Abstract
Objectives:
To analyze the frequency of NMDA receptor (NMDAR) antibodies in patients with various inflammatory demyelinating diseases of the CNS and to determine their clinical correlates.
Methods:
Retrospective case-control study from 2005 to 2014 with the detection of serum IgG antibodies to NMDAR, aquaporin-4, and myelin oligodendrocyte glycoprotein by recombinant live cell-based immunofluorescence assays. Fifty-one patients with acute disseminated encephalomyelitis, 41 with neuromyelitis optica spectrum disorders, 34 with clinically isolated syndrome, and 89 with multiple sclerosis (MS) were included. Due to a known association of NMDAR antibodies with seizures and behavioral symptoms, patients with those clinical manifestations were preferentially included and are therefore overrepresented in our cohort. Nine patients with NMDAR encephalitis, 94 patients with other neurologic diseases, and 48 healthy individuals were used as controls.
Results:
NMDAR antibodies were found in all 9 patients with NMDAR encephalitis but in only 1 of 215 (0.5%) patients with inflammatory demyelination and in none of the controls. This patient had relapsing-remitting MS with NMDAR antibodies present at disease onset, with an increase in NMDAR antibody titer with the onset of psychiatric symptoms and cognitive deficits.
Conclusion:
In demyelinating disorders, NMDAR antibodies are uncommon, even in those with symptoms seen in NMDAR encephalitis.
NMDA receptor (NMDAR) encephalitis is characterized by the presence of IgG autoantibodies against NMDAR in serum and CSF and affects mainly pediatric and young adult female patients. The first symptoms are frequently psychiatric, followed by seizures, dyskinesias, memory deficits, and speech problems, often leading to autonomic instabilities.1 Autoantibodies against NMDAR are also found in cases of CNS demyelinating diseases such as acute disseminated encephalomyelitis (ADEM),2 optic neuritis (ON),2 neuromyelitis optica spectrum disorders (NMOSDs),3 or multiple sclerosis (MS).4 Recent data point to a possible association of NMDAR antibodies with demyelination in some patients, particularly when IgG antibodies against glial surface antigens aquaporin-4 (AQP4) or myelin oligodendrocyte glycoprotein (MOG) are present.5
This is a unique study to systematically investigate the seroprevalence of NMDAR antibodies in patients with CNS demyelinating diseases. We retrospectively screened a cohort of patients with demyelinating diseases for IgG antibodies against NMDAR, AQP4, and MOG.
METHODS
Patients and controls.
Serum samples for this retrospective case-control study were collected in the Clinical Department of Neurology Innsbruck between 2005 and 2014 and stored at −80°C until use. All patients fulfilled diagnostic criteria as described below.
Serum samples from 366 individuals were included: 51 patients with ADEM, 41 patients with NMOSD, 34 patients with clinically isolated syndrome (CIS), and 89 patients with MS. Nine patients with NMDAR encephalitis, 94 patients with other neurologic diseases, and 48 healthy individuals were included as controls. Most of the patients have previously been included in a study on NMDAR antibody assay validation6 and in other studies on MOG and/or AQP4 antibodies.7,8 NMOSDs were diagnosed as described by Wingerchuk et al. in 2007, ADEM was diagnosed according to the criteria of the International Pediatric MS Study Group, and MS and CIS were diagnosed according to the 2005 revisions to the McDonald criteria, as previously described.6–8 Diagnosis of NMDAR encephalitis was based on clinical assessment (e.g., new onset of neuropsychiatric symptoms) and demonstration of antibodies in serum or CSF, as recommended recently.9 The relative frequency of symptoms suggestive of NMDAR encephalitis was selectively increased in our cohort of patients with demyelinating diseases (figure).
Figure. Overview of patients with demyelinating diseases, presence of clinical symptoms frequently associated with NMDAR encephalitis, and antibody status.
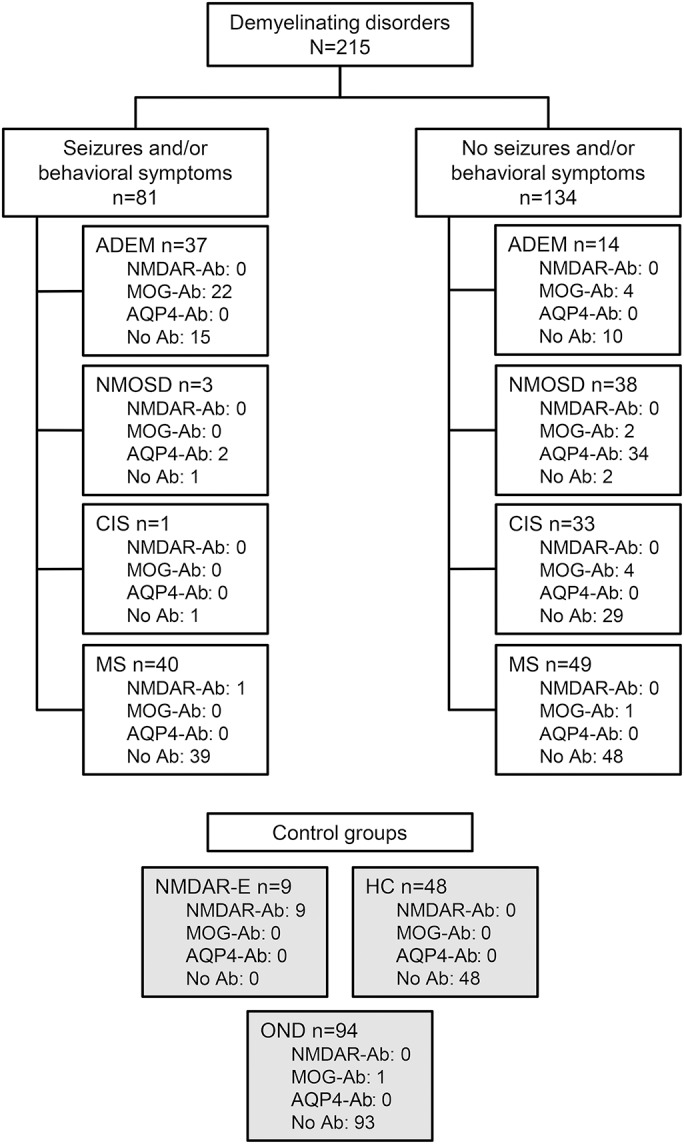
We included 215 patients with demyelinating CNS disorders. Of these, 81 presented with seizures and/or behavioral symptoms whereas 134 did not. As expected, all 9 patients with NMDA receptor (NMDAR) encephalitis presented with seizures and/or behavioral symptoms. Ab = antibody; ADEM = acute disseminated encephalomyelitis; AQP4 = aquaporin-4; CIS = clinically isolated syndrome; HC = healthy controls; MOG = myelin oligodendrocyte glycoprotein; MS = multiple sclerosis; NMDAR-E = NMDAR encephalitis; NMOSD = neuromyelitis optica spectrum disorder; OND = other neurologic diseases.
Standard protocol approvals, registrations, and patient consents.
The present study was approved by the ethical committee of Innsbruck Medical University (study numbers AM3041A and AM4059). All patients or their legal representatives gave written informed consent to the study protocol.
Live cell-based assays for antibody detection.
For the detection of autoantibodies against NMDAR, AQP4, and MOG we used live HEK293A cells transfected with the respective complementary DNA, as described previously.6,7 The following cutoff values were used: ≥1:20 for NMDAR antibodies, ≥1:160 for MOG antibodies, and ≥1:20 for AQP4 antibodies.
To prove the specificity of antibody binding, serum samples were preincubated with HEK293A cells expressing NMDAR or nontransfected HEK293A.
One CSF sample was tested for NMDAR antibodies as described above, except CSF was applied undiluted but supplemented with MK-801 (Sigma-Aldrich, St. Louis, MO) and further diluted (1:2, 1:4, etc.) in washing buffer containing MK-801.
NMDAR antibody testing with fixed cell-based assay (CBA) and rat brain immunohistochemistry (IHC) was done as described recently.9
RESULTS
Demographic data and the NMDAR, MOG, and AQP4 antibody seroprevalence of all patients and controls are shown in the table.
Table.
Demographic data and prevalence of antibodies in patients with demyelinating diseases and controls
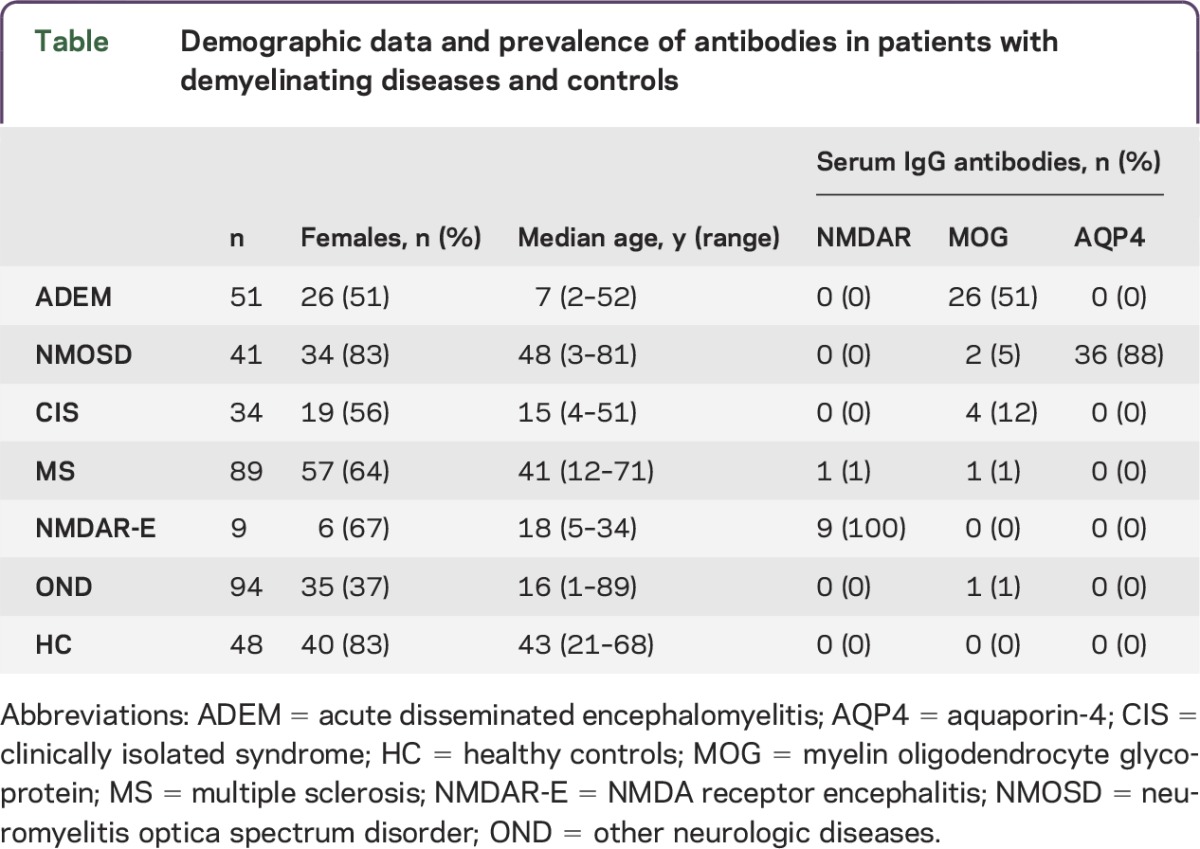
Serum NMDAR antibodies were present in 9/9 (100%) patients with NMDAR encephalitis, in only 1/89 (1%) patients with MS, and in none of the other cases. CSF positivity of NMDAR antibodies was confirmed in all patients with NMDAR encephalitis by fixed CBA and/or tissue IHC. CSF from the patient with MS with low titer (1:20) serum NMDAR antibodies at disease onset was negative for NMDAR antibodies in our live CBA but positive in the fixed CBA and IHC. In contrast, serum was negative in the fixed CBA despite being positive in live CBA and weakly positive in IHC.
At the age of 23, this woman presented with paresthesia of the left hand. MRI showed multiple MS-typical T2 lesions, and investigation of CSF showed the presence of oligoclonal bands and 15 cells/μL. The patient was diagnosed with MS, and complete remission was observed after treatment with high-dose methylprednisolone. Three months later, ON coincided with a clear increase in T2 lesions on MRI, and she was again treated with methylprednisolone followed by complete remission. However, ON reoccurred 3 years after disease onset, and the patient had residual visual deficit despite methylprednisolone treatment. The patient repeatedly declined disease-modifying therapy. Nine months later she gave birth to a healthy child. Three months postpartum, at the age of 26, the patient presented with psychiatric (dysphoria, logorrhea) and cognitive (dyscalculia, frontal executive disorder, psychomotor slowing) symptoms. MRI showed dramatic progression of T2 lesion load and serum was positive for low titer antinuclear antibodies.
Absorption of patient serum with NMDAR-transfected cells abolished the NMDAR-specific signal in the live CBA, whereas incubation with nontransfected cells did not, proving the specificity of antibodies against NMDAR. Antibodies against MOG or AQP4 were absent at any time point. Before the patient could be treated for an autoimmune cause of the psychiatric symptoms, she was transferred to an outside psychiatric hospital and no further clinical data were available.
Demographic, clinical, and antibody data of all patients with demyelinating CNS disorders with seizures and/or behavioral symptoms, which are the major initial symptoms of NMDAR encephalitis, are shown in the figure.
DISCUSSION
Overall, we found serum NMDAR antibodies in 1 of 215 patients with CNS demyelinating diseases, suggesting that the frequency of those autoantibodies is very low (<1%). Although a low false-positive rate of IgG NMDAR antibodies was reported recently,10 in our study serum IgG NMDAR antibodies were only present in patients with symptoms suggestive of NMDAR encephalitis.
The major limitation of our study was the small number of available CSF samples, which could have possibly yielded a higher number of NMDAR antibody–positive patients with demyelinating diseases when using suitable testing methods.
As demonstrated here, live CBA might be more sensitive for detecting low titer serum NMDAR antibodies but not for testing CSF samples, as currently used.
The disease course of the 1 patient with MS and NMDAR antibodies was initially typical for relapsing-remitting MS with residual visual deficits after the second episode of ON. Because analysis of NMDAR antibodies was done retrospectively, the occurrence of antibodies during the first episode of demyelination was not known at that time point. CSF was positive for NMDAR antibodies at disease onset, in line with studies suggesting CSF to be more sensitive than serum.1,9 With the onset of psychiatric and cognitive symptoms, serum NMDAR antibody titers increased. Unfortunately, CSF was not available from the second time point.
This case demonstrates that NMDAR autoantibodies can be linked with demyelination, which has been shown in a recent study investigating a cohort of 691 patients with NMDAR encephalitis, identifying a subgroup of patients with demyelinating episodes.5
Most reported cases (18/23) of demyelinating syndromes overlapping with the presence of NMDAR antibodies were positive for at least 1 antibody associated with demyelination, namely antibodies against MOG or AQP4.5 In our case, those antibodies were not found. Of note, NMDAR encephalitis–related symptoms occurred 3 months after the patient gave birth to a healthy child, paralleling a recently described case,4 although our case showed a less severe disease course.
We found NMDAR antibodies to be rare in demyelinating diseases, even when focusing on patients with symptoms suggestive of NMDAR encephalitis. In the case described here, the presence of NMDAR antibodies preceded the occurrence of symptoms associated with NMDAR encephalitis. Thus, serum NMDAR antibodies could have diagnostic value for an overlapping NMDAR encephalitis–related disease course in patients with demyelinating diseases.
ACKNOWLEDGMENT
The authors thank Dr. Josep Dalmau (Barcelona, Spain) for carrying out the CSF NMDAR antibody IHC testing on the patient with MS positive for NMDAR antibodies and Ingrid Gstrein for technical support.
GLOSSARY
- ADEM
acute disseminated encephalomyelitis
- AQP4
aquaporin-4
- CBA
cell-based assay
- CIS
clinically isolated syndrome
- IHC
immunohistochemistry
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NMDAR
NMDA receptor
- NMOSD
neuromyelitis optica spectrum disorder
- ON
optic neuritis
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: M. Ramberger, G.B., M. Reindl. Performed the experiments: M. Ramberger, K.S., R.H., M. Reindl. Analyzed the data: M. Ramberger, M. Reindl. Contributed reagents/materials/analysis: G.B., R.H., K.R., M.B., F.A.-D., A.L., F.D., T.B. Wrote the manuscript: M. Ramberger, G.B., M. Reindl.
STUDY FUNDING
This study was supported by research grants from the Fonds zur Förderung der wissenschaftlichen Forschung, Austria (FWF graduate program W1206 SPIN) and the Austrian Federal Ministry of Science and Economy (grant BIG WIG MS).
DISCLOSURE
M. Ramberger, G. Bsteh, K. Schanda, and R. Höftberger report no disclosures. K. Rostásy is on the scientific advisory board for Novartis and received speaker honoraria from Merck-Serono. M. Baumann and F. Abouleneim-Djamshidian report no disclosures. A. Lutterotti received travel funding from European Charcot Foundation and Fundacio GAEM and received research support from Austrian MS Society. F. Deisenhammer is on the scientific advisory board for Biogen-Idec, Novartis Pharma, Genzyme, Merck, and Teva; received travel funding and/or speaker honoraria from Bayer Healthcare, Biogen-Idec, Novartis, and Genzyme; was a section editor for Multiple Sclerosis and Related Disorders; and received research support from Biogen-Idec and Innovative Medicine Initiatives. T. Berger reports no disclosures. M. Reindl is an academic editor for PLoS One, was on the editorial board for Current Medicinal Chemistry and Autoimmune Diseases, and received research support from Fonds zur Förderung der wissenschaftlichen Forschung and Austrian Federal Ministry of Science, Research and Economy. Dr. Reindl and Medical University of Innsbruck offer testing for MOG antibodies for research purposes only (free of charge). The University Hospital and Medical University of Innsbruck receive payments for antibody assays (NMDAR, AQP4, and other autoantibodies) and for AQP4 antibody validation experiments organized by Euroimmun. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011;10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hacohen Y, Absoud M, Woodhall M, et al. Autoantibody biomarkers in childhood-acquired demyelinating syndromes: results from a national surveillance cohort. J Neurol Neurosurg Psychiatry 2014;85:456–461. [DOI] [PubMed] [Google Scholar]
- 3.Kruer MC, Koch TK, Bourdette DN, et al. NMDA receptor encephalitis mimicking seronegative neuromyelitis optica. Neurology 2010;74:1473–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischmann R, Pruss H, Rosche B, et al. Severe cognitive impairment associated with intrathecal antibodies to the NR1 subunit of the N-methyl-D-aspartate receptor in a patient with multiple sclerosis. JAMA Neurol 2015;72:96–99. [DOI] [PubMed] [Google Scholar]
- 5.Titulaer MJ, Hoftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;75:411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramberger M, Peschl P, Schanda K, et al. Comparison of diagnostic accuracy of microscopy and flow cytometry in evaluating N-methyl-d-aspartate receptor antibodies in serum using a live cell-based assay. PLoS One 2015;10:e0122037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mader S, Gredler V, Schanda K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 2011;8:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann M, Sahin K, Lechner C, et al. Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry 2015;86:265–272. [DOI] [PubMed] [Google Scholar]
- 9.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014;13:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahm L, Ott C, Steiner J, et al. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol 2014;76:82–94. [DOI] [PubMed] [Google Scholar]


