splenectomy or distal pancreatectomy. In this series, a higher dose of cisplatin during HIPEC, duration of CRS, extent of CRS, and need for either splenectomy or gastrectomy were independently associated with POPF. These authors also reported frequent grade C POPF (39 %). In another study, duration of operation, need for more than 6 units of blood transfused, and splenectomy were independent factors associated with POPF. Although POPF was associated with longer hospital stay, it did not increase procedure-specific morbidity. These authors also found a high percentage (65 %) of severe fistulas.27
In our series, the development of POPF did not appear to increase the risk of other postoperative complications or to impact significantly the short-term clinical course of patients. However, in concordance with the two larger series described, we did demonstrate a higher frequency of severe fistulas. Like Kusamura et al.,7 we hypothesize that the use of HIPEC may predispose to more severe fistulas due to its cytotoxic effects and possible impact on wound healing, which is supported by their finding that increasing the dose of cisplatin is independently associated with the development of POPF.
The management of patients with POPF in our study was similar to that described in the literature for patients who experience POPF after pancreatic resection alone. External drainage is the treatment of choice, with the possible need for antibiotic, octreotide, and total parenteral nutrition.28-32 In studies reporting POPF in the setting of CRS/HIPEC, fistula drainage also was the mainstay of management, with the addition of the adjuncts previously described when necessary.7,9,10,27
Our data demonstrated increased recurrence in the first year after CRS/HIPEC when POPFs were encountered in patients with CRC, although no adverse impact on OS was seen. An inability to start adjuvant chemotherapy after CRS/HIPEC may be an underlying reason for this higher recurrence rate given the nutritional depletion with the physiologic and physical deconditioning often associated with the development of POPF. Although Kehoe et al.10 reported no delay in the use of chemotherapy for patients with POPF, they were limited by the very few patients with POPF in their series (n = 4). In clinical practice, prolonged use of an intraabdominal drain (median time of 39 days in our study) or the need for total parenteral nutrition would most certainly delay initiation of adjuvant chemotherapy.
We recognize several limitations to this study. First, our study was retrospective, and although the database of HIPEC patients was prospectively maintained, our second cohort of patients arose from a retrospective database, and the patients were not matched. Moreover, we would expect that the extent of surgery and the physiologic impact of CRS/HIPEC is inherently greater compared with distal pancreatectomy alone. Therefore, these two groups were compared only to the extent of POPF rates and severity. Further comparisons were avoided in light of the disparate procedures, as mentioned earlier.
Our sample size was small because CRS/HIPEC is only selectively used as a treatment method. Furthermore, splenectomy or distal pancreatectomy is performed only for a small percentage of patients undergoing CRS/HIPEC. Finally, the ISGPF system was not developed to grade fistulas occurring in the setting of CRS/HIPEC and therefore may not be the ideal metric for evaluating POPF after HIPEC. However, given that it currently is the gold standard for evaluating POPF, it is the best system currently available.
In summary, POPFs after CRS/HIPEC are more severe than when they occur after distal pancreatectomy alone despite a similar frequency of occurrence. We advocate a low threshold for the use of drains after distal pancreatectomy during CRS/HIPEC because POPFs usually are high grade. However, the development of POPF after CRS/HIPEC does not confer increased risk of other postoperative complications or have an impact on the clinical course of patients in the short term. Although POPFs may predispose to early recurrence rates in CRC patients, they do not appear to have an impact on survival. Selective use of distal pancreatectomy is important because POPF may increase disease recurrence for patients with CRC. In the future, the focus should be on techniques for preventing the more severe grade B and C POPFs in the setting of CRS/HIPEC.
ACKNOWLEDGEMENT
S. Downs-Canner and D. Magge were supported by grant T32CA113263 from the National Cancer Institute. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
DISCLOSURE None.
REFERENCES
- 1.Ho CK, Kleeff J, Friess H, Buchler MW. Complications of pancreatic surgery. HPB Oxford. 2005;7:99–108. doi: 10.1080/13651820510028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berberat PO, Friess H, Kleeff J, et al. Prevention and treatment of complications in pancreatic cancer surgery. Dig Surg. 1999;16:327–36. doi: 10.1159/000018743. [DOI] [PubMed] [Google Scholar]
- 3.Verwaal VJ, Bruin S, Boot H, et al. 8-Year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–32. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 4.Chua TC, Robertson G, Liauw W, et al. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol. 2009;135:1637–45. doi: 10.1007/s00432-009-0667-4. [DOI] [PubMed] [Google Scholar]
- 5.Yan TD, Welch L, Black D, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol. 2007;18:827–34. doi: 10.1093/annonc/mdl428. [DOI] [PubMed] [Google Scholar]
- 6.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 7.Kusamura S, Baratti D, Antonucci A, et al. Incidence of postoperative pancreatic fistula and hyperamylasemia after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2007;14:3443–52. doi: 10.1245/s10434-007-9551-0. [DOI] [PubMed] [Google Scholar]
- 8.Austin F, Mavanur A, Sathaiah M, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms. Ann Surg Oncol. 2012;19:1386–93. doi: 10.1245/s10434-012-2241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato K, Tate S, Nishikimi K, Shozu M. Management of pancreatic fistulas after a splenectomy as part of cytoreductive surgery for ovarian cancer. Int J Gynecol Cancer. 2013;23:1506–11. doi: 10.1097/IGC.0b013e3182a0fa66. [DOI] [PubMed] [Google Scholar]
- 10.Kehoe SM, Eisenhauer EL, Abu-Rustum NR, et al. Incidence and management of pancreatic leaks after splenectomy with distal pancreatectomy performed during primary cytoreductive surgery for advanced ovarian, peritoneal, and fallopian tube cancer. Gynecol Oncol. 2009;112:496–500. doi: 10.1016/j.ygyno.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Ihemelandu CU, McQuellon R, Shen P, et al. Predicting postoperative morbidity following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CS + HIPEC) with preoperative FACT-C (Functional Assessment of Cancer Therapy) and patient-rated performance status. Ann Surg Oncol. 2013;20:3519–26. doi: 10.1245/s10434-013-3049-8. [DOI] [PubMed] [Google Scholar]
- 12.Stewart JHt, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13:624–34. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 13.Sugarbaker PH, Kern K, Lack E. Malignant pseudomyxoma peritonei of colonic origin: natural history and presentation of a curative approach to treatment. Dis Colon Rectum. 1987;30:772–9. doi: 10.1007/BF02554625. [DOI] [PubMed] [Google Scholar]
- 14.Tabrizian P, Shrager B, Jibara G, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: outcomes from a single tertiary institution. J Gastrointest Surg. 2014;18(5):1024–31. doi: 10.1007/s11605-014-2477-5. [DOI] [PubMed] [Google Scholar]
- 15.Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863–9. doi: 10.1245/aso.2003.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Levine EA, Stewart JHt, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218:573–85. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magge D, Gooding W, Choudry H, et al. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg. 2013;148:525–31. doi: 10.1001/jamasurg.2013.1673. [DOI] [PubMed] [Google Scholar]
- 18.Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer J. 2009;15:204–11. doi: 10.1097/PPO.0b013e3181a9c6f0. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Portilla AG, Shigeki K, Dario B, Marcello D. The intraoperative staging systems in the management of peritoneal surface malignancy. J Surg Oncol. 2008;98:228–31. doi: 10.1002/jso.21068. [DOI] [PubMed] [Google Scholar]
- 22.Arakelian E, Gunningberg L, Larsson J, et al. Factors influencing early postoperative recovery after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2011;37:897–903. doi: 10.1016/j.ejso.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Raue W, Tsilimparis N, Bloch A, et al. Volume therapy and cardiocircular function during hyperthermic intraperitoneal chemotherapy. Eur Surg Res. 2009;43:365–72. doi: 10.1159/000248164. [DOI] [PubMed] [Google Scholar]
- 24.Rankovic VI, Masirevic VP, Pavlov MJ, et al. Hemodynamic and cardiovascular problems during modified hyperthermic intraperitoneal perioperative chemotherapy. Hepatogastroenterology. 2007;54:364–6. [PubMed] [Google Scholar]
- 25.Esquivel J, Angulo F, Bland RK, et al. Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open “coliseum technique.”. Ann Surg Oncol. 2000;7:296–300. doi: 10.1007/s10434-000-0296-2. [DOI] [PubMed] [Google Scholar]
- 26.Miao N, Pingpank JF, Alexander HR, et al. Cytoreductive surgery and continuous hyperthermic peritoneal perfusion in patients with mesothelioma and peritoneal carcinomatosis: hemodynamic, metabolic, and anesthetic considerations. Ann Surg Oncol. 2009;16:334–44. doi: 10.1245/s10434-008-0253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena A, Chua TC, Yan TD, Morris DL. Postoperative pancreatic fistula after cytoreductive surgery and perioperative intraperitoneal chemotherapy: incidence, risk factors, management, and clinical sequelae. Ann Surg Oncol. 2010;17:1302–10. doi: 10.1245/s10434-009-0898-2. [DOI] [PubMed] [Google Scholar]
- 28.Goh BK, Tan YM, Chung YF, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg. 2008;143:956–65. doi: 10.1001/archsurg.143.10.956. [DOI] [PubMed] [Google Scholar]
- 29.Kazanjian KK, Hines OJ, Eibl G, Reber HA. Management of pancreatic fistulas after pancreaticoduodenectomy: results in 437 consecutive patients. Arch Surg. 2005;140:849–54. doi: 10.1001/archsurg.140.9.849. discussion 854-6. [DOI] [PubMed] [Google Scholar]
- 30.Aranha GV, Aaron JM, Shoup M, Pickleman J. Current management of pancreatic fistula after pancreaticoduodenectomy. Surgery. 2006;140:561–8. doi: 10.1016/j.surg.2006.07.009. discussion 568-9. [DOI] [PubMed] [Google Scholar]
- 31.Pannegeon V, Pessaux P, Sauvanet A, et al. Pancreatic fistula after distal pancreatectomy: predictive risk factors and value of conservative treatment. Arch Surg. 2006;141:1071–6. doi: 10.1001/archsurg.141.11.1071. discussion 1076. [DOI] [PubMed] [Google Scholar]
- 32.Li-Ling J, Irving M. Somatostatin and octreotide in the prevention of postoperative pancreatic complications and the treatment of enterocutaneous pancreatic fistulas: a systematic review of randomized controlled trials. Br J Surg. 2001;88:190–9. doi: 10.1046/j.1365-2168.2001.01659.x. [DOI] [PubMed] [Google Scholar]