Abstract
IMPORTANCE
We previously showed that detection of androgen receptor splice variant 7 (AR-V7) in circulating tumor cells (CTCs) from men with castration-resistant prostate cancer (CRPC) was associated with primary resistance to enzalutamide and abiraterone therapy, but the relevance of AR-V7 status in the context of chemotherapy is unknown.
OBJECTIVE
To investigate whether AR-V7–positive patients would retain sensitivity to taxane chemotherapy and whether AR-V7 status would have a differential impact on taxane-treated men compared with enzalutamide- or abiraterone-treated men.
DESIGN, SETTING, AND PARTICIPANTS
We examined CTCs for AR-V7 mRNA using a reverse-transcription polymerase chain reaction assay. From January 2013 to July 2014, we prospectively enrolled patients with metastatic CRPC initiating taxane chemotherapy (docetaxel or cabazitaxel) at a single academic institution (Johns Hopkins). Our prespecified statistical plan required a sample size of 36 taxane-treated men.
MAIN OUTCOMES AND MEASURES
We evaluated associations between AR-V7 status and prostate-specific antigen (PSA) response rates. PSA progression-free survival (PSA PFS), and clinical and/or radiographic progression-free survival (PFS). After incorporating updated data from our prior study of 62 patients treated with enzalutamide or abiraterone, we also investigated the interaction between AR-V7 status (positive or negative) and treatment type (taxane vs enzalutamide or abiraterone).
RESULTS
Of 37 taxane-treated patients enrolled. 17 (46%) had detectable AR-V7 in CTCs. Prostate-specific antigen responses were achieved in both AR-V7–positive and AR-V7–negative men (41% vs 65%; P = .19) Similarly, PSA PFS (hazard ratio [HR], 1.7, 95% CI, 0.6-5.0; P = .32) and PFS (HR, 2.7, 95% CI, 0.8-8.8; P = .11) were comparable in AR-V7–positive and AR-V7–negative patients. A significant interaction was observed between AR-V7 status and treatment type (P < .001). Clinical outcomes were superior with taxanes compared with enzalutamide or abiraterone therapy in AR-V7–positive men, whereas outcomes did not differ by treatment type in AR-V7–negative men. In AR-V7–positive patients. PSA responses were higher in taxane-treated vs enzalutamide- or abiraterone-treated men (41% vs 0%; P < .001), and PSA PFS and PFS were significantly longer in taxane-treated men (HR, 0.19 [95% CI, 0.07-0.52] for PSA PFS, P = .001; HR, 0.21 [95% CI, 0.07-0.59] for PFS, P = .003).
CONCLUSIONS AND RELEVANCE
Detection of AR-V7 in CTCs from men with metastatic CRPC is not associated with primary resistance to taxane chemotherapy. In AR-V7–positive men, taxanes appear to be more efficacious than enzalutamide or abiraterone therapy, whereas in AR-V7–negative men, taxanes and enzalutamide or abiraterone may have comparable efficacy. Circulating tumor cell–based AR-V7 detection may serve as a treatment selection biomarker in CRPC.
There are currently 6 available therapies for the treatment of castration-resistant prostate cancer (CRPC), all of which have produced survival improvements.1 These therapies fall into 4 classes: androgen receptor (AR)-directed therapies (abiraterone acetate,2 enzalutamide3), taxane chemotherapies (docetaxel,4 cabazitaxel5), immunotherapies (sipuleucel-T6), and bone-targeting radiopharmaceuticals (radium-223).7 Of these, the most widely used are the AR-targeting therapies and the chemotherapies. However, mechanisms of response and resistance to these therapies remain poorly understood.8,9 Furthermore, predictive biomarkers aiding in treatment selection (ie, selecting for or against a particular therapy) are still lacking, although prognostic markers are abundant.10
We have recently shown that AR splice variants, in particular AR variant 7 (AR-V7), are strongly associated with primary resistance to abiraterone and enzalutamide therapy in men with CRPC.11 Androgen receptor variants (AR-Vs) are alternatively spliced isoforms of the AR that encode a truncated AR protein lacking the C-terminal ligand-binding domain but retaining the transactivating N-terminal domain.12-14 Although these AR-Vs are unable to bind to the ligand (eg, dihydrotestosterone), they are constitutively active and capable of promoting transcription of target genes.14-16 To investigate the clinical relevance of AR-Vs in CRPC, we previously developed a circulating tumor cell (CTC)-based assay to interrogate AR-V7 in men undergoing therapy with abiraterone (an androgen synthesis inhibitor) or enzalutamide (an AR antagonist). We demonstrated that detection of AR-V7 in CTCs from such patients was associated with lack of a prostate-specific antigen (PSA) response and that AR-V7–positive patients had shorter progression-free survival (PFS) and overall survival (OS) than AR-V7–negative men.11
Recent preclinical data have emerged suggesting that taxane chemotherapies may exert their antitumor activity in CRPC (at least partially) by impairing AR signaling along the microtubule network, thereby sequestering AR in the cytoplasm.17-20 In addition, it has been shown that in patients with taxane-sensitive disease, treatment produces microtubule bundling resulting in exclusion of the AR from the nucleus. Conversely, AR often remains capable of trafficking into the nucleus despite therapy in patients with taxane-resistant disease.19,21 Furthermore, in certain xenograft mouse models it has been suggested that some AR splice variants may promote resistance to taxane chemotherapies while others may be compatible with taxane sensitivity.22 However, the clinical significance of AR-Vs in patients receiving taxanes is unknown.
The present study aimed to prospectively evaluate the predictive impact of AR-Vs in men with CRPC undergoing taxane chemotherapy. We hypothesized that men with detectable CTC-derived AR-V7 would retain sensitivity to taxanes and that AR-V7 status would have a differential effect on taxane-treated men vs enzalutamide- or abiraterone-treated men. Herein, we show that detection of AR-V7 is not associated with primary resistance to taxane chemotherapy and that taxanes may have superior efficacy compared with AR-targeting agents in AR-V7–positive patients.
Methods
Patients
The study enrolled men with metastatic CRPC who were beginning standard-of-care treatment with docetaxel or cabazitaxel. Patients were required to have histologically confirmed prostate adenocarcinoma, progressive disease despite “castration levels” of serum testosterone (<50 ng/dL), and documented radiographic metastases on computed tomography (CT) or technetium-99 bone scans. Patients were required to have at least 3 increasing serum PSA values taken at least 2 weeks apart with the last value being at least 2.0 ng/mL, consistent with the Prostate Cancer Working Group (PCWG2) guidelines.23 Patients were excluded if they planned to receive additional concurrent anticancer therapies (standard or investigational) during the course of taxane treatment. Prior treatment with abiraterone and/or enzalutamide was permitted, as was previous treatment with docetaxel among men starting cabazitaxel therapy (consistent with the labeled indication5). The study was approved by the Johns Hopkins University institutional review board, and patients provided written informed consent.
Study Design
This was a prospective study evaluating the ability of baseline AR-V7 status to predict sensitivity or resistance to taxane agents. Patients who were about to begin docetaxel or cabazitaxel chemotherapy were enrolled and underwent peripheral blood CTC sampling at up to 3 time points: at baseline, at the time of a clinical and/or biochemical response (if a response occurred), and at the time of clinical and/or radiographic progression. Docetaxel was administered at a dose of 75 mg/m2 intravenously every 3 weeks, and cabazitaxel was given at a dose of 25 mg/m2 intravenously every 3 weeks (both with prednisone 5 mg twice daily).
Follow-up was prospectively defined: patients had PSA measurements every 1 to 2 months, as well as CT (chest/abdomen/pelvis) and technetium-99 bone scans every 2 to 4 months. Therapy with docetaxel or cabazitaxel was continued until PSA progression or clinical and/or radiographic progression, or until patients developed unmanageable drug-related toxic effects.
CTC-Based AR-V7 Detection
The CTC analyses were conducted using a modification of the commercially available AdnaTest platform (Qiagen), as previously described.11 Isolation and enrichment of CTCs was per-formed using the ProstateCancerSelect kit, and mRNA expression analyses were performed using the ProstateCancerDetect kit with multiplexed reverse-transcription polymerase chain reaction primers to establish the presence or absence of CTCs. Custom primers were used to detect the full-length AR (AR-FL) mRNA and AR-V7 mRNA, as previously described.11 The relative abundance of AR-V7 was determined by calculating the ratio of AR-V7 transcript to AR-FL transcript.
Outcome Measures
The primary end point was PSA response: the proportion of patients who achieved at least a 50% PSA level decline from baseline at any time point after therapy (and maintained it for ≥3 weeks). Secondary end points included PSA PFS and clinical and/or radiographic PFS (referred to hereafter as PFS). Overall survival was an exploratory end point. Prostate-specific antigen progression was defined as at least a 25% increase in PSA level from nadir (and by ≥2 ng/mL), requiring confirmation at least 3 weeks later (PCWG2 criteria).23 Clinical and/or radiographic progression was defined as symptomatic progression (worsening disease-related symptoms or new cancer-related complications), radiologic progression (on CT scan, ≥20% enlargement in sum diameter of soft-tissue target lesions [RE-CIST {Response Evaluation Criteria in Solid Tumors} criteria24]; on bone scan, ≥2 new bone lesions), or death, whichever occurred first.23 Overall survival was defined as the time to death from any cause.
Statistical Analyses
Sample size was determined on the basis of the primary end point of PSA response, assuming that 30% of men would be AR-V7 positive at baseline. In our prior study,11 enzalutamide- or abiraterone-treated patients showed a difference in PSA response rates between AR-V7–positive and AR-V7–negative patients of 61% (95% CI, 43%-80%). Because we hypothesized here that the impact of AR-V7 status would be smaller in the context of taxane-treated patients compared with enzalutamide- or abiraterone-treated patients, we sought a much smaller difference in PSA response rates such that the upper bound of the 95% CI for the difference was less than 61% (the point estimate from our previous study). Accordingly, a sample size of 36 patients produced a 2-sided 95% CI for the difference in PSA response rates between AR-V7–positive and AR-V7–negative patients with an upper bound of 60%, when the observed absolute difference is 30% (45% PSA response rate in AR-V7–negative men and 15% in AR-V7–positive men).
Clinical outcomes in taxane-treated men were compared between AR-V7–positive and AR-V7–negative patients. The PSA response rates were compared using the Fisher exact test. Time-to-event outcomes (PSA PFS, PFS, OS) were evaluated using Kaplan-Meier analysis, and survival time differences were compared using the log-rank test. Univariate and multivariable logistic regression analyses (for PSA response) and Cox regression analyses (for time-to-event end points) were used to assess the effect of AR-V7 status in predicting clinical outcomes. Because of the small sample size and limited number of events, each multivariable model included only 3 covariates (AR-V7 status, AR-FL expression levels, and prior use of abiraterone and/or enzalutamide). These 3 variables were strongly associated with clinical outcomes in our prior study of AR-V7.11
We then incorporated updated data on PSA responses, PSA PFS, PFS, and OS from our prior study of enzalutamide- or abiraterone-treated patients (n = 62) to compare the impact of AR-V7 status (ie, its ability to differentiate patients with a poor prognosis from those with a good prognosis) in the context of taxane chemotherapy vs AR-directed therapy. Specifically, we tested the interaction between AR-V7 status (positive or negative) and treatment type (taxane vs enzalutamide or abiraterone) with respect to PSA responses, PSA PFS, PFS, and OS. Univariate and multivariable Cox regression analyses were used to assess the interaction of AR-V7 status and treatment type with respect to the time-to-event outcomes; each multivariable model included 6 covariates (AR-V7 status, treatment type, AR-FL expression levels, prior use of chemotherapy, prior use of enzalutamide or abiraterone, and the interaction of AR-V7 status and treatment type).
After observing significant results from the interaction tests, we performed subgroup analyses to evaluate the efficacy of different treatment types (taxane vs abiraterone or enzalutamide) in AR-V7–positive and AR-V7–negative men separately. Univariate and multivariable Cox regression analyses were used to assess the independent effect of treatment type within each AR-V7 subgroup. Multivariable models (constructed separately for each AR-V7 subgroup) included 3 covariates: treatment type, AR-FL expression levels, and prior use of enzalutamide or abiraterone.
All statistical tests were 2-sided, and P ≤ .05 was considered significant. Statistical analyses were performed using the R software, version 2.15.1.
The clinical investigators were blinded to the AR-V7 data. The laboratory investigators were blinded to the clinical information when determining AR-V7 status. The study statisticians were the first to unblind the data, after at least 36 patients had been enrolled.
Results
Patients
From January 2013 to July 2014, we prospectively enrolled 37 CTC-positive patients; 30 received docetaxel and 7 received cabazitaxel. Forty-three patients were screened to identify 37 men with detectable CTCs (86% yield; CTC-negative patients were excluded from further analysis). At the data cutoff date (September 1, 2014), median (range) follow-up among all taxane-treated patients was 7.7 (0.7-19.0) months. Seventeen (46%) of the 37 men had detectable AR-V7 in their baseline CTC samples. In these patients, the median (range) AR-V7/AR-FL ratio was 23% (range, 3%-69%) (eFigure in the Supplement). The prevalence of AR-V7 was influenced by prior use of enzalutamide or abiraterone: in men who had not previously received enzalutamide or abiraterone, AR-V7 was detected in 2 (25%) of 8 cases; in men who had received either enzalutamide or abiraterone, AR-V7 was detected in 7 (50%) of 14 cases; and in men who had received both enzalutamide and abiraterone, AR-V7 was detected in 8 (53%) of 15 cases.
Table 1 shows baseline characteristics for the taxane-treated population as a whole, and separated by AR-V7 status. The AR-V7–positive men were more likely to have younger age, Gleason score at least 8, prior enzalutamide or abiraterone treatment, at least 6 bone metastases, higher PSA levels, higher alkaline phosphatase levels, and higher AR-FL levels (although most of these differences were not statistically significant).
Table1.
Baseline Characteristics of the 37 Taxane-Treated Patients
| Baseline Characteristic | All Patients (n = 37) |
AR-V7 Negative (n = 20) |
AR-V7 Positive (n = 17) |
P Valuea |
|---|---|---|---|---|
| Age, median (range), y | 67 (46-82) | 68 (46-82) | 64 (50-77) | .11 |
|
| ||||
| Race, No. (%)b | ||||
|
| ||||
| White | 32 (86) | 16 (80) | 16 (94) | .35 |
|
| ||||
| Nonwhite | 5 (14) | 4 (20) | 1 (6) | |
|
| ||||
| Time since diagnosis, median (range), y | 5 (1-12) | 5 (1-12) | 4 (1-11) | .60 |
|
| ||||
| Tumor stage at diagnosis, No. (%) | ||||
|
| ||||
| T1/T2 | 14 (38) | 7 (35) | 7 (41) | .75 |
|
| ||||
| T3/T4 | 23 (62) | 13 (65) | 10 (59) | |
|
| ||||
| Gleason sum at diagnosis, No. (%) | ||||
|
| ||||
| ≤7 | 6 (17) | 4 (22) | 2 (12) | .66 |
|
| ||||
| ≥8 | 29 (83) | 14 (78) | 15 (88) | |
|
| ||||
| Type of local treatment, No. (%) | ||||
|
| ||||
| Surgery | 14 (38) | 7 (35) | 7 (41) | .99 |
|
| ||||
| Radiation therapy | 9 (24) | 5 (25) | 4 (24) | |
|
| ||||
| None | 14 (38) | 8 (40) | 6 (35) | |
|
| ||||
| Current taxane therapy, No. (%) | ||||
|
| ||||
| Docetaxel | 30 (81) | 15 (75) | 15 (88) | .42 |
|
| ||||
| Cabazitaxel | 7 (19) | 5 (25) | 2 (12) | |
|
| ||||
| No. of prior hormonal therapies, median (range) | 4 (2-7) | 4 (2-7) | 4 (2-6) | .92 |
|
| ||||
| Prior use of abiraterone, No. (%) | ||||
|
| ||||
| Yes | 29 (78) | 14 (70) | 15 (88) | .25 |
|
| ||||
| No | 8 (22) | 6 (30) | 2 (12) | |
|
| ||||
| Prior use of enzalutamide, No. (%) | ||||
|
| ||||
| Yes | 15 (41) | 7 (35) | 8 (47) | .52 |
|
| ||||
| No | 22 (59) | 13 (65) | 9 (53) | |
|
| ||||
| Prior use of docetaxel, No. (%) | ||||
|
| ||||
| Yes | 7 (19) | 5 (25) | 2 (12) | .42 |
|
| ||||
| No | 30 (81) | 15 (75) | 15 (88) | |
|
| ||||
| Presence of bone metastases, No. (%) | ||||
|
| ||||
| Yes | 35 (95) | 18 (90) | 17 (100) | .49 |
|
| ||||
| No | 2 (5) | 2 (10) | 0 | |
|
| ||||
| No. of bone metastases, No. (%) | ||||
|
| ||||
| ≤5 | 6 (16) | 5 (25) | 1 (6) | .19 |
|
| ||||
| ≥6 | 31 (84) | 15 (75) | 16 (94) | |
|
| ||||
| Presence of visceral metastases, No. (%) | ||||
|
| ||||
| Yes | 13 (35) | 7 (35) | 6 (35) | .99 |
|
| ||||
| No | 24 (65) | 13 (65) | 11 (65) | |
|
| ||||
| ECOG performance status, No. (%) | ||||
|
| ||||
| 0 | 20 (54) | 8 (40) | 12 (71) | .10 |
|
| ||||
| 1 or 2 | 17 (46) | 12 (60) | 5 (29) | |
|
| ||||
| Baseline PSA level, median (range), ng/mL | 126 (0.1-2270) | 102 (5-534) | 189 (0.1-2270) | .07 |
|
| ||||
| Baseline alkaline phosphatase level, median (range), U/L |
161 (53-1243) | 111 (53-930) | 291 (53-1243) | .07 |
|
| ||||
| Baseline AR-FL level, copy number, median (range) |
16 (0-4567) | 4 (0-55) | 88 (4-4567) | <.01 |
Abbreviations: AR-FL, full-length androgen receptor; AR-V7, androgen receptor splice variant 7; ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen.
SI conversion factors: To convert PSA level to micrograms per liter, multiply by 1.0; to convert alkaline phosphatase to microkatals per liter, multiply by 0.017.
P values are based on Fisher exact test and Wilcoxon Mann-Whitney test for categorical and continuous variables, respectively.
Race was self-reported by participants (although options were defined by the investigators).
Table 2 compares baseline characteristics of the 37 taxane-treated patients and the 62 enzalutamide- or abiraterone-treated patients incorporated from our prior study.11 These 62 men were emolled between December 2012 and September 2013, and their clinical outcomes were updated using the cutoff date of September 1, 2014. In this updated analysis, median (range) follow-up among all enzalutamide- or abiraterone-treated patients was 13.0 (1.4-19.8) months. Eighteen (29%) of these 62 men had detectable AR-V7 at baseline. Compared with taxane-treated patients, enzalutamide- or abiraterone-treated men were more likely to have Gleason scores less than or equal to 7, fewer prior hormonal therapies, no more than 5 bone metastases, Eastern Cooperative Oncology Group (ECOG) performance status of 0, lower PSA levels, lower alkaline phosphatase levels, and lower AR-FL levels (although not all of these differences were statistically significant).
Table 2.
Comparison of Baseline Characteristics of the 37 Taxane-Treated Patients and the 62 Enzalutamide- or abiraterone-treated Patients
| Baseline Characteristic | Taxane-Treated Patients (n = 37) |
Enzalutamide- or abiraterone-treated Patients (n = 62) |
P Valuea |
|---|---|---|---|
| Age, median (range), y | 67 (46-82) | 69 (48-84) | .32 |
|
| |||
| Race, No. (%)b | |||
|
| |||
| White | 32 (86) | 51 (82) | .78 |
|
| |||
| Nonwhite | 5 (14) | 11 (18) | |
|
| |||
| Time since diagnosis, median (range), y | 5 (1-12) | 5 (1-21) | .59 |
|
| |||
| Tumor stage at diagnosis, No. (%) | |||
|
| |||
| T1/T2 | 14 (38) | 29 (47) | .41 |
|
| |||
| T3/T4 | 23 (62) | 33 (53) | |
|
| |||
| Gleason sum at diagnosis, No. (%) | |||
|
| |||
| ≤7 | 6 (17) | 20 (33) | .10 |
|
| |||
| ≥8 | 29 (83) | 40 (67) | |
|
| |||
| Type of local treatment, No. (%) | |||
|
| |||
| Surgery | 14 (38) | 27 (44) | .68 |
|
| |||
| Radiation therapy | 9 (24) | 17 (27) | |
|
| |||
| None | 14 (38) | 18 (29) | |
|
| |||
| No. of prior hormonal therapies, median (range) |
4 (2-7) | 3 (2-6) | <.01 |
|
| |||
| Prior use of enzalutamide or abiraterone, No. (%) |
|||
|
| |||
| Yes | 29 (78) | 24 (39) | <.01 |
|
| |||
| No | 8 (22) | 38 (61) | |
|
| |||
| Prior use of docetaxel, No. (%) | |||
|
| |||
| Yes | 7 (19) | 25 (40) | .04 |
|
| |||
| No | 30 (81) | 37 (60) | |
|
| |||
| Presence of bone metastases, No. (%) | |||
|
| |||
| Yes | 35 (95) | 52 (84) | .20 |
|
| |||
| No | 2 (5) | 10 (16) | |
|
| |||
| No. of bone metastases, No. (%) | |||
|
| |||
| ≤5 | 6 (16) | 37 (60) | <.01 |
|
| |||
| ≥6 | 31 (84) | 25 (40) | |
|
| |||
| Presence of visceral metastases, No. (%) | |||
|
| |||
| Yes | 13 (35) | 18 (29) | .66 |
|
| |||
| No | 24 (65) | 44 (71) | |
|
| |||
| ECOG performance status, No. (%) | |||
|
| |||
| 0 | 20 (54) | 47 (76) | .03 |
|
| |||
| 1 or 2 | 17 (46) | 15 (24) | |
|
| |||
| Baseline PSA level, median (range), ng/mL | 126 (0.1-2270) | 42 (2.2-3204) | <.01 |
|
| |||
| Baseline alkaline phosphatase level, median (range), U/L |
161 (53-1243) | 111 (58-1348) | .04 |
|
| |||
| Baseline AR-FL level, copy number, median (range) |
16 (0-4567) | 7 (0-734) | .05 |
Abbreviations: AR-FL, full-length androgen receptor; ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen.
SI conversion factors: To convert PSA level to micrograms per liter, multiply by 1.0; to convert alkaline phosphatase to microkatals per liter, multiply by 0.017.
P values are based on Fisher exact test and Wilcoxon Mann-Whitney test for categorical and continuous variables, respectively.
Race was self-reported by participants (although options were defined by the investigators).
Clinical Outcomes in Taxane-Treated Patients According to AR-V7 Status
PSA Responses
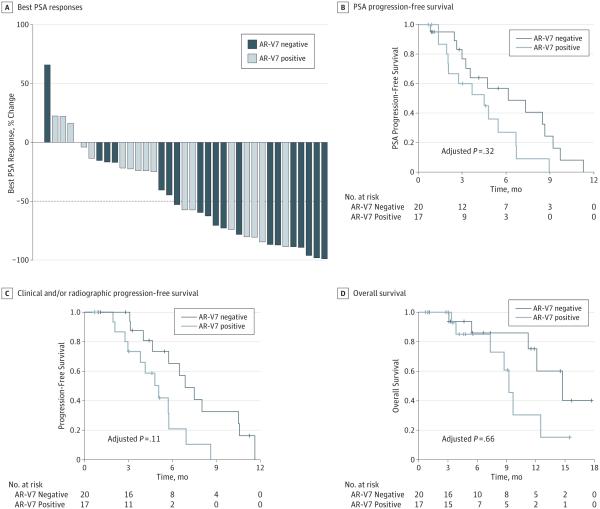
The overall proportion of patients who achieved a PSA response during taxane treatment was 54% (20 of 37 men; 95% CI, 37%-71%), and there was no significant difference according to AR-V7 status. The PSA response rates were 41% (7 of 17 men; 95% CI, 18%-67%) in AR-V7–positive patients and 65% (13 of 20 men; 95% CI, 41%-85%) in AR-V7–negative patients, a non-significant difference of 24% (P = .19; 95% CI for the difference, −13% to 60%). Best PSA responses according to AR-V7 status are depicted in Figure 1A. In multivariable logistic regression modeling, AR-V7 status remained nonpredictive for PSA response (odds ratio, 0.39 [95% CI, 0.06-2.32]; P = .31) after adjusting for AR-FL expression and previous use of enzalutamide or abiraterone.
Figure 1. Clinical Outcomes in 37 Taxane-Treated Patients, According to Circulating Tumor Cell Androgen Receptor Splice Variant 7 (AR-V7) Status.
A, Waterfall plot depicting best prostate-specific antigen (PSA) responses, according to AR-V7 status. The dotted line shows the threshold for defining a PSA response (≥50% PSA reduction from baseline). Among patients who achieved a PSA response, 35% (7 of 20 men) were AR-V7 positive, whereas in those patients without a PSA response, 59% (12 of 17 men) were AR-V7 positive.
B, Kaplan-Meier curves showing PSA progression-free survival in 37 taxane-treated patients, according to AR-V7 status. C, Kaplan-Meier curves showing clinical and/or radiographic progression-free survival in taxane-treated patients, according to AR-V7 status. D, Kaplan-Meier curves showing overall survival in taxane-treated patients, according to AR-V7 status.
PSA PFS
Prostate-specific antigen PFS did not differ significantly according to AR-V7 status. Median PSA PFS was 4.5 months in AR-V7–positive men and 6.2 months in AR-V7–negative men (hazard ratio [HR], 2.1 [95% CI, 0.9-4.9]; P = .06). In a multivariable Cox model adjusting for AR-FL expression and prior enzalutamide or abiraterone use, AR-V7 status remained non-significant in its ability to predict PSA PFS (HR, 1.7 [95% CI, 0.6-5.0]; P = .32) (Figure 1B); AR-FL levels (HR, 1.0 [95% CI, 0.9-1.2]) and previous enzalutamide or abiraterone use (HR, 1.4 [95% CI, 0.4-4.2]) were also nonpredictive of PSA PFS in this multivariable analysis.
PFS
Clinical and/or radiographic PFS also did not differ significantly depending on AR-V7 status. Median PFS was 5.1 months in AR-V7–positive men and 6.9 months in AR-V7–negative men (HR, 2.8 [95% CI, 1.2-6.9]; P = .02). Although this difference appeared significant, in a multivariable Cox model adjusting for AR-FL expression and prior enzalutamide or abiraterone use, AR-V7 status lost its ability to predict PFS (HR, 2.7 [95% CI, 0.8-8.8]; P = .11) (Figure 1C); AR-FL levels (HR, 1.0 [95% CI, 0.9-1.1]) and previous enzalutamide or abiraterone use (HR, 1.7 [95% CI, 0.5-6.2]) were also nonpredictive of PFS.
OS (Exploratory)
Overall survival also did not differ significantly according to AR-V7 status. Median OS was 9.2 months in AR-V7–positive men and 14.7 months in AR-V7–negative men (HR, 2.5 [95% CI, 0.8-8.1]; P = .11). In a multivariable Cox model adjusting for AR-FL expression, AR-V7 status remained nonsignificant in its ability to predict OS (HR, 0.7 [95% CI, 0.1-3.8]; P = .66) (Figure 1D); AR-FL levels were also nonpredictive of OS (HR, 1.3 [95% CI, 0.9-1.8]).
Differential Effect of AR-V7 in Men Treated With Taxanes vs AR-Directed Therapies
PSA Responses
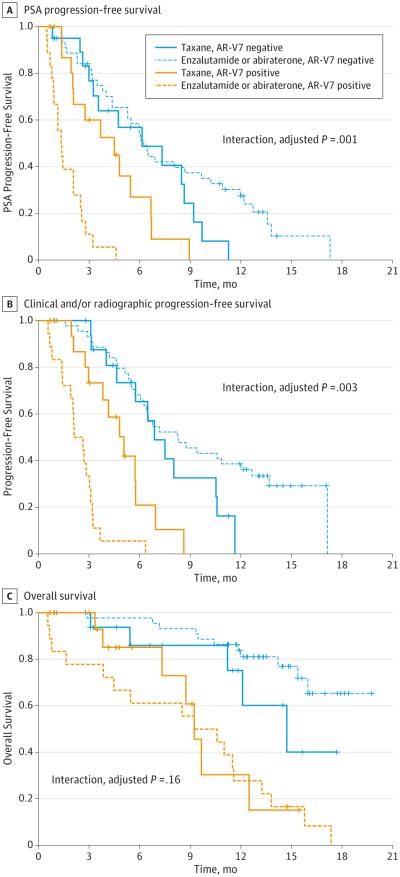
A significant interaction between AR-V7 status and treatment type was observed in the unadjusted linear model (P = .002). In an adjusted model also accounting for AR-FL levels, prior chemotherapy use, and prior enzalutamide or abiraterone use, the interaction remained significant (P = .006).
PSA PFS
A significant interaction between AR-V7 status and treatment type was observed in the unadjusted Cox model (P < .001). In an adjusted model also accounting for AR-FL levels, prior chemotherapy, and prior use of enzalutamide or abiraterone, the interaction remained significant (P = .001) (Figure 2A).
Figure 2. Interaction Between Androgen Receptor Splice Variant 7 (AR-V7) Status and Treatment Type, After Including Data From Enzalutamide- or Abiraterone-Treated Patients.
Kaplan-Meier analysis in 37 taxane-treated patients and 62 enzalutamide- or abiraterone-treated patients, separated according to AR-V7 status.
A, Prostate-specific antigen (PSA) progression-free survival. A positive interaction between AR-V7 status and treatment type was observed (adjusted P = .001). B, Clinical and/or radiographic progression-free survival. A positive interaction between AR-V7 status and treatment type was observed (adjusted P = .003). C, Kaplan-Meier analysis showing overall survival in taxane-treated patients and enzalutamide- or abiraterone-treated patients, according to AR-V7 status. A significant interaction between AR-V7 status and treatment type was not observed (adjusted P = .16).
PFS
A significant interaction between AR-V7 status and treatment type was observed in the unadjusted Cox model (P < .001). In the adjusted model, the interaction remained significant (P = .003) (Figure 2B).
OS (Exploratory)
A significant interaction between AR-V7 status and treatment type was not observed either in the unadjusted Cox model (P = .18) or the adjusted model (P = .16) (Figure 2C).
Clinical Outcomes With Taxanes vs AR-Directed Therapies According to AR-V7 Status
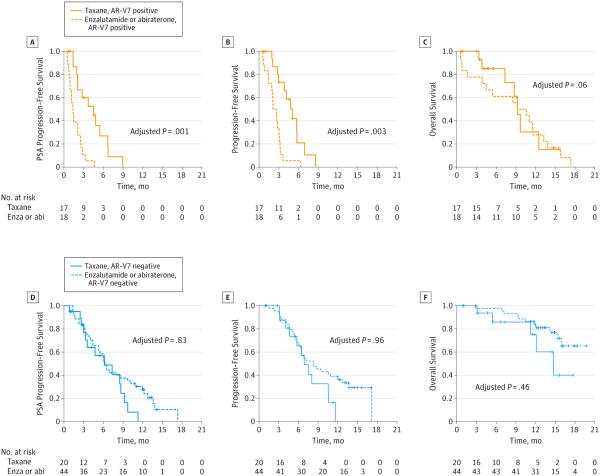
AR-V7–Positive Patients
Treatment with taxanes appeared superior to AR-directed therapy in AR-V7–positive men. The PSA responses were 41% (7 of 17) in taxane-treated patients and 0% (0 of 18) in enzalutamide- or abiraterone-treated patients (P < .001). In a multivariable linear model adjusting for AR-FL level, prior chemotherapy, and prior enzalutamide or abiraterone, treatment with taxanes remained superior to enzalutamide or abiraterone (P < .001). Moreover, median PSA PFS was longer in taxane-treated men compared with enzalutamide- or abiraterone-treated men (HR, 0.22 [95% CI, 0.09-0.53]; P < .001). In a multivariable Cox model adjusting for AR-FL level and prior enzalutamide or abiraterone therapy, taxane therapy remained superior to AR-directed therapy (HR, 0.19 [95% CI, 0.07-0.52]; P = .001) (Figure 3A). Similarly, median PFS was longer in taxane-treated compared with enzalutamide- or abiraterone-treated men (HR, 0.26 [95% CI, 0.11-0.59]; P = .001). In a multivariable Cox model adjusting for AR-FL level and prior enzalutamide or abiraterone therapy, taxane therapy remained superior (HR, 0.21 [95% CI, 0.07-0.59]; P = .003) (Figure 3B). Finally, median OS (exploratory) was numerically superior in taxane-treated compared with enzalutamide- or abiraterone-treated patients (HR, 0.83 [95% CI, 0.34-2.00]; P = .76). In a multivariable Cox model adjusting for AR-FL level and prior use of enzalutamide or abiraterone, there was numerically superior survival with taxane therapy (HR, 0.28 [95% CI, 0.07-1.00]; P = .06) (Figure 3C).
Figure 3. Clinical Outcomes With Taxanes vs Androgen Receptor–Directed Therapies for Androgen Receptor Splice Variant 7 (AR-V7)–Positive and AR-V7–Negative Patients.
Kaplan-Meier analyses comparing taxane-treated patients vs enzalutamide (enza)- or abiraterone (abi)-treated patients. A, Prostate-specific antigen progression-free survival, focusing only on AR-V7–positive men. B, Clinical and/or radiographic progression-free survival, focusing only on AR-V7–positive men. C, Overall survival, focusing only on AR-V7–positive men. D, Prostate-specific antigen progression-free survival, focusing only on AR-V7–negative men. E, Clinical and/or radiographic progression-free survival, focusing only on AR-V7–negative men. F, Overall survival, focusing only on AR-V7–negative men.
AR-V7–Negative Patients
There were no significant differences between taxane treatment and AR-directed therapy with respect to any clinical outcomes in AR-V7–negative men. Prostate-specific antigen responses were 65% (13 of 20) in taxane-treated patients and 64% (28 of 44) in enzalutamide- or abiraterone-treated patients (P = .60); this difference remained nonsignificant after adjusting for AR-FL level, prior chemotherapy, and prior enzalutamide or abiraterone treatment in a multivariable linear model (P = .36). Median PSA PFS was not significantly different in taxane-treated patients compared with enzalutamide- or abiraterone-treated patients (HR, 1.61 [95% CI, 0.84-3.06]; P = .15), even after adjusting for AR-FL level and prior enzalutamide or abiraterone treatment in the multivariable Cox model (HR, 1.09 [95% CI, 0.51-2.31]; P = .83) (Figure 3D). Similarly, median PFS was not significantly different in taxane-treated compared with enzalutamide- or abiraterone-treated patients (HR, 1.68 [95% CI, 0.84-3.33]; P = .14), even after adjusting for AR-FL and prior enzalutamide or abiraterone treatment in multivariable Cox analysis (HR, 1.02 [95% CI, 0.46-2.25]; P = .96) (Figure 3E). Finally, median OS (exploratory) was not significantly different between the 2 treatment groups, either in the univariate (HR, 2.26 [95% CI, 0.78-6.62]; P = .13 or the multivariable (HR, 1.55 [95% CI, 0.49-4.95]; P = .46) analyses (Figure 3F).
AR-V7 Conversions at Taxane Progression
Twenty-one taxane-treated patients had paired CTC samples collected at baseline and at the time of progression that were evaluable for AR-V7. Among men with initially undetectable AR-V7 (n = 9), 1 patient (11%) subsequently converted to AR-V7 positive during the course of taxane treatment whereas 8 patients (89%) remained AR-V7 negative at progression. Conversely, among men with detectable AR-V7 at baseline (n = 12), 7 patients (58%) converted to AR-V7 negative during taxane therapy whereas 5 patients (42%) remained AR-V7 positive at progression. The clinical significance of these conversions in AR-V7 status is currently unknown.
Discussion
Although there are multiple available therapies for men with metastatic CRPC, there are currently no molecular biomarkers to help guide optimal treatment choices in these patients. We have previously shown that detection of AR-V7 is associated with primary resistance to abiraterone and enzalutamide therapy, as manifested by inferior PSA responses, shorter PFS, and shorter OS.11 Here we show that men with detectable AR-V7 retain sensitivity to taxane chemotherapies, that the impact of AR-V7 is greater in the context of AR-directed therapies than with chemotherapies, and that taxanes may have superior efficacy to enzalutamide or abiraterone in AR-V7–positive men (but not in AR-V7–negative men). The present study represents the first prospective analysis of AR-V7 in patients receiving taxane chemotherapy, and the totality of our data suggests that AR-V7 may represent a treatment selection marker in CRPC.
Although the principal mechanism of action of taxane agents is the disruption of microtubules, inducing mitotic arrest, it is increasingly understood that taxanes may also mediate their antitumor effects in CRPC by disrupting cytoplasmic-to-nuclear trafficking of AR along the microtubule network,17-20 while other mechanisms have also been postulated.25,26 Therefore, some degree of cross-resistance has been suggested between AR-targeting therapies and taxane chemotherapies, although this cross-resistance may be less substantial with cabazitaxel than with docetaxel.27 Recently, work on a particular mouse model of CRPC has also suggested that certain AR-Vs may be associated with sensitivity to taxanes whereas others may mediate taxane resistance.22 To this end, AR-V7 was shown to result in taxane resistance in at least 1 preclinical model, due to deletion of the AR hinge region that is thought to be necessary for microtubule binding.22 However, our clinical data do not recapitulate the observations from this mouse model. In fact, we show here that in AR-V7–positive patients, PSA response rates to taxanes are 41% and median PFS is 5.1 months. Although clinical outcomes to taxanes may appear inferior in AR-V7–positive compared with AR-V7–negative men, these differences were not statistically significant after multivariable adjustments. More importantly, we demonstrate that AR-V7 detection is not associated with primary resistance to taxane agents (as seen with abiraterone and enzalutamide11).
An important observation from our present study is the suggestion that taxane therapy may be more efficacious than AR-directed therapy for men with AR-V7–positive CRPC. Conversely, clinical outcomes did not seem to differ significantly on the basis of the type of therapy used among AR-V7–negative patients. If these results are confirmed by additional prospective biomarker-stratified clinical trials, this observation might suggest that AR-V7–positive men may fare better receiving taxanes than AR-targeting therapies, whereas in AR-V7–negative men both treatment approaches might be reasonable. However, our study has important limitations. Because of the small sample size, we were unable to perform a comprehensive multivariable analysis to determine the independent contribution of AR-V7 status to prognosis, and we were not able to define subpopulations in which the utility of the biomarker may be greatest. It remains possible that AR-V7 is simply a marker of more advanced disease or higher disease burden. Second, the comparison of clinical outcomes between taxane-treated and enzalutamide- or abiraterone-treated patients is confounded by the fact that treatment selection was not randomly assigned and that baseline patient characteristics (including numbers and types of prior therapies received) were different in the 2 cohorts. Confirmation of these findings will require larger biomarker-driven studies randomizing patients to taxane chemotherapy vs AR-directed therapy. To this end, prospective validation of the AR-V7 biomarker will be pursued in the PRIMCAB study (NCT02379390), a multicenter randomized phase 2 trial of abiraterone or enzalutamide vs cabazitaxel therapy in men with primary resistance to prior enzalutamide or abiraterone therapy.
Finally, an intriguing finding from our study was the fact that certain patients with detectable AR-V7 at baseline converted to AR-V7–negative status during the course of taxane therapy. Notably, in our prior analysis of AR-V7 in enzalutamide- or abiraterone-treated patients, all men with detectable AR-V7 at baseline remained AR-V7 positive throughout treatment with abiraterone and enzalutamide.11 Biologically, a conversion from AR-V7–positive to AR-V7–negative status might imply decreased selection pressure on the AR axis exerted by taxanes, allowing a resumption of canonical AR signaling and a lack of requirement for aberrant AR-V–mediated signaling. An alternative hypothesis is that effective taxane therapy may have decreased the burden of CTCs, thereby making it more difficult to detect AR-V7 present in low abundance. The clinical significance of these AR-V7 conversions remains unclear and is the subject of ongoing investigations.
Conclusions
Our findings suggest that detection of AR-V7 in CTCs from men with CRPC is not associated with primary resistance to taxane chemotherapy and that AR-V7–positive patients may respond better to taxanes than to AR-targeting drugs. If confirmed in larger-scale clinical trials, AR-V7 status could emerge as the first treatment selection biomarker for CRPC.
Supplementary Material
At a Glance.
Androgen receptor splice variant 7 (AR-V7) is associated with resistance to enzalutamide and abiraterone, but its relevance in the context of taxane chemotherapy is unknown.
Detection of AR-V7 in circulating tumor cells from men with metastatic castration-resistant prostate cancer is not associated with primary resistance to taxane chemotherapy; AR-V7–positive patients may retain sensitivity to taxanes.
In AR-V7–positive men, taxanes may be more efficacious than AR-directed agents (enzalutamide and abiraterone).
In AR-V7–negative men, taxanes appear to have comparable efficacy to AR-directed agents.
Androgen receptor splice variant 7 may serve as a treatment selection marker in metastatic castration-resistant prostate cancer.
Acknowledgments
Funding/Support: This research was supported by the Prostate Cancer Foundation, the Department of Defense Prostate Cancer Research Program grant W81XWH-12-1-0605, the Patrick C. Walsh Fund, the Johns Hopkins Prostate SPORE grant P50 CA058236, and National Institutes of Health grant P30 CA006973.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank the patients and their families who participated in this study.
Footnotes
Supplemental content at jamaoncology.com
Author Contributions: Drs Antonarakis and Luo had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Antonarakis, Luber, Wang, Carducci, Eisenberger, Luo.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Antonarakis, Luber, Chen, Nakazawa, Carducci, Luo.
Critical revision of the manuscript for important intellectual content: Antonarakis, Lu, Wang, Nadal, Paller, Denmeade, Carducci, Eisenberger, Luo.
Statistical analysis: Antonarakis, Luber, Wang, Luo.
Obtained funding: Antonarakis, Carducci, Luo.
Administrative, technical, or material support: Antonarakis, Lu, Chen, Nakazawa, Paller, Denmeade, Carducci, Luo.
Study supervision: Antonarakis, Eisenberger, Luo.
Conflict of Interest Disclosures: Dr Antonarakis has served as a paid consultant/advisor for Janssen, Astellas, Sanofi, Dendreon, Essa, and Medivation; received research funding from Janssen, Johnson & Johnson, Sanofi, Dendreon, Exelixis, Genentech, Novartis, and Tokai; and is a co-inventor of a technology that has been licensed to Tokai. Dr Luo has served as a paid consultant/advisor for Astellas, has been a speaker for Sanofi and Gilead, has received research funding from Sanofi and Mirati, and is also a co-inventor of a technology that has been licensed to Tokai. Relevant disclosures have been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies. No other disclosures are reported.
REFERENCES
- 1.Basch E, Loblaw DA, Oliver TK, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014;32(30):3436–3448. doi: 10.1200/JCO.2013.54.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bono JS, Logothetis CJ, Molina A, et al. COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Fizazi K, Saad F, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Oudard S, Ozguroglu M, et al. TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, et al. IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 7.Parker C, Nilsson S, Heinrich D, et al. ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 8.Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8(1):12–23. doi: 10.1038/nrclinonc.2010.136. [DOI] [PubMed] [Google Scholar]
- 9.Karantanos T, Evans CP, Tombal B, Thompson TC, Montironi R, Isaacs WB. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015;67(3):470–479. doi: 10.1016/j.eururo.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong AJ, Eisenberger MA, Halabi S, et al. Biomarkers in the management and treatment of men with metastatic castration-resistant prostate cancer. Eur Urol. 2012;61(3):549–559. doi: 10.1016/j.eururo.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72(14):3457–3462. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17(18):5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73(2):483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan L, Chen S, Wang Y, et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res. 2009;69(21):8386–8394. doi: 10.1158/0008-5472.CAN-09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70(20):7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darshan MS, Loftus MS, Thadani-Mulero M, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71(18):6019–6029. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thadani-Mulero M, Nanus DM, Giannakakou P. Androgen receptor on the move: boarding the microtubule expressway to the nucleus. Cancer Res. 2012;72(18):4611–4615. doi: 10.1158/0008-5472.CAN-12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby BJ, Jodari M, Loftus MS, et al. Functional characterization of circulating tumor cells with a prostate-cancer-specific microfluidic device. PLoS One. 2012;7(4):e35976. doi: 10.1371/journal.pone.0035976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thadani-Mulero M, Portella L, Sun S, et al. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. Cancer Res. 2014;74(8):2270–2282. doi: 10.1158/0008-5472.CAN-13-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher HI, Halabi S, Tannock I, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.de Leeuw R, Berman-Booty LD, Schiewer MJ, et al. Novel actions of next-generation taxanes benefit advanced stages of prostate cancer. Clin Cancer Res. 2015;21(4):795–807. doi: 10.1158/1078-0432.CCR-14-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plymate SR, Bhatt RS, Balk SP. Taxane resistance in prostate cancer mediated by AR-independent GATA2 regulation of IGF2. Cancer Cell. 2015;27(2):158–159. doi: 10.1016/j.ccell.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 27.van Soest RJ, de Morrée ES, Kweldam CF, et al. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur Urol. 2015;67(6):981–985. doi: 10.1016/j.eururo.2014.11.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.