Abstract
Objective
Obesity is becoming a worldwide health problem. The genome wide association (GWA) study particularly for body mass index (BMI) has not been successfully conducted in the Chinese. In order to identify novel genes for BMI variation in the Chinese, an initial GWA study and a follow up replication study were performed.
Methods
Affymetrix 500K SNPs were genotyped for initial GWA of 597 Northern Chinese. After quality control, 281 533 SNPs were included in the association analysis. Three SNPs were genotyped in a Southern Chinese replication sample containing 2 955 Chinese Han subjects. Association analyses were performed by Plink software.
Results
Eight SNPs were significantly associated with BMI variation after false discovery rate (FDR) correction (P=5.45×10−7−7.26×10−6, FDR q=0.033–0.048). Two adjacent SNPs (rs4432245 & rs711906) in the eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) gene were significantly associated with BMI (P=6.38×10−6 & 4.39×10−6, FDR q=0.048). In the follow-up replication study, we confirmed the associations between BMI and rs4432245, rs711906 in the EIF2AKE gene (P=0.03 & 0.01, respectively).
Conclusion
Our study suggests novel mechanisms for BMI, where EIF2AK4 has exerted a profound effect on the synthesis and storage of triglycerides and may impact on overall energy homeostasis associated with obesity. The minor allele frequencies for the two SNPs in the EIF2AK4 gene have marked ethnic differences between Caucasians and the Chinese. The association of the EIF2AK4 gene with BMI is suggested to be ‘ethnic specific’ in the Chinese.
Keywords: Body mass index, Genome wide association, EIF2AK4, Replication
INTRODUCTION
Obesity is becoming a global health problem, affecting people in both developing and developed countries[1–2]. Approximately 250 million adults, nearly 7% of the world adult population, are estimated to suffer from obesity[3–4]. According to statistical data (2004) from the Ministry of Health of China, approximately 7.1% of the total 1.3 billion population are affected with obesity, and the prevalence of obesity in adults in large cities account for 12.3%. Body mass index (BMI), a key index of body composition, is widely used for defining morbid obesity and assessing risks to cardiovascular disease and type 2 diabetes[5–6]. BMI is a complex quantitative trait, determined by multiple genetic and/or environmental factors[7]. The estimated heritability of BMI ranges from 0.50 to 0.90[8–9].
Many quantitative trait loci or candidate genes underlying BMI variation have been identified using genome wide linkage (GWL) scans or candidate gene approaches[10–29], though both of these methods have their own limitations. GWL studies have identified many genomic regions associated with BMI[10,12–18,20–22,29], however, few of these regions have been replicated in other populations. Genomic regions identified in GWL scans are typically fairly large and few follow-up fine mapping studies have been successfully pursued. Candidate gene association mapping approaches are considered more powerful than GWL studies, nevertheless, the selection of these genes is based on prior knowledge of gene function so these approaches are not designed to identify novel genes influencing BMI, and the number of genes successfully tested is limited.
Recent technological advances in single-nucleotide polymorphism (SNP) genotyping in conjunction with increased knowledge of linkage disequilibrium (LD) patterns in major human populations, as revealed by the HapMap project, have enhanced the practicality of assessing the entire human genome by assaying hundreds of thousands of SNPs simultaneously. Consequently, genome wide association (GWA) study is becoming an important approach toward identifying common variants associated with complex diseases or quantitative traits. Recent GWA studies have identified several significant genes associated with obesity-related phenotypes, primarily on BMI in Caucasians[30–35]. For example, our group conducted a GWA study for BMI and fat mass in Caucasians and identified a novel gene, CTNNBL1, which may play an important role in the development of obesity[30].
Ethnic disparity in the genetic background has been proposed as an important factor contributing to the variation of obesity-related traits across different populations, thereby justifying genetic studies in distinct ethnic populations (details in Discussion). To the best of our knowledge, except for one GWA study for BMI conducted in Koreans[36] and the other conduced in the Japanese[37], no more GWA studies particularly for BMI on population based samples have been previously performed in the Chinese, the largest population in the world. In order to identify novel genes and search for potential ethnic-specific genes for BMI in the Chinese, we performed a modest GWA study in 597 unrelated Northern Chinese adults using a highly dense Affymetrix 500K SNP array examining about 500 000 SNPs and a follow-up replication study in an independent sample of 2 955 unrelated Southern Chinese.
MATERIALS AND METHODS
Subjects
The study was approved by the Institutional Review Board or Research Administration of the involved institutions. Signed informed-consent documents were obtained from all study participants before they were enrolled in the study.
The Northern Chinese Sample for GWA
The study was initially performed with a GWAS discovery stage for SNPs of potential significance for bone mineral density in a Chinese Han sample in Xi’an City and the surrounding areas of Northern China. Subjects with serious metabolic diseases (diabetes, hypo-and hyper-parathyroidism, hyperthyroidism, etc.) or chronic use of drugs affecting metabolism were excluded. The detailed procedure of exclusion was presented by a previous study[38]. The sample included 258 men and 339 women. Weight was measured on electronic scales to the nearest 0.1 kg and height was measured to the nearest 0.1 cm with a wall-mounted stadiometer with subjects wearing light clothing and no shoes. BMI was calculated as body weight (in kilograms) divided by the square of height (in meters).
The Southern Chinese Sample for Replication
These unrelated subjects were randomly selected from the extended database of an unrelated sample in the Changsha City and the surrounding area located in Southern China. The detailed sampling method can be found by a previous study of our group[61]. A total of 2 955 Chinese Han subjects (1 518 women and 1 437 men) aged 19–88 years were included in the final replication study. The basic characteristics of all the studied subjects are presented in Table 1.
Table 1.
Basic Characteristics of the Study Subjects
| Study | Trait | Total | Female | Male |
|---|---|---|---|---|
| GWA Study | Sample size | 597 | 339 | 258 |
| Age (years) | 70.4±7.4 | 69.7±7.8 | 71.1±6.8 | |
| Height (cm) | 160.8±8.9 | 155.5±6.3 | 167.8±6.9 | |
| Weight (kg) | 59.4±11.4 | 56.3±10.7 | 63.6±11.1 | |
| BMI (kg/m2) | 22.9±3.8 | 23.2±4.0 | 22.5±3.4 | |
| Replication Study |
Sample size | 2955 | 1518 | 1437 |
| Age (years) | 33.1±14.5 | 35.4±15.7 | 30.5±12.5 | |
| Height (cm) | 163.7±7.9 | 158.1±5.3 | 169.6±5.6 | |
| Weight (kg) | 58.6±10.2 | 53.4±8.1 | 64.0±9.3 | |
| BMI (kg/m2) | 21.8±3.1 | 21.4±3.2 | 22.2±2.9 | |
Genotyping
Affymetrix 500K SNPs in 597 Chinese in Northern China
Genomic DNA was extracted from whole human blood using a commercial isolation kit (Gentra systems, Minneapolis, MN, USA) according to the protocols of the kit. For each sample, genotyping with GeneChip® Human Mapping 500K set containing 250K Nsp array and 250K Sty array (Affymetrix, Santa Clara, CA, USA) was performed using the standard protocols recommended by the manufacturer. Briefly, for each array, 250 ng of DNA was digested with restriction enzyme (Nspl or Styl) and ligated to adapters. A single PCR primer that recognizes the adapter sequence was used to amplify the ligated product. The amplified DNA (200–1100 bp) was fragmented into approximately 50 bp size, then labeled with biotin and hybridized to the arrays. After 16–18 h of hybridization, the arrays were washed with Wash Buffer A (6xSSPE, 0.1% Tween20) and Wash Buffer B (0.6xSSPE, 0.1% Tween20), in turn, on an Affymetrix Fluidics Station FS450. Then they were stained with the Streptavidin Phycoerythrin (SAPE, 10 µg/mL) and the signals were amplified with Anti-streptavidin antibody. The stained arrays were scanned with an Affymetrix GeneChip® 3000 7G scanner at 0.7 urn solution and generated relevant signal images. SNPs genotypes from the scanned images were extracted using GCOS and GTYPE software (Affymetrix).
Quality control procedures were as follows. First, only samples with a minimum of 95% call rate were included. The final mean BRLMM call rate of the entire sample reached a high level of 99.02%. Second, out of the initial full-set of 500 568 SNPs, we discarded: 1) SNPs with a call rate <90% in the total sample (n=54 845); 2) those deviating from Hardy-Weinberg equilibrium (HWE) (P<0.001, n=22 002); and; 3) those having a minor allele frequency (MAF) <0.05 in the total sample (n=142 188). Therefore, 281 533 SNPs were available for the subsequent analyses.
Replication Genotyping in 2 955 Subjects in Southern China
Three selected SNPs were genotyped for replication study in the unrelated Southern Chinese sample. Genotyping of subjects was performed using a primer extension method with MALDI-TOF mass spectrometry for multiplexed genotyping of SNPs on a MassARRAY system as suggested by the manufacturer (Sequenom, Inc., San Diego, CA). The method was described by Braun et al.[39]. The SNP genotyping success rate was 97% and the duplicate concordance rate was 99%. The three SNPs were all in HWE (P>0.10), and the MAFs of the SNPs were consistent with the MAFs in initial GWA (Table 3).
Table 3.
Analyzed SNPs in EIF2AK4 Gene for the GWA Sample
| Associated Gene |
dbSNP | Physical Position |
Role | AlleleA | MAFB | MAFC | P ValueD | FDR q ValueE |
|---|---|---|---|---|---|---|---|---|
| EIF2AK4 (15q15.1) |
rs534757 | 38014169 | Intron 1 | T/C | 0.167 | 0.144 | 0.624 814 | NS |
| rs518770 | 38017564 | Intron 1 | T/C | 0.166 | 0.144 | 0.677 616 | NS | |
| rs489508 | 38033071 | Intron 4 | T/C | 0.016 | 0.022 | 0.484 009 | NS | |
| rs16970033 | 38033092 | Intron 4 | A/G | 0.195 | 0.211 | 0.377 959 | NS | |
| rs16970035 | 38033128 | Intron 4 | C/A | 0.284 | 0.211 | 0.432 093 | NS | |
| rs8041785 | 38039033 | Intron 6 | G/A | 0.46 | 0.456 | 0.469 773 | NS | |
| rs7174767 | 38057092 | Intron 12 | T/C | 0.232 | 0.267 | 0.639 034 | NS | |
| rs7173301 | 38057135 | Intron 12 | G/A | 0.25 | 0.211 | 0.254 551 | NS | |
| rs12442713 | 38068058 | Intron 15 | C/A | 0.294 | 0.244 | 0.227 739 | NS | |
| rs16970132 | 38069064 | Intron 15 | G/A | 0.221 | 0.267 | 0.199 312 | NS | |
| rs2291626 | 38077998 | Intron 19 | T/C | 0.265 | 0.167 | 0.048 587 | NS | |
| rs2412462 | 38088462 | Intron 25 | G/A | 0.291 | 0.289 | 0.166 460 | NS | |
| rs4432245 | 38111773 | Intron 36 | C/T | 0.481 (0.473) | 0.489 | 6.39×10−6 (0.03) | 0.048 | |
| rs711906 | 38112983 | Intron 37 | A/G | 0.47 (0.471) | 0.489 | 4.38×10−6 (0.01) | 0.048 |
Note.
The former allele represents the minor one of each locus;
Minor allele frequency calculated in initial GWA sample; data presented in parenthesis were the MAFs calculated in replication sample;
Minor allele frequency reported for the Chinese or Asians in the public database such as HapMap or dbSNP;
P value for association using single-SNP test in initial GWA sample; data presented in parenthesis were the P values for association in replication sample;
FDR q value is FDR correction value for multiple testing at the genome-wide level for initial GWA analysis; ‘NS’ means not significant.
Statistical Analyses
Initial GWA
The two significant covariates, gender and age, were used to adjust raw BMI values for subsequent analyses. Then HelixTree 5.3.1 (Golden Helix, Bozeman, MT) was used to perform genotypic association analyses and haplotype association analyses. The linkage disequilibrium (LD) [standardized D’ (D/Dmax)] patterns for genes of interest were analyzed and plotted using the Haploview program[40]. Genotypic association analyses were used to compare the difference of mean BMI values among three genotypic groups for each SNP. Haplotype association or block association detected the different mean BMI values among the haplotype groups within haplotype blocks. We also performed marker-marker interaction analyses for those significant markers identified in single SNP analyses using the two-loci genetic association analysis implemented in Helixtree. Marker-marker interaction was used to compare difference of mean BMI values among the new categorical variable formed by the combination of the two markers.
Multiple testing is a perplexing issue in GWA studies. As Bonferroni adjustment for multiple-testing in a GWA study is usually considered to be extremely conservative, we used QVALUE software developed by Storey and Tibshirani[41] to calculate a FDR (false discovery rate) based q value to measure the statistical significance at the genome wide level for the association results. The cutoff for significant association at the genome wide level was set at FDR q value ≤0.05.
To detect spurious association results that may be brought by potential population stratification, we used STRUCTURE 2.2 software to investigate the potential substructure of our sample. The program uses a Markov chain Monte Carlo (MCMC) algorithm to cluster individuals into different cryptic sub-populations on the basis of multilocus genotype data[42] . We performed an independent analysis under the assumed number of population strata k=2 and a set of 1 000 un-linked markers randomly selected from about 280 000 eligible SNPs of the whole genome. We also used the method of genome control to detect potential population stratification of this sample. It can estimated the inflation factor (λ) based on the genome-wide SNP information. We further performed the principal component analysis (PCA) implemented in EIGENSTRAT to guard against possible population stratification.
In order to analyze and predict the function of the significant SNPs identified, and other interesting SNPs, we utilized the FASTSNP program to provide up-to-date information about known and potential functional effects of SNPs[43].
Replication Analyses
First, we adjusted age and sex for the raw BMI values, then transformed them into normal distribution with box-cox transformation. Plink software[44] was used to perform the general genotype-based association test.
Finally, the P values for significant SNPs from initial GWA study and the follow-up replication study were combined using Fisher’s method[45] to quantify the overall evidence for association with BMI variation. It is a ‘meta-analysis’ technique for combining the results from a variety of independent tests bearing upon the same overall hypothesis as if in a single test. Fisher’s method combines extreme value probabilities from each test, called ‘P-values’, into one test statistic (χ2) having a chi-square distribution using the formula , whereas k is the degrees of freedom of the χ2 statistic combining pi from the study[45].
RESULTS
Initial GWAS Study
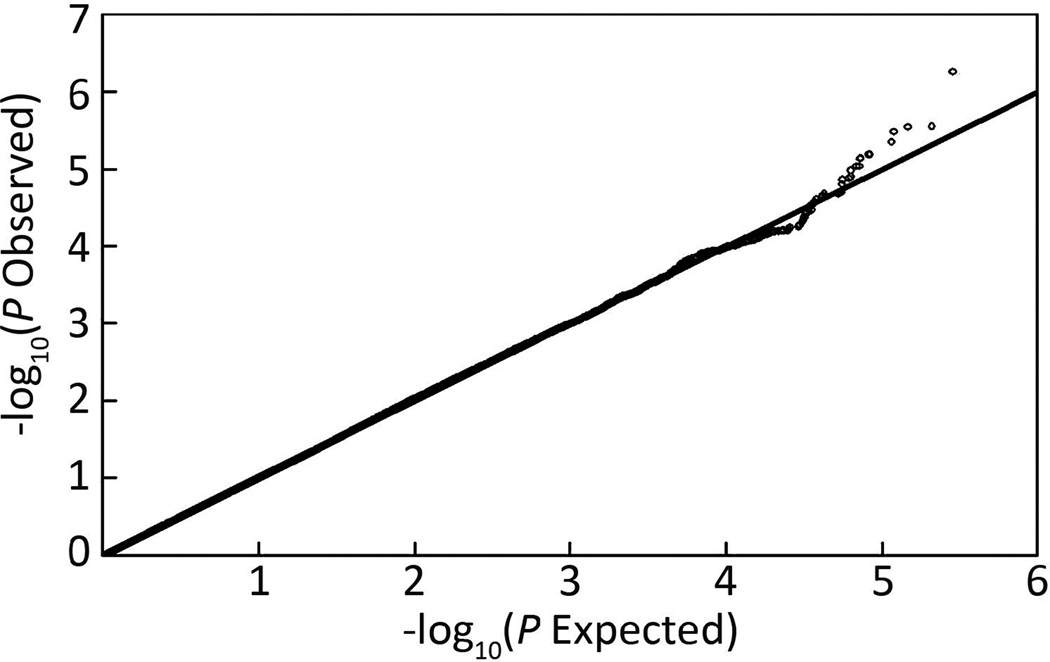
Using about 280 000 eligible SNPs, we examined the quantile-quantile (Q–Q) plot for the distribution of P values involving all SNPs tested in our sample (Figure 1). We observed a fraction of SNPs associated with BMI compared to that expected P values based on chance alone. These results indicate that the most strongly associated SNPs are likely to have true associations with BMI.
Figure 1.
Quantile-quantile (Q–Q) plots for BMI association. The Y-axis is the-LOG10(p) values for the GWA study SNPs and the X-axis is the-LOG10(p) values expected under the null distribution for the GWA study SNPs.
The SNPs with significant association signals in our initial association analyses, after FDR correction (q value ≤0.05), are summarized in Table 2. Eight SNPs in seven genes showed significant association signals with BMI (P=5.45×10−7 −7.26×10−6 , FDR q=0.033–0.048). The most significant SNP is rs4633, located in the exon of the catechol-O-methyltransferase (COMT) gene (P=5.45×10−7 , FDR q=0.033). Two adjacent SNPs (rs4432245 & rs711906) in the eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) gene were significantly associated with BMI (P=4.38×10−6 and 6.39×10−6 , respectively, FDR q=0.048).
Table 2.
Top Eight SNPs Showing Significant Evidence for Associations with BMI (FDR q values≤0.05) in GWA Analyses
| dbSNP ID | Chromosome Position |
Physical Position |
Associated GeneA |
Role | Allele | MAFB | MAFC | P Value |
q Value |
|---|---|---|---|---|---|---|---|---|---|
| rs4633 | 22q11.21 | 18330235 | COMT | Exon3 | T/C | 0.254 | 0.233 | 5.45×10−7 | 0.033 |
| rs3213523 | 22q13.32 | 48131411 | FU44385 | Upstream | C/T | 0.352 | 0.422 | 2.75×10−6 | 0.048 |
| rs1438168 | 3p25.3 | 10412280 | ATP2B2 | Intron4 | G/A | 0.409 | 0.389 | 2.82×10−6 | 0.048 |
| rs10904363 | 10p15.1 | 4910413 | AKR1CL2 | Intron5 | C/G | 0.417 | 0.356 | 3.24×10−6 | 0.048 |
| rs711906 | 15q15.1 | 38112983 | EIF2AK4 | Intron37 | A/G | 0.470 | 0.489 | 4.38×10−6 | 0.048 |
| rs4432245 | 15q15.1 | 38111773 | EIF2AK4 | Intron36 | C/T | 0.481 | 0.489 | 6.39×10−6 | 0.048 |
| rs7623901 | 3q13.33 | 123011701 | IQCB1 | Intron5 | T/C | 0.053 | 0.056 | 6.41×10−6 | 0.048 |
| rs12496318 | 3q13.33 | 123044190 | EAF2 | Intron1 | T/G | 0.054 | 0.056 | 7.26×10−6 | 0.048 |
Note.
Abbreviations: COMT (catechol-O-methyltransferase), EIF2AK4 (eukaryotic translation initiation factor 2 alpha ki nase 4), FU44385 (uncharacterized), ATP2B2 (plasma membrane Ca(2+)-ATPase), AKR1CL2 (aldo-keto reductase family 1, member C-like 2), IQCB1 (IQ motif containing B1), EAF2 (ELL associated factor 2);
Minor allele frequency calculated in initial GWA sample;
Minor allele frequency reported for the Chinese or Asians in the public database such as HapMap or dbSNP.
Replication Study
We further genotyped SNPs rs4432245, rs711906, and rs4633 in an independent Southern Chinese sample. We selected these three SNPs because: 1) of the 8 SNPs which had significant q-value, the three SNPs in these two genes might have some known direct or indirect function in the pathogenesis of obesity; 2) genotyping budget for replication was limited. Two SNPs, rs4432245 and rs711906 in EIF2AK4 gene, showed significant association with BMI (P=0.03 & 0.01, respectively) under genotypic test model. However, no significant association (P=0.52) between rs4633 and BMI was detected.
Combined P values from the initial GWA study and the replication study for rs4432245 and rs711906 in EIF2AK4 gene (P=1.12xl0−6 and 2.21× 10−6, respectively) supported the above significant associations between these SNPs and BMI.
Analyses within EIF2AK4 Gene
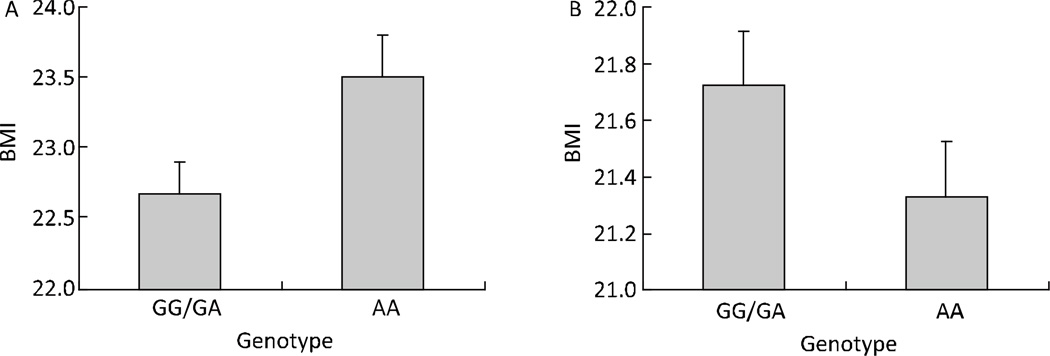
Table 3 presents information for all analyzed SNPs in the EIF2AK4 gene, and their single-SNP association signals with BMI. Among the 14 SNPs analyzed in the EIF2AK4 gene only rs4432245 and rs711906, located at introns 36 and 37 respectively, were significantly associated with BMI at the genome-wide level; moreover they were in strong LD. rs711906, with a polymorphic G→A nucleotide change, has a MAF of 47.0% according to our own data and 48.9% according to the HapMap CHB database (Table 3). Individuals having one or two copies of the ‘G’ allele of rs711906 had a BMI that was, on average, 0.9 kg/m2 lower than that of non-carriers of G (Figure 2A). But in replication studies, individual having one or two copies of the ‘G’ allele of rs711906 had a BMI that was, on average, 0.4 kg/m2 higher than that of non-carriers of G (Figure 2B).
Figure 2.
The distribution of age and sex adjusted BMI (least square means) in different genotype groups of rs711906 in EIF2AK4 gene for GWA study (A) and replication study (B). The data are presented as mean (SE).
For significant variants in the EIF2AK4 gene, we also observed evidence of significant marker-marker interactions between rs4432245 and rs711906 (P=4.76×10−6). These interactions between markers illustrate the importance of considering them jointly in BMI genetic analysis and suggest potential patterns of biological interaction contributing to BMI variation.
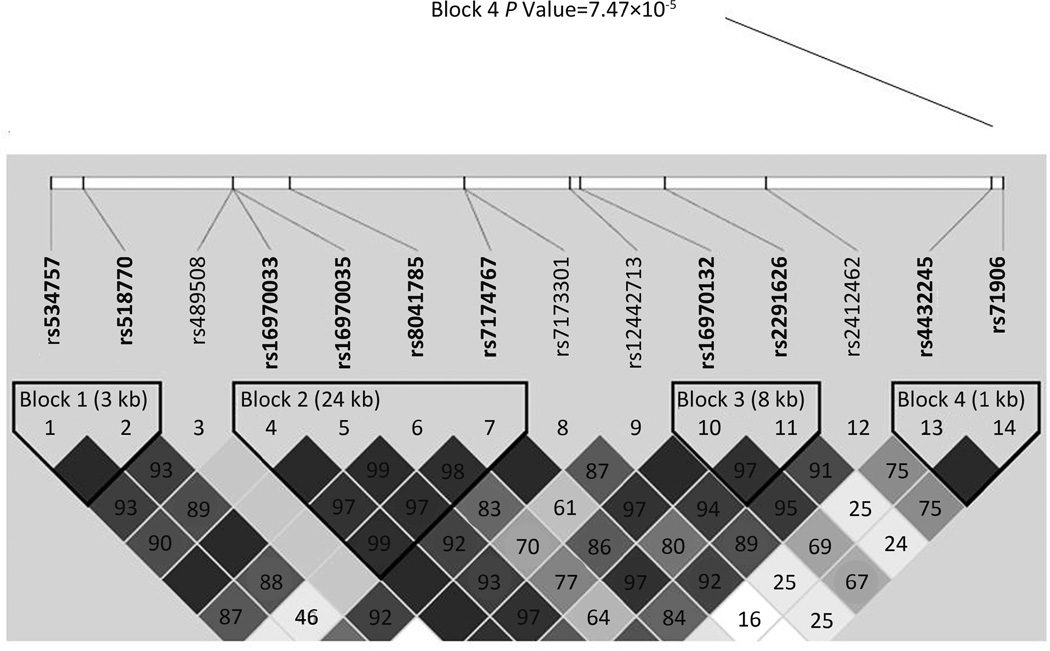
Figure 3 shows the LD pattern and haplotype block structure of the EIF2AK4 gene. In the EIF2AK4 gene blocks, the two significant SNPs (rs4432245 & rs711906) were in strong LD and formed one haplotype block. The haplotypes in this block structure were strongly associated with BMI (P=7.47×10−5).
Figure 3.
Haplotype blocks within EIF2AK4 gene and the P value of association between block 4 and BMI.
Population Stratification
For testing the potential population stratification of our sample, we randomly selected 1000 unlinked markers to cluster our subjects. From the triangle plot generated by STRUCTURE, all 597 subjects were tightly clustered together and could not be assigned to any subgroup. This structure analysis suggests that there is no significant population stratification in our sample. The population stratification analyses using genome control and PCA were in consistent with the results by STRUCTURE, which can be found by another published research using the same subjects in our group[38], (no details here). All of these results indicated that potential population stratification in this homogeneous Chinese population was very minimal.
Comparison of our GWA with Previous GWLs and GWAs
Table 4 lists regions identified in previous linkage studies that were confirmed by strong associaton signals (P<10−4) in the current GWA study[10,16–18,20,46--58]; in instances where multiple SNPs within a region were associated with BMI, we only presented data for the SNP with the highest association signal. The strong association signals that we detected for these previously implicated linkage regions partially suggested the reasonable power and utility of our association analyses for identifying genes that influence BMI variation.
Table 4.
Genomic Regions Linked to BMI in Previous Studies, Confirmed by Results of the Current GWA Study
| Genome Wide Linkage (GWL) |
Genome Wide Association (GWA) |
||||||
|---|---|---|---|---|---|---|---|
| markerB | LODA | Populaton | Ref | dbSNP RS ID | Associated GeneB | P ValueC | Cytoband |
| D4S2632 | 6.1 | utah | 45 | ||||
| D4S3350 | 9.2 | rs16883786 | PCDH7 | 8.20×10−5 | 4p15.1 | ||
| D4S2912 | 4.5 | Mexican Americans | 49 | ||||
| D12S2070 | 2.98 | European American | 54 | rs206952 | MSI1 | 0.0001 | 12q24.31 |
| D6S287 | 4.06 | French | 18 | rs11968468 | PLN | 0.0002 | 6q22.31 |
| D17S949 | 3 | rs3809700 | ST6GALNAC2 | 7.22×10−5 | 17q25.1 | ||
| D5S433 | 2.28 | French | 16 | rs10514384 | - | 0.0001 | 5q15 |
| D10S587 | 2.9 | rs10885378 | VTI1A | 2.25×10−5 | 10q25.2 | ||
| D19S418 | 3.59 | rs2544785 | RPL18 | 0.0004 | 19q13.32 | ||
| D3S2427 | 3.3 | White American | 46 | rs9833131 | EPHB3 | 0.001 | 3q27.1 |
| D3S1764 | 3.45 | Black American | rs2369949 | ACAD11 | 0.0003 | 3q22.1 | |
| D3S1259 | White American | rs4680792 | RBMS3 | 0.003 | 3p24.1 | ||
| D7S3051 | rs2711028 | OSBPL3 | 0.0001 | 7p15.2 | |||
| D7S2847 | >2 | 53 | rs42172 | PIK3CG | 0.007 | 7q22.3 | |
| D14S617 | Japan, China | rs4344663 | C14orf118 | 0.0003 | 14q24.3 | ||
| GATA67G11 | rs13337356 | - | 0.003 | 16q12.2 | |||
| D17S947 | rs2273026 | SHMT1 | 0.002 | 17p11.2 | |||
| D7S817 | 3.8 | Nigeria, Yoruba | 20 | rs7798775 | HECW1 | 0.0001 | 7p14.1 |
| D11S2000 | 3.3 | rs7945321 | TRPC6 | 8.05×10−5 | 11q22.1 | ||
| D7S1804 | 3.2 | White American | 52 | rs125095 | PLXNA4B | 0.001 | 7q32.3 |
| D13S257 | 3.2 | rs4942014 | ELF1 | 0.001 | 13q14.11 | ||
| D2S1788 | 3.08 | rs848641 | FEZ2 | 0.0008 | 2p22.2 | ||
| D7S3056-D7S2477 | 2.53 | White | 51 | rs10951131 | CARD11 | 6.81×10−5 | 7p22.2 |
| D12S1052-D12S1064 | 3.41 | rs17110690 | TPH2 | 0.0002 | 12q21.1 | ||
| D4S1647 | 2.63 | rs2567397 | PPP3CA | 0.009 | 4q23 | ||
| GATA8B01 | 2.56 | African American | 50 | rs10958163 | LOC138046 | 0.0003 | 8q21.13 |
| D10S212 | 2.06 | rs1917847 | C10orf120 | 6.10×10−5 | 10q26.13 | ||
| D12PAH | 2.6 | rs7953150 | PTPN11 | 0.003 | 12q24.13 | ||
| D7S2557 | 2.9 | White American | 47 | rs2529754 | SP8 | 0.0005 | 7p15.3 |
| D7S484 | 2.4 | rs6463435 | - | 7.29×10−5 | 7p12.3 | ||
| D5S1505 | 2.2 | rs17141793 | - | 0.0003 | 5q23.1 | ||
| D2S347 | 4.04 | European American | 10 | rs3931840 | RALB | 0.0005 | 2q14.2 |
| D5S1725-D5S1462 | 2.4 | rs423449 | AP3B1 | 2.95×10−5 | 5q14.1 | ||
| D8S556-D8S592 | 2 | White American | 48 | rs4147527 | SAMD12 | 0.0003 | 8q24.12 |
| D10S1435-D10S189 | 2.3–2.7 | rs10904363 | AKR1CL2 | 3.24×10−6 | 10p15.1 | ||
| D14S283-D14S742-D14S1280 | 2.2–2.4 | rs1188538 | OR11G2 | 0.0009 | 14q11.2 | ||
| SE30 | 2.13 | Dutch | 17 | rs6917225 | LYRM4 | 0.0002 | 6p25.1 |
| D7S3056-D7S2477 | 2.4 | rs12055909 | FERD3L | 0.0004 | 7p21.1 | ||
| - | 2.23 | Chinese | 55 | rs10952332 | GALNTL5 | 6.24×10−5 | 7q36.1 |
| D13S265 | 2.09 | Samoans | 56 | rs9301947 | GPC6 | 0.001 | 13q31.3 |
| AFMb035xb9 | 2.18 | European American | 57 | Rs9640008 | ZFAND2A | 0.0009 | 7p22.3 |
| AAT013 | 2.03 | FAM44B | 0.001 | 5q35.2 | |||
Note.
Peak LOD scores >2.0 in previous GWLs.
‘-’ represents not available.
Our peak associaton signals in the corresponding regions identfied in GWLs.
Table 5 lists several genes that were associated with BMI in previous GWA studies. Our association results provided supporting replication association evidence for some of these genes (e.g. INSIG2 on chromosome 2q14.1, PFKP on 10p15, FTO on 16q12, MC4R on18q22, MRPS22 on 3q22, CDKAL1 on 6p22.3, and KLF9 on 9q21). For others, however (e.g. CTNNBL1 on 20q11), the association with BMI could not be replicated in our Chinese sample.
Table 5.
Comparison of Current Study Results with Previously Published Original GWA Studies for BMI
| Results of the Published Original GWA StudiesA |
Results of Current GWA StudyB |
|||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Associated Gene | Cytoband | p Value | Ref. | SNP | Associated Gene | Cytoband | P Value |
| rs7566605 | INSIG2 | 2q14.1 | 0.0026 | 33 | rs1866407 | INSIG2 | 2q14.1 | 0.002 |
| rs17782313 | MC4R | 18q22 | 2.8 ×10−15 | 35 | rs1943229 | MC4R | 18q22 | 0.005 |
| rs7638110 | MRPS22 | 3q23 | 4.6×10−8 | 34 | rs10460842 | MRPS22 | 3q23 | 6.9 ×10−5 |
| rs6013029 | CTNNBL1 | 20q11.23-q12 | 2.7 ×10−7 | 30 | rs4811211 | CTNNBL1 | 20q11.23-q12 | 0.08 |
| rs9930506 | FTO | 16q12.2 | 8.6 ×10−7 | 32 | rs13337356 | FTO | 16q12.2 | 0.003 |
| rs9939609 | FTO | 16q12.2 | 3 ×10−10 | 31 | ||||
| rs9939609 | FTO | 16q12.2 | 1.5 ×10−7 | 36 | ||||
| rs6602024 | PFKP | 10p15 | 4.9 ×10−6 | 32 | rs2388384 | PFKP | 10p15 | 0.001 |
| rs2206734 | CDKAL1 | 6p22.3 | 1.4 × 10−11 | 37 | rs9350257 | CDKAL1 | 6p22.3 | 0.001 |
| rs11142387 | KLF9 | 9q21 | 1.3 × 10−9 | 37 | rs6560130 | KLF9 | 9q21 | 0.01 |
Note.
Only studies conducted for BMI on population-based samples were included.
The most significant SNP in current GWA study within the gene identified by published GWA studies.
DISCUSSION
We identified a novel gene that might influence BMI variation in the Chinese by a powerful GWA study. In particularly, the two significant SNPs, rs4432245 and rs711906, identified by the GWA study were successfully replicated by a different Chinese sample. The major lines of evidence supported the significance of the SNPs, rs4432245 and rs711906, within EIF2AK4 to BMI.
EIF2AK4 belongs to a family of kinases that phosphorylate the alpha subunit of eukaryotic translation initiation factor-2 leading to down-regulation of protein synthesis in response to a variety of cellular stresses. Our GWA study in the Chinese provided the first evidence of association between EIF2AK4 gene and obesity. Currently, this gene was not shown to have any function directly relevant to obesity in humans. Guo et al. have shown that in knock-out mice study, there is no significant body weight difference against wild-type mice under regular chaw. However, during leucine deprivation in mice, significant differences of adiposity was shown as EIF2AK4 down-regulated genes and enzyme activity related to triglyceride synthesis[59]. Through regulating genes related to the synthesis of fatty acids, EIF2AK4 has a profound effect on the synthesis and storage of triglycerides and overall energy homeostasis. So it is speculated that EIF2AK4 may act as a master regulator of metabolic adaptation to nutrient deprivation, resulting in the process of fat accumulation. These biological evidences, together with the significant associations found in our initial GWAS and in our replication study, strongly support EIF2AK4 as a novel candidate gene influencing the variation of BMI. Functional analysis by FASTSNP suggested that rs4432245 and rs711906 might serve as binding sites for intronic enhancers in the EIF2AK4 gene. A ‘C → T’ change at rs4432245 may potentially delete one binding site for transcription factor MZF1, whereas an ‘A → G’ change at rs711906 may delete binding sites for three distinct transcription factors (CdxA, S8, and Nkx-2).
The opposite effect of allele ‘A’ of rs711906 on BMI variation may be caused by several factors. First, the direction of allelic association may flip when the target risk allele is inversely correlated with another risk allele at another locus, or positively associated with a protective allele at another locus. And the flip-flop associations depend on allele frequency and interlocus correlation (Abstract of the presentation at 11th International Congress of Human Genetics. 2006, Australia). In our study, the unknown inverse correlation of rs711906 with other risk locus may exist. Czarnomska et al. have also shown that a set of genes control the impact of the ApcMin mutation in both organs but with opposite effects[60]. Second, some studies have shown that environmental covariates will influence the effect direction of gene variants. Eder et al. discovered opposite effects of CD14/-260 on serum IgE levels in children raised in different environments[61]. Reneland et al. found that rs1498608 in PDE4D gene showed an opposite relationship with BMD variation, indicating that the variant’s effect may be context-dependent[62]. As our replication sample comes from south China, which is significant different from the initial GWA study sample recruited in north China, the different environment, like living and dietary habit may influence the effect direction of the allele. However, it needs to be confirmed in other independent samples.
The most significant association of rs4633 was not successfully confirmed in the replication study. This may be explained in two aspects. First, it was just a false-positive signal in the GWA study. Second, the two samples for initial GWA study and follow-up replication study differed largely by age. Tworoger et al. found that postmenopausal women exercisers with the COMT Val/Val genotype had a smaller decrease in BMI than women with neither allele (−1.0 vs. +0.1 kg/m2, P=0.009)[63]. The genetic variability of COMT gene can affect estrogen and androgen[64]. As the old people suffered an obvious loses of estrogen and androgen, while the young could maintain these hormones at a steady level, we may guess that this gene’s effect on fat regulation through estrogen and androgen could only be observed in the old people. However, to ascertain this assumption further in depth investigations will be needed.
The prevalence of obesity has been shown to vary widely across different ethnics/populations[65–66], and ethnic disparity in the genetic background has been considered as an important contributing factor that helps explain this variation. Henderson et al. found a statistically significant interaction between race/ethnicity and obesity status (P=0.005) in a multivariate regression of IGF-I levels[67]. Li et al. showed that variants in FTO gene, which was significantly and consistently associated with BMI in populations of European origin, were not associated with BMI in a Chinese Han population[68]. Similarly, the association of the CTNNBL1 gene with BMI identified by our research group in Caucasian subjects[30] could not be replicated in the present study in Chinese. Collectively, these data support the concept that the genetic determinants for BMI or obesity related phenotypes may partially vary across different ethnic groups.
Using the software Genetic Power Calculator[69], the estimated power of the present GWA sample to detect a gene accounting for 1.5% of BMI variation is 86.4% at a threshold P<0.05 (used in the replication study). We can confirm some common genes important for both ethnic populations, but the present study suggests that the EIF2AK4 gene may potentially be an ethnic-specific gene regulating BMI variation in the Chinese. First, the MAFs of the two significant SNPs in the EIF2AK4 gene are distinctly different between the Chinese and the Caucasians, (0.49 and 0.05, respectively), according to the dbSNP database. Second, in the Chinese we found a significant association between the EIF2AK4 gene and BMI in our initial GWA and follow-up replication studies; we did not, however, find a significant association between EIF2AK4 and BMI in our GWA study of 1000 Caucasians (data not shown).
In summary, our GWA study has identified a novel candidate gene, EIF2AK4, that is significantly associated with BMI variation in Chinese. The association of the EIF2AK4 gene is suggested to be ‘ethnic specific’ in Chinese. Follow-up studies could be pursued by replicating in other larger samples and populations to validate the specific associations, genotyping denser SNPs or re- sequencing the novel genomic region containing the gene to identify the causal variants and performing molecular functional studies to define the exact roles that the gene plays in regulating fat metabolism.
ACKNOWLEDGEMENTS
We thank Dr LEI Shu Feng for his constructive comments and suggestions in writing this paper.
Biography
YANG Fang, female, born in 1981, PhD, majoring in statistical genetics.
Footnotes
This work was supported by grants from Natural Science Foundation of China (30600364, 30470534, 30771222, 30731160618, 30230210, and 81101655), the grant from the China Postdoctoral Science Foundation (2011M501282).
REFERENCES
- 1.Doll S, Paccaud F, Bovet P, et al. Body mass index, abdominal adiposity and blood pressure: consistency of their association across developing and developed countries. Int J Obes Relat Metab Disor. 2002;26:48–57. doi: 10.1038/sj.ijo.0801854. [DOI] [PubMed] [Google Scholar]
- 2.Shetty P, Schmidhuber J. Introductory lecture the epidemiology and determinants of obesity in developed and developing countries. Int J Vitam Nutr Res. 2006;76:157–162. doi: 10.1024/0300-9831.76.4.157. [DOI] [PubMed] [Google Scholar]
- 3.Seidell JC. Obesity: a growing problem . Acta Paediatr. 1999;88(suppl):46–50. doi: 10.1111/j.1651-2227.1999.tb14350.x. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Menke A, Muntner P, Wildman RP, et al. Measures of adiposity and cardiovascular disease risk factors. Obesity (Silver.Spring) 2007;15:785–795. doi: 10.1038/oby.2007.593. [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Lv JH, Lei SF, et al. Receiver-operating characteristic analyses of body mass index, waist circumference and waist-to-hip ratio for obesity: Screening in young adults in central south of China. Clin Nutr. 2006;25:1030–1039. doi: 10.1016/j.clnu.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Comuzzie AG, Williams JT, Martin LJ, et al. Searching for genes underlying normal variation in human adiposity. J Mol Med. 2001;79:57–70. doi: 10.1007/s001090100202. [DOI] [PubMed] [Google Scholar]
- 8.Deng FY, Lei SF, Li MX, et al. Genetic determination and correlation of body mass index and bone mineral density at the spine and hip in Chinese Han ethnicity. Osteoporos Int. 2006;17:119–124. doi: 10.1007/s00198-005-1930-4. [DOI] [PubMed] [Google Scholar]
- 9.Deng HW, Lai DB, Conway T, et al. Characterization of genetic and lifestyle factors for determining variation in body mass index, fat mass, percentage of fat mass, and lean mass. J Clin Densitom. 2001;4:353–361. doi: 10.1385/jcd:4:4:353. [DOI] [PubMed] [Google Scholar]
- 10.Deng HW, Deng H, Liu YJ, et al. A genomewide linkage scan for quantitative-trait loci for obesity phenotypes. Am J Hum Genet. 2002;70:1138–1151. doi: 10.1086/339934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo YF, Xiong DH, Shen H, et al. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006;43:798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai F, Keighley ED, Sun G, et al. Genome-wide scan for adiposity-related phenotypes in adults from American Samoa. Int J Obes (Lond) 2007;31:1832–1842. doi: 10.1038/sj.ijo.0803675. [DOI] [PubMed] [Google Scholar]
- 13.Groves CJ, Zeggini E, Walker M, et al. Significant linkage of BMI to chromosome 10p in the U.K population and evaluation of GAD2 as a positional candidate. Diabetes. 2006;55:1884–1889. doi: 10.2337/db05-1674. [DOI] [PubMed] [Google Scholar]
- 14.Cornes BK, Medland SE, Ferreira MA, et al. Sex-limited genome-wide linkage scan for body mass index in an unselected sample of 933 Australian twin families. Twin Res Hum Genet. 2005;8:616–632. [PubMed] [Google Scholar]
- 15.Chen G, Adeyemo AA, Johnson T, et al. A genome-wide scan for quantitative trait loci linked to obesity phenotypes among West Africans. Int J Obes (Lond) 2005;29:255–259. doi: 10.1038/sj.ijo.0802873. [DOI] [PubMed] [Google Scholar]
- 16.Bell CG, Benzinou M, Siddiq A, et al. Genome-wide linkage analysis for severe obesity in french caucasians finds significant susceptibility locus on chromosome 19q. Diabetes. 2004;53:1857–1865. doi: 10.2337/diabetes.53.7.1857. [DOI] [PubMed] [Google Scholar]
- 17.Heijmans BT, Beem AL, Willemsen G, et al. Further evidence for a QTL influencing body mass index on chromosome 7p from a genome-wide scan in Dutch families. Twin Res. 2004;7:192–196. doi: 10.1375/136905204323016177. [DOI] [PubMed] [Google Scholar]
- 18.Meyre D, Lecoeur C, Delplanque J, et al. A genome-wide scan for childhood obesity-associated traits in French families shows significant linkage on chromosome 6q22.31-q23.2. Diabetes. 2004;53:803–811. doi: 10.2337/diabetes.53.3.803. [DOI] [PubMed] [Google Scholar]
- 19.Suviolahti E, Oksanen LJ, Ohman M, et al. The SLC6A14 gene shows evidence of association with obesity. J Clin Invest. 2003;112:1762–1772. doi: 10.1172/JCI17491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adeyemo A, Luke A, Cooper R, et al. A genome-wide scan for body mass index among Nigerian families. Obes Res. 2003;11:266–273. doi: 10.1038/oby.2003.40. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Cooper RS, Luke A, et al. A genome-wide scan for obesity in African-Americans. Diabetes. 2002;51:541–544. doi: 10.2337/diabetes.51.2.541. [DOI] [PubMed] [Google Scholar]
- 22.Hsueh WC, Mitchell BD, Schneider JL, et al. Genome-wide scan of obesity in the Old Order Amish. J Clin Endocrinol Metab. 2001;86:1199–1205. doi: 10.1210/jcem.86.3.7358. [DOI] [PubMed] [Google Scholar]
- 23.Eshraghi P, Hedayati M, Daneshpour MS, et al. Association of body mass index and Trp64Arg polymorphism of the beta3-adrenoreceptor gene and leptin level in Tehran Lipid and Glucose Study. Br J Biomed Sci. 2007;64:117–120. doi: 10.1080/09674845.2007.11732769. [DOI] [PubMed] [Google Scholar]
- 24.Um JY, Chung HS, Song MY, et al. Association of interleukin-1beta gene polymorphism with body mass index in women. Clin Chem. 2004;50:647–650. doi: 10.1373/clinchem.2003.025858. [DOI] [PubMed] [Google Scholar]
- 25.Shima Y, Nakanishi K, Odawara M, et al. Association of the SNP-19 genotype 22 in the calpain-10 gene with elevated body mass index and hemoglobin A1c levels in Japanese. Clin Chim Acta. 2003;336:89–96. doi: 10.1016/s0009-8981(03)00320-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhao LJ, Guo YF, Xiong DH, et al. Is a gene important for bone resorption a candidate for obesity? An association and linkage study on the RANK (receptor activator of nuclear factor-kappaB) gene in a large Caucasian sample. Hum Genet. 2006;120:561–570. doi: 10.1007/s00439-006-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu YJ, Rocha-Sanchez SM, Liu PY, et al. Tests of linkage and/or association of the LEPR gene polymorphisms with obesity phenotypes in Caucasian nuclear families. Physiol Genomics. 2004;17:101–106. doi: 10.1152/physiolgenomics.00213.2003. [DOI] [PubMed] [Google Scholar]
- 28.Deng HW, Li J, Li JL, et al. Association of estrogen receptor-alpha genotypes with body mass index in normal healthy postmenopausal Caucasian women. J Clin Endocrinol Metab. 2000;85:2748–2751. doi: 10.1210/jcem.85.8.6728. [DOI] [PubMed] [Google Scholar]
- 29.Almasy L, Goring HH, Diego V, et al. A novel obesity locus on chromosome 4q: the Strong Heart Family Study. Obesity (Silver Spring) 2007;15:1741–1748. doi: 10.1038/oby.2007.207. [DOI] [PubMed] [Google Scholar]
- 30.Liu YJ, Liu XG, Wang L, et al. Genome-wide association scans identified CTNNBL1 as a novel gene for obesity. Hum Mol Genet. 2008;17:1803–1813. doi: 10.1093/hmg/ddn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbert A, Gerry NP, McQueen MB, et al. A common genetic variant is associated with adult and childhood obesity. Science. 2006;312:279–283. doi: 10.1126/science.1124779. [DOI] [PubMed] [Google Scholar]
- 34.Melka MG, Bernard M, Mahboubi A, et al. Genome-wide scan for loci of adolescent obesity and their relationship with blood pressure. J Clin Endocrinol Metab. 2012;97:145–150. doi: 10.1210/jc.2011-1801. [DOI] [PubMed] [Google Scholar]
- 35.Loos RJ, Lindgren CM, Li S, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho YS, Go MJ, Kim YJ, et al. large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet. 2009;41:527–534. doi: 10.1038/ng.357. [DOI] [PubMed] [Google Scholar]
- 37.Okada Y, Kubo M, Ohmiya H, et al. Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nat Genet. 2012;44:302–306. doi: 10.1038/ng.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y, Tan LJ, Lei SF, et al. Genome-wide association study identifies ALDH7A1 as a novel susceptibility gene for osteoporosis. PLoS Genet. 2010:6. doi: 10.1371/journal.pgen.1000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braun A, Roth R, McGinniss MJ. Technology challenges in screening single gene disorders. Eur J Pediatr. 2003;162(Suppl 1):13–6. doi: 10.1007/s00431-003-1343-3. [DOI] [PubMed] [Google Scholar]
- 40.Barrett JC, Fry B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 41.Storey JD, Tibshirani R. Statistical significance for genomewide studies Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan HY, Chiou JJ, Tseng WH, et al. FASTSNP: an always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res. 2006;34:635–641. doi: 10.1093/nar/gkl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher RA. Statistical Methods for Research Workers. New York: Hafner; 1925. [Google Scholar]
- 46.Stone S, Abkevich V, Hunt SC, et al. A major predisposition locus for severe obesity, at 4p15-p14. Am J Hum Genet. 2002;70:1459–1468. doi: 10.1086/340670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kissebah AH, Sonnenberg GE, Myklebust J, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci USA. 2000;97:14478–14483. doi: 10.1073/pnas.97.26.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Li S, Cook NR, et al. An autosomal genome scan for loci influencing longitudinal burden of body mass index from childhood to young adulthood in white sibships: The Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:462–469. doi: 10.1038/sj.ijo.0802610. [DOI] [PubMed] [Google Scholar]
- 49.Chagnon YC, Rice T, Perusse L, et al. Genomic scan for genes affecting body composition before and after training in Caucasians from HERITAGE. J Appl Physiol. 2001;90:1777–1787. doi: 10.1152/jappl.2001.90.5.1777. [DOI] [PubMed] [Google Scholar]
- 50.Arya R, Duggirala R, Jenkinson CP, et al. Evidence of a novel quantitative-trait locus for obesity on chromosome 4p in Mexican Americans. Am J Hum Genet. 2004;74:272–282. doi: 10.1086/381717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer LJ, Buxbaum SG, Larkin EK, et al. Whole genome scan for obstructive sleep apnea and obesity in African-American families. Am J Respir Crit Care Med. 2004;169:1314–1321. doi: 10.1164/rccm.200304-493OC. [DOI] [PubMed] [Google Scholar]
- 52.Palmer LJ, Buxbaum SG, Larkin E, et al. A whole-genome scan for obstructive sleep apnea and obesity. Am J Hum Genet. 2003;72:340–350. doi: 10.1086/346064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feitosa MF, Borecki IB, Rich SS, et al. Quantitative-trait loci influencing body-mass index reside on chromosomes 7 and 13: the National Heart, Lung, and Blood Institute Family Heart Study. Am J Hum Genet. 2002;70:72–82. doi: 10.1086/338144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X, Cooper RS, Borecki I, et al. A combined analysis of genomewide linkage scans for body mass index from the National Heart, Lung, and Blood Institute Family Blood Pressure Program. Am J Hum Genet. 2002;70:1247–1256. doi: 10.1086/340362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li WD, Dong C, Li D, et al. An obesity-related locus in chromosome region 12q23–24. Diabetes. 2004;53:812–820. doi: 10.2337/diabetes.53.3.812. [DOI] [PubMed] [Google Scholar]
- 56.Chiu YF, Chuang LM, Kao HY, et al. Sex-specific genetic architecture of human fatness in Chinese: the SAPPHIRe Study. 2010;128:501–513. doi: 10.1007/s00439-010-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai F, Sun G, Aberg K, et al. A whole genome linkage scan identifies multiple chromosomal regions influencing adiposity-related traits among Samoans. Ann Hum Genet. 2008;72:780–792. doi: 10.1111/j.1469-1809.2008.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He LN, Liu YJ, Xiao P, et al. Genomewide linkage scan for combined obesity phenotypes using principal component analysis. Ann Hum Genet. 2008;72:319–326. doi: 10.1111/j.1469-1809.2007.00423.x. [DOI] [PubMed] [Google Scholar]
- 59.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–114. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Czarnomska A, Krysiak E, Piskorowska J, et al. Opposite effects of modifiers of the ApcMin mutation in intestine and mammary gland. Cancer Res. 2003;63:4533–4537. [PubMed] [Google Scholar]
- 61.Eder W, Klimecki W, Yu L, et al. Opposite effects of CD 14/-260 on serum IgE levels in children raised in different environments. J Allergy Clin Immunol. 2005;116:601–607. doi: 10.1016/j.jaci.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Reneland RH, Mah S, Kammerer S, et al. Association between a variation in the phosphodiesterase 4D gene and bone mineral density. BMC Med Genet. 2005;6:9. doi: 10.1186/1471-2350-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tworoger SS, Chubak J, Aiello EJ, et al. The effect of CYP19 and COMT polymorphisms on exercise-induced fat loss in postmenopausal women. Obes Res. 2004;12:972–981. doi: 10.1038/oby.2004.119. [DOI] [PubMed] [Google Scholar]
- 64.Tworoger SS, Chubak J, Aiello EJ, et al. Association of CYP17, CYP19, CYP1B1, and COMT polymorphisms with serum and urinary sex hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:94–101. doi: 10.1158/1055-9965.epi-03-0026. [DOI] [PubMed] [Google Scholar]
- 65.Palep-Singh M, Picton HM, Barth JH, et al. Ethnic variations in the distribution of obesity and biochemical metabolic abnormalities in fertility clinic attendees. J Reprod Med. 2008;53:117–123. [PubMed] [Google Scholar]
- 66.Myles S, Davison D, Barrett J, et al. Worldwide population differentiation at disease-associated SNPs. BMC Med Genomics. 2008;1:22. doi: 10.1186/1755-8794-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henderson KD, Goran MI, Kolonel LN, et al. Ethnic disparity in the relationship between obesity and plasma insulin-like growth factors: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:2298–2302. doi: 10.1158/1055-9965.EPI-06-0344. [DOI] [PubMed] [Google Scholar]
- 68.Li H, Wu Y, Loos RJ, et al. Variants in the fat mass- and obesity-associated (FTO) gene are not associated with obesity in a Chinese Han population. Diabetes. 2008;57:264–268. doi: 10.2337/db07-1130. [DOI] [PubMed] [Google Scholar]
- 69.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]