Abstract
Background
Over the last three decades, the epidemiological profile of visceral leishmaniasis (VL) has changed with epidemics occurring in large urban centers of Brazil, an increase in HIV/AIDS co-infection, and a significant increase in mortality. The objective of this study was to identify the risk factors associated with death among adult patients with VL from an urban endemic area of Brazil.
Methodology
A prospective cohort study included 134 adult patients with VL admitted to the University Hospital of the Federal University of Mato Grosso do Sul between August 2011 and August 2013.
Principal Findings
Patients ranged from 18 to 93 years old, with a mean age of 43.6 (±15.7%). Of these patients, 36.6% were co-infected with HIV/AIDS, and the mortality rate was 21.6%. In a multivariate analysis, the risk factors associated with death were secondary bacterial infection (42.86, 5.05–363.85), relapse (12.17, 2.06–71.99), edema (7.74, 1.33–45.05) and HIV/AIDS co-infection (7.33, 1.22–43.98).
Conclusions/Significance
VL has a high mortality rate in adults from endemic urban areas, especially when coinciding with high rates of HIV/AIDS co-infection.
Author Summary
Visceral Leishmaniasis (VL) is considered a neglected disease by the World Health Organization (WHO). Over the last two decades, the epidemiological profile of VL has changed with epidemics occurring in large urban centers of Brazil with increased HIV/AIDS co-infection and mortality. Understanding the factors that lead to death in these patients is important to improve public health strategies and reduce mortality. We performed a prospective cohort study between August 2011 and August 2013 of 134 adult patients with VL from Campo Grande urban area. Approximately 40% had AIDS and 22% died during the 12-month follow-up. Relapses, bacterial infection, AIDS and edema were strongly associated with death among VL patients in the Brazilian urban centers. All VL patients should be screened for HIV and bacterial infection as well as to prevent VL relapses.
Introduction
Over 90% of visceral leishmaniasis (VL) cases occur in six countries: Bangladesh, Brazil, Ethiopia, India, South Sudan and Sudan [1]. In Brazil, an increase in incidence over the past three decades has coincided with epidemics in large urban centers such as Campo Grande, Teresina, São Luis, Natal and Belo Horizonte [2] and a significant increase in mortality [3].
Despite increased mortality, there are few studies assessing risk factors associated with death among VL patients and the majority involves populations of all age groups in urban and non-urban areas with low rates of HIV co-infection. In non HIV infected patients, jaundice, bleeding, and associated infections were most frequent risk factors [4–8], while being older than 45 years of age was reported by one study [9].
In addition, over the last three decades, Brazil has had an increase in the HIV/AIDS epidemic [10]. Due to the changes in the epidemiological profile of VL and its expansion into urban areas with higher prevalence of HIV/AIDS, prospective studies are necessary to better understand the determinants associated with death and to propose future interventions. This study aims to identify risk factors associated with death from VL in adult patients from endemic urban areas of Brazil.
Materials and Methods
Type of study, location and population
This prospective cohort study was conducted between August 2011 and August 2013 with adult patients with VL. Patients were monitored by the Reference Service for Infectious and Parasitic Diseases of the University Hospital in the Federal University of Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brazil. Clinical, sociodemographic and laboratory variables were recorded on a standardized form and. The criteria for inclusion in the study were for patients to be aged 18 years or older and have a confirmed laboratory diagnosis of VL.
Diagnostic procedures
The VL diagnosis was performed according to the recommendations of the laboratory of the Ministry of Health [11]. Parasitological diagnoses were performed based on detection of amastigotes of the parasite in Giemsa stained bone marrow smears by an experienced microscopist and by isolation of promastigotes from culture media (Mc Neal, Novy & Nicolle-NNN and Brain Heart Infusion-BHI). Immunological tests were performed using an immunochromatographic test (Kala-azar Detect, INBIOS, WA). Cases were considered confirmed if they presented at least one positive laboratory test, parasitological or immunochromatographic test. HIV screening was performed by enzyme-linked immunosorbent assay and confirmed with an immunofluorescence antibody test, or Western Blot assay [11].
Protocol of treatment
The treatment protocol was defined according to the guidelines of the Ministry of Health and considered severity signs (jaundice, bleeding hemorrhages, generalized edema, signs of toxemia and severe malnutrition), co-infection with HIV/AIDS, age, renal failure and heart disease [12, 13].
Patients with less than 50 years, without HIV infection, without renal failure or heart disease and without severity signs were treated with antimoniate of N-methylglucamine, which contains pentavalent antimony (Sbv), at a dose of 20 mg/kg/day of Sbv for 30 days. Patients with severity signs received amphotericin B deoxycholate at a dose of 1 mg/kg/day for 14 to 20 days [14].
Patients with more than 50 years or with HIV/AIDS or renal or cardiac failure, with or without severity sign, received liposomal amphotericin B at 4 mg/kg/day for 5 days or 3 mg/kg/day for 7 days with 20 mg/kg of total dose [13].
Follow-up
After discharge, patients were instructed to return after 1, 3, 6 and 12 months for clinical and laboratory revaluation.
Protocol of secondary prophylaxis
After treatment, patients with AIDS received secondary prophylaxis of liposomal amphotericin B, administered at 3 mg/Kg every 15 days to avoid relapse [12, 15].
Relapse definition
Relapse was defined as showing clinical signs and symptoms suggestive of VL within 12 months in successfully treated patients [11]. Successful therapy was achieved if there was improvement of general condition, resolution of fever, regression of splenomegaly and recovery of blood counts [15].
Statistical analysis
Data were tabulated in a spreadsheet (S1 Data). SAS version 9.2 (SAS Institute, Cary, NC) and SPSS version 22.2 were used to analyze bivariate and multivariable models. Dichotomy or categorical data were analyzed with the chi-square test or Fisher’s exact test. For continuous variables, the t-test or Anova was utilized. Missing data were excluded of analyses. Univariate and multivariate logistic regressions were conducted to identify factors associated with death. Variables were included in the model if they achieved a significance level of p<0.20 in the univariate analysis. Correlated variables were tested individually, and the Wald test was used to evaluate the significance level of risk factors in the final model. The results were expressed as relative risk (RR) with 95% confidence intervals (95% CI). A correlation matrix was used to assess confounding variables and correlations between variables. Kaplan-Meier survival curves were constructed for each variable using the long-rank test. Statistical significance was set at p<0.05.
Ethical considerations
The present study was approved by the Ethics Committee of the Federal University of Mato Grosso do Sul under protocol number 2179 and case number 02480049000–11. All patients voluntarily signed a statement of informed consent for the collection of data.
Results
One hundred thirty-seven patients were eligible for the study, however, three not agreed to participate. Of the 134 participants in the present study, 94 (70.1%) were male. The age of participants ranged from 18 to 93 years, with a mean age of 43.6 (SD 41 ± 15.7). Most of the individuals (95%) were from urban areas, 107 (79.8%) from Campo Grande, and the remainder from 17 other cities.
The most common comorbidity in VL patients was AIDS (49;36.6%), followed by tuberculosis (3;2.3%), erythematosus systemic lupus (1;0.75%) and chronic myelocytic leukemia (1;0.75%). At the time of diagnosis of VL in patients with HIV/AIDS, the mean T-CD4+ cell count of was 68/mm3. VL and HIV/AIDS were diagnosed simultaneously in 32.7% of the patients.
Parasitological examination (direct and culture) was performed in 126 (94.0%) patients, with 93 positive results (73.84%). Of the 119 (88.8%) samples tested by immunochromatographic test, 96 (80.7%) were positive. In 57 (42.5%) patients, diagnosis was confirmed by two methods (parasitological and immunochromatographic test), only by parasitological in 36 (26.9%), and only by immunochromatographic test in 41 (30.6%) patients.
The time between the first symptoms and diagnosis of VL ranged from one to 421 days, with a median of 31 days. The period between diagnosis and death ranged from three to 431 days, with a median of 346 days. The mortality rate was 21.6% (29/134). Differences in demographic, clinical, laboratory and therapeutic relation to the evolution of the disease are shown in Tables 1 and 2.
Table 1. Adult patients with visceral leishmaniasis according to the following aspects: demographic; clinical; therapeutic; co-morbidities and evolution to death (n = 134).
| Variable | Death | RR | CI 95% | P value | ||
|---|---|---|---|---|---|---|
| Total | Yes | No | ||||
| (n = 134) | (n = 29) | (n = 105) | ||||
| Gender | ||||||
| Male | 70.1 (94) | 18.1 (17) | 81.9 (77) | 1 | 0.192 | |
| Female | 29.9 (40) | 30.0 (12) | 70.0 (28) | 1.66 | 0.88–3.15 | |
| Age in years | ||||||
| 18 to 50 | 74.6 (100) | 19.0 (19) | 81.0 (81) | 1 | 0.302 | |
| ≥ 50 | 25.4 (34) | 29.4 (10) | 70.6 (24) | 1.55 | 0.80–2.99 | |
| Fever | ||||||
| No | 9.1 (12) | 50.0 (6) | 50.0 (6) | 1 | 0.029 | |
| Yes | 90.9 (120) | 18.3 (22) | 81.7 (98) | 0.37 | 0.19–0.72 | |
| Edema | ||||||
| No | 65.9 (87) | 11.5 (10) | 88.5 (77) | 1 | <0.001 | |
| Yes | 34.1 (45) | 37.8 (17) | 62.2 (28) | 3.29 | 1.64–6.57 | |
| Splenomegaly | ||||||
| No | 34.6 (46) | 39.1 (18) | 60.9 (28) | 1 | <0.001 | |
| Yes | 65.4 (87) | 11.5 (10) | 88.5 (77) | 0.29 | 0.15–0.58 | |
| Hepatomegaly | ||||||
| No | 29.1 (39) | 35.9 (14) | 64.1 (25) | 1 | 0.019 | |
| Yes | 70.9 (95) | 15.8 (15) | 84.2 (80) | 0.44 | 0.24–0.82 | |
| Cough | ||||||
| No | 37.6 (50) | 16.0 (8) | 84.0 (42) | 1 | 0.298 | |
| Yes | 62.4 (83) | 25.3 (21) | 74.7 (62) | 1.58 | 0.76–3.30 | |
| Weight loss | ||||||
| No | 9.2 (12) | 0.0 (0) | 100.0 (12) | 1 | 0.127 | |
| Yes | 90.8 (119) | 23.5 (28) | 76.5 (91) | - | - | |
| Relapse | ||||||
| No | 82.8 (111) | 15.3 (17) | 84.7 (94) | 1 | <0.001 | |
| Yes | 17.2 (23) | 52.2 (12) | 47.8 (11) | 3.41 | 1.89–6.13 | |
| HIV | ||||||
| No | 63.4 (85) | 11.8 (10) | 88.2 (75) | 1 | <0.001 | |
| Yes | 36.6 (49) | 38.8 (19) | 61.2 (30) | 3.30 | 1.67–6.51 | |
| Bacterial infection | ||||||
| No | 64.9 (87) | 6.9 (6) | 93.1 (81) | 1 | <0.001 | |
| Yes | 35.1 (47) | 48.9 (23) | 51.1 (24) | 7.10 | 3.11–16.20 | |
| Pneumonia | ||||||
| No | 87.3 (117) | 15.4 (18) | 84.6 (99) | 1 | <0.001 | |
| Yes | 12.7 (17) | 64.7 (11) | 35.3 (6) | 4.21 | 2.42–7.30 | |
| Tuberculosis | ||||||
| No | 97.8 (131) | 19.8 (26) | 80.2 (105) | 1 | 0.001 | |
| Yes | 2.2 (3) | 100.0 (3) | 0.0 (0) | 5.04 | 3.57–7.11 | |
| Treatment | ||||||
| Pentavalent Antimonial | 4.5 (6) | 0.0 (0) | 100.0 (6) | 0.00 | - | 1.000 |
| Amphotericin B deoxycholate | 15.7 (21) | 14.3 (3) | 85.7 (18) | 1.31 | 0.35–4.99 | 0.698 |
| Liposomal Amphotericin B | 45.5 (61) | 34.4 (21) | 65.6 (40) | 3.17 | 1.29–7.77 | 0.010 |
| Association of two drugs | 34.3 (46) | 10.9 (5) | 89.1 (41) | 1 | ||
The results are presented as relative frequency (absolute frequency). RR = relative risk. CI 95% = Confidence interval of 95%. P values in the chi-square test. Significant risks are indicated by p values in bold.
Table 2. Adult patients with visceral leishmaniasis according to the results of laboratory tests at admission stratified by death (n = 134).
| Variable | Death | p value | |
|---|---|---|---|
| Yes | No | ||
| Mean ± SD | Mean ± SD | ||
| Erythrocytes (million/mm3) | 3.07±0.15 | 3.48±0.08 | 0.014 |
| Leukocytes (mil/mm3) | 6,64±2,10 | 2,64±0,21 | 0.068 |
| Rod cell (%) | 12.31±1.56 | 15.51±1.22 | 0.110 |
| Segmented neutrophils (%) | 54.24±4.01 | 39.85±1.50 | 0.002 |
| Eosinophils (%) | 2.00±0.87 | 1.07±0.26 | 0.311 |
| Basophils (%) | 0.03±0.03 | 0.18±0.05 | 0.023 |
| Lymphocytes (%) | 23.72±3.45 | 32.41±1.43 | 0.009 |
| Monocytes (%) | 6.07±0.71 | 7.79±0.45 | 0.067 |
| Platelets (million/mm3) | 111,17±15,81 | 97,90±6,90 | 0.393 |
| Albumin (g/dl) | 2.67±0.20 | 2.98±0.07 | 0.060 |
| Creatinine (g/dl) | 1.28±0.17 | 1.02±0.07 | 0.165 |
| Urea (mg/dl) | 56.49±8.60 | 30.59±2.35 | 0.007 |
| ALT (U/L) | 75.55±35.12 | 53.19±5.30 | 0.534 |
| AST (U/L) | 88.03±14.91 | 67.75±7.52 | 0.216 |
| Globulin | 2.67±0.20 | 5.68±2.70 | 0.563 |
| Total bilirubin | 3.64±0.31 | 4.24±0.19 | 0.139 |
| Na (mEq/ml) | 137.00±1.22 | 135.69±0.50 | 0.259 |
| K (mmol/L) | 3.82±0.14 | 4.78±0.58 | 0.379 |
| Glucose (mg/dl) | 115.75±9.76 | 105.77±5.26 | 0.371 |
The results are presented as the mean ± standard error (SD). P values in the students t-test. The significant differences between patients who died and those that survived are shown with P values in bold.
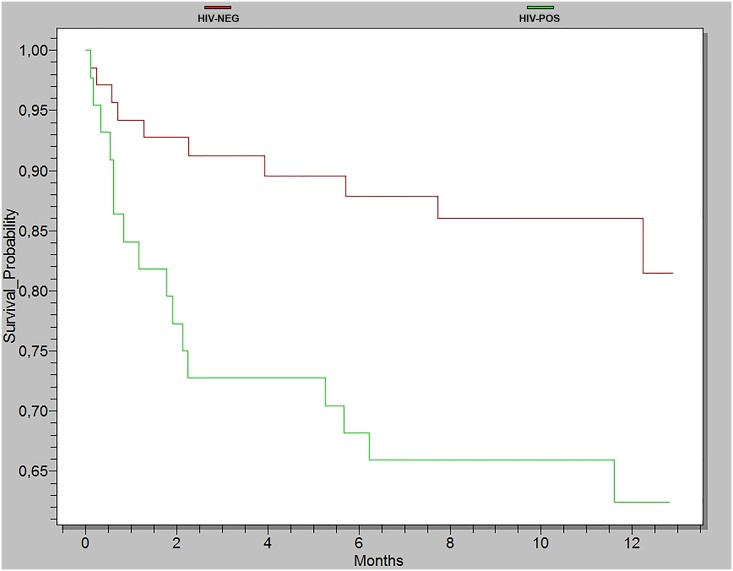
Patients with VL and HIV/AIDS exhibited a mortality rate of 36.7% (18/49) (Fig 1). Variables identified as risk factors for mortality were HIV/AIDS, relapse, secondary bacterial infection and edema (Table 3). Although splenomegaly presented a p<0.05 in the univariate analysis, it showed a strong correlation with HIV and when included in the final model and a reduction in the Wald test value when included in the multivariable model. Therefore, we only HIV variable was evaluated in the final model.
Fig 1. Adult survival, positive for visceral leishmaniasis, stratified by the presence (n = 49) or absence (n = 105) of HIV/AIDS infection during a 12-month follow-up period.
Log-rank test = 11, 2534; p = 0,0008.
Table 3. Univariate and multivariate analysis and progress to death in adult patients with visceral leishmaniasis (n = 134).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | RR | 95% CI | p value | RR | 95% CI | p value |
| Secondary bacterial infection | 7,10 | 3,11–16,20 | <0,001 | 2.57 | 1.31–3.83 | <0,001 |
| Relapse | 3,41 | 1,89–6,13 | <0,001 | 5.87 | 1.39–24.75 | 0.016 |
| Edema | 3,29 | 1,64–6,57 | <0,001 | 5.20 | 1.55–17.49 | 0.008 |
| HIV/AIDS | 3,30 | 1,67–6,51 | <0,001 | 4.63 | 1,35–15.90 | 0.015 |
RR = relative risk. CI 95% = Confidence interval of 95%.
Multivariate analysis was followed by the Wald test and correlation matrix.
Among 23 patients who relapsed, 15 (65.2%) were HIV-positive. Although there was an association between HIV and relapse (p<0.01), the two variables in the final model had a higher Wald test value than when only one of the variables was included in the model. Of the 8 patients without HIV infection who relapsed, one had systemic erythematous lupus, one had leukemia, one had cirrhosis, and two were older than 85 years of age.
Of the 49 patients with AIDS, 7(14.3%) died during hospitalization and only 15/42 (35.7%) of them regularly adhered to secondary prophylaxis. No difference in relapse and death rates was observed among of those who adhered and those did not adhere to prophylaxis [33.3% versus 29.6%; p = 0.92 and 40.0% versus 22.2%; p = 0.38, respectively].
Discussion
Most studies that evaluated risk factors for death among VL patients are retrospective design, secondary data analysis and involving different age groups [4, 6, 7, 8]. Our study differs by providing a 12-month follow-up of a cohort of adult patients from an urban center with a high co-infection rate of HIV/AIDS. In this way, relapses and deaths that occurred after the first episode of VL in this particular group of patients could also be documented.
The mortality rate (21.6%) observed in the present study is higher than the rates normally detected in Brazil [2, 3, 5]. This high rate could be associated with the clinical differences of patients included in the study who were mostly over 50 years of age and were co-infected with HIV/AIDS [4, 16, 17]. Herein, we identified four variables associated with death among VL patients: secondary bacterial infection, edema, HIV/AIDS co-infection and VL relapse.
Secondary bacterial infection is a well-known risk factor related to the severity of sepsis and described in previous studies [7, 8, 18, 19, 20]. The presence of edema, which may reflect protein malnutrition, liver or renal failure, has also been described as a risk factor for an unfavorable outcome [7, 8].
Recent studies have also identified that co-infection with HIV/AIDS is a risk factor for death [4, 5, 20]. The 36% mortality rate among HIV/AIDS patients, in this study, is much higher than studies previously conducted in Brazil [21, 22, 23, 24]. It is known that patients who are HIV/AIDS-positive are more likely to develop VL due to the depletion of both cellular and humoral immune responses against Leishmania [25] and, moreover, VL/HIV co-infection can accelerate the evolution of both diseases [26], given that the two agents are located in the same host cell. The co-infection can also enhance pathogenic effects and impair the correct function of macrophages [27, 28]. This synergism favors a fatal outcome of patients with VL.
Liposomal amphotericin B is the first choice of treatment in Brazil for the following individuals: VL positive patients over 50 years of age, patients co-infected with HIV/AIDS, patients with immunosuppressive diseases, and those with renal or cardiac disorders [13, 29]. Therefore, patients who used this drug were those affected with greater severity and a higher propensity towards death.
In the present study, the follow-up period was 12 months, which enabled the detection of relapses, deaths during relapse episodes and a correlation between HIV and relapses of VL. In general, co-infection with HIV/AIDS has a strong association with VL relapses [30] and the frequency of relapses in patients co-infected with VL-HIV/AIDS ranges from 10 to 60% [31]. A systematic review identified that the predictors of VL relapses in HIV-infected patients as: the absence of an increase in CD4+ T cells at follow-up, a lack of secondary prophylaxis, previous history of VL relapses, and CD4+ T cell counts below 100 cells/μl at the time of primary VL diagnosis [32].
Altered immunity may persist in co-infected patients, and the lymphocyte T-CD4+ count can be low, despite the administration of HAART (Highly Active Antiretroviral Therapy) and anti-leishmania therapy [33]. The cell activation increases the transcription of the integrated HIV that results in CD4+ cell death. It is induced by the activation of CD4+ and CD8+T cell memory population, resulting in an exhaustion of immune resources. After the treatment some parasites remains inside macrophages and leishmania antigens was thought to be responsible for the cellular activation observed in AIDS patients [27]. This additional monocyte/macrophage activation in VL/AIDS patients has been also associated with increased microbial translocation [34, 35].
According to the recommendations of the WHO, patients with HIV/AIDS should receive secondary prophylaxis to prevent a relapse of VL [32, 36, 37]. Although secondary prophylaxis was indicated, in our study, for all patients with a T-CD4+ count lower than 350 cell/mm3, adherence to secondary prophylaxis was not associated with protection of relapse or death during the follow-up. Other studies have shown that secondary prophylaxis is not completely effective in prevent relapses [38, 39].
It is possible that the VL treatment used in patients with HIV infection has been insufficient to reduced parasitic load and cure. During the study period, the recommended liposomal amphotericin B total dose was 20mg/kg. However the current recommendation by the Ministry of Health of Brazil is 40mg/kg, instead of 20mg/kg, in patients co-infected with HIV [15, 40]. Although the cure criteria are essentially clinical, many clinical and laboratory changes are still present, including splenomegaly, anemia and hypergammaglobulinemia, at the end of treatment in co-infections patients. There is no recommendation to repeat the parasitological examination at the end of treatment [41] and Real-time PCR has been proposed as a suitable tool for monitoring the parasite load during follow-up of co-infected patients and predict the risk of relapses after treatment [42].
In conclusion, this study demonstrated that VL is a serious disease with high mortality rates in adult patients from urban areas, especially when co-infected with HIV/AIDS. Further studies are needed to define the best therapeutic options for effective treatment and prevent VL relapses in these patients.
Supporting Information
(XLS)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso Do Sul-FUNDECT-http://sigfundect.ledes.net (AMMP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO http://www.who.int/leishmaniasis/burden/magnitude/burden_magnitude/en/index.html. World Health Organization, 2014. Accessed on March, 25, 2014.
- 2. Maia-Elkhoury AN, Alves WA, Sousa-Gomes ML, Sena JM, Luna EA. (2008) Visceral leishmaniasis in Brazil: trends and challenges. Cad Saude Publica 24 (12): 2941–2947. [DOI] [PubMed] [Google Scholar]
- 3. Martins-Melo FR, Lima Mda S, Ramos AN Jr, Alencar CH, Heukelbach J.(2014) Mortality and case fatality due to visceral leishmaniasis in Brazil: a nationwide analysis of epidemiology, trends and spatial patterns. PLoS One 3; 9 (4): e93770 10.1371/journal.pone.0093770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Araújo VE, Morais MH, Reis IA, Rabello A, Carneiro M. (2012) Early clinical manifestations associated with death from visceral leishmaniasis. PLoS Negl Trop Dis 6 (2): e1511 10.1371/journal.pntd.0001511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Madalosso G, Fortaleza CM, Ribeiro AF, Cruz LL, Nogueira PA, et al. (2012) American visceral leishmaniasis: factors associated with lethality in the state of São Paulo, Brazil. J Trop Med 2012: 281572 10.1155/2012/281572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Werneck GL, Batista MS, Gomes JR, Costa DL, Costa CH. (2003) Prognostic factors for death from visceral leishmaniasis in Teresina, Brazil. Infection 31 (3): 174–177. [DOI] [PubMed] [Google Scholar]
- 7. Costa CH, Werneck GL, Costa DL, Holanda TA, Aguiar GB, et al. (2010) Is severe visceral leishmaniasis a systemic inflammatory response syndrome? A case control study. Rev Soc Bras Med Trop 43 (4): 386–392. [DOI] [PubMed] [Google Scholar]
- 8. Sampaio MJ, Cavalcanti NV, Alves JG, Filho MJ, Correia JB. (2010) Risk factors for death in children with visceral leishmaniasis. PLoS Negl Trop Dis 2; 4 (11): e877 10.1371/journal.pntd.0000877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mueller Y, Mbulamberi DB, Odermatt P, Hoffmann A, Loutan L,et al. (2009) Risk factors for in-hospital mortality of visceral leishmaniasis patientes in eastern Uganda. Tropical Medicine and International Health 14 (8): 910–917. 10.1111/j.1365-3156.2009.02305.x [DOI] [PubMed] [Google Scholar]
- 10. Brasil (2014) Boletim epidemiológico HIV/AIDS no Brasil Departamento de DST/AIDS e Hepatites Virais. Ministério da Saúde. [Google Scholar]
- 11. Brasil (2010) Guia de vigilância epidemiológica Secretaria de Vigilância em Saúde; Brasilia: Ministério da Saúde. [Google Scholar]
- 12. Brasil (2011) Manual de recomendações para diagnóstico, tratamento e acompanhamento de pacientes com a coinfecção Leishmania-HIV Secretaria de Vigilância em Saúde. Brasília: Ministério da Saúde. [Google Scholar]
- 13. Brasil (2011) Leishmaniose visceral: recomendações clínicas para redução da letalidade Secretaria de Vigilância em Saúde. Brasilia: Ministério da Saúde: 120 p. [Google Scholar]
- 14. Brasil (2006) Manual de leishmaniose visceral grave: normas e condutas Secretaria de Vigilância em Saúde. Brasilia: Ministério da Saúde: 60 p. [Google Scholar]
- 15.WHO (2010) Control of the leishmaniases: Report of a meeting of the WHO expert committee on the control of leishmaniases. Geneva: World Health Organization.
- 16. Martins-Melo FR, Lima Mda S, Alencar CH, Ramos AN Jr, Heukelbach J. (2014) Epidemiological patterns of mortality due to visceral leishmaniasis and HIV/AIDS co-infection in Brazil, 2000–2011. Trans R Soc Trop Med Hyg 108 (6): 338–347. 10.1093/trstmh/tru050 [DOI] [PubMed] [Google Scholar]
- 17. Werneck GL. (2014) Visceral leishmaniasis in Brazil: rationale and concerns related to reservoir control. Rev Saude Publica 48 (5): 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliveira JM, Fernandes AC, Dorval ME, Alves TP, Fernandes TD, et al. (2010) Mortality due to visceral leishmaniasis: clinical and laboratory characteristics. Rev Soc Bras Med Trop 43 (2): 188–193. [DOI] [PubMed] [Google Scholar]
- 19. Oliveira AL, Paniago AM, Dorval ME, Oshiro ET, Leal CR, et al. (2006) Emergent outbreak of visceral leishmaniasis in Mato Grosso do Sul State. Rev Soc Bras Med Trop 39 (5): 446–450. [DOI] [PubMed] [Google Scholar]
- 20. Belo VS, Struchiner CJ, Barbosa DS, Nascimento BW, Horta MA, et al. (2014) Risk factors for adverse prognosis and death in American visceral leishmaniasis: a meta-analysis. PLoS Negl Trop Dis 24; 8 (7): e2982 10.1371/journal.pntd.0002982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lima IP, Müller MC, Holanda TA, Harhay M, Costa CH et al. (2013) Human immunodeficiency virus/Leishmania infantum in the first foci of urban American visceral leishmaniasis: clinical presentation from 1994 to 2010. Rev Soc Bras Med Trop 46 (2): 156–160. [DOI] [PubMed] [Google Scholar]
- 22. Albuquerque LC, Mendonca IR, Cardoso PN, Baldaçara LR, Borges MR, et al. (2014) HIV/AIDS-related visceral leishmaniasis: a clinical and epidemiological description of visceral leishmaniasis in northern Brazil. Rev Soc Bras Med Trop 47 (1): 38–46. 10.1590/0037-8682-0180-2013 [DOI] [PubMed] [Google Scholar]
- 23. Nascimento ET, Moura ML, Queiroz JW, Barroso AW, Araujo AF, et al. (2011) The emergence of concurrent HIV-1/AIDS and visceral leishmaniasis in Northeast Brazil. Trans R Soc Trop Med Hyg 105(5):298–300. 10.1016/j.trstmh.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coura–Vital W, De Araújo VE, Reis IA, Amanacio FF, Reis AB, et al. (2014) Prognostic Factors and Scoring System for Death from Visceral Leishmaniasis: An Historical Cohort Study in Brazil. PLoS Negl Trop Dis 118(12): e3374 10.1371/journal.pntd.0003374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moreno J, Cañavate C, Chamizo C, Laguna F, Alvar J. (2000) HIV-Leishmania infantum co-infection: humoral and cellular immune responses to the parasite after chemotherapy. Trans R Soc Trop Med Hyg 94(3):328–32. [DOI] [PubMed] [Google Scholar]
- 26. Lindoso JA, Cota GF, da Cruz AM, Goto H, Maia-Elkhoury AN, et al. (2014) Visceral Leishmaniasis and HIV Coinfection in Latin America. PLoS Negl Trop Dis 18; 8 (9): e3136 10.1371/journal.pntd.0003136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santos-Oliveira JR, Giacoia-Gripp CB, Alexandrino de Oliveira P, Amato VS, Lindoso JA, et al. (2010) High levels of T lymphocyte activation in Leishmania-HIV-1 co-infected individuals despite low HIV viral load. BMC Infect Dis 20; 10: 358 10.1186/1471-2334-10-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Andreani G, Lodge R, Richard D, Tremblay MJ. (2012) Mechanisms of interaction between protozoan parasites and HIV. Curr Opin HIV AIDS 7 (3): 276–82. 10.1097/COH.0b013e32835211e9 [DOI] [PubMed] [Google Scholar]
- 29. Cota GF, de Sousa MR, de Freitas Nogueira BM, Gomes LI, Oliveira E, et al. (2013) Comparison of parasitological, serological, and molecular tests for visceral leishmaniasis in HIV-infected patients: a cross-sectional delayed-type study. Am J Trop Med Hyg 89(3):570–7. 10.4269/ajtmh.13-0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, et al. (2008)The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 21 (2): 334–59. 10.1128/CMR.00061-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diro E, Lynen L, Ritmeijer K, Boelaert M, Hailu A,et al. (2014) Visceral Leishmaniasis and HIV coinfection in East Africa. PLoS Negl Trop Dis 26;8(6):e2869 10.1371/journal.pntd.0002869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cota GF, de Sousa MR, Rabello A. (2011) Predictors of visceral leishmaniasis relapse in HIV-infected patients: a systematic review. PLoS Negl Trop Dis 5(6): e1153 10.1371/journal.pntd.0001153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cota GF, de Sousa MR, de Mendonça AL, Patrocinio A, Assunção LS,et al. (2014) Leishmania-HIV co-infection: clinical presentation and outcomes in an urban area in Brazil. PLoS Negl Trop Dis 17;8(4):e2816 10.1371/journal.pntd.0002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Casado JL, Abad-Fernández M, Moreno S, Pérez-Elías MJ, Moreno A, et al. (2015) Visceral leishmaniasis as an independent cause of high immune activation, T-cell senescence, and lack of immune recovery in virologically suppressed HIV-1-coinfected patients. HIV Med 16(4):240–8. 10.1111/hiv.12206 [DOI] [PubMed] [Google Scholar]
- 35. Santos-Oliveira JR, Eduardo G. Regis, Giacoia-Gripp Carmem B.W., Valverde Joanna G., Alexandrino-de-Oliveira Priscilla, Lindoso Jose Ângelo L., Goto Hiro, Oliveira-Neto Manoel P., Guerra Jorge, Grinsztejn Beatriz, Jerônimo Selma Maria B., Morgado Mariza G., and Da-Cruz Alda M.. Microbial translocation induces an intense proinflammatory response in visceral leishmaniasis patients co-infected with HIV-1 J Infect Dis. first published online March 28, 2013. 10.1093/infdis/jit135 [DOI] [PubMed] [Google Scholar]
- 36. Pasquau F, Ena J, Sanchez R, Cuadrado JM, Amador C, et al. (2005) Leishmaniasis as an opportunistic infection in HIV-infected patients: determinants of relapse and mortality in a collaborative study of 228 episodes in a Mediterreanean region. Eur J Clin Microbiol Infect Dis 24 (6): 411–8. [DOI] [PubMed] [Google Scholar]
- 37. Ter Horst R, Collin SM, Ritmeijer K, Bogale A, Davidson RN. (2008) Concordant HIV infection and visceral leishmaniasis in Ethiopia: the influence of antiretroviral treatment and other factors on outcome. Clin Infect Dis 1; 46 (11): 1702–9. 10.1086/587899 [DOI] [PubMed] [Google Scholar]
- 38. De la Rosa R, Pineda JA, Delgado J, Macias J, Morillas F, et al. (2002) Incidence of and risk factors for symptomatic visceral leishmaniasis among human immunodeficiency virus type 1-infected patients from Spain in the era of highly active antiretroviral therapy. J Clin Microbiol 40 (3): 762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bourgeois N, Bastien P, Reynes J, Makinson A, Rouanet I, et al. (2010) Active chronic visceral leishmaniasis’ in HIV-1-infected patients demonstrated by biological and clinical long-term follow-up of 10 patients. HIV Med 11: 670–3. 10.1111/j.1468-1293.2010.00846.x [DOI] [PubMed] [Google Scholar]
- 40. Brasil (2015) Guia de Vigilância Epidemiológica Secretaria de Vigilância em Saúde. Brasilia; Ministério da Sapude; [Google Scholar]
- 41. Del Giudice P, Mary-Krause M, Pradier C, Grabar S, Dellamonica P, et al. (2002) Impact of highly active antiretroviral therapy on the incidence of visceral leishmaniasis in a french cohort of patients infected with human immunodeficiency virus. J Infect Dis 1; 186 (9): 1366–70. [DOI] [PubMed] [Google Scholar]
- 42. Monge-Maillo B, Norman FF, Cruz I, Alvar J, López-Vélez R. (2014) Visceral leishmaniasis and HIV coinfection in the Mediterranean region. PLoS Negl Trop Dis 21; 8 (8): e3021 10.1371/journal.pntd.0003021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.