Abstract
Objective
Cannabis use has been examined as a predictor of psychosis in clinical high-risk (CHR) samples, but little is known about the impact of other substances on this relationship.
Method
Substance use was assessed in a large sample of CHR participants (N = 370, mean age = 18.3) enrolled in the multisite North American Prodrome Longitudinal Study Phase 1 project. Three hundred and forty-one participants with cannabis use data were divided into groups: No Use (NU, N = 211); Cannabis Use without impairment (CU, N = 63); Cannabis Abuse/Dependence (CA/CD, N = 67). Participants (N = 283) were followed for ≥2 years to determine psychosis conversion.
Results
Alcohol (45.3%) and cannabis (38.1%) were the most common substances. Cannabis use groups did not differ on baseline attenuated positive symptoms. Seventy-nine of 283 participants with cannabis and follow-up data converted to psychosis. Survival analysis revealed significant differences between conversion rates in the CA/CD group compared with the No Use (P = 0.031) and CU group (P = 0.027). CA/CD also significantly predicted psychosis in a regression analysis, but adjusting for alcohol use weakened this relationship.
Conclusion
The cannabis misuse and psychosis association was confounded by alcohol use. Non-impairing cannabis use was not related to psychosis. Results highlight the need to control for other substance use, so as to not overstate the cannabis/psychosis connection.
Keywords: cannabis use, alcohol use, substance use, clinical high-risk, prodromal states, psychosis
Introduction
A large body of research examining the relationship between substance use and psychosis has been generated over the past several decades. Much of this literature has focused on cannabis use, in particular, given that high rates of cannabis use disorders have been found in clinical samples of patients with psychosis (1). A recent meta-analysis found that this is especially true of younger, first episode psychosis cohorts (2). In addition, population- based studies have shown that persons who use cannabis are at increased risk for the development of psychotic symptoms and psychotic disorders (3, 4). In some studies, a dose-dependent relationship was observed, with twice the risk for those individuals with the highest frequency cannabis use (3). Given these findings, it was expected that a similar association would be found in adolescent and young adult clinical samples that are enriched for the development of psychosis based upon clinical and familial characteristics. Approximately 19–39% of these clinical high-risk (CHR) individuals will go on to develop a psychotic disorder (5–7). Thus, CHR individuals, who present to specialized research programmes before the onset of psychosis, but when symptoms are beginning to develop, provide a unique opportunity to study the purported cannabis/psychosis connection.
However, results from CHR studies have been inconsistent. The first study (8) conducted in Australia with 100 ‘ultra high-risk’ young people did not find a significant relationship between self-reported cannabis use or dependence and risk for conversion to psychosis at a 1 year follow-up. Similar negative findings were found in high-risk studies in North America (9–11) and Europe (12, 13). However, a study conducted in California (14) did find that CHR subjects with lifetime cannabis abuse or dependence were significantly more likely to develop psychosis, and Valmaggia et al. (15) found that frequent use beginning before the age of 15 was associated with a higher rate of transition. Some of the studies conducted thus far are compromised by relatively low rates of cannabis use (8, 10) and others by small sample sizes (9, 12–14), indicating that power issues may make drawing conclusions about cannabis use and psychosis risk difficult. In addition, some research groups excluded subjects with current cannabis dependence and rates of lifetime cannabis abuse/dependence were low in most studies, leaving open the possibility that level of use may mitigate the relationship between cannabis use and psychosis.
On a larger scale, two independent CHR consortia have reported findings on the predictors of psychosis conversion in their respective samples. These studies looked at DSM-IV diagnoses of substance abuse more broadly and did not single out any one substance. Reports from the European Prediction of Psychosis Study (7, 16, 17) did not find substance abuse to be a significant predictor of psychosis conversion. The other consortia, utilizing a large sample of CHR individuals, the North American Prodrome Longitudinal Study (NAPLS) (6, 18), found that substance abuse in general, but not cannabis abuse specifically, was one of five significant predictors of psychosis conversion in that sample. This suggests that the relationship between cannabis and psychosis may be overestimated when not examined in conjunction with other substance use. Most high-risk studies to date have made little mention of possible confounding by other substances, although confounding factors such as this have been implicated in the inconsistent results found in several observational population-based studies of cannabis and psychosis (19). Alcohol use in particular appears to be the most likely potential confounder given high lifetime prevalence rates of alcohol use disorders (median = 20.6%) in patients with schizophrenia (20). Baseline rates of alcohol use are also high in CHR samples [16.7%–44.3%; (21)], and some studies have reported high rates of comorbid alcohol and cannabis use (9, 17).
Aims of the study
Therefore, the aims of this study were to more closely examine (i) the rates of substance use, in particular cannabis, in the North American Prodrome Longitudinal Study Phase 1 sample, (ii) the relationship between level of cannabis use and attenuated positive and negative symptoms of psychosis, (iii) the impact of level of cannabis use on conversion to psychosis, and (iv) the impact of other substance use (especially alcohol use) on the association between cannabis and conversion to psychosis.
Material and methods
Overview
The NAPLS 1 collaborative database was formed in 2007 and contains data on a number of clinical, cognitive, and functioning variables collected from eight independent research centers: Emory University, Harvard University, University of California – Los Angeles, University of California – San Diego, University of Calgary, University of North Carolina, Yale University, and The Zucker Hillside Hospital. The centers included in the collaborative database were all funded by NIMH between 2000 and 2003, and the data were collected between 1998 and 2005. This multisite collaboration was possible given that all sites utilized the same diagnostic instrument for high-risk categorization, the Structured Interview for Prodromal Syndromes (SIPS) (22, 23), had established interrater reliability on this instrument after completing a rater-training workshop, and were investigating many similar clinical and functioning domains (18). All sites received Institutional Review Board approval to contribute de-identified data to the common NAPLS database.
Sample
In total, 888 subjects were contributed to the baseline database by the eight sites. Of these, 370 were characterized as CHR based upon the SIPS interview [same sample as Cannon et al. (6)]. Although the SIPS measure contains four subscales, the positive symptom scale, assessing unusual thoughts, suspiciousness, grandiose ideas, perceptual abnormalities, and conceptual disorganization, is the most relevant to diagnosing a high-risk state. To be included in the CHR sample, subjects had to meet the criteria for one of the following syndromes: (i) attenuated positive symptom (APS) syndrome, that is, one or more positive symptoms in the prodromal severity range (moderate to severe, but not psychotic), occurring at a frequency of least once per week and that began or worsened in the year prior to ascertainment; (ii) brief intermittent psychotic syndrome (BIPS), that is, one psychotic level positive symptom that has begun in the 3 months prior to baseline that occurs at least once per month, but less than four times per week, and that spontaneously remits; or (iii) genetic high-risk and deterioration (GRD) syndrome, that is, subject has schizotypal personality disorder or a first degree relative with a psychotic disorder, and a 30% decline in Global Assessment of Functioning (GAF) score over the year prior to ascertainment.
Of 370 participants who met CHR criteria at baseline, 29 (7.8%) did not have data on cannabis use and were excluded from the current analyses. Of the remaining 341 subjects, the majority met criteria for APS (96.8%, n = 330), 10 subjects met BIPS criteria (2.9%), and one met GRD criteria (0.3%). The mean age of the sample is 18.3 (SD = 4.77). Fifty-four APS subjects and four BIPS subjects did not have outcome data (17%), leaving 283 subjects in the outcome analyses. There were more males without outcome data (20.5% vs. 11.5%; χ2 = 4.66, P = 0.031), but no differences between those with and without outcome data on age, race, and rates of substance use (P = 0.09– 0.90). About a third of the sample (28.7%) was included in earlier published reports (10, 14).
Measures
The SIPS subscale items are rated on 7-point anchored scales with scores of 0–2 representing none to mild level severity, scores of 3–5 representing moderate to severe level of severity, and scores of 6 indicating psychotic/extreme level of severity. Other clinical and demographic variables were collected at all sites using similar, although not always the same instruments. Careful attention was paid to the recoding of variables when instruments differed (18, 24).
All participants were interviewed with semistructured interviews using the Structured Clinical Interview for DSM (SCID) (25) or the Kiddie-Schedule for Affective Disorders and Schizophrenia (KSADS) (26) to determine rates of lifetime substance use and substance use disorders. Substances assessed included the following: alcohol, cannabis, hallucinogens, cocaine, stimulants, opioids, and hypnotics. Information on frequency or recency of substance use was not available. Based upon reported level of lifetime use, participants were divided into the following subgroups: no use, use without impairment, abuse and dependence. The latter two categories are based on DSM-IV (27) criteria. The ‘use without impairment’ classification refers to individuals who reported substance use that was not impairing enough to meet the criteria for a substance use disorder.
Conversion to psychosis was based upon meeting the full criteria for Presence of Psychotic Syndrome (POPS) on the SIPS which is defined as having a psychotic level positive symptom that is either seriously disorganizing or dangerous or that occurs for at least 1 h/day for an average frequency of 4 days in the past month. IQ estimates were based on the Wechsler intelligence scales (28–30).
Statistical analyses
Statistical analyses were performed with SPSS version 16 (SPSS Inc, Chicago, IL, USA) statistical software. One-way ANOVA and chi-square tests were conducted to compare groups on demographics and clinical variables. Significant ANOVAs were followed by post hoc tests with Bonferroni correction for multiple comparisons (P = 0.05).
The cumulative probabilities of transition to psychosis were estimated using the Kaplan–Meier method (31) and were compared for three cannabis groups using the log-rank test: No Use (NU), Cannabis Use without impairment (CU), and a combined group of Cannabis Abuse (CA)/Cannabis Dependence (CD). Kaplan–Meier survival method estimated the shape of the survival function during the follow-up interval, the cumulative rate of conversion, and the incidence rates of conversion within successive 6-month epochs. A Cox regression also was conducted to examine the impact of level of cannabis use (compared with NU) on conversion to psychosis after adjusting for potential confounding variables including age at baseline, gender, SOPS positive symptom total, SOPS negative symptom total, and use of alcohol and drugs other than cannabis (P < 0.05).
Results
Baseline comparisons
Alcohol was the most frequently reported substance used (45.3%) followed by cannabis use (38.1%; See Table 1). 85% of cannabis users also reported alcohol use (63.6% alcohol use without impairment and 36.4% alcohol abuse/dependence). Use of drugs other than alcohol and cannabis was infrequent with 87–96% of subjects reporting no use of these substances.
Table 1.
Rates of lifetime substance use in high-risk subjects
| No use | Use without impairment | Abuse | Dependence | |
|---|---|---|---|---|
| Substance | N (%) | N (%) | N (%) | N (%) |
| Alcohol | 187 (54.7) | 110 (32.2) | 34 (9.9) | 11 (3.2) |
| Cannabis | 211 (61.9) | 63 (18.5) | 45 (13.2) | 22 (6.5) |
| Hallucinogens | 298 (87.4) | 36 (10.6) | 4 (1.2) | 3 (0.9) |
| Cocaine | 317 (92.7) | 15 (4.4) | 4 (1.2) | 6 (1.8) |
| Stimulants | 319 (93.5) | 13 (3.8) | 5 (1.5) | 4 (1.2) |
| Opioids | 328 (96.2) | 12 (3.5) | 1 (0.3) | 0 |
| Hypnotics | 329 (96.5) | 8 (2.3) | 3 (0.9) | 1 (0.3) |
| Other | 321 (94.1) | 14 (4.1) | 4 (1.2) | 2 (0.6) |
Total N = 341 for all substances except for alcohol and cocaine (N = 342).
Among cannabis users, 18.5% reported cannabis use without impairment (CU group), 13.2% met criteria for cannabis abuse (CA group), and 6.5% met criteria for cannabis dependence (CD group). The NU group was significantly younger than all three cannabis using groups (P ≤ 0.01), and this group had significantly lower IQ estimates than the CU group (P < 0.01). The CD group had significantly fewer parents with greater than high school level of education compared with the CA and NU groups. There were no significant differences on gender or race (see Table 2). Given the relatively small sample sizes for the CA and CD groups, these two groups were combined for the rest of the analyses.
Table 2.
Demographic characteristics comparing high-risk subjects based on level of lifetime cannabis use
| No cannabis use | Cannabis use w/o impairment |
Cannabis abuse | Cannabis dependence |
||||
|---|---|---|---|---|---|---|---|
| (N = 211) | (N = 63) | (N = 45) | (N = 22) | Statistic | P | Post hoc comparisons | |
| Age At baseline – Mean (SD) | 17.16 (4.49) | 19.77 (4.28) | 19.87 (5.08) | 21.82 (4.63) | F(3,340) = 12.83 | <0.001 | NU < CU, CA, CD |
| Gender – % Male | 59.2% | 60.3% | 73.3% | 63.6% | χ2 = 3.20 | 0.362 | – |
| Estimated IQ – Mean (SD)* | 103.20 (16.43) | 113.20 (16.88) | 107.00 (15.14) | 106.00 (20.76) | F(3,243) = 4.14 | 0.007 | NU < CU |
| Parental education – % > High school† | 83.2% | 71.4% | 85.3%% | 52.6% | χ2 = 12.51 | 0.006 | CD < CA, NU |
| Race – % Caucasian | 74.4% | 81% | 86.7% | 72.7% | χ2 = 3.97 | 0.265 | – |
NU, no cannabis use; CU, cannabis use without impairment; CA, cannabis abuse; CD, cannabis dependence.
Estimated IQ: Total N = 244.
Parental education: Total N = 276.
In terms of SIPS high-risk symptoms, there were no significant differences between cannabis use groups on attenuated positive symptoms at baseline (see Table 3). For attenuated negative symptoms, the NU group had significantly higher scores on Social Anhedonia compared with the CU group and significantly higher scores on the Decreased Expression of Emotion item compared with the CU and the combined CA/CD group (Table 3). However, these results did not remain significant with correction for multiple comparisons (P = 0.074; P = 0.057; P = 0.090 respectively). The CU group had significantly higher scores than the NU group on Experience of Emotion which remained significant with Bonferroni correction (P = 0.038).
Table 3.
Baseline cannabis use and SIPS attenuated positive and negative symptoms
| No use (N = 211) |
Cannabis use w/o impairment (N = 63) |
Cannabis abuse or dependence (N = 67) |
F | P | |
|---|---|---|---|---|---|
| Unusual Thought Content | 3.08 | 3.37 | 3.09 | 0.88 | 0.417 |
| M (SD) | (1.57) | (1.35) | (1.67) | ||
| Suspiciousness | 2.80 | 3.11 | 2.76 | 1.22 | 0.298 |
| M (SD) | (1.48) | (1.44) | (1.60) | ||
| Grandiosity | 1.08 | 1.11 | 1.27 | 0.51 | 0.601 |
| M (SD) | (1.29) | (1.43) | (1.52) | ||
| Perceptual Abnormalities | 2.88 | 2.87 | 3.21 | 0.97 | 0.379 |
| M (SD) | (1.79) | (1.69) | (1.58) | ||
| Conceptual Disorganization | 1.97 | 1.48 | 1.90 | 2.90 | 0.056 |
| M (SD) | (1.45) | (1.29) | (1.51) | ||
| Total Positive Symptoms | 11.80 | 11.94 | 12.22 | 0.28 | 0.757 |
| M (SD) | (3.86) | (3.97) | (4.68) | ||
| Social Anhedonia | 2.72 | 2.10 | 2.30 | 3.09 | 0.047 |
| M (SD) | (1.98) | (1.78) | (1.84) | ||
| Avolition | 2.41 | 2.08 | 2.22 | 0.99 | 0.374 |
| M (SD) | (1.69) | (1.81) | (1.77) | ||
| Decreased Expression of Emotion | 1.66 | 1.13 | 1.18 | 4.16 | 0.016 |
| M (SD) | (1.66) | (1.37) | (1.46) | ||
| Decreased Experience of Emotion | 1.35 | 1.97 | 1.58 | 3.21 | 0.042 |
| M (SD) | (1.60) | (1.82) | (1.86) | ||
| Decreased Ideational Richness | 1.37 | 1.21 | 1.16 | 0.59 | 0.555 |
| M (SD) | (1.55) | (1.33) | (1.51) | ||
| Decline in Occupational Functioning | 3.12 | 2.71 | 3.09 | 1.17 | 0.313 |
| M (SD) | (1.81) | (1.82) | (2.03) | ||
| Total Negative Symptoms | 12.46 | 11.19 | 11.54 | 1.03 | 0.357 |
| M (SD) | (6.83) | (6.71) | (7.46) |
Social Anhedonia: Total N = 338; Avolition, Ideational Richness, and Occupational Functioning: Total N = 335; Decreased Expression of Emotion: Total N = 334; and Decreased Experience of Emotion: Total N = 333.
Follow-up comparisons
Of the 341 high-risk subjects with cannabis use data, 283 (82.9%) had outcome data (NU: N = 174, CU: N = 51, and CA/CD: N = 58). The mean time to follow up (in months) did not differ between the groups: NU = 18.06 (SD = 9.65), CU = 16.19 (SD = 9.79), and CA/CD = 16.44 (SD = 9.96; F(2) = 1.07, P = 0.35).
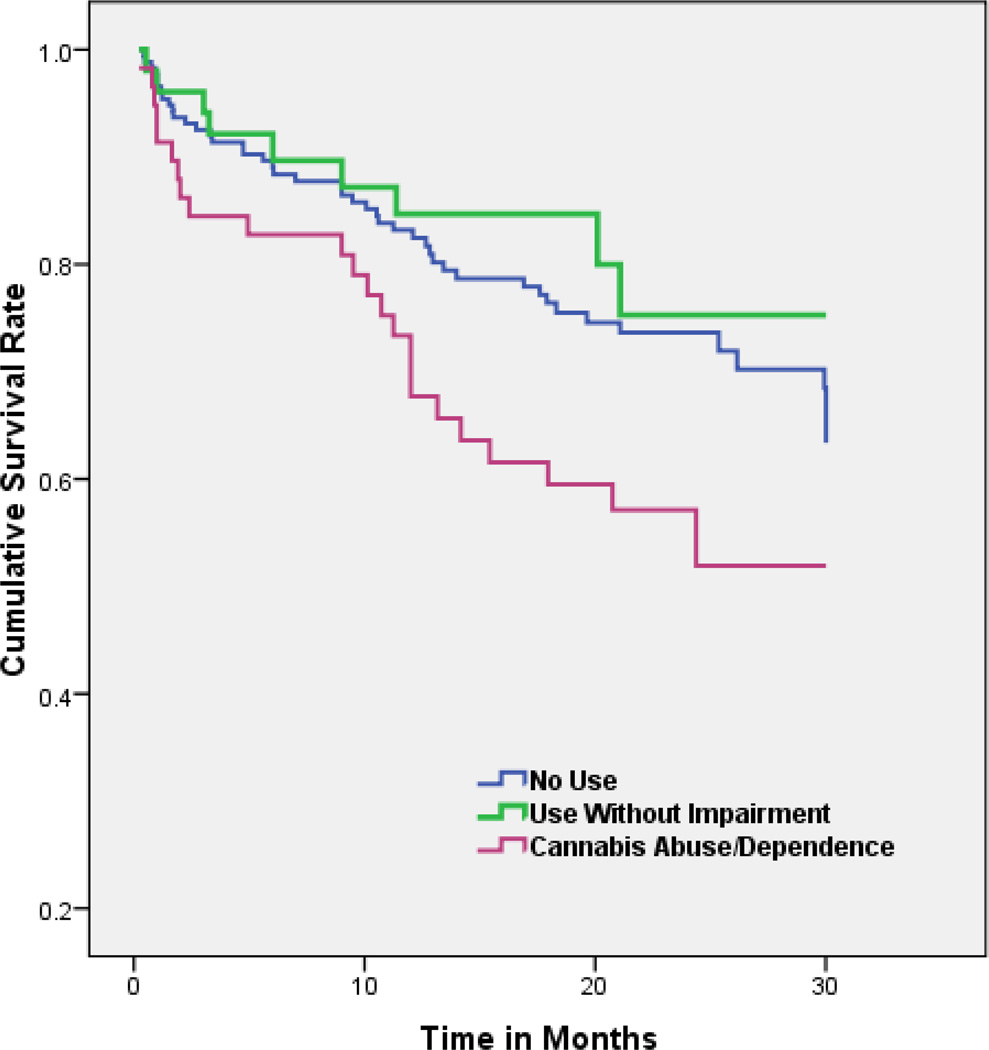
Of the 283 high-risk subjects with outcome data, 79 converted to psychosis during the follow-up period: 46 (26.4%) from the NU group, 9 (17.6%) from the CU group, and 24 (41.4%) from the CA/ CD group. A Kaplan–Meier survival analysis revealed a significant difference between the rates of conversion in the CA/CD group compared with both the NU (log-rank test − χ2 = 4.67, P = 0.031) and CU group (log-rank test − χ2 = 4.92, P = 0.027; See Fig. 1). At 6 months follow-up, 17.2% (SE = 0.05) of the CA/CD group had converted to psychosis compared with 10.3% (SE = 0.02) in the NU group and 7.8% (SE = 0.04) in the CU group. At 12 months follow- up, the conversion rates were 26.6% (SE = 0.06) for the CA/CD group compared with 16.8% (SE = 0.03) for the NU and 15.3% (SE = 0.05) for the CU group. At 18 months, conversion rates were 40.5% (SE = 0.07) for CA/CD, 23.6% (SE = 0.03) for NU and remained the same for the CU group. By 24 months, conversion rates were as follows: 42.9% (SE = 0.07) CA/CD vs. 26.4% (SE = 0.04) NU vs. 24.7% (SE = 0.08) CU.
Fig. 1.
Kaplan–Meier survival analysis depicting time to conversion (in months) for high-risk subjects with no cannabis use vs. cannabis use without impairment vs. cannabis abuse/dependence.
A Cox regression was also carried out to look at the contribution of level of cannabis to conversion after adjusting for other predictor variables. Predictors in the model included age at baseline, gender, SIPS positive symptom total, SIPS negative symptom total, and the three levels of cannabis use. Results revealed that SIPS positive and negative symptom totals were significantly related to conversion (P < 0.001 and P = 0.001 respectively) as was CA/CD (P = 0.015). Gender, age at baseline, and CU did not significantly predict conversion to psychosis (P = 0.165, P = 0.929, and P = 0.369 respectively).
However, when three levels of alcohol use (no use, use without impairment, and abuse/dependence) were included in the model, alcohol use was not significantly related to conversion (P ≥ 0.34) but CA/CD was no longer significantly related to conversion (P = 0.064; See Table 4). In addition, when level of alcohol use was examined without cannabis use, neither alcohol use nor alcohol abuse/dependence was significant predictor of psychosis in regression analysis (P = 0.31 and P = 0.06 respectively). The inclusion of ‘any drug use’ other than cannabis (i.e., hallucinogens, cocaine, stimulants, opioids, or hypnotics; P ≥ 0.706) did not alter the significant association between cannabis and conversion for those with CA/CD (P = 0.043).
Table 4.
Cox Regression predicting conversion to psychosis including both cannabis and alcohol use
| 95% CI for Exp(β) | |||||||
|---|---|---|---|---|---|---|---|
| SE | Wald | df | P | HR | Lower | Upper | |
| Age at baseline | 0.028 | 0.014 | 1 | 0.905 | 1.003 | 0.950 | 1.059 |
| Gender | 0.238 | 1.966 | 1 | 0.161 | 0.716 | 0.449 | 1.142 |
| SIPS total positive symptoms | 0.027 | 12.576 | 1 | 0.000 | 1.100 | 1.044 | 1.159 |
| SIPS total negative symptoms | 0.017 | 10.613 | 1 | 0.001 | 1.057 | 1.022 | 1.093 |
| Cannabis overall | – | 4.526 | 2 | 0.104 | – | – | – |
| Cannabis use w/o impairment | 0.417 | 0.145 | 1 | 0.703 | 0.853 | 0.377 | 1.930 |
| Cannabis abuse/dependence | 0.340 | 3.419 | 1 | 0.064 | 1.875 | 0.963 | 3.651 |
| Alcohol overall | – | 1.266 | 2 | 0.531 | – | – | – |
| Alcohol use w/o impairment | 0.340 | 0.910 | 1 | 0.340 | 0.723 | 0.372 | 1.407 |
| Alcohol abuse/dependence | 0.436 | 0.015 | 1 | 0.902 | 1.055 | 0.449 | 2.477 |
HR, Hazard ratio (Exp(β)).
Discussion
In this large sample of CHR participants, cannabis use or cannabis use disorders were not related to more severe attenuated positive symptoms at baseline. However, there was an association over follow- up between more impairing cannabis use and conversion to psychosis. Specifically, participants with cannabis use disorders – cannabis abuse and cannabis dependence – had a higher rate of conversion in a shorter period of time compared with the non-using subjects and subjects with cannabis use without impairment. When level of cannabis use was added to a regression adjusting for baseline clinical (positive and negative) symptoms and demographic variables, having a cannabis use disorder was a significant predictor of conversion while cannabis use without impairment was not a predictor. These findings are consistent with some clinical cohort and epidemiological findings (3, 4, 14) and suggest that it is the impairing effects of cannabis that account for the significant relationship between cannabis misuse and psychosis. However, a second possible interpretation is that youngsters closest to converting are most likely to self-medicate, a possibility supported by the confounding effect of alcohol use. While not significantly related to conversion itself, taking alcohol use into account weakened the relationship between cannabis abuse/dependence and psychosis.
It is likely that earlier discrepant reports about the relationship of cannabis to psychosis onset in CHR samples have resulted from lack of attention to confounding factors such as level of cannabis use and simultaneous abuse of other substances. Most of the studies that did not find a relationship between cannabis and psychosis focused on cannabis use in general and did not take more severe levels of use into account that, in fact, were often exclusionary criteria for studies (8, 10). The findings from the current study highlight the importance of controlling for confounding factors that could lead to an overestimation of the association between cannabis and psychosis (3, 19). Adjustment for other substance use has been found to weaken cannabis and psychosis associations in population-based studies (32, 33) and has been largely overlooked in CHR studies (21).
Alcohol is the predominant substance used in this sample, and rates of use are comparable to other North American high-risk samples (9–11) and to US population rates for 18–20-year olds (34). In reference to the latter, 43.8% of those 18- to 20-year olds surveyed reported alcohol use in the past month, 29.1% reported binge drinking, and approximately 8.5% reported heavy alcohol use. European samples have reported even higher rates of heavy alcohol use. For example, Dragt et al. (17) reported that 48% of the cannabis-using high-risk participants in their sample also had a diagnosed alcohol use disorder. The significance of this is seen in studies that show an association between heavy alcohol use in adolescence and emotional, behavioural, and psychosocial problems in young adulthood across cultures (35, 36). Given the prevalence of alcohol use in teenagers and young adults, examination of cannabis use is likely always confounded by the use of this substance. This is especially relevant given that alcohol use in adolescence has been associated with cognitive deficits (37, 38) and decreased white matter integrity in neuroimaging studies (39–41). Furthermore, while detrimental effects on white matter integrity are seen for alcohol use (and even more so for heavy use), this same relationship was not found for cannabis use in one longitudinal study of adolescents (42).
Although this sample evidenced low rates of substance use other than alcohol and cannabis and including other drugs in the regression did not alter the results, it is important to consider the impact of other drugs on the development of psychiatric symptoms. For example, a longitudinal population study of adolescents in Germany found associations between psychotic symptoms and cocaine and psychedelics (43). Furthermore, a recent cohort study found that methamphetamine use was associated with risk for schizophrenia equivalent to cannabis use (44). The findings from Kristensen et al. (14) also point to the importance of looking at tobacco use as this was found to be a significant predictor of conversion to psychosis in that high-risk sample. Additionally, van Gastel et al. (45) found that cigarette smoking confounded the relationship between cannabis use and distress related to psychotic-like experiences in a population survey study in the Netherlands.
Limitations
It should be noted that the majority of CHR subjects have never used cannabis and about 50% of those who do use cannabis do not report any impairing effects of use. In those participants who use cannabis but are not impaired to the point that a diagnosis is warranted, cannabis use is not related to psychosis development. However, cannabis use (and other substance use) in this sample reflects lifetime use and substance use was not recorded over the follow-up period, both of which constrain interpretation. For example, it is not known whether incident use or continued cannabis use over follow-up would have impacted the results (4, 15, 46). Frequency and quantity of cannabis use was also not recorded, which is a further limitation of the study and prevented us from examining a dose response relationship directly. A related issue is the lack of an objective measure of substance use such as urine analysis. Rates of other substance use aside from cannabis and alcohol were low in this sample (although consistent with the population rates), which limited our ability to look at levels of other drug abuse. Additionally, we did not have data on tobacco use, so the relationship between tobacco use and psychosis which has been found in other relevant studies could not be explored in this sample. Lastly, there are some demographic factors specific to this study (e.g., parental education, race) that may limit generalizability to other samples.
Nonetheless, this study utilizing a large multisite database sheds light on the purported connection between cannabis and psychosis by identifying that abuse of cannabis with impairing effects, rather than general recreational use, appears to place one at risk for psychosis when examined in isolation, although this relationship is mitigated when concomitant alcohol use is considered. Studies that do not control for other substance use may overestimate the relationship between cannabis use and psychosis. Furthermore, this study highlights the need for the field to focus on not only cannabis misuse, but also alcohol misuse, as both are highly prevalent in adolescent samples. This will have important implications when assessing high-risk symptoms and designing treatment interventions.
Significant outcomes.
Alcohol and cannabis are the most frequently reported substances used by clinical high-risk individuals.
Cannabis use that is not impairing does not predict conversion to psychosis.
Cannabis misuse (abuse or dependence) is associated with psychosis conversion when examined in isolation, but this relationship is weakened when alcohol use is also considered.
Limitations.
Information on substance use frequency, recency, and use over follow-up was not available and may have impacted outcomes.
Rates of alcohol and cannabis use may be most applicable to North American samples.
This sample had low rates of substance use other than alcohol and cannabis, which limited our ability to examine the impact of other substances on conversion to psychosis.
Acknowledgements
This study was supported by the National Institute of Mental Health (grant R01 MH061523, U01 MH081857 to Dr. Cornblatt; grants R18 MH43518, U01 MH081928, and P50 MH080272 to Dr. Seidman; grant P50 MH066286 and R01 MH65079 to Drs. Cannon and Bearden; grants RO1 MH062066 and 5U01 MH081988 to Dr. Walker; grants R01 MH60720 and K24 MH76191 to Dr. Cadenhead; grant K05 MH01654 to Dr. McGlashan; grant U01MH081902-05 to Dr. Perkins; grant R01 MH081861 and R01 MH085560 to Dr. Tsuang; grants U01 MH082022 and U01 MH 066160 to Dr. Woods; grant U01 MH066134 to Dr. Addington).
Dr. Woods reports that in the past two years he has received investigator-initiated research funding support from Pfizer and sponsor-initiated support from Auspex. He has been granted US patent no. 8492418 B2 for a method of treating prodromal schizophrenia with glycine agonists, is an inventor on a patent pending for a method of predicting psychosis risk using blood biomarker analysis, and has received royalties from Oxford University Press. Dr. McGlashan has received royalties from Oxford University Press. Dr. Cannon is a consultant to the Los Angeles County Department of Mental Health on the implementation of programmes for early detection and intervention in psychosis. This activity is unrelated to the study. Dr. Perkins receives grant funding from Genentech and is a consultant for Genentech, Otsuka, Sunovion, Lunbeck, Telesage, and Janssen.
Footnotes
Declaration of interest
No other authors report conflict of interests.
References
- 1.Green B, Young R, Kavanagh D. Cannabis use and misuse prevalence among people with psychosis. Br J Psychiatry. 2005;187:306–313. doi: 10.1192/bjp.187.4.306. [DOI] [PubMed] [Google Scholar]
- 2.Koskinen J, Löhönen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull. 2010;36:1115–1130. doi: 10.1093/schbul/sbp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 4.Kuepper R, van Os J, Lieb R, Wittchen H-U, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruhrmann S, Schultze-Lutter F, Salokangas RKR, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67:241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- 8.Phillips LJ, Curry C, Yung AR, Yuen HP, Adlard S, McGorry PD. Cannabis use is not associated with the development of psychosis in an “ultra” high-risk group. Aust N Z J Psychiatry. 2002;36:800–806. doi: 10.1046/j.1440-1614.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 9.Corcoran CM, Kimhy D, Stanford A, et al. Temporal association of cannabis use with symptoms in individuals at clinical high risk for psychosis. Schizophr Res. 2008;106:286–293. doi: 10.1016/j.schres.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auther AM, McLaughlin D, Carrión RE, Nagachandran P, Correll CU, Cornblatt BA. Prospective study of cannabis use in adolescents at clinical high risk for psychosis: impact on conversion to psychosis and functional outcome. Psychol Med. 2012;42:2485–2497. doi: 10.1017/S0033291712000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchy L, Perkins D, Woods SW, Liu L, Addington J. Impact of substance use on conversion to psychosis in youth at clinical high risk of psychosis. Schizophr Res. 2014;156:277–280. doi: 10.1016/j.schres.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragt S, Nieman DH, Becker HE, et al. Age of onset of cannabis use is associated with age of onset of high-risk symptoms for psychosis. Can J Psychiatry. 2010;55:165–171. doi: 10.1177/070674371005500308. [DOI] [PubMed] [Google Scholar]
- 13.Korver N, Nieman DH, Becker HE, et al. Symptomatology and neuropsychological functioning in cannabis using subjects at ultra-high risk for developing psychosis and healthy controls. Aust N Z J Psychiatry. 2010;44:230–236. doi: 10.3109/00048670903487118. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen K, Cadenhead KS. Cannabis abuse and risk for psychosis in a prodromal sample. Psychiatry Res. 2007;151:151–154. doi: 10.1016/j.psychres.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valmaggia LR, Day FL, Jones C, et al. Cannabis use and transition to psychosis in people at ultra-high risk. Psychol Med. 2014;44:2503–2512. doi: 10.1017/S0033291714000117. [DOI] [PubMed] [Google Scholar]
- 16.Klosterkötter J, Ruhrmann S, Schultze-Lutter F, et al. The European Prediction of Psychosis Study (EPOS): integrating early recognition and intervention in Europe. World Psychiatry. 2005;4:161–167. [PMC free article] [PubMed] [Google Scholar]
- 17.Dragt S, Nieman DH, Schultze-Lutter F, et al. Cannabis use and age at onset of symptoms in subjects at clinical high risk for psychosis. Acta Psychiatr Scand. 2012;125:45–53. doi: 10.1111/j.1600-0447.2011.01763.x. [DOI] [PubMed] [Google Scholar]
- 18.Addington J, Cadenhead KS, Cannon TD, et al. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33:665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zammit S, Moore THM, Lingford-Hughes A, et al. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193:357–363. doi: 10.1192/bjp.bp.107.046375. [DOI] [PubMed] [Google Scholar]
- 20.Koskinen J, Löhönen J, Koponen H, Isohanni M, Miettunen J. Prevalence of alcohol use disorders in schizophrenia – a systematic review and meta-analysis. Acta Psychiatr Scand. 2009;120:85–96. doi: 10.1111/j.1600-0447.2009.01385.x. [DOI] [PubMed] [Google Scholar]
- 21.Addington J, Case N, Saleem MM, Auther AM, Cornblatt BA, Cadenhead KS. Substance use in clinical high risk for psychosis: a review of the literature. Early Interv Psychiatry. 2014;8:1–9. doi: 10.1111/eip.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller TJ, McGlashan TH, Woods SW, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- 23.McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L. Instrument for the assessment of prodromal symptoms and states. In: Miller T, Mednick SA, McGlashan TH, Libiger J, Johannessen JO, editors. Early Intervention in psychotic disorders. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2001. pp. 135–149. [Google Scholar]
- 24.Cornblatt BA, Auther AM, Niendam T, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JB, Gibbon M, First MB, et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 26.Orvaschel H, Puig-Antich J. Schedule for affective disorders and schizophrenia for school-age children – Epidemiologic version. Fort Lauderdale: Nova Southeastern University; 1994. [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 28.Wechsler D. WAIS-R manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 29.Wechsler D. Wechsler intelligence scale for children. 3rd edn. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- 30.Wechsler D. WASI (Wechsler abbreviated scale of intelligence) San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. JASA. 1958;53:457–481. [Google Scholar]
- 32.Tien AY, Anthony JC. Epidemiological analysis of alcohol and drug use as risk factors for psychotic experiences. J Nerv Ment Dis. 1990;178:473–480. [PubMed] [Google Scholar]
- 33.Van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156:319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- 34.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: summary of national findings (NSDUH Series H-48, HHS Publication No. (SMA) 14-4863) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- 35.Steinhausen H-C, Eschmann S, Heimgartner A, Metzke CW. Frequency, course and correlates of alcohol use from adolescence to young adulthood in a Swiss community survey. BMC Psychiatry. 2008;8:5. doi: 10.1186/1471-244X-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinhausen HC. Developmental psychopathology in adolescence: findings from a Swiss study – the NAPE Lecture 2005. Acta Psychiatr Scand. 2006;113:6–12. doi: 10.1111/j.1600-0447.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 37.Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2010;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeigler DW, Wang CC, Yoast RA, et al. The neurocognitive effects of alcohol on adolescents and college students. Prev Med (Baltim) 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 39.Elofson J, Gongvatana W, Carey KB. Alcohol use and cerebral white matter compromise in adolescence. Addict Behav. 2013;38:2295–2305. doi: 10.1016/j.addbeh.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. 2013;214:374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobus J, McQueeny T, Bava S, et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res. 2013;37(Suppl 1):E181–E189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuzenko N, Sareen J, Beesdo-Baum K, et al. Associations between use of cocaine, amphetamines, or psychedelics and psychotic symptoms in a community sample. Acta Psychiatr Scand. 2011;123:466–474. doi: 10.1111/j.1600-0447.2010.01633.x. [DOI] [PubMed] [Google Scholar]
- 44.Callaghan RC. Methamphetamine use and schizophrenia: a population-based cohort study in California. Am J Psychiatry. 2012;169:389. doi: 10.1176/appi.ajp.2011.10070937. [DOI] [PubMed] [Google Scholar]
- 45.VanGastel WA, MacCabe JH, Schubart CD, et al. Cigarette smoking and cannabis use are equally strongly associated with psychotic-like experiences: a cross-sectional study in 1929 young adults. Psychol Med. 2013;43:2393–2401. doi: 10.1017/S0033291713000202. [DOI] [PubMed] [Google Scholar]
- 46.Yücel M, Bora E, Lubman DI, et al. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull. 2012;38:316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]