A committee was established at the request of the Canadian Urological Association to develop guidelines for the investigation and management of azoospermia. Members of the committee, all of whom have special expertise in the investigation and management of male infertility, were chosen from different communities across Canada. The members represent different practices in different communities.
Introduction and background
Infertility or subfertility affects 15% of couples in Canada, with a male factor contributing to the fertility problem in close to 50% of these couples. Of the men presenting for fertility investigation, up to 20% are found to be azoospermic. These men can be categorized as having either:
- Pre-testicular azoospermia (2% of men with azoospermia);
- Due to a hypothalamic or pituitary abnormality (diagnosed with hypo-gonadotropic-hypogonadism).
- Testicular failure or non-obstructive azoospermia (49%–93% of men with azoospermia); or
A further group of men have a failure to ejaculate. These may be men with spinal cord injury, psychogenic failure to ejaculate, or neurological damage (sympathetic nerve damage from, for example, a retroperitoneal lymph node dissection).
To understand the management of azoospermia, it is important to also understand the role of assisted reproductive technologies (ARTs) (i.e., in-vitro fertilization) in the treatment of azoospermia. Since the 1970s, breakthroughs in the ARTs have allowed us to offer potentially successful treatments for up to 98% of couples with male factor infertility.6 These significant advances had little to do with techniques to improve the sperm quality, but relied on the use of ARTs to “treat” the male infertility. These programs used techniques to increase the number of mature eggs produced by the women by manipulating the hormonal environment in the women using exogenous hormones (ovulation induction) then used either:
Timed insemination to optimize the pregnancy rates either through intercourse or intra-uterine insemination of the partners washed sperm;
In-vitro fertilization (IVF); or oocytes are retrieved from the ovaries then are either incubated with the sperm in a dish; or
Intra-cytoplasmic sperm injection (ICSI), by injecting the sperm directly into the cytoplasm of the oocyte.
All of the above techniques are widely used to treat couples with male factor infertility. In the United States in 2012, over 165 000 IVF/ICSI cycles were performed.7 Worldwide the numbers of IVF/ICSI cycles were high. In 2013, the International Committee for Monitoring Assisted Reproductive Technology reported that in 2004 there were 954 743 IVF or ICSI cycles worldwide, with 237 809 babies born.8
Using ICSI, it is now possible to produce a pregnancy with any live sperm (motile or not), from either the semen or any site within the male reproductive tract. Even men with azoospermia can be offered sperm retrieval with ICSI. Sperm could be retrieved from any site in the reproductive tract and used for ICSI. These are the men who previously had very limited chances to ever have biologically related children. Pregnancy rates of close to 50% per cycle of ICSI (women under 35 years of age) are expected, with the pregnancy rates independent of the site of the origin of the sperm.6
History, physical exam and initial investigations for men with azoospermia
After at least two semen analyses have confirmed azoospermia, the men should be investigated with a thorough history (Table 1) and physical examination (Table 2). Most men will also require laboratory and imaging studies.
Table 1.
Type of information to gather during a patient’s history
| General information | Examples and areas of focus |
|---|---|
| 1. Infertility history |
|
| 2. The general health of the man |
|
| 3. Any proven or suspected genitourinary infections, testicular infections or inflammation8 |
|
| 4. Any surgery of the reproductive tract |
|
| 5. Exposure to medications and therapies which might have an adverse impact on spermatogenesis |
|
| 6. Environmental exposures |
|
| 7. Any recreational drugs |
|
| 8. History of any genetic abnormalities in the patient or his family |
Table 2.
Type of information to gather during a patient’s physical examination
| Type of examination | Examples and areas of focus |
|---|---|
| 1. State of virilization | |
| 2. Scrotal examination |
|
| 3. Abdominal examination |
|
If the man has had exposure to any of the gonadotoxic agents (Table 1), he should be taken off these medications and his semen should be retested in 3 to 6 months. If the man has had a recent serious medical illness or injury or he has evidence of a recent reproductive tract infection, semen testing should be repeated at least 3 months following recovery from the illness.
The initial investigations will depend on the information obtained from the history and physical examination, in addition to the results of the semen analyses.
Azoospermia with reduced semen volume
If the semen volume is reduced (<1.5 mL) and documented on repeat testing, careful questioning should elicit whether this is an artifact (i.e., missed the container, difficulty providing specimen) or truly a low semen volume.9
Since most of the semen comes from the seminal vesicles and prostate (>90%), low semen volume means that either:
the seminal vesicles are abnormal or obstructed;
the ejaculatory ducts are obstructed; or
there is an ejaculatory dysfunction (either failure of emission or retrograde ejaculation).
Physical examination will help determine if the vas deferens is present in the scrotum. Absence of the vas deferens in the scrotum is usually associated with absence of the seminal vesicles and is a cause for low semen volume and azoospermia.
The first laboratory test is to determine if there is retrograde ejaculation by testing the post-ejaculate urine for the presence of sperm. The presence of any sperm in the urine, post-ejaculation in men with azoospermia, is diagnostic of retrograde ejaculation. Since retrograde ejaculation may be due to a failure of the bladder neck to close with orgasm, use of an alpha agonist (pseudoephedrine or other alpha agonist) before ejaculation may close the bladder neck and convert retrograde into ante-grade ejaculation. Diabetic men often have retrograde ejaculation or failure of emission.10
If there is no evidence of retrograde ejaculation, diagnostic imaging of the reproductive tract is usually required to identify reproductive tract obstruction or abnormalities. A transrectal ultrasound (TRUS) will determine if the seminal vesicles and vas deferens close to the prostate are normal. Obstruction of the ejaculatory duct is usually detected by a TRUS and is usually accompanied by dilation of the seminal vesicles (typically >1.5 cm wide).11 If absence of the vas deferens and/or the seminal vesicle is identified, the man has about an 80% chance of carrying a genetic alteration associated with cystic fibrosis.12 Cystic fibrosis testing should be performed on all men without vas deferens/seminal vesicles (Grade A Recommendation). Men with congenital bilateral absence of the vas deferens (CBAVD) typically have normal spermatogenesis and a diagnostic biopsy is usually not required to diagnose active spermatogenesis. An abdominal ultrasound to assess the kidneys is indicated in men with CBAVD who are not carriers of cystic fibrosis mutations, since these men have a higher chance of having absence of one of their kidneys (Fig. 1).11,13
Fig. 1.
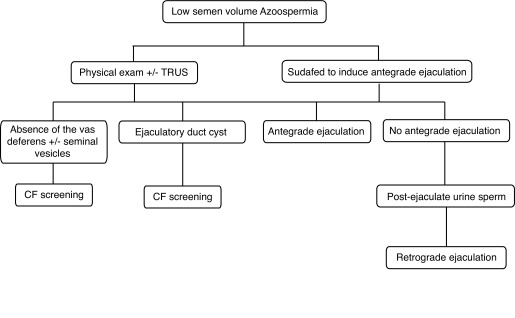
Algorithm for the investigation of low volume azoospermia. CF: cystic fibrosis; TRUS: transurethral ultrasound.
Vasography is not required and should be discouraged for men with an ejaculatory duct obstruction (Level of Evidence 3, Grade C Recommendation). If an ejaculatory duct obstruction is identified, the man has about a 25% chance of carrying a genetic alteration associated with cystic fibrosis.12 Cystic fibrosis testing should be performed on all men with ejaculatory duct cysts.
Normal semen volume azoospermia
As stated above, the categories of azoospermia are:
Pre-testicular azoospermia;
Testicular failure or non-obstructive azoospermia; and
The category of azoospermia may often be determined by the luteinizing hormone (LH) and follicular stimulating hormone (FSH) levels without the need for a testicular biopsy.
The diagnosis of pre-testicular azoospermia is relatively uncomplicated. LH and FSH levels will be low and the testosterone levels will be either low or normal.14
Men with elevated FSH and LH and small testis bilaterally have non-obstructive azoospermia.
However, men with normal levels of FSH and LH could have either non-obstructive azoospermia or obstructive azoospermia.14 Unfortunately, there is no non-invasive method to differentiate obstructive from non-obstructive azoospermia in this group of men. A testicular biopsy is usually required to provide a definitive diagnosis (Fig. 2).
Fig. 2.
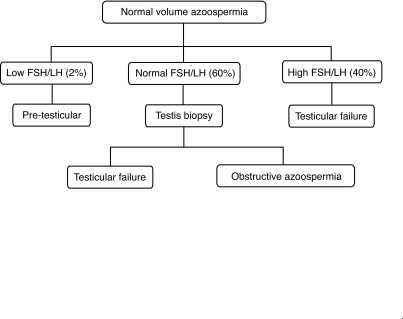
Algorithm for determining the category of normal semen volume azoospermia. FSH: follicular stimulating hormone; LH: luteinizing hormone.
Failure to ejaculate
In men with a clear neurological cause (e.g., spinal cord injury, retroperitoneal lymph node surgery), no further investigations are required prior to treatment. Men with idiopathic failure to ejaculate (particularly those with a failure to orgasm) should be seen by a sex therapist.
Genetic investigations for men with azoospermia
All men with hypogonadotropic hypogonadism should be referred for genetics counselling, as almost all of the congenital abnormalities of the hypothalamus are due to a genetic alteration.12
All men with absence (absence of the vas deferens) or obstruction (epididymal or ejaculatory duct) of the reproductive tract ductal structures have an increased risk of carrying a genetic alteration associated with cystic fibrosis.12,15 We recommend that not only the man, but also his partner, be offered cystic fibrosis testing in this situation.16
If a genetic alteration is identified, then genetic counselling is suggested (Level of Evidence 2, Grade of Recommendation B). Men with obstructive azoospermia do not require any other routine genetic testing.12
All men with testicular failure should be offered karyotype and Y-micro-deletion testing, then referred for genetics counselling if an abnormality is identified15 (Level of Evidence 1, Grade of Recommendation A). Men with non-obstructive azoospermia do not require cystic fibrosis testing (Table 3).17
Table 3.
Common Genetic Abnormalities found in Different Categories of Azoospermia
| Cystic fibrosis | Karyotype | Y-microdeletion | |
|---|---|---|---|
| Absence or obstruction of the vas deferens, epididymis or ejaculatory ducts | 25–80% | ||
| Testicular failure | 14% | 1–30% |
Management options for men with azoospermia
Couples have many ways to achieve their goal of completing their family. The options of adoption, donor sperm, and child-free living should always be discussed with the couple. The treatment options discussed below are those which allow a couple to have children biologically related to the man. These options depend on the diagnosis.
Hypogonadotropic-hypogonadism or pre-testicular azoospermia
This is best treated with the use of FSH/LH or gonadotropin-releasing hormone (GnRH) analogues to stimulate spermatogenesis.18 In over 90% of the cases, spermatogenesis is induced and the men have ejaculated sperm. However, therapy may take more than 6 months to be effective.
Retrograde ejaculation
Use of pseudoephedrine (use 60 mg prior to ejaculation) or a similar alpha agonist may convert retrograde ejaculation into antegrade ejaculation. If this is not successful, it is often possible to retrieve sperm from the bladder (either using a post-ejaculatory voided or catherized urine specimen). This sperm could then be used for one of the ARTs.
Obstructive azoospermia
This is managed with either of the following:
Sperm retrieved from the reproductive tract (close to 100% chance of finding sperm) to be used in an ICSI program. The method of sperm retrieval used may be a percutaneous or an open microscopic aspiration of sperm from the epididymis or a percutaneous or open biopsy of the testis. Any of the retrieval methods listed above are acceptable.15
Bypass/repair of the obstructed area of the reproductive tract. This is a realistic therapy for most men with obstructive azoospermia.15 The most common area of obstruction is within the epididymis. With the present microsurgical techniques, centres with expertise in performing vaso-epididymostomies report over 85% patency of the anastomosis (sperm in the ejaculate is the measure of patency), with over a 50% spontaneous pregnancy rate.19 However, this is surgery requiring micro-surgical expertise and experience and should only be performed in centres with this kind of expertise. We recommend that all men be offered the option to cryo-bank sperm retrieved during the course of the operation in case the surgery is not successful (Level of Evidence 3, Grade of Recommendation C).
Transurethral resection (TUR) of the ejaculatory duct. Men with an ejaculatory duct obstruction may be candidates for a TUR ejaculatory duct.20 This is best performed using TRUS guidance to allow the TUR to precisely unroof the ejaculatory duct cyst. It is important to warn the men of the potential complications associated with a TUR of the prostate.
Non-obstructive azoospermia
Testicular sperm extraction (TESE) may be used to identify sperm (reported success up to 75%, mean 52%), which could then be processed for use in an ICSI program.14,21–27 At present, the optimum way to identify these pockets of sperm is to perform an extensive, surgical dissection of the seminiferous tubules (a testicular sperm extraction) (Level of Evidence 2, Grade of Recommendation B). Large sections of the seminiferous tubules of the testis are examined with an operating microscope. Those tubules, which are larger in size, are more likely to have spermatogenesis than smaller diameter tubules. The advantage of this technique over the regular random biopsy method is the ability to identify areas of the seminiferous tubules, which are more likely to contain sperm before the tissue is removed from the testicle. Using this technique the chance of finding sperm is higher than the older technique of taking random testicular biopsies alone (in one series 63% compared to 45%); while the procedure is laborious (surgical time may exceed 3 hours), the damage to the testicle is minimal due to the minimal amount of testis tissue eventually taken.26 ICSI pregnancy rates using sperm from a testicular sperm extraction program are between 19% and 50%.15,21,24,25,28 The testicular sperm extraction procedure should be offered to all men with non-obstructive azoospermia, but should only be undertaken in a centre with expertise in micro-TESE and where an ICSI laboratory with expertise in handling these samples is available.
Failure to ejaculate
Men with a neurological cause for a failure to ejaculate should be offered either vibro-stimulation or electro-ejaculation.29 Both procedures may cause autonomic dysreflexia in men with high spinal cord injuries. The semen specimen may be used for one of the ARTs. It is common that multiple (i.e., 2–3) procedures several weeks apart may be needed to optimize the semen quality. Occasionally these men may also have a concomitant obstruction of the epididymis, so occasionally sperm aspiration is required.
Which azoospermic men might need a diagnostic testis biopsy?
As mentioned above, men having normal FSH and LH levels could have either obstructive azoospermia or non-obstructive azoospermia. Currently, the only way to make this diagnosis is by using a testicular biopsy. However, a testis biopsy should only be offered to men in whom this diagnosis would alter management. For example, we would discourage a man from having a testis biopsy if he and his partner are not interested in any of the potential management options that follow, such as sperm aspiration plus ICSI or vaso-epididymostomy.
If the couple is interested in considering the other fertility treatments mentioned above, then the biopsy could be performed either:
As a diagnostic procedure alone (either a percutaneous or an open biopsy are acceptable methods of testicular biopsies) or as a combination of a diagnostic and a therapeutic biopsy (some of the tissue is cryo-preserved for later use). The biopsy results then guide the next treatments; or
As the initial part of the larger fertility treatment. Once the biopsy results are available as a quick section, the surgery would then proceed with a reconstruction and/or sperm retrieval (if active spermatogenesis is detected) or a testicular sperm extraction (if a pattern of testicular failure is detected). This should only be performed in centres with the expertise to perform the needed microsurgery and with a laboratory with the capacity to cryopreserve sperm.
A bilateral diagnostic testicular biopsy is generally not required. If there is a discrepancy in testicular size, the larger of the two testes should be biopsied.
What is the role of varicocelectomy in men with azoospermia?
The role of varicocelectomy in men with azoospermia remains controversial. There is some evidence that a small percentage of men with azoospermia due to testicular failure may benefit from treatment of a clinical varicocele.30 Schlegel and colleagues reported that close to 20% of men with azoospermia had sperm in the ejaculation following a varicocele repair.30 It is considered reasonable to offer a varicocele repair to men with clinical varicoceles and testicular failure, but men should be warned that there is a low probability that this will result in any improvement in his semen parameters. Most men will still need ICSI to help conceive (Level of Evidence 4, Grade of Recommendation D).
What is the role of hormone therapy for men with azoospermia?
Apart from the management of men with hypo-gonadotropic hypogonadism, the use of hormones to treat men with azoospermia remains controversial.
Based on a multicentre study, Hussein and colleagues reported that in men with non-obstructive azoospermia given clomiphene and/or human chorionic gonadotropin (HCG) to increase FSH to a target level of 1.5-fold times the initial FSH levels and a target serum testosterone of 600 to 800 ng/dL, the yield of sperm on a micro-TESE was 57% compared to 33.6% in the control group.31
Further support for the concept that hormonal therapies might benefit men with non-obstructive azoospermia came from Shiraishi and colleagues. The authors studied the effects of HCG 5000 U 3 times/week with FSH for men with a declining FSH and with no sperm found on initial micro-TESE surgery. In total, 6 of the 28 men who received the hormone therapy had sperm retrieved on a subsequent micro-TESE, while none of the men not receiving hormone therapy had sperm on the second micro-TESE.32
Conversely, Reifsnyder and colleagues reported on men with non-obstructive azoospermia having initially lower serum testosterone levels (<300 ng/dL) and who took hormone therapy with increases in serum testosterone levels. These men had no different results in their testicular sperm extraction (sperm retrieval or pregnancy rates) than men who had higher testosterone levels initially.33
We cannot presently recommend the use of any hormone therapy for men with non-obstructive azoospermia.
The use of androgens is contraindicated in men with azoospermia34 (Level of Evidence 1, Grade of Recommendation A).
Footnotes
Competing interests: Dr. Jarvi is a member of the advisory board for Eli Lilly. Dr. Grober is a member of the advisory boards and speaker’s bureau for Eli Lilly, Abbott, and Paladin. He has also received a grant from Eli Lilly and Paladin. Dr. Mak has received a grant from Amgen and Janssen. Dr. Chow is on the advisory boards for Astellas and Johnson & Johnson. Dr. Domes has received speaker honoraria from Eli Lilly and Abbott. Dr. Lo, Dr. Fischer, Dr. Grantmyre, Dr. Zini, Dr. Chan, and Dr. Patry declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Fogle RH, Steiner AZ, Marshall FE, et al. Etiology of azoospermia in a large nonreferral inner-city population. Fertil Steril. 2006;86:197–9. doi: 10.1016/j.fertnstert.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 2.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142:62–5. doi: 10.1016/s0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- 3.Fedder J, Cruger D, Oestergaard B, et al. Etiology of azoospermia in 100 consecutive nonvasectomized men. Fertil Steril. 2004;82:1463–5. doi: 10.1016/j.fertnstert.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, So SY, Wong KK, et al. Chronic sinopulmonary disease in Chinese patients with obstructive azoospermia. J Androl. 1987;8:225–9. doi: 10.1002/j.1939-4640.1987.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 5.Matsumiya K, Namiki M, Takahara S, et al. Clinical study of azoospermia. Int J Androl. 1994;17:140–2. doi: 10.1111/j.1365-2605.1994.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 6. Services, U.D.o.H.a.H. Assisted Reproductive Technology Success Rates 2003: National Summary and Fertility Clinic Reports. US Department of Health and Human Services, C.f.D.C.a.P.; 2005. [Google Scholar]
- 7.Society for Assisted Reproductive Technology https://www.sartcorsonline.com. Accessed July 14, 2015.
- 8.Sullivan EA, Zegers-Hochschild F, Mansour R, et al. International Committee for Monitoring Assisted Reproductive Technologies (ICMART) world report: Assisted reproductive technology 2004. Hum Reprod. 2013;28:1375–90. doi: 10.1093/humrep/det036. [DOI] [PubMed] [Google Scholar]
- 9.Bachir BG, Jarvi K. Infectious, inflammatory, and immunologic conditions resulting in male infertility. Urol Clin North Am. 2014;41:67–81. doi: 10.1016/j.ucl.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Samplaski MK, Lo K, Grober E, et al. Finasteride use in the male infertility population: Effects on semen and hormone parameters. Fertil Steril. 2013;100:1542–6. doi: 10.1016/j.fertnstert.2013.07.2000. [DOI] [PubMed] [Google Scholar]
- 11.Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 12.Delfino M, Imbrogno N, Elia J, et al. Prevalence of diabetes mellitus in male partners of infertile couples. Minerva Urol Nefrol. 2007;59:131–5. [PubMed] [Google Scholar]
- 13.Jhaveri KS, Mazrani W, Chawla TP, et al. The role of cross-sectional imaging in male infertility: A pictorial review. Can Assoc Radiol J. 2010;61:144–55. doi: 10.1016/j.carj.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Mak V, Jarvi KA. The genetics of male infertility. J Urol. 1996;156:1245–56. [PubMed] [Google Scholar]
- 15.Jarvi K, McCallum S, Zielenski J, et al. Heterogeneity of reproductive tract abnormalities in men with absence of the vas deferens: Role of cystic fibrosis transmembrane conductance regulator gene mutations. Fertil Steril. 1998;70:724–8. doi: 10.1016/S0015-0282(98)00247-7. [DOI] [PubMed] [Google Scholar]
- 16.Von Eckardstein S, Simoni M, Bergmann M, et al. Serum inhibin B in combination with serum follicle-stimulating hormone (FSH) is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrinol Metab. 1999;84:2496–501. doi: 10.1210/jc.84.7.2496. [DOI] [PubMed] [Google Scholar]
- 17.Schlegel PN. Causes of azoospermia and their management. Reprod Fertil Dev. 2004;16:561–72. doi: 10.1071/RD03087. [DOI] [PubMed] [Google Scholar]
- 18.Mak V, Zielenski J, Tsui LC, et al. Proportion of cystic fibrosis gene mutations not detected by routine testing in men with obstructive azoospermia. JAMA. 1999;281:2217–24. doi: 10.1001/jama.281.23.2217. [DOI] [PubMed] [Google Scholar]
- 19.Mak V, Zielenski J, Tsui LC, et al. Cystic fibrosis gene mutations and infertile men with primary testicular failure. Hum Reprod. 2000;15:436–9. doi: 10.1093/humrep/15.2.436. [DOI] [PubMed] [Google Scholar]
- 20.Jungwirth A, Giwercman A, Tournaye H, et al. European Association of Urology guidelines on Male Infertility: The 2012 update. Eur Urol. 2012;62:324–32. doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 21.Chawla A, O’Brien J, Lisi M, et al. Should all urologists performing vasectomy reversals be able to perform vasoepididymostomies if required? J Urol. 2004;172:1048–50. doi: 10.1097/01.ju.0000135118.43383.b1. [DOI] [PubMed] [Google Scholar]
- 22.Smith JF, Walsh TJ, Turek PJ. Ejaculatory duct obstruction. Urol Clin North Am. 2008;35:221–7. doi: 10.1016/j.ucl.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Chan PT, Palermo GD, Veeck LL, et al. Testicular sperm extraction combined with intracytoplasmic sperm injection in the treatment of men with persistent azoospermia postchemotherapy. Cancer. 2001;92:1632–7. doi: 10.1002/1097-0142(20010915)92:6<1632::AID-CNCR1489>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Gil Salom M. Spermatic recovery techniques for intracytoplasmic spermatozoid injection (ICSI) in male infertility. Arch Esp Urol. 2004;57:1035–46. [PubMed] [Google Scholar]
- 25.Kim ED, Gilbaugh JH, Patel VR, et al. Testis biopsies frequently demonstrate sperm in men with azoospermia and significantly elevated follicle-stimulating hormone levels. J Urol. 1997;157:144–6. doi: 10.1016/S0022-5347(01)65308-4. [DOI] [PubMed] [Google Scholar]
- 26.Raman JD, Schlegel PN. Testicular sperm extraction with intracytoplasmic sperm injection is successful for the treatment of nonobstructive azoospermia associated with cryptorchidism. J Urol. 2003;170:1287–90. doi: 10.1097/01.ju.0000080707.75753.ec. [DOI] [PubMed] [Google Scholar]
- 27.Schiff JD, Palermo GD, Veeck LL, et al. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90:6263–7. doi: 10.1210/jc.2004-2322. [DOI] [PubMed] [Google Scholar]
- 28.Schlegel PN. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–5. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 29.Schlegel PN, Palermo GD, Goldstein M, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology. 1997;49:435–40. doi: 10.1016/S0090-4295(97)00032-0. [DOI] [PubMed] [Google Scholar]
- 30.Colpi GM, Piediferro G, Nerva F, et al. Sperm retrieval for intra-cytoplasmic sperm injection in non-obstructive azoospermia. Minerva Urol Nefrol. 2005;57:99–107. [PubMed] [Google Scholar]
- 31.Brackett NL, Lynne CM, Ibrahim E, et al. Treatment of infertility in men with spinal cord injury. Nat Rev Urol. 2010;7:162–72. doi: 10.1038/nrurol.2010.7. [DOI] [PubMed] [Google Scholar]
- 32.Schlegel PN, Kaufmann J. Role of varicocelectomy in men with nonobstructive azoospermia. Fertil Steril. 2004;81:1585–8. doi: 10.1016/j.fertnstert.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 33.Hussein A, Ozgok Y, Ross L, et al. Optimization of spermatogenesis-regulating hormones in patients with non-obstructive azoospermia and its impact on sperm retrieval: A multicentre study. BJU Int. 2013;111:E110–4. doi: 10.1111/j.1464-410X.2012.11485.x. [DOI] [PubMed] [Google Scholar]
- 34.Shiraishi K, Ohmi C, Shimabukuro T, et al. Human chorionic gonadotrophin treatment prior to microdissection testicular sperm extraction in non-obstructive azoospermia. Hum Reprod. 2012;27:331–9. doi: 10.1093/humrep/der404. [DOI] [PubMed] [Google Scholar]


