Abstract
Background
New therapies are being explored as therapeutic options for men with biochemically recurrent prostate cancer (BRPC) who wish to defer androgen deprivation therapy. MPX is pulverized muscadine grape (Vitis rotifundolia) skin that contains ellagic acid, quercetin, and resveratrol and demonstrates preclinical activity against prostate cancer cells in vitro.
Methods
In the phase I portion of this phase I/II study non-metastatic BRPC patients were assigned to increasing doses of MPX (Muscadine Naturals Inc., Clemmons, NC) in cohorts of 2 patients, with 6 patients at the highest dose, using a modified continual reassessment method. Initial dose selection was based on preclinical data showing the equivalent of 500 to 4,000 mg of MPX to be safe in mouse models. The primary end point was the recommended phase II dosing regimen.
Results
The cohort (n=14, 71% Caucasian, 29% black) had a median follow-up of 19.2 (6.2 – 29.7) months, median age 61 years, and median Gleason of 7. Four patients had possibly related gastrointestinal symptoms, including grade 1 flatulence, grade 1 soft stools, and grade 1 eructation. No other related adverse events were reported and one patient reported improvement of chronic constipation. Six of 14 patients came off study for disease progression (5 metastatic, 1 rising PSA) after exposure for a median of 15 months. One patient came off for myasthenia gravis that was unrelated to treatment. Seven patients remain on study. The lack of dose limiting toxicities led to the selection of 4000 mg/d as the highest dose for further study. Median within-patient PSADT increased by 5.3 months (non-significant, p = 0.17). No patients experienced a maintained decline in serum PSA from baseline.
Conclusions
These data suggest that 4000 mg of MPX is safe, and exploratory review of a lengthening in PSADT of a median of 5.3 months supports further exploration of MPX. Both low dose (500 mg) and high dose (4,000 mg) MPX are being further investigated in a randomized, multicenter, placebo-controlled, dose evaluating phase II trial.
Keywords: prostate cancer, rising PSA, muscadine grape skin, PSADT, complementary therapy
INTRODUCTION
Prostate carcinoma is the most common solid tumor among men. In 2015 an estimated 220,800 new cases of prostate cancer will be diagnosed and 27,540 men will die of the disease [1]. Although local therapy is curative in the majority of patients, 33 to 50% of patients who undergo prostatectomy or radiation treatment as primary therapy for localized prostate cancer (PCA) experience biochemical recurrence (BCR) of the disease [2, 3]. Rising serum prostate specific antigen (PSA) levels are the earliest indication of recurrence, and median time from PSA recurrence to radiologic evidence of metastatic disease ranges from 7.6 to 14.0 years [4]. Treatment options for PCA patients with PSA recurrence after local therapy, without evidence of metastatic disease, include salvage local radiation in the proper setting, androgen deprivation therapy, or observation. These patients often have a desire to avoid the adverse effects of androgen deprivation therapy and may be ideal candidates for clinical trials of novel agents with the goal of delaying development of metastatic disease [5, 6].
Preclinical studies of pulverized muscadine grape skin offer evidence that it may extend the time between biochemical recurrence and development of metastatic disease [7, 8]. Muscadine grapes are indigenous to the southeastern United States and have been cultivated continuously since the 16th century [9]. Muscadine grape skin is a source of polyphenols such as ellagic acid, quercetin, and resveratrol, that have demonstrated antioxidant and anticancer activity in preclinical studies [10]. The principal metabolite of ellagic acid, urolithin-A [8], is preferentially absorbed by the prostate [11] and inhibits the protein complex NF-κB, potentially leading to increased rates of apoptosis and decreases in cancer cell proliferation [12]. Quercetin is a flavonoid that has been shown to inhibit growth in many different cancer cell lines in vitro and in vivo [13]. Quercetin inhibits expression of oncogenes and upregulates expression of tumor suppression genes in aggressive PC-3 and DU-145 prostate cancer cells. Quercetin also inhibits expression of genes controlling G1, S, G2, and M cell cycle phases [14]. Quercetin down-regulates oncogene expression, including c-myc and K-ras [15], and induces wildtype p53 [16]. In vitro synergistic effects were discovered when quercetin was combined with resveratrol, another polyphenol found in MPX. The effect of the combination on mitochondrial cytochrome c release and caspase-3 activity was greater than the expected additive response, supporting earlier data showing that these agents act on different pathways, and also demonstrating interactions between the pathways that may result in synergistic interactions [17]. Randomized phase II trials of other natural products rich in polyphenols showed encouraging results, meeting their primary endpoints [18–21].
In this study, we evaluated the safety and tolerability of MPX and determined the recommended phase II dosing in prostate cancer patients with rising PSA following local therapy. We measured ellagic acid, quercetin, and resveratrol pharmacokinetics to explore the relationships among serum drug concentrations and dosage. Although not an endpoint of this study, we also report preliminary antitumor activity of MPX, as measured by changes in PSA doubling time (PSADT). Notably, it has been shown that the slowing of PSADT by non-hormonal agents in patients with BRPC is associated with improved metastasis-free survival and overall survival [22, 23].
MATERIALS AND METHODS
Study Population
This is a phase I/II study with study participants in the phase I portion recruited at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins between October 2011 and August 2012. Eligible participants were ≥18 years of age, had ≥6 months life expectancy, and had histologically confirmed prostate adenocarcinoma, biochemical recurrence following definitive therapy (surgery, surgery with radiation therapy, cryotherapy, radiation therapy or brachytherapy) for the primary prostate cancer and no radiographic evidence of metastases. Biochemical recurrence was defined as a rising PSA over a minimum of 3 time points, at least 21 days apart, within the 12 months prior to study enrollment. Participants had to have baseline level of PSA >0.4 ng/mL following surgery and following multiple treatment modalities (e.g., surgery + radiation, radiation + cryotherapy, etc.). A rise by 2 ng/mL or more above the nadir PSA was used to define biochemical failure after radiation therapy with or without hormonal therapy per Prostate Cancer Working Group 2 criteria [24]. Men with negative PSADT (declining PSA values) were excluded as were men who received any therapies that modulate testosterone levels (e.g., androgen ablative/anti-androgen therapy, 5 alpha reductase inhibitors) during the 6 months prior to study, or who had prior or concomitant treatment with experimental drugs, high dose steroids, or any other cancer treatment within 4 weeks prior to the first dose of the study product. In addition men were excluded if they had ECOG performance status > 2, had testosterone levels <150 ng/dL at screening, or had consumed any muscadine grape-derived products over the past 2 months. Men who had leukocytes <3,000/mcL or platelets <100,000/mcL were also excluded. Participants agreed to abstain from commercially available muscadine grape products while on study and to maintain the same dosing of any other dietary supplements they had been consuming during the 2 months prior to study initiation.
Study Design
The primary objectives of the phase I portion of this phase I/II study were to evaluate the safety and tolerability of MPX and to determine dosing levels for the phase II portion of the study in prostate cancer patients with rising PSA following definitive local therapy. Although not an endpoint of this study, preliminary antitumor activity of MPX, as measured by changes in PSADT, was also examined.
MPX is a commercial product manufactured by Muscadine Naturals Inc. in Warsaw, North Carolina. In preparing the drug MPX, the skin of Vitis rotundifolia (muscadine grapes) of the Noble cultivar is dried, pulverized and packed in 500 mg capsules. Each capsule contained polyphenols including approximately 1.2 mg of ellagic acid, 9.2 μg of quercetin, and 4.4 μg of trans resveratrol [7, 8].
Treatment cycles consisted of once daily oral dosing of MPX on days 1 through 28. Patients were seen in clinic weekly during the first month and every 3 months thereafter. Adherence was verified at each study visit by reviewing drug diaries and counting remaining pills. Changes in concomitant medications and supplements were also recorded at each study visit.
Employing a modified continual reassessment method (mCRM) with 2 patients per cohort, dose escalation continued from 500 mg/d through 1000 mg/d, 2000 mg/d, and 3000 mg/d up to the maximum dose of 4000 mg/d and that cohort was expanded to 6 patients for a total of 14 patients. The 6 patients in the 4000 mg/d cohort participated in pharmacokinetic (PK) studies. Prior to the first dose of MPX, patients fasted for 8 hours before and 1 hour after drug administration to enable effective PK measurement. For all other doses, MPX was to be taken 1 hour prior to eating or 2 hours after eating, to avoid potential food effects.
The starting dose and an upper limit dosing of 4000 mg/d was predetermined after converting the No Observed Adverse Effect Level (NOAEL) to a Human Equivalent Dose based on the maximum NOAEL doses explored in preclinical studies conducted at NIH (unpublished animal studies included in the Investigational New Drug Application, #109605) [25]. Additionally, there were concerns about adherence when patients are asked to consume more than 8 capsules per day and thus the 4000 mg/d dose level (8 capsules) was selected as the highest dose level in the study, even if patients at that dose level did not develop dose limiting toxicities (DLTs).
The Johns Hopkins Institutional Review Board approved the study and all patients provided written informed consent. The manufacturer of MPX provided descriptive information about the manufacturing process for the Investigational New Drug application, but played no role in the study design or in the collection, analysis or interpretation of the data.
Toxicity and Response
Toxicities were assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE v4.0). A DLT was defined as any grade 3 or higher toxicity thought by the investigators to be related to MPX. In the event of MPX-related grade 3 toxicities, dose reductions were allowed by protocol. However, no related grade 3 or 4 toxicities were experienced. Toxicity assessment was conducted at weekly clinic visits during the first 4 weeks and every 3 months thereafter until use of the drug was discontinued. Toxicity assessments were recorded on protocol-specific case report forms and were based on physical examination by the treating physician or nurse during clinic visits, complete blood cell counts, and blood chemistries.
Patients were assessed using serum PSA levels and PSADT as markers of clinical benefit. Serum PSA levels were measured after 4 weeks of treatment and every 3 months thereafter until the drug was discontinued. Patients were allowed to continue on the study drug until progression or until they wished to discontinue the drug. Progression was defined by PSA, radiographic or clinical changes. Patients who achieved an initial ≥50% decline in PSA, confirmed by a second PSA at least six weeks later, were considered PSA responders. For this group, PSA progression was defined as an increase in PSA ≥50% over the nadir after at least 6 months on study, and minimum PSA rise of 5 ng/dl. For patients whose PSA did not decrease by 50%, progression was defined as an increase in PSA value ≥50% of baseline or PSA nadir, whichever was lowest, after at least 6 months of treatment, with a minimum change of 5ng/dl. Progression could also be defined as the appearance of radiographic evidence of metastatic disease or clinical progression. To assess for metastatic disease, bone scans and computed tomography scans of the abdomen and pelvis were performed every 12 months per routine clinical practice. PSADT was calculated as ln 2 (0.693)/β (slope of the linear regression fit to ln PSA vs. time in months) [26], using baseline values (3 time points within the 12 months prior to study at least 21 days apart) and at least 2 on study PSA values separated by no less than 14 days.
Pharmacokinetic Sampling, Assay, and Analysis
Pharmacokinetic analysis was performed for resveratrol, quercetin, ellagic acid, and ellagic acid’s metabolite urolithin A in patients treated with the 4000 mg/d dose of MPX. Serial blood samples for pharmacokinetic analysis were collected during pre-treatment and post-treatment on Cycle 1 Day 1 at 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 24 hours with an additional trough sample obtained on Cycle 1 Day 14. Since flavonoids are extensively metabolized to conjugated forms, glucuronide and sulfate conjugates were analyzed after a 2 hour incubation of 0.2 mL of plasma with 0.1 mL β-glucuronidase and sulfatase (Sigma G7017), which demonstrated maximal enzymatic hydrolysis. Briefly, 0.2 mL plasma or 0.3 mL β-glucuronidase and sulfatase-treated plasma was treated with saturated L-ascorbic acid prior to extraction with ethyl acetate followed by a second extraction with acetone. Analytes were separated on a Waters X-Terra MS C18 (20 × 2.1mm, 3.5μm; Milford, MA, USA) column with 0.1% formic acid in acetonitrile (solvent A) and 5mM ammonium acetate (solvent B) using a gradient flow of 0.2 mL/min. The initial mobile phase composition was 30% solvent A and increased linearly to 50% by 5 min. Between 5 and 8 minutes, the percentage of solvent A was increased linearly to 65%. Between 8 and 8.2 minutes, the percentage of solvent A was decreased linearly to 30% and maintained through the 10 minute run to re-equilibrate the column for the next injection. The column effluent was monitored using an AB Sciex 5500 triple quadrapole™ 5500 mass-spectrometric detector (Applied Biosystems, Foster City, CA, USA) using electrospray ionization operating in negative mode. The spectrometer was programmed to monitor the following MRM transition 227.0 → 184.9, 301.0 → 150.9, 301.0 → 144.8, and 226.9 → 198.0 for resveratrol, quercetin, ellagic acid, and ellagic acid’s metabolite urolithin A. Plasma concentrations of resveratrol (10–2000 ng/mL), quercetin (10–2000 ng/mL), ellagic acid (20–1000 ng/mL), and ellagic acid’s metabolite urolithin A (2–400 ng/mL) were measured over the range of concentrations indicated in parentheses. The values for precision and accuracy for all components during the in-study evaluation were within 15%. Since we anticipated patients having detectable conjugated metabolites, we explored the long-term stability of spiked plasma samples. We observed more than 15% degradation in the spiked samples of quercetin and ellagic acid at approximately 7 days, but were able to document plasma stability to 41 days for resveratrol and urolithin A.
Statistics
This study used a modified continual reassessment method (mCRM) [27] with 2 patients at each dosing. The mCRM was chosen, rather than the more common “3+3” design, because mCRM is a more accurate method of determining the dose associated with target toxicity of 33% [28]. Cohort size was 2 patients, and we targeted a 33% risk of DLT. Since no patient experienced DLTs, escalation continued to the highest dose level of 4000 mg/d.
Determination of MPX safety included all subjects who receive at least 1 dose of MPX. A Wilcoxon signed-rank test was used to determine statistical significance of changes from baseline PSADT to post-baseline PSADT in patients on study. As has been done previously, 1 patient with a negative post-baseline PSADT was assigned a value of 100 months [29]. Pharmacokinetic parameters were summarized by descriptive statistics.
RESULTS
Patients
Between October 2011 and August 2012, a total of 14 patients were enrolled, and were evaluable for toxicities. Their baseline characteristics are shown in Table 1. The median age of the patients was 61.0 (range 51–74). Four of the 14 patients (29%) were black. Median PSA at baseline was 3.5 ng/mL (range 0.9–75.1) and median PSADT was 9.4 months (range 2.1–35.2). Median Gleason score was 7 (range 6–9).
Table 1.
Patient Baseline Characteristics
| Total Number of Patients (n) | 14 |
|
| |
| Age | |
| Mean (SD) | 62.6 (7.5) |
| Median (Range) | 61 (51–74) |
|
| |
| Race, n% | |
| White | 10 (71%) |
| Black | 4 (29%) |
|
| |
| PSA Values at Screening (ng/ml) | |
| Mean (SD) | 12.1 (20.1) |
| Median (Range) | 3.5 (0.9 – 75.1) |
|
| |
| PSADT at Screening (months) | |
| Mean (SD) | 13.0 (10.1) |
| Median (Range) | 9.4 (2.1 – 35.2) |
|
| |
| Pre-Operative Gleason Score, n (%) | |
| 6 | 3 (21%) |
| 7 | 7 (50%) |
| 8 | 1 (7%) |
| 9 | 3 (21%) |
|
| |
| Prior treatment | |
| Surgery | 4 (29%) |
| Radiation | 1 (7%) |
| Both | 9 (64%) |
Adverse events
All study-related adverse events (AEs) are shown in Table 2. All AEs were reported as grade 1 events, and patients continued on therapy for a median of 19.8 months (range 6.9, 29.7+) with no tolerability issues. Study related AEs during all cycles were primarily gastrointestinal and were reported by 5 of 14 (36%) patients, 4 of whom were in the highest dose group. Flatulence was reported by 4 patients, and soft stools, abdominal distension, and eructation were reported by 1 patient each. Among the AE’s unrelated to the study drug, grade 3 ptosis was observed in 1 patient, and grade 2 leg pain, back pain, incisional pain, fatigue, bronchial infection, bronchitis, increased glucose and myasthenia gravis were each reported by 1 patient.
Table 2.
All Treatment-Related Adverse Events
| Adverse Event | Grade | Number of Patients | Dose Level |
|---|---|---|---|
| Flatulence | 1 | 4 | 1 at 1,000 mg 3 at 4,000 mg |
| Soft Stools | 1 | 1 | 4,000 mg |
| Abdominal distension | 1 | 1 | 4,000 mg |
| Eructation | 1 | 1 | 4,000 mg |
No serious AEs were reported during the study, and there were no study-related hospitalizations. No new cancer diagnoses were reported for patients on study. As shown in Table 3, 5 patients were removed from the study due to metastatic disease on their annual bone and CT scans (3 bone, 2 lymph node). One additional patient came off study for disease progression (rising PSA), and 1 patient came off for myasthenia gravis, which was unrelated to his prostate cancer or the study drug. The remaining 7 patients with no evidence of radiographic progression remained on the study drug for 21–30+ months.
Table 3.
Pre- and Post-treatment PSADT, Gleason Scores and Reasons for Discontinuation
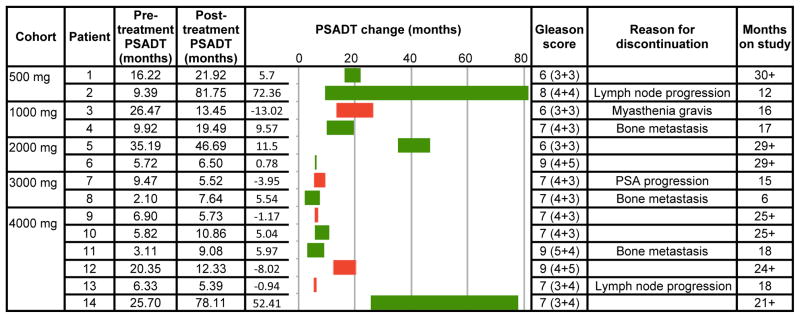
|
The graphic element of this table displays changes in PSADT. The green bars show lengthening of PSADT from pre-treatment (left end) to post-treatment PSADT (right end). The red bars show shortening of PSADT from pre-treatment (right end) to post-treatment (left end).
PSA Kinetics
Table 3 shows pre and post treatment PSADT and PSADT change for each of the 14 patients, by dose cohort. PSADT increased in 64% of the patients and 44% of those patients had > 6 month increase in PSADT. PSADT shortened in 5 of the patients (36%) including 3 of 6 patients in the highest dose cohort, but without significant change in the patients’ prognosis based on PSADT. Median within-patient PSADT increased by 5.3 months (non-significant, p = 0.17). Median pre-treatment PSADT was 9.4 months and median post treatment PSADT was 11.6 months (range 5.4–81.8). Pre-treatment PSADT was measured using values available during the 12 months prior to randomization. Post treatment PSADT was estimated over 6 months or longer based on when the patient came off study. Median follow-up was 19.2 months (range 6.2 – 29.7); mean was 19.6 months (SD 6.2). No patients experienced a sustained decrease in serum PSA from baseline. Seven patients who demonstrated no evidence of radiographic progression were thought to be achieving continued clinical benefit and have remained on study for a range of 21 to 30+ months, despite increasing PSA levels.
Pharmacokinetics
For the 6 patients enrolled at the 4000 mg dose level, we quantified the plasma concentrations of conjugated and unconjugated resveratrol, quercetin, ellagic acid, and urolithin A. Conjugated and unconjugated resveratrol and quercetin were not detected in the plasma. While conjugated and unconjugated ellagic acid also were not detected, the ellagic acid metabolite urolithin A was detected. Unconjugated urolithin A was detected in 1 sample in 1 patient at 30 minutes post-dose. Conjugated urolithin A was detected in 3 of 6 patients at baseline which was consistent with their dietary intake of fruits, but the resultant concentration-time profile was consistent with the administration of an oral dose of a drug. Conjugated urolithin A was detected in 5 of 6 patients at 24 hours and 4 of 5 patients at day 14 which is suggestive of a lag time to formation of this metabolite. Since the samples were analyzed in batch at the end of the phase I, only qualitative data are presented.
DISCUSSION
Dietary supplement use is widespread among men diagnosed with prostate cancer with 26–35% reporting consumption of vitamin, mineral and/or herbal supplements [30, 31]. The lack of rigorous testing of the safety and efficacy of dietary supplements means that there is little to no evidence for physicians to make recommendations to patients. As a result, patients often make decisions based on anecdotes or on information found on the Internet where many pages touting the unsubstantiated benefits of dietary supplements are sponsored by organizations that have an economic stake in the sale of those supplements [32]. Further, some highly promoted supplements like Vitamin E, have been associated with increased rather than decreased incidence of prostate cancer in large scale studies [33]. Thus, evidence-based testing of these supplements is an important step toward protecting cancer patients and toward identifying natural products that may provide benefits that may be complementary to standard of care.
In this phase I safety and dose finding evaluation, we demonstrate that MPX, a commercially available product composed of pulverized muscadine grape skin, is safe and tolerable up to a dose of 4000 mg/d which was the maximum dose tested. The study accrued rapidly, partly because patients with PSA recurrence as the only evidence of disease progression are often disposed to avoid the toxicities associated with androgen deprivation therapy [5, 34]. One patient came off study before completing 12 months of treatment with MPX, and the 6 patients came off study after 12 months because of disease progression (5 patients) or another toxicity unrelated to the study drug (1 patient). Seven patients with no evidence of radiographic progression remained on MPX for 21–30+ months due to perceived ongoing clinical benefit. The lack of dose limiting toxicities led to the selection of 4,000 mg/day as the recommended phase II dosing.
A goal of the study was to characterize the plasma pharmacokinetics of MPX components in the conjugated and unconjugated forms at the maximum tested dose. At the 4000 mg dose level, ellagic acid, quercetin, and resveratrol were undetectable in the plasma. This result was not unexpected since these polyphenols are known for poor bioavailability and extensive first-pass metabolism to conjugated metabolites [35–37]. However, we were able to more consistently detect conjugated urolithin A, which is a metabolite of ellagic acid [36]. Baseline levels of conjugated urolithin-A were observed in 3 patients, each of whom had been consuming pomegranate extract supplements for at least 2 months prior to initiation of the MPX study. Absorption from MPX is suggested by the observation of urolithin-A in 2 of the 3 non-baseline-urolithin-A patients at 24 hours and 1 of the 3 non-baseline-urolithin-A patients at 14 days.
Given the variability in the detection at a 4000 mg, it is unlikely that urolithin A would have been detected in the plasma from patients treated at the lower dose levels. The lack of quantitative pharmacokinetic data may also be confounded by long term storage of samples at −80° C prior to analysis. In subsequent studies we determined that these polyphenols are not stable in plasma after long-term storage at −80° C. Based on these results from this phase I study we developed a method to stabilize ellagic acid, quercetin, resveratrol, and urolithin A in plasma utilizing ascorbic and formic acid. This method will be applied to pharmacokinetic plasma samples collected in the phase II portion of the study.
This phase I study, as designed, had a small sample size, and PSADT change was not the primary endpoint of the study. We did not see a dose effect. The lack of statistically significant within-patient change in PSADT, and shortening in PSADT in 36% of the patients raise concerns about the efficacy of MPX. It is possible that 4000 mg/d is insufficient to result in a sustained decline in PSA. Despite our concern that patient adherence would decline at more than 8 pills per day, there may be value in conducting a study that escalates MPX dosage to get a concentration that increases likelihood of benefit. This concern is countered by 29 % of the patients experiencing greater than 5 months increase in PSADT.
The median increase in PSADT on the trial was 5.3 months which is at the threshold of clinical relevance in a population of BCR prostate cancer patients with rising PSA. In studies of patients with rising PSA on placebo or on observation, PSADT increases of approximately 3.9 months were observed in the absence of treatment. On this basis a minimal level of 6 month increase in PSADT has been recommended for clinical relevance [38]. However, several patients on the study experienced large increases in PSADT while on MPX. Based on the favorable safety profile at doses up to 4000 mg/day, we moved ahead with the expanded 125-patient, randomized placebo-controlled, phase II portion of the trial of MPX in PSA-recurrent prostate cancer patients. This study was conducted at multiple sites through the Department of Defense Prostate Cancer Clinical Trials Consortium and completed accrual in October 2014. The phase II trial randomized patients to placebo, 500 mg/d and 4000 mg/d arms and includes correlative analysis to evaluate the differential effect of MPX on patients with a single nucleotide polymorphism known to be associated with aggressive prostate cancer in men [39]. The goal of the phase II study is to determine whether MPX has a clinically significant effect, and, if so, its optimal dosing and whether a potential biomarker may be predictive of response to MPX.
Acknowledgments
The project described was supported by an ASCO YIA and ECOG Paul Carbone Fellowship, as well as the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1 TR 001079, and the Shared Instrument Grant (1S10RR026824-01)). In addition the clinical trial was supported by a grant from the Community Foundation of the National Capital Region.
Footnotes
ClinicalTrials.gov Identifier: NCT01317199
Disclosure: Dr. Wagner has an ownership interest in a manufacturer of muscadine grape skin products and holds a patent on the manufacturing process. None of the other authors have potential conflicts.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Paller CJ, Antonarakis ES, Eisenberger MA, Carducci MA. Management of patients with biochemical recurrence after local therapy for prostate cancer. Hematol Oncol Clin North Am. 2013;27(6):1205–19. viii. doi: 10.1016/j.hoc.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchio EM, Aslan M, Wells CK, Calderone J, Concato J. Impact of biochemical recurrence in prostate cancer among US veterans. Arch Intern Med. 2010;170(15):1390–5. doi: 10.1001/archinternmed.2010.262. [DOI] [PubMed] [Google Scholar]
- 4.Schweizer MT, Zhou XC, Wang H, Yang T, Shaukat F, Partin AW, Eisenberger MA, Antonarakis ES. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann Oncol. 2013;24(11):2881–6. doi: 10.1093/annonc/mdt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson CA, Shanafelt TD, Loprinzi CL. Andropause: symptom management for prostate cancer patients treated with hormonal ablation. Oncologist. 2003;8(5):474–87. doi: 10.1634/theoncologist.8-5-474. [DOI] [PubMed] [Google Scholar]
- 6.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson TS, Hartle DK, Hursting SD, Nunez NP, Wang TT, Young HA, Arany P, Green JE. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007;67(17):8396–405. doi: 10.1158/0008-5472.CAN-06-4069. [DOI] [PubMed] [Google Scholar]
- 8.Yi W, Fischer J, Akoh CC. Study of anticancer activities of muscadine grape phenolics in vitro. J Agric Food Chem. 2005;53(22):8804–12. doi: 10.1021/jf0515328. [DOI] [PubMed] [Google Scholar]
- 9.Raz AVB, Heath E. Galectin-3: A possible complementary marker to the PSA blood test. JCO. 2013;31(S abstr e22168) doi: 10.18632/oncotarget.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia EQ, Deng GF, Guo YJ, Li HB. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010;11(2):622–46. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP, Sartippour M, Harris DM, Rettig M, Suchard MA, Pantuck AJ, Belldegrun A, Heber D. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55(19):7732–7. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 12.Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54(3):980–5. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- 13.Xing N, Chen Y, Mitchell SH, Young CY. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2001;22(3):409–14. doi: 10.1093/carcin/22.3.409. [DOI] [PubMed] [Google Scholar]
- 14.Nair HK, Rao KV, Aalinkeel R, Mahajan S, Chawda R, Schwartz SA. Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin Diagn Lab Immunol. 2004;11(1):63–9. doi: 10.1128/CDLI.11.1.63-69.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber G, Shen F, Prajda N, Yang H, Li W, Yeh A, Csokay B, Olah E, Look KY. Regulation of the signal transduction program by drugs. Adv Enzyme Regul. 1997;37:35–55. doi: 10.1016/s0065-2571(96)00025-8. [DOI] [PubMed] [Google Scholar]
- 16.Plaumann B, Fritsche M, Rimpler H, Brandner G, Hess RD. Flavonoids activate wild-type p53. Oncogene. 1996;13(8):1605–14. [PubMed] [Google Scholar]
- 17.Mouria M, Gukovskaya AS, Jung Y, Buechler P, Hines OJ, Reber HA, Pandol SJ. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int J Cancer. 2002;98(5):761–9. doi: 10.1002/ijc.10202. [DOI] [PubMed] [Google Scholar]
- 18.Barber NJ, Zhang X, Zhu G, Pramanik R, Barber JA, Martin FL, Morris JD, Muir GH. Lycopene inhibits DNA synthesis in primary prostate epithelial cells in vitro and its administration is associated with a reduced prostate-specific antigen velocity in a phase II clinical study. Prostate Cancer Prostatic Dis. 2006;9(4):407–13. doi: 10.1038/sj.pcan.4500895. [DOI] [PubMed] [Google Scholar]
- 19.Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TW, Ferketich AK, Monk JP, Gong MC, Bahnson RR, DeGroff VL, Clinton SK. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60(2):145–54. doi: 10.1080/01635580701621338. [DOI] [PubMed] [Google Scholar]
- 20.Paller CJ, Ye X, Wozniak PJ, Gillespie BK, Sieber PR, Greengold RH, Stockton BR, Hertzman BL, Efros MD, Roper RP, Liker HR, Carducci MA. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013;16(1):50–5. doi: 10.1038/pcan.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas R, Williams M, Sharma H, Chaudry A, Bellamy P. A double-blind, placebo-controlled randomised trial evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer--the U.K. NCRN Pomi-T study. Prostate Cancer Prostatic Dis. 2014;17(2):180–6. doi: 10.1038/pcan.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonarakis ES, Zahurak ML, Lin J, Keizman D, Carducci MA, Eisenberger MA. Changes in PSA kinetics predict metastasis- free survival in men with PSA-recurrent prostate cancer treated with nonhormonal agents: combined analysis of 4 phase II trials. Cancer. 2012;118(6):1533–42. doi: 10.1002/cncr.26437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzman DL, Zhou XC, Zahurak ML, Lin J, Antonarakis ES. Change in PSA velocity is a predictor of overall survival in men with biochemically-recurrent prostate cancer treated with nonhormonal agents: combined analysis of four phase-2 trials. Prostate Cancer Prostatic Dis. 2014 doi: 10.1038/pcan.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M Prostate Cancer Clinical Trials Working G. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpentier A, Brandes A, Kesari S, et al. Safety interim data from a 3-arm phase 2 study evaluating safety and pharmacokinetics of the oral transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with glioblastoma at first progression. J Clin Oncol. 2013;31(suppl):abstr 2061. [Google Scholar]
- 26.Arlen PM, Bianco F, Dahut WL, D’Amico A, Figg WD, Freedland SJ, Gulley JL, Kantoff PW, Kattan MW, Lee A, Regan MM, Sartor O. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol. 2008;179(6):2181–5. doi: 10.1016/j.juro.2008.01.099. discussion 2185–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onar A, Kocak M, Boyett JM. Continual reassessment method vs. traditional empirically based design: modifications motivated by Phase I trials in pediatric oncology by the Pediatric Brain Tumor Consortium. J Biopharm Stat. 2009;19(3):437–55. doi: 10.1080/10543400902800486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrett-Mayer E. The continual reassessment method for dose-finding studies: a tutorial. Clin Trials. 2006;3(1):57–71. doi: 10.1191/1740774506cn134oa. [DOI] [PubMed] [Google Scholar]
- 29.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 30.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol. 2008;26(4):665–73. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- 31.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103(3):323–8. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 32. [Accessed March 21, 2015]; https://www.ftc.gov/news-events/press-releases/2013/01/ftc-commissioners-uphold-trial-judge-decision-pom-wonderful-llc.
- 33.Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306(14):1549–56. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 35.Cai X, Fang Z, Dou J, Yu A, Zhai G. Bioavailability of quercetin: problems and promises. Curr Med Chem. 2013;20(20):2572–82. doi: 10.2174/09298673113209990120. [DOI] [PubMed] [Google Scholar]
- 36.Lipinska L, Klewicka E, Sojka M. The structure, occurrence and biological activity of ellagitannins: a general review. Acta Sci Pol Technol Aliment. 2014;13(3):289–99. doi: 10.17306/j.afs.2014.3.7. [DOI] [PubMed] [Google Scholar]
- 37.Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 38.Paller CJ, Olatoye D, Xie S, Zhou X, Denmeade SR, Eisenberger MA, Antonarakis ES, Carducci MA, Rosner GL. The effect of the frequency and duration of PSA measurement on PSA doubling time calculations in men with biochemically recurrent prostate cancer. Prostate Cancer Prostatic Dis. 2014;17(1):28–33. doi: 10.1038/pcan.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikhak B, Hunter DJ, Spiegelman D, Platz EA, Wu K, Erdman JW, Jr, Giovannucci E. Manganese superoxide dismutase (MnSOD) gene polymorphism, interactions with carotenoid levels and prostate cancer risk. Carcinogenesis. 2008;29(12):2335–40. doi: 10.1093/carcin/bgn212. [DOI] [PMC free article] [PubMed] [Google Scholar]


