Abstract
Staphylococcus epidermidis is a common commensal of healthy conjunctiva and it can cause endophthalmitis, however its presence in conjunctivitis, keratitis and blepharitis is unknown. Molecular genotyping of S. epidermidis from healthy conjunctiva could provide information about the origin of the strains that infect the eye. In this paper two collections of S. epidermidis were used: one from ocular infection (n = 62), and another from healthy conjunctiva (n = 45). All isolates were genotyped by pulsed field gel electrophoresis (PFGE), multilocus sequence typing (MLST), staphylococcal cassette chromosome mec (SCCmec), detection of the genes icaA, icaD, IS256 and polymorphism type of agr locus. The phenotypic data included biofilm production and antibiotic resistance. The results displayed 61 PFGE types from 107 isolates and they were highly discriminatory. MLST analysis generated a total of 25 STs, of which 11 STs were distributed among the ocular infection isolates and lineage ST2 was the most frequent (48.4%), while 14 STs were present in the healthy conjunctiva isolates and lineage ST5 was the most abundant (24.4%). By means of a principal coordinates analysis (PCoA) and a discriminant analysis (DA) it was found that ocular infection isolates had as discriminant markers agr III or agr II, SCCmec V or SCCmec I, mecA gene, resistance to tobramycin, positive biofilm, and IS256+. In contrast to the healthy conjunctiva isolates, the discriminating markers were agr I, and resistance to chloramphenicol, ciprofloxacin, gatifloxacin and oxacillin. The discriminant biomarkers of ocular infection were examined in healthy conjunctiva isolates, and it was found that 3 healthy conjunctiva isolates [two with ST2 and another with ST9] (3/45, 6.66%) had similar genotypic and phenotypic characteristics to ocular infection isolates, therefore a small population from healthy conjunctiva could cause an ocular infection. These data suggest that the healthy conjunctiva isolates do not, in almost all cases, infect the eye due to their large genotypic and phenotypic difference with the ocular infection isolates.
Introduction
Staphylococcus epidermidis is a common inhabitant of the skin and mucous membranes, however in recent decades it has gained interest due to its high frequency of isolation in hospital acquired infections [1]. The main vehicle to cause infection is by medical device, such as catheters, heart valves, contact lenses, etc. [2–3]. Different strategies have been applied in order to find determinant biomarkers for S. epidermidis isolates from nosocomial. Some determinant biomarkers include biofilm formation, antibiotic resistance, the presence of the ica genes (encoding exopolysaccharide for biofilm formation) which partly explain their opportunistic pathogenicity to humans [4]. However the molecular genotyping of S. epidermidis isolates by pulsed-field gel electrophoresis (PFGE), multilocus sequence typing (MLST), staphylococcal cassette chromosome mec (SCCmec) type assignment [5] and the whole genome sequencing [6] have exhibited a high diversity; which makes difficult to discriminate between opportunistic pathogenic strains of Staphylococcus epidermidis from the commensal strains.
In the eye, S. epidermidis can cause infection and the most common examples include conjunctivitis, blepharitis, corneal ulcers and endophthalmitis. The main cause of ocular infection is the use of contact lenses (in the case of conjunctivitis and corneal ulcer) [7] or during ocular surgery (endophthalmitis). High incidences of ocular infection by S. epidermidis have been reported [8], even in some cases superior than those achieved by S. aureus [9]. These reports are considerable because S. epidermidis have not a variety of virulence factors such as S. aureus. Furthermore, the eye is an organ continuously exposed to the environment, and it is also in contact with various microorganisms. Despite this high exposure, the hazard of ocular infection during a person’ life is low. One possible explanation against bacterial infection in the eye is the production of molecules associated to innate immunity; such as lysozyme, lactotransferrin, secretoglobin family 2A [10], antimicrobial peptides [11], cytokine modulators [12] and TLRs [13], which help to remove the bacteria on the ocular surface.
Additionally, S. epidermidis inhabits the ocular surface specifically into the conjunctival sac [14]. Innate immunity of the conjunctiva recognizes the commensal population of S. epidermidis, with specific feature to regulate their growth. S. epidermidis strains infecting the eye must evade the innate immunity of the host. Probably the virulent pathogenic strains have elements, which would be absent in commensal strains. The origin of infective strains of S. epidermidis in the eye could be the conjunctiva sac, as in some studies have suggested [15–16], or could be from different origin. The presence of S. epidermidis in endophthalmitis had shown that the primary source of infection is the healthy conjunctiva [17]. However, in a study of keratitis and endophthalmitis caused by S. epidermidis was proven otherwise [18]. It has also been documented that S. epidermidis from healthy conjunctiva is polyclonal because genotyping by PFGE of S. epidermidis isolates from the conjunctiva of a healthy subject are different [19]. Currently it is unclear the involvement of S. epidermidis from healthy conjunctiva in ocular infection and the limited research on molecular genotyping of these isolates fail to clarify this point. In this paper, we propose that healthy conjunctiva isolates of S. epidermidis could provide more information on the source of infective strains to the eye. Therefore, a genetic and phenotypic comparison of S. epidermidis isolates from healthy conjunctiva and ocular infection may answer this question.
Materials and Methods
2.1 Isolates
S. epidermidis isolates from ocular infection were obtained from 1995 to 2000 in the Institute of Ophthalmology Conde de Valenciana at Mexico City, DF. Samples were obtained from patients diagnosed with conjunctivitis (n = 21), corneal ulcers (n = 7), endophthalmitis (n = 14) or staphylococcal blepharitis (n = 20). Corneal ulcer samples were obtained by scraping, and conjunctivitis and staphylococcal blepharitis samples were obtained by swabbing. The vitreous samples of patients with endophthalmitis were obtained mainly by vitrectomy. Swabbing samples were obtained from the conjunctiva of healthy individual (n = 45). All clinical samples were spreading onto chocolate blood, and mannitol salt agar plates, and incubated to 37°C for 12 h. For ocular infection isolates, it was considered plates that exhibited single colony morphology and these were re-growth onto mannitol salt agar plates. S. epidermidis identification was performed by the Vitek Jr computerized system (BioMériux, Craponne, France), using the GPS-101 and V-1305 identification cards for Gram-positive bacteria. Based on the Declaration of Helsinki, all patients signed medical informed consent forms to participate in this study, and the study was approved by the ethics committee of National School of Biological Sciences of the “Instituto Politécnico Nacional” Mexico.
2.2 Pulsed-Field Gel Electrophoresis (PFGE)
The SmaI DNA restriction fragments were separated by PFGE [20] and the resulting patterns were analyzed using the BioNumerics software (version 4.61 of Applied Maths, Saint-Martens-Latem, Belgium) with previously optimized settings for S. epidermidis [21].
2.3 Genomic DNA extraction
Bacterial cells were grown overnight in tryptic soy broth (TSB; Becton Dickinson, NJ, USA), harvested by centrifugation and resuspended in 200 μL of lysis solution (20% sucrose, 10 mM Tris-HCl pH 8 and 10 μg/mL lysozyme). Cells were incubated at 37°C for 40 min and 200 μL of Whinston solution (2% Triton X-100, 1% SDS, 10 nM NaCl, 10 mM Tris base pH 8.0 and 1 mM EDTA) was added. DNA was extracted with phenol/chloroform/isoamyl alcohol (25:24:1). DNA was subsequently precipitated with a volume of isopropanol and purified by the addition of two volumes of ethanol 70%. Finally, DNA was resuspended in sterile distilled water.
2.4 SCCmec typing
SCCmec typing was performed using the PCR schemes previously published [22–23]. A single PCR was performed for each gene. For isolates in which SCCmec could not be typed, classes of the mec gene complex and the ccr gene complex (ccrAB1, ccrAB2, ccrAB3 and ccrC1) were examined by additional PCRs using the primers described previously [22]. SCCmec types were assigned based on the mec complex classes and the ccr gene types according to the criteria set for S. aureus [22–23].
2.5 Molecular Typing by Multilocus Sequence Typing (MLST)
Genomic DNAs of the isolates were subjected to MLST according to the procedure described by Thomas et al., (2007) [24]. Briefly, PCRs were performed to amplify fragments (approximately 450 bp) of arcC, aroE, gtr, mutS, pyr, tpi and yqiL genes. The amplified products were purified and sequenced by the BigDye terminator fluorescence kit (Applied Biosystems, Foster City, CA, USA). The sequences and allelic profiles assignments were performed through S. epidermidis MLST database (http://www.mlst.net).
2.6 PCR amplification of the icaA and icaD, and IS256
Genomic DNAs isolates were used as templates for the amplification of icaA and icaD genes, and IS256 with primers described previously [25]. The PCR reactions were performed with 1 μL of DNA (100 ng), 1X buffer, 1 mM MgCl2, 200 μM of each dNTPs, 1 U of Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) and 0.2 μM of each specific primer. The optimal PCR conditions were 30 cycles of 30 s at 92°C, 40 s at 60°C and 30 s at 72°C. The PCR products were analyzed on agarose gels.
2.7 Determination of biofilm formation
A semi-quantitative determination of biofilm formation was performed in 96-well tissue culture plates (Nunc, Rochester, NY, USA) based on the method reported by Christensen et al. (1985) [26]. Bacteria were grown in individual wells of 96-well plates at 37°C in tryptic soy broth (TSB; Becton Dickinson, NJ, USA) medium. After 24 h of growth, the plates were washed vigorously with 1X phosphate buffed saline (PBS), dried for 30 min at 55°C, and stained with 0.5% (w/v) crystal violet solution. After staining, the plates were washed with 1X PBS. A492 nm of the adhered, stained cells was measured using a Multiskan EX Microplate Photometer (Thermo Fisher Scientific, Lenexa, KS, USA). The criterion outlined by Chistensen et al. (1985) [26] was used to determine whether isolates were non-adherent and biofilm-negative (A492 < 0.12) or strongly biofilm-positive (A492 > 0.12). Assays were repeated six times, and the mean biofilm absorbance values were used. The results were analyzed using a one-way ANOVA with a Tukey’s test.
2.8 Genetic polymorphism of agr by PCR
Genetic polymorphisms of agr were determined according to Li et al. (2004) [27]. Briefly, A primers were designed from conserved sequences of agr, which are common to agr groups I, II and III (1,022 bp). B primers were designed from the hypervarible region, which is common in agr groups II and III but not group I (453 bp). C primers are specific for group II (615 bp). The PCR assay was performed following conditions as previously described by Li et al. (2004) [27].
2.9 Statistical analysis
To analyze the above data, it was used a principal coordinates analysis (PCoA) and then a discriminant analysis (DA), using the PAST program v2.15 [28]. The PCoA is a sorting method, also known as Metric Multidimensional Scaling; PCoA routine calculated eigenvalues (eigenvalues) and eigenvectors (eigenvectors) of a matrix containing Jaccard distances of all data. The routine was processed with default values and the eigenvalues, which provides a measurement of the variance displayed by the corresponding eigenvectors [28]. The discriminant analysis (DA) was carried out, also with default values and the result was used to confirm or reject the hypothesis that the two groups are different. Subsequently, the discriminant function was calculated, which is a linear equation, for classifying data according to the score obtained in the discriminant function. Additionally, the values of the coefficients of the discriminant function can be the contribution of each variable in the DA because the sign variable load (positive or negative) [28] is known. All data phenotypic and genotypic of two isolation sources are included in the supplementary material (S1 and S2 Tables).
Results
3.1 Diversity of STs in strains of S. epidermidis isolated from healthy conjunctiva and ocular infection
A total of 107 isolates were distributed in 25 STs. Ocular infection isolates (n = 62) were distributed in 11 STs, and lineage ST2 was the most prevalent (30 isolates, 48.4%), followed by ST9 (15 isolates, 24.2%) and ST23 (9 isolates, 14.5%) (Table 1). ST16, ST10, ST87, ST38, ST71, ST21, ST57 and ST46 types were only represented by a single isolate (this group was designated as "Other STs"; Table 1).
Table 1. Relationship of ST with genotypic and phenotypic data from the two sources.
| STs (n, %) | PFGE types | SCCmec Type (%) | Biofilm + (%) | agr I (%) | agr II (%) | agr III (%) | icaA (%) | icaD (%) | IS256 (%) |
|---|---|---|---|---|---|---|---|---|---|
| Ocular infection (n = 62) | |||||||||
| ST2 (30, 48.4) | 16 | I, II, IV (26.6) | 9 (30) | 2 (6.6) | 14 (46.6) | 14 (46.6) | 6 (20) | 6 (20) | 9 (30) |
| ST9 (15, 24.2) | 7 | V (46.6) | 7 (46.6) | 1 (6.6) | 4 (26.6) | 10 (66.6) | 6 (40) | 3 (20) | 3 (20) |
| ST23 (9, 14.5) | 5 | II, IV, V (33.3) | 6 (66.6) | 0 | 2 (22.2) | 7 (77.7) | 2 (22.2) | 2 (22.2) | 1 (11) |
| Others STs a (8,13) | 2 | I (50) | 6 (75) | 1 (12.5) | 2 (25) | 5 (62.5) | 1 (12.5) | 3 (37.5) | 2 (25) |
| Healthy conjunctiva (n = 45) | |||||||||
| ST5 (11, 24.4) | 10 | III (36.4) | 0 | 6 (54.5) | 3 (27.3) | 2 (18.2) | 0 | 2 (18.2) | 1 (9) |
| ST10 (9, 20) | 8 | IV (33.3) | 0 | 4 (44.4) | 2 (22.2) | 3 (33.3) | 0 | 0 | 1 (11) |
| Others STs b (25, 55) | 24 | II (40) | 8 (32) | 8 (32) | 12 (48) | 5 (20) | 7 (28) | 10 (40) | 4 (16) |
a Represented by ST16, ST10, ST87, ST38, ST71, ST21, ST57 and ST46, each type with a single isolate.
b Represented by lineage ST238 and ST118 (4 isolates for each one, 8.8%), ST2, ST4, ST9 (3 isolates for each one, 6.6%), ST23 (2 isolates for each one, 4.4%), ST26, ST135, ST494, ST43, ST48 and ST173 (one isolate for each one).
In comparing with ocular infection isolates, 14 STs were found in the healthy conjunctiva isolates (n = 45), lineage ST5 was the most prevalent (11 isolates, 24.4%), followed by lineage ST10 (9 isolates, 20%). Lineages ST238 and ST118 (4 isolates of each one, 8.8%), ST2, ST4, ST9 (3 isolates of each one, 6.6%), ST23 (2 isolates, 4.4%), ST26, ST135, ST494, ST43, ST48 and ST173 (1 isolate of each one) had the lowest frequency and they are named as the group "Others STs' (Table 1).
3.2 PFGE typing
S. epidermidis isolates were widely spread in 61 PFGE types. Healthy conjunctiva isolates had more genetic diversity than the ocular infection isolates, 31 PFGE types was presented in healthy conjunctiva isolates, 19 PFGE types in ocular infection, and only 11 PFGE types were shared between both sources of isolation (Table 1). Relationship between ST with PFGE types exhibited a greater diversity of PFGE patterns compared with ST lineages, this implies that PFGE was more discriminatory than MLST and that PFGE increased the genotypic diversity. For the above reason, in this study the STs lineages of the isolates were used to correlate them with additional genotypic and phenotypic data.
3.3 Relationship between types of STs and type of SCCmec
With regard to ocular infection isolates, the mecA gene was 75.8% and SCCmec type II was the most recurrent (25.8%). Isolates associated with lineage ST2 from ocular infection were SCCmec type I, II and IV (each 26.6%), whereas isolates with lineage ST9 were SCCmec type V (46.6%) and lineage ST23 were SCCmec types II, IV, and V (each 33%; Table 1). In relation to healthy conjunctiva isolates, the mecA gene was 66.6% and SCCmec type II (31.1%) was the most recurrent, whereas lineage ST5 was related to SCCmec type III (36.4%) and ST10 with the SCCmec type IV (33.3%; Table 1).
3.4 Relationship between ST, biofilm formation, the presence of icaA and icaD genes and IS256 in S. epidermidis
Ocular infection isolates produced more biofilm (28/62, 45.16%) than healthy conjunctiva isolates (8/45, 17.7%) (p < 0.05). However, ocular infection isolates grouped in "others STs" are the largest biofilm producers, while ocular infection isolates with lineage ST2 were the minor biofilm producers (Table 1). In addition, the isolates included in "other STs" from healthy conjunctiva were the largest biofilm producers in their respective groups (Table 1).
Regarding icaA and icaD genes, a predominance of 20 to 40% distributed homogenously between the STs lineages of the two sources of isolation were determined, and no statistical significance was shown. Similar results were found with IS256 (Table 1).
3.5 Relation between ST and type of agr in strains of S. epidermidis
Ocular infection isolates with lineage ST2 were predominantly agr types II and III. Ocular infection isolates with lineages ST9 and ST23 were mainly agr type III. ST5 and ST10 from healthy conjunctiva have a tendency to agr types I and II (Table 1).
3.6 ST relationship with resistance to antibiotics
In general, it was observed that healthy conjunctiva isolates are more resistant to antibiotics than those from ocular infection. Healthy conjunctiva isolates were more multiresistant (14/45; 31.1%) than ocular infection isolates (3/62; 4.8%). Healthy conjunctiva isolates with lineage ST5 were different to ocular infection isolates with lineage ST2, in terms of resistance to oxacillin (p < 0.0058), ciprofloxacin, levofloxacin and chloramphenicol (p < 0.05). In contrast, ocular infection isolates were only resistant to tobramycin (p < 0.05) (Table 2).
Table 2. Antibiotic resistance of healthy conjunctiva and ocular infection isolates.
| ST (n, %) | Oxa n (%) | Cip n (%) | Ofl n (%) | Lev n (%) | Mox n (%) | Gat n (%) | Tob n (%) | Chl n (%) | Van n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Healthy Conjunctiva | |||||||||
| ST5 (11, 24.4) | 6 (54.5) | 5 (45.4) | 4 (36.4) | 2 (18.1) | 0 (0) | 0 (0) | 0 (0) | 5 (45.4) | 0 (0) |
| ST10 (9, 20) | 2 (22.2) | 3 (33.3) | 3 (33.3) | 1 (11.1) | 0 (0) | 1 (11.1) | 0 (0) | 2 (22.2) | 1 (11.1) |
| Others STs (25, 55.5) | 11 (44) | 3 (12) | 3 (12) | 3 (12) | 1 (4) | 2 (8) | 3 (12) | 8 (32) | 0 (0) |
| Ocular Infection | |||||||||
| ST2 (30, 48.4) | 3 (10) | 0 (0) | 6 (20) | 0 (0) | 0 (0) | 0 (0) | 11 (36.6) | 0 (0) | 1 (3.3) |
| ST9 (15, 24.2) | 3 (20) | 2 (13.3) | 4 (26.6) | 0 (0) | 0 (0) | 0 (0) | 5 (33.3) | 0 (0) | 0 (0) |
| ST23 (9, 14.5) | 1 (11.1) | 0 (0) | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 2 (22.2) | 0 (0) | 0 (0) |
| Others STs (8, 13) | 1 (12.5) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 5 (62.5) | 0 (0) | 0 (0) |
Oxa: oxacillin; Cip: ciprofloxacin; Ofl: ofloxacin; Lev: levofloxacin; Mox: moxifloxacin; Gat: gatifloxacin; Tob: tobramycin; Chl: chloramphenicol; Van: vancomycin.
3.7 Statistical differentiation between isolates from healthy conjunctiva, and ocular infection
It was observed that PCoA exhibited a clear separation between the two groups of isolates (using 2 axis had the highest percentage of representation, with 31% of explained variance) (Fig 1A). Similarly, with the discriminant analysis (DA), ocular infection isolates were grouped within the more negative values of the graph while healthy conjunctiva isolates among the most positive values (Fig 1B). With both methods, the isolates were separated in two groups. Subsequently, in order to find the preponderant biomarkers of the isolates, the coefficient of the discriminant function was determined, and a preponderant biomarker was considered when a value equal to 0.5 or greater than 0.5 was reached. Based on the above, ocular infection isolates are discriminating in the biomarkers of agr III (-5.5711) or agr II (-3.5108), SCCmec V (-3.3422) or SCCmec I (-3.2048), mecA gene (-2.2384), resistance to tobramycin (-1.7989), positive biofilm (-1.2738), and IS256+ (-0.94603). In contrast, healthy conjunctiva isolates were discriminating in agr I (3.8668), and resistance to chloramphenicol (3.7826), ciprofloxacin (2.5111), gatifloxacin (2.2844), oxacillin (1.49). Besides, it was found that the discriminant function properly assigned to 77% of healthy conjunctiva isolates and 84% of ocular infection isolates. Later, these data were present in a dendrogram to search a relationship with the lineage ST (Fig 2), two major groups were observed, the first includes all the isolates from healthy conjunctiva and their corresponding STs (ST5 and ST10 were predominant) and some isolates from ocular infection, and the second grouped all the ocular infection isolates with lineage ST2 and ST9.
Fig 1. Statistical analysis of phenotypic and genotypic data.
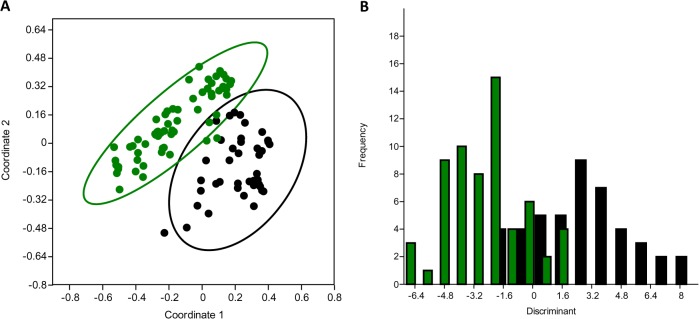
Principal Coordinates Analysis (PCoA) of S. epidermidis (A) from Ocular Infection (OI, green) and isolates from healthy conjunctiva (HC, black). The circles represent the confidence interval 95%. The first two coordinate axes represent 31% of the variance. Discriminant analysis (B) of the isolates of S. epidermidis from Ocular Infection (OI, green) and healthy conjunctiva (HC, black). Negative values belong to OI isolates and positive to HC isolates. The discriminant function properly allocated to 77% of isolates from HC and 84% from OI.
Fig 2. Cluster analyses of the isolates from Ocular Infection and healthy conjunctiva.
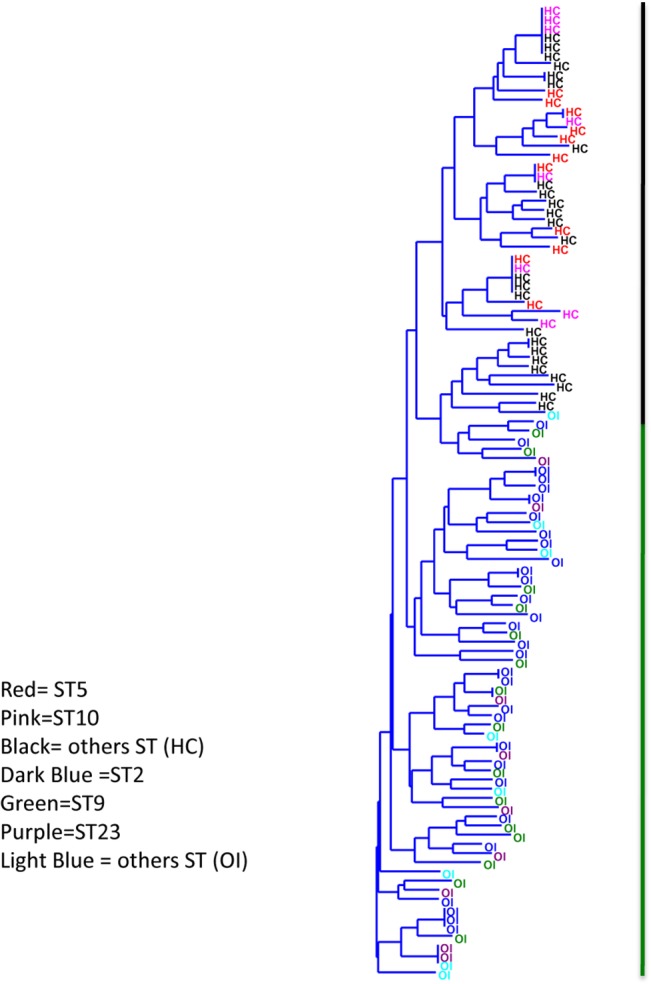
The colors represent the ST's. The black vertical line represents the isolates from healthy conjunctiva (HC) and green vertical line isolates from ocular infection (OI).
Discussion
S. epidermidis provides a helpful symbiotic relationship with the skin and conjunctiva of humans. Currently, S. epidermidis is recognized as an opportunistic pathogen in patients with low immune response and frequently cause infections linked to medical devises, particularly in cases of prosthetic cardiac valves, cerebrospinal fluid shunts, intravascular catheters and orthopedic implants [2]. To explain the ability of S. epidermidis as an opportunistic pathogen, some studies at genetic level had been performed. Genome sequencing of 17 isolates of S. epidermidis obtained from different parts of the body of a healthy individual showed significant differences [6], indicating the high genetic variation of this bacterium. Moreover, it have been documented the isolation of different strains of S. epidermidis from acquired infections at the same hospital. With regard to ocular infection isolates, the comparison of 42 isolates of keratitis and endophthalmitis with 14 healthy conjunctiva isolates, revealed 11profiles of fluorescence-amplified fragment lentgth polymorphism (FAFLP); a clear separation of groups was observed between ocular infection isolates (keratitis and endophthalmitis) and commensal isolates [18]. In this work, the PFGE generated a high discrimination level between isolates of the two sources of isolation and no accurate data that allow the separation between them were observed. Typing by MLST STs generated lineages that were shared between the two sources of isolation but some lineages were not shared. The lineage ST2 was the most frequent in ocular infection isolates (48.4%); however this lineage was not common in healthy conjunctiva isolates (6.6%), in which the ST5 lineage (24.4%) was the most abundant. This suggests that at STs level, there is a substantial difference between isolates from ocular infection and healthy conjunctiva, and that S. epidermidis that inhabits the conjunctiva probably could not infect the eye. Using a similar approach to this work in the hospital in Shangai, China compared clinical isolates from peripheral blood with nasal isolates from healthcare staffs and with community nasal isolates from healthy people, these authors found that the lineage ST2 was the most frequent in clinical isolates but this was absent in isolates from healthy patients [29]. The same result was reported in another hospital in Belgium [4]. Some studies have pointed out that the ST2 lineage of S. epidermidis is associated with virulent strains [30–32]. However, in a recent study, 36 isolates from keratitis (eye infection) generated lineages ST59, ST5 and ST6 mainly and these authors suggested that these lineages are occasional and could be specific for the eye infection [15]. The ST5 lineage has also been detected in clinical isolates of US hospitals [33] or in bone and joint infections [34]. These studies had demonstrated that there is an association between lineage ST5 and infections.
Biofilm production in S. epidermidis is a virulence factor and it is recognized that clinical isolates produce more biofilm than commensal isolates [4, 29]. Besides, the commensal isolates from conjunctiva produce more biofilm than the skin isolates [35]. Our results are consistent with these findings, because ocular infection isolates with lineage ST2 produced more biofilm than healthy conjunctiva isolates with ST5. The presence of the icaA and icaD genes (involved in the production of exopolysaccharide N-acetyl-glucosamine) and IS256 exhibited a low frequency in the isolates of the two sources of isolation; which it was according to S. epidermidis isolates from keratitis [15] and also icaA gene was not useful for discriminating between the isolates of keratitis from the healthy conjunctiva isolates [18]. However in S. epidermidis isolates from peripheral blood there was a correlation between the high production biofilm and the presence of icaA and icaD genes [30]. Furthermore quorum sensing activates the agr operon and it negatively regulates the biofilm production. This operon has three types of polymorphisms. In ocular infection isolates there was a major tendency toward agr III than in commensal isolates. Ocular infection isolates with lineage ST2 presented agr II and III. Li, et al. (2010) found that clinical isolates of S. epidermidis harbored agr I while commensal isolates were agr II [27]. Our results were in agreement with that study, in which the isolates with lineage ST2 of prosthetic joint infections (PJI) presented mainly agr I, but isolates with lineage ST215 were agr III [36]. In S. epidermidis isolates from peripheral blood with lineage ST2 were predominantly agr III [32]. Isolates with lineage ST215 were found in patients at hospitals in Sweden and Norway [37], indicating that ST215 could be associated with an increased pathogenicity and/or greater ability to survive in hospital environment [37]. Conversely, in this study ST215 is not present in any of the isolates.
Another relevant virulence factor of S. epidermidis is the resistance to antibiotics. Healthy conjunctiva isolates showed higher resistance to antibiotics than ocular infection isolates. Healthy conjunctiva isolates with ST5 lineage were more resistant to oxacillin than ocular infection isolates with ST2 lineage. Contrary to another studies where it was exhibited that S. epidermidis isolates from different clinical sources are more resistant to antibiotics than commensal isolates [32, 34]. However, it has been documented that near to 50% of healthy conjunctiva isolates of S. epidermidis have mutations in the gyrA and parC genes, which have been associated with resistance to fluoroquinolones [38]; contrary, ocular infection isolates harbored fewer mutations and reduced resistant to these antibiotics [39–40]. Moreover, healthy conjunctiva isolates significantly increased their resistance to azithromycin and fluoroquinolone after repeated exposure to them [41]. This suggests that the native healthy conjunctiva strains have been under increasing pressure by the use of antibiotics for prophylaxis or during the treatment of ocular infection caused by S. epidermidis, or by another bacteria.
Based on the statistical analysis (PCoA and DA) of genetic and phenotypic data, it was found that the discriminant biomarkers for ocular infection isolates were: agr III or agr II, SCCmec V or SCCmec I, mecA gene, resistance to tobramycin, positive biofilm and IS256+, and by MLST was the lineage ST2. In contrast, the discriminant biomarkers in healthy conjunctiva isolates were agr I, and resistance to chloramphenicol, ciprofloxacin, gatifloxacin, oxacillin, and by MLST was the lineage ST5. This result shows that ocular infection isolates are markedly different from healthy conjunctiva isolates, and suggests that S. epidermidis from healthy conjunctiva do not infect the eye. However, we do not reject that the isolates from healthy conjunctiva could infect the eye. This opinion is supported by the work of Bispo et al. (2014) who found that S. epidermidis isolates from keratitis gave ST59 or ST5 lineages, are high producers of biofilm, absence of icaA gene and IS256, agr I and a high resistance to antibiotics [15]. These biomarkers were very similar to our healthy conjunctiva isolates with lineage ST5. Furthermore, the authors of that work suggested that S. epidermidis that inhabits the healthy conjunctiva is responsible for infecting the eye, but this suggestion was based only on the hypothesis of the proximity of the conjunctiva to the eye, because they did not work with healthy conjunctiva isolates of S. epidermidis. Another study has shown that isolates of S. epidermidis that colonize the conjunctiva and the eye are responsible of the post-operative endophthalmitis [16]. In contrast, it has been shown a clear separation between the healthy conjunctiva isolates with the isolates from keratitis and endophthalmitis by RFLP analysis [18]. Another characteristic of S. epidermidis isolates that inhabit the healthy conjunctiva is that they are polyclonal and can change their genotypic traits in a short time (months) [19]. Probably, in the healthy conjunctiva there is a common ancestor of S. epidermidis (lineage ST5), which generates, by mutations, different genotypic variants (other lineages STs) in response to changes in the conjunctiva over time. Based on the above information and in order to explain the origin of S. epidermidis isolates that infect the eye, in this paper, discriminant biomarkers of ocular infection were examined in healthy conjunctiva isolates, and it was found that 3 healthy conjunctiva isolates [two with ST2 and another with ST9] (3/45, 6.66%) had similar genotypic and phenotypic characteristics to ocular infection isolates, therefore a small population from healthy conjunctiva could cause an ocular infection. Alternatively, isolates of S. epidermidis that inhabit the skin of body parts could be those infecting the eye because it has been documented that these are genotypically different [6].
Conclusion
With these results we conclude that in the healthy conjunctiva (6.66%) inhabit a low population of S. epidermidis, which possess discriminant and similar biomarkers to that found in ocular infection isolates. This small population might potentially infect the eye. The major population (with different characteristics to ocular infection isolates) inhabiting conjunctiva probably do not infect the eye; in part by the innate immune system of this organ, which recognize, control and select these isolates for their survival.
Supporting Information
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
CFVM, SRM, JJR, MECD, and JCCD were supported by the COFAA-IPN, EDI-IPN and SNI-CONACyT fellowships. JCCD also received support from Consejo Nacional de Ciencia y Tecnología, Mexico, 153268.
References
- 1. Rogers KL, Fey PD, Rupp ME. Coagulase-negative staphylococcal infections. Infect Dis Clin North Am. 2009; 23: 73–98. 10.1016/j.idc.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 2. McCann MT, Gilmore BF, Gorman SP. Staphylococcus epidermidis device-related infections: pathogenesis and clinical management. J Pharm Pharmacol. 2008; 60: 1551–1571. 10.1211/jpp/60.12.0001 [DOI] [PubMed] [Google Scholar]
- 3. Leshem R, Maharshak I, Ben Jacob E, Ofek I, Kremer I. The effect of nondialyzable material (NDM) cranberry extract on formation of contact lens biofilm by Staphylococcus epidermidis . Invest Ophthalmol Vis Sci. 2011; 52: 4929–4934. 10.1167/iovs.10-5335 [DOI] [PubMed] [Google Scholar]
- 4. Cherifi S, Byl B, Deplano A, Nagant C, Nonhoff C, Denis O, et al. Genetic characteristics and antimicrobial resistance of Staphylococcus epidermidis isolates from patients with catheter-related bloodstream infections and from colonized healthcare workers in a Belgian hospital. Ann Clin Microbiol Antimicrob. 2014; 13: 20–27. 10.1186/1476-0711-13-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cherifi S, Byl B, Deplano A, Nonhoff C, Denis O, Hallin M. Comparative epidemiology of Staphylococcus epidermidis isolates from patients with catheter-related bacteremia and from healthy volunteers. J Clin Microbiol. 2013; 51: 1541–1547. 10.1128/JCM.03378-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conlan S, Mijares LA, NISC Comparative Sequencing Program, Becker J, Blakesley RW, Bouffard GG, et al. Staphylococcus epidermidis pan-genome sequence analysis reveals diversity of skin commensal and hospital infection-associated isolates. Genome Biol. 2012; 13: R64 10.1186/gb-2012-13-7-r64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park YM, Kwon HJ, Lee JS. Microbiological study of therapeutic soft contact lenses used in the treatment of recurrent corneal erosion syndrome. Eye Contact Lens. 2015; 41: 84–86. 10.1097/ICL.0000000000000068 [DOI] [PubMed] [Google Scholar]
- 8. Moloney TP, Park J. Microbiological isolates and antibiotic sensitivities in culture-proven endophthalmitis: a 15-year review. Br J Ophthalmol. 2014; 98: 1492–1497. 10.1136/bjophthalmol-2014-305030 [DOI] [PubMed] [Google Scholar]
- 9. Bhoomibunchoo C, Ratanapakorn T, Sinawat S, Sanguansak T, Moontawee K, Yospaiboon Y. Infectious endophthalmitis: review of 420 cases. Clin Ophthalmol. 2013; 7: 247–252. 10.2147/OPTH.S39934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ubels JL, Gipson IK, Spurr-Michaud SJ, Tisdale AS, Van Dyken RE, Hatton MP. Gene expression in human accessory lacrimal glands of Wolfring. Invest Ophthalmol Vis Sci. 2012; 53: 6738–6747. 10.1167/iovs.12-10750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez-Martinez S, Cancino-Diaz ME, Cancino-Diaz JC. Expression of CRAMP via PGN-TLR-2 and of alpha-defensin-3 via CpG-ODN-TLR-9 in corneal fibroblasts. Br J Ophthalmol. 2006; 90: 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Talreja D, Singh PK, Kumar A. In vivo role of TLR2 and MyD88 signaling in eliciting innate immune responses in staphylococcal endophthalmitis. Invest Ophthalmol Vis Sci. 2015; 56: 1719–1732. 10.1167/iovs.14-16087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodríguez-Martínez S, Cancino-Díaz ME, Jiménez-Zamudio L, García-Latorre E, Cancino-Díaz JC. TLRs and NODs mRNA expression pattern in healthy mouse eye. Br J Ophthalmol. 2005; 89: 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Y, Liu ZR, Chen H, Fan YC, Duo J, Zheng H, et al. Comparison on conjunctival sac bacterial flora of the seniors with dry eye in Ganzi autonomous prefecture. Int J Ophthalmol. 2013; 6: 452–457. 10.3980/j.issn.2222-3959.2013.04.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bispo PJ, Hofling-Lima AL, Pignatari AC. Characterization of ocular methicillin-resistant Staphylococcus epidermidis isolates belonging predominantly to clonal complex 2 subcluster II. J Clin Microbiol. 2014; 52: 1412–1417. 10.1128/JCM.03098-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kenchappa P, Duggirala A, Ahmed N, Pathengay A, Das T, Hasnain SE, et al. Fluorescent amplified fragment length polymorphism (FAFLP) genotyping demonstrates the role of biofilm-producing methicillin-resistant periocular Staphylococcus epidermidis strains in postoperative endophthalmitis. BMC Ophthalmol. 2006; 6: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bannerman TL, Rhoden DL, McAllister SK, Miller JM, Wilson LA. The source of coagulase-negative staphylococci in the endophthalmitis vitrectomy study. A comparison of eyelid and intraocular isolates using pulsed-field gel electrophoresis. Arch Ophthalmol. 1997; 115: 357–361. [DOI] [PubMed] [Google Scholar]
- 18. Duggirala A, Kenchappa P, Sharma S, Peeters JK, Ahmed N, Garg P, et al. High-resolution genome profiling differentiated Staphylococcus epidermidis isolated from patients with ocular infections and normal individuals. Invest Ophthalmol Vis Sci. 2007; 48: 3239–3245. [DOI] [PubMed] [Google Scholar]
- 19. Ueta M, Iida T, Sakamoto M, Sotozono C, Takahashi J, Kojima K, et al. Polyclonality of Staphylococcus epidermidis residing on the healthy ocular surface. J Med Microbiol. 2007; 56: 77–82. [DOI] [PubMed] [Google Scholar]
- 20. Chung M, de Lencastre H, Matthews P, Tomasz A, Adamsson I, Aires de Sousa M, et al. Molecular typing of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis: comparison of results obtained in a multilaboratory effort using identical protocols and MRSA strains. Microb Drug Resist. 2000; 6: 189–198. [DOI] [PubMed] [Google Scholar]
- 21. Miragaia M, Carriço JA, Thomas JC, Couto I, Enright MC, de Lencastre H. Comparison of molecular typing methods for characterization of Staphylococcus epidermidis: proposal for clone definition. J Clin Microbiol. 2008; 46: 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus . J Clin Microbiol. 2005; 43: 5026–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghaznavi-Rad E, Shamsudin MN, Sekawi Z, van Belkum A, Neela V. A simplified multiplex PCR assay for fast and easy discrimination of globally distributed staphylococcal cassette chromosome mec types in meticillin-resistant Staphylococcus aureus . J Med Microbiol. 2010; 59: 1135–1139. 10.1099/jmm.0.021956-0 [DOI] [PubMed] [Google Scholar]
- 24. Thomas JC, Vargas MR, Miragaia M, Peacock SJ, Archer GL, Enright MC. Improved multilocus sequence typing scheme for Staphylococcus epidermidis . J Clin Microbiol. 2007; 45: 616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Catalanotti P, Lanza M, Del Prete A, Lucido M, Catania MR, Gallè F, el al. Slime-producing Staphylococcus epidermidis and S. aureus in acute bacterial conjunctivitis in soft contact lens wearers. New Microbiol; 2005; 28: 345–354. [PubMed] [Google Scholar]
- 26. Christensen GD, Simpson WA, Younger J.J, Baddour LM, Barrett FF, Melton DM, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985; 22: 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li M, Guan M, Jiang XF, Yuan FY, Xu M, Zhang WZ, et al. Genetic polymorphism of the accessory gene regulator (agr) locus in Staphylococcus epidermidis and its association with pathogenicity. J Med Microbiol. 2004; 53: 545–549. [DOI] [PubMed] [Google Scholar]
- 28. Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 2001; 4: 9 Available: http://folk.uio.no/ohammer/past. [Google Scholar]
- 29. Du X, Zhu Y, Song Y, Li T, Luo T, Sun G, et al. Molecular analysis of Staphylococcus epidermidis strains isolated from community and hospital environments in China. PLoS One. 2013; 8: e62742 10.1371/journal.pone.0062742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giormezis N, Kolonitsiou F, Foka A, Drougka E, Liakopoulos A, Makri A, et al. Coagulase-negative staphylococcal bloodstream and prosthetic-device-associated infections: the role of biofilm formation and distribution of adhesin and toxin genes. J Med Microbiol. 2014; 63: 1500–1508. 10.1099/jmm.0.075259-0 [DOI] [PubMed] [Google Scholar]
- 31. Ahlstrand E, Hellmark B, Svensson K, Söderquist B. Long-term molecular epidemiology of Staphylococcus epidermidis blood culture isolates from patients with hematological malignancies. PLoS One. 2014; 9: e99045 10.1371/journal.pone.0099045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mertens A, Ghebremedhin B. Genetic determinants and biofilm formation of clinical Staphylococcus epidermidis isolates from blood cultures and indwelling devises. Eur J Microbiol Immunol (Bp). 2013; 3: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mendes RE, Deshpande LM, Costello AJ, Farrell DJ. Molecular epidemiology of Staphylococcus epidermidis clinical isolates from U.S. hospitals. Antimicrob Agents Chemother. 2012; 56: 4656–4661. 10.1128/AAC.00279-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cremniter J, Sivadon-Tardy V, Caulliez C, Bauer T, Porcher R, Lortat-Jacob A, et al. Genetic analysis of glycopeptide-resistant Staphylococcus epidermidis strains from bone and joint infections. J Clin Microbiol. 2013; 51: 1014–1019. 10.1128/JCM.02608-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suzuki T, Kawamura Y, Uno T, Ohashi Y, Ezaki T. Prevalence of Staphylococcus epidermidis strains with biofilm-forming ability in isolates from conjunctiva and facial skin. Am J Ophthalmol. 2005; 140: 844–850. [DOI] [PubMed] [Google Scholar]
- 36. Hellmark B, Söderquist B, Unemo M, Nilsdotter-Augustinsson Å. Comparison of Staphylococcus epidermidis isolated from prosthetic joint infections and commensal isolates in regard to antibiotic susceptibility, agr type, biofilm production, and epidemiology. Int J Med Microbiol. 2013; 303: 32–39. 10.1016/j.ijmm.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 37. Widerström M, Monsen T, Karlsson C, Edebro H, Johansson A, Wiström J. Clonality among multidrug-resistant hospital-associated Staphylococcus epidermidis in northern Europe. Scand J Infect Dis. 2009; 41: 642–649. 10.1080/00365540903146987 [DOI] [PubMed] [Google Scholar]
- 38. Yamada M, Yoshida J, Hatou S, Yoshida T, Minagawa Y. Mutations in the quinolone resistance determining region in Staphylococcus epidermidis recovered from conjunctiva and their association with susceptibility to various fluoroquinolones. Br J Ophthalmol. 2008; 92: 848–851. 10.1136/bjo.2007.129858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bispo PJ, Alfonso EC, Flynn HW, Miller D. Emerging 8-methoxyfluoroquinolone resistance among methicillin-susceptible Staphylococcus epidermidis isolates recovered from patients with endophthalmitis. J Clin Microbiol. 2013; 51: 2959–2963. 10.1128/JCM.00846-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Betanzos-Cabrera G, Juárez-Verdayes MA, González-González G, Cancino-Díaz ME, Cancino-Díaz JC. Gatifloxacin, moxifloxacin, and balofloxacin resistance due to mutations in the gyrA and parC genes of Staphylococcus epidermidis strains isolated from patients with endophthalmitis, corneal ulcers and conjunctivitis. Ophthalmic Res. 2009; 42: 43–48. 10.1159/000219684 [DOI] [PubMed] [Google Scholar]
- 41. Dave SB, Toma HS, Kim SJ. Changes in ocular flora in eyes exposed to ophthalmic antibiotics. Ophthalmology. 2013; 120: 937–941. 10.1016/j.ophtha.2012.11.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


