Abstract
Alcohol use disorder, anxiety disorders, and post-traumatic stress disorder (PTSD) are highly comorbid, and exposure to chronic stress during adolescence may increase the incidence of these conditions in adulthood. Efforts to identify the common stress-related mechanisms driving these disorders have been hampered, in part, by a lack of reliable preclinical models that replicate their comorbid symptomatology. Prior work by us, and others, has shown that adolescent social isolation increases anxiety-like behaviors and voluntary ethanol consumption in adult male Long-Evans rats. Here we examined whether social isolation also produces deficiencies in extinction of conditioned fear, a hallmark symptom of PTSD. Additionally, as disrupted noradrenergic signaling may contribute to alcoholism, we examined the effect of anxiolytic medications that target noradrenergic signaling on ethanol intake following adolescent social isolation. Our results confirm and extend previous findings that adolescent social isolation increases anxiety-like behavior and enhances ethanol intake and preference in adulthood. Additionally, social isolation is associated with a significant deficit in the extinction of conditioned fear and a marked increase in the ability of noradrenergic therapeutics to decrease ethanol intake. These results suggest that adolescent social isolation not only leads to persistent increases in anxiety-like behaviors and ethanol consumption, but also disrupts fear extinction, and as such may be a useful preclinical model of stress-related psychopathology. Our data also suggest that disrupted noradrenergic signaling may contribute to escalated ethanol drinking following social isolation, thus further highlighting the potential utility of noradrenergic therapeutics in treating the deleterious behavioral sequelae associated with early life stress.
Keywords: Duloxetine, Fear-Potentiated Startle, Prazosin, Propranolol, Social Isolation
1. Introduction
Alcohol use disorder, anxiety disorders, and post-traumatic stress disorder (PTSD) frequently co-occur (Brady and Sinha, 2005; Kushner et al., 2000), and exposure to chronic stress in early life has been linked to the incidence of each of these conditions (Brady and Sinha, 2005; Enoch, 2011; Gillespie et al., 2009; Huot et al., 2001; Kaufman et al., 2007; Roman and Nylander, 2005). Individuals suffering from anxiety or trauma- and stressor-related disorders may drink to self-medicate, as acute alcohol relieves anxiety and transiently alleviates the symptoms of PTSD (Davis et al., 2013; Leeies et al., 2010; Robinson et al., 2011). In contrast, alcohol withdrawal enhances anxiety and exacerbates PTSD symptomatology, thereby promoting further intake (Becker, 2012; Driessen et al., 2001; Koob and Le Moal, 2005; Robinson et al., 2011). As alcohol dependence is characterized by cycles of intoxication and withdrawal, anxiety-related drinking has been implicated in advancing the progression of alcoholism (Breese et al., 2011; Koob, 2013), and individuals suffering from comorbid PTSD, alcohol use disorder, and anxiety disorders exhibit worsened clinical profiles and experience poorer treatment outcomes (Bruce et al., 2005; Jacobsen et al., 2001). As such, determining the shared mechanisms driving these commonly comorbid conditions may prove crucial to effectively preventing and reversing their co-occurrence.
Clinical research suggests that dysregulation of the noradrenergic system may contribute to the etiology of these disorders, as potentiated noradrenergic signaling has been observed in individuals suffering from each condition (Brawman-Mintzer and Lydiard, 1997; Geracioti et al., 2001; Patkar et al., 2004) and putative noradrenergic inhibitors have been shown to decrease anxiety, ethanol intake, and PTSD symptomatology (Boehnlein and Kinzie, 2007; Famularo et al., 1988; Fox et al., 2012; Lader, 1988; Petrakis et al., 2012; Simpson et al., 2009). Noradrenergic signaling is an integral component of the stress response system, and sustained activation of locus coeruleus (LC) noradrenergic afferents in response to prolonged stress exposure has been linked to persistent increases in anxiety (Jedema et al., 2001; Ressler and Nemeroff, 2001). LC afferents project diffusely throughout the neuraxis, targeting many regions involved in generating and terminating appropriate behavioral and neuroendocrine responses to stressors (Berridge and Waterhouse, 2003); as such, noradrenergic pharmacotherapeutics likely exert their effects by acting on a number of LC targets. In support of this, chronic stress or ethanol exposure have been shown to disrupt noradrenergic signaling in many brain regions implicated in the maintenance of fear- and anxiety-related behaviors, including the hypothalamus, basolateral amygdala (BLA), and bed nucleus of the stria terminalis (BNST) (Aston-Jones and Harris, 2004; Morilak et al., 2005; Smith and Aston-Jones, 2008). Despite the potential importance of understanding how the noradrenergic system is disrupted by chronic stress or ethanol exposure and righted by treatment with drugs thought to decrease noradrenergic signaling, the specific neurobiological changes underlying these phenomena have yet to be identified. These efforts have been partially hampered by a lack of reliable animal models that engender enduring increases in anxiety-like behaviors, PTSD-like symptoms, and excessive ethanol intake.
Toward the goal of developing such a model, we have recently begun characterizing the effects of adolescent social isolation on subsequent ethanol consumption and anxiety measures in adult male Long-Evans rats. Preclinical models have demonstrated that exposure to chronic stress during development increases anxiety-like behaviors and ethanol intake in adulthood (Cruz et al., 2008; Huot et al., 2001; Roman and Nylander, 2005), and adolescence is arguably a particularly vulnerable developmental period during which the brain is especially sensitive to the effects of stress (Heim and Nemeroff, 2001; Jankord et al., 2011; Spear, 2009). In support of this, adolescent social isolation has been shown to increase anxiety-like behaviors (Chappell et al., 2013; Hall et al., 1998b; Hellemans et al., 2004; Yorgason et al., 2013) and ethanol self-administration (Chappell et al., 2013; Deehan et al., 2007; Ehlers et al., 2007; Hall et al., 1998a; McCool and Chappell, 2009) in adult rats. The experiments described herein extend previous studies by using the fear-potentiated startle paradigm, a highly translational and clinically relevant measure of fear learning (Grillon, 2008; Jovanovic et al., 2013), to examine whether adolescent isolation also produces disruptions in fear and extinction learning. Specifically, we hypothesized that adolescent social isolation would disrupt extinction memory acquisition following fear conditioning, as extinction learning is known to be disrupted among individuals suffering from PTSD (Grillon and Morgan, 1999; Jovanovic et al., 2010). Our results support this hypothesis, suggesting that exposure to chronic stress during adolescent development disrupts the ability to extinguish fear memories in later life.
Additionally, we examined the effect of three anxiolytic medications that target the noradrenergic system (the α1-adrenoreceptor (AR) antagonist prazosin, the β1/2-AR antagonist propranolol, and the serotonin-norepinephrine reuptake inhibitor (SNRI) duloxetine) on home-cage ethanol drinking in singly housed adult animals exposed to either social isolation or group housing during adolescence. Our findings confirm and extend our previous results, demonstrating that adolescent social isolation results in a robust and long-lasting increase in ethanol intake in adulthood, even relative to adolescent group housed subjects that were isolated during the adult drinking regimen. Furthermore, we report that all three modulators of noradrenergic signaling dose-dependently decrease ethanol intake in animals that were isolated throughout adolescence, while having little to no effect on intake among adolescent group housed conspecifics. Additionally, we found preliminary evidence that fear conditioning promotes greater ethanol intake among socially isolated animals, without effecting group-reared conspecifics.
2. Materials and Methods
2.1. Subjects
A total of sixty-eight male Long Evans rats from four temporally distinct cohorts were used in these studies, all obtained from Harlan Laboratories (Indianapolis, IN). All animals were treated identically, except where indicated (Fig. 1). Animals arrived at postnatal day 21, and were group housed (4 animals/cage) in large, clear plexiglass cages (33.0 cm × 59.7 cm; Nalgene, Rochester, NY) for one week. Following this, socially isolated animals (SI n = 35) were moved to smaller clear cages (25.4 cm × 45.7 cm), while group housed animals (GH n = 33) remained in their original cages; animals stayed in these housing conditions with minimal handling (one cage change/week) for six weeks prior to behavioral testing. Rats had ad libitum access to food (Prolab RMH 3000, LabDiet; PMI Nutrition International, St. Louis, MO) and water throughout, and were maintained on a 12-hour light/dark cycle in the same colony room. Animal care procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Wake Forest University Animal Care and Use Committee.
Figure 1.
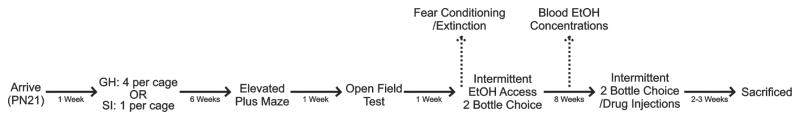
Experimental Design. Animals arrived on postnatal day 21 and were housed in groups of four for one week. Following this, animals were randomly assigned to housing conditions, and either remained group housed (GH n = 33) or were socially isolated (SI n = 35) for the remainder of their adolescent development. At the end of six weeks, anxiety-like behavior and locomotor activity were assessed using the elevated plus-maze and open field tests. After this, 57 animals (GH n = 28; SI n = 29) were individually housed and began intermittent two-bottle choice ethanol self-administration in their home cages. A subset of animals (GH n = 13; SI n = 14) also underwent fear conditioning and extinction learning prior to ethanol access. All animals were allowed to self-administer ethanol on this schedule for eight weeks. A separate subset of animals (GH n = 15; SI n = 16) were used to determine blood ethanol concentrations three weeks into the drinking study. At the end of eight weeks, animals began receiving injections of either prazosin, propranolol, or duloxetine once per week to assess the dose-dependent effects of these drugs on drinking. Animals were sacrificed at the end of these studies.
2.2. Anxiety-Like Behavior
Anxiety-like behavior was assessed in all animals during the seventh week of the adolescent housing manipulation using standard elevated plus-mazes (Med Associates, St. Albans, VT) raised 72.4 cm from floor level, with runways measuring 10.2 cm wide by 50.8 cm long. Open runways had 1.3 cm high lips and closed runways were enclosed in 40.6 cm high black polypropylene walls. Exits and entries from runways were detected via infrared sensors attached to the opening of each arm of the maze. Data were obtained and recorded via personal computer interfaced with control units and MED-PC programming (Med Associates). Animals were placed at the junction of the four arms at the beginning of the session, and activity was measured for five minutes. Anxiety-like behavior was assessed by measuring the total time spent on the open arms of the maze as well as the number of entries into the open arms. General locomotor activity was assessed by measuring the number of closed arm entries.
One week later, general locomotion in a novel environment was measured in all animals using an open field test, conducted in standard activity chambers (model-RXYZCM, Digiscan animal activity monitors, Omnitech, Columbus, OH). At the start of the test, animals were placed in the center of acrylic plastic chambers (42 * 42 * 30 cm) equipped with eight photobeam arrays of infrared photodectors located at regular intervals along each wall of the chamber (2.5 cm above the floor). The chamber walls were solid, and the room was dimly lit. Exploratory activity in this environment was measured for one hour, and data were stored in five minute time bins. Locomotion was assessed by measuring the total distance traveled during this time, while anxiety-like behavior was determined by comparing the percentage of time spent in the center of the chamber relative to the perimeter.
2.3. Fear Conditioning and Extinction
Fear conditioning tests were conducted on a subset of animals (from two temporally distinct cohorts) after the open field test, but before ethanol access commenced. Fear-potentiated startle was trained and assessed using standard acoustic startle chambers (San Diego Instruments, CA) containing plexiglass tubes with grid floors capable of delivering footshocks. All procedures were carried out during the light cycle. The plexiglass tubes were large enough for animals to turn around freely and animals were allowed to acclimate to the tubes and chambers for five minutes prior to each training or testing session. Group housed and socially isolated animals (GH n = 13; SI n = 14) were trained to pair a light (conditioned stimulus (CS)) with a 0.5 mA footshock (unconditioned stimulus (US)) in a single session consisting of 10 CS/US pairings. During each CS/US presentation, the light appeared for two seconds and was paired with a co-terminating 500 ms footshock. Acquisition of fear learning was assessed 24 hours later. During test sessions, subjects were presented with 10 startle-inducing noise bursts (100 dB) delivered alone and 10 additional noise bursts delivered in the presence of the CS in a pseudorandom order. Fear learning was determined by measuring the change in startle responding in the presence of the CS relative to noise-alone trials. Following confirmation of fear learning, animals were exposed to three consecutive extinction sessions, separated by 24 hours, consisting of 30 presentations of the CS alone (without shock or noise bursts). Twenty-four hours after the last extinction session, a test session identical to that used to measure the acquisition of fear conditioning was used to assess extinction learning. The persistence of a potentiated startle response in the presence of the CS during this test session indicated failure to acquire extinction learning.
2.4. Ethanol Access
Following the conclusion of the aforementioned behavioral tests, 11 animals were removed from the study (GH n = 5; SI n = 6) to be used for electrophysiological analysis (data not presented here). All remaining group housed animals (n = 28) were separated into single cages identical to those used to house the socially isolated rats (n = 29). Ethanol intake was then assessed using a home-cage, intermittent access two–bottle choice procedure. Animals were given access to two bottles containing 20% ethanol (v/v) or water Monday, Wednesday and Friday of each week with only water available on the remaining days. The placement of the ethanol and water bottles was alternated with each presentation to control for side preferences. On ethanol drinking days, fluid intake was measured after 30 minutes of access and again 24 hours later. It should be noted that the animals used for fear conditioning and extinction experiments were initiated to the ethanol access regimen at a later timepoint than animals that were only exposed to the elevated plus-maze and open field tests (two week delay).
2.5. Blood Ethanol Determination
Following three weeks of intermittent ethanol access, blood ethanol concentrations were measured in a subset of animals (GH n = 15; SI n = 16). Subjects were given access to ethanol and water for 30 minutes and then a 10 μl blood sample was collected from a tail snip. Blood ethanol concentrations were determined using a commercially available alcohol dehydrogenase enzymatic assay kit (Diagnostic Chemicals, Oxford, CT).
2.6. Drug Administration
Propranolol (1.25 and 2.5 mg/kg), prazosin (0.5, 1.0, and 1.5 mg/kg), and duloxetine (0.75 and 1.25 mg/kg) were obtained from Tocris Bioscience (Minneapolis, MN) and dissolved in sterile 0.9% NaCl. Following eight weeks of intermittent access two-bottle choice ethanol access, rats received weekly intraperitoneal injections of vehicle or one dose of the above pharmacotherapeutics 30 minutes prior to daily ethanol access. Cohorts were divided into three treatment groups; 16 animals (8 per housing condition) received prazosin injections, 16 animals (8 per housing condition) received propranolol injections, and 25 animals (GH n =12; SI n = 13) received duloxetine injections. For one week prior to injections, animals were removed from their cages and gently poked in the abdomen with a blunt object at the injection site prior to daily ethanol access in order to acclimate them to the handling procedure. During drug delivery weeks, animals received vehicle injections (0.9% NaCl) on Monday and drug injections on Wednesday. All drug injections were separated by 7 days, and each drug effect was compared to the average of all vehicle injections.
2.7. Statistics
Anxiety-like behavior on the elevated plus-maze was analyzed using unpaired t-tests, or Mann-Whitney Rank Sum Tests in the event of non-normally distributed data. Unpaired t-tests were used to compare blood ethanol concentrations (BECs) and ethanol intake between groups on the day that this assay was performed, and Pearson Product Moment Correlations were used to assess the relationship between BECs and ethanol intake within each group. Locomotor activity in the open field, fear conditioning and extinction learning, ethanol intake and preference, and drug effects on drinking were analyzed using repeated measures two-way analyses of variance (ANOVA) followed by Newman-Keuls post-hoc tests. The significance level for all statistical analyses was set at p < 0.05.
3. Results
3.1. Social isolation increases anxiety-like behavior
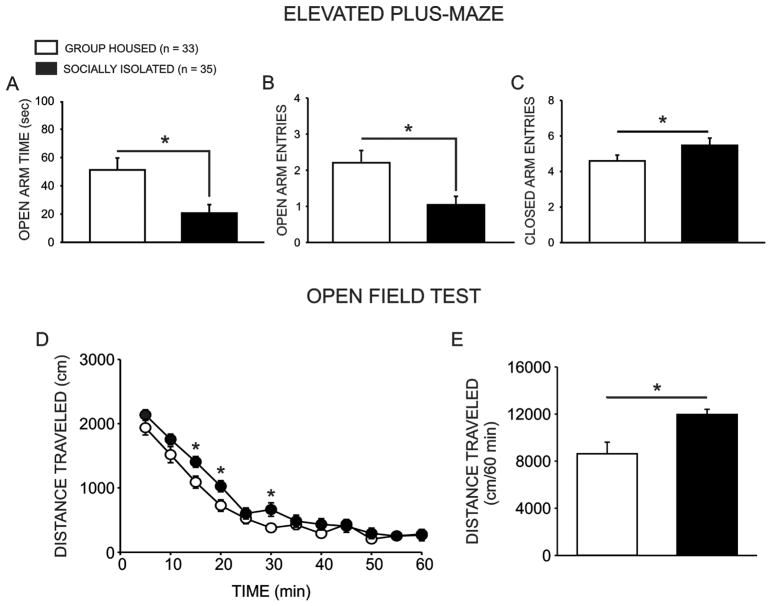
Baseline anxiety-like behavior was assessed following six weeks of exposure to either group housed or socially isolated rearing conditions. Consistent with previous reports from our lab (Chappell et al., 2013; McCool and Chappell, 2009; Yorgason et al., 2013), group housed animals (GH n = 33) exhibited less anxiety-like behavior than socially isolated conspecifics (SI n = 35), as evidenced by more time spent in the open arms of the elevated plus-maze (Fig. 2a; U(66) = 403, p = 0.029, two-tailed) and more open arm entries (Fig. 2b; U(66) = 368.5, p = 0.008, two-tailed). Socially isolated and group housed animals also showed a modest, albeit significant, difference in closed arm entries (Fig. 2c), a measure of non-specific locomotion (t(66) = 2.020, p = 0.048, two-tailed). Socially isolated rats also exhibited significantly greater locomotor activity in response to a novel environment (Fig. 2d,e), as evidenced by a significant main effect of housing condition (F(1,605) = 5.548, p = 0.026) and time (F(11,605) = 127.068, p < 0.001); no interaction of group and time was observed (F(11,605) = 1.398, p = 0.173). No differences in the time spent in the center of the open field were observed on this assay (F(1,605) = 0.008, p = 0.925; GH: M = 19.554, SEM = 2.189; SI: M = 19.842, SEM = 2.151).
Figure 2.
Social isolation increases anxiety-like behavior on the elevated plus-maze and locomotor activity in the open field test. Group housed (GH n = 33) animals exhibited less anxiety-like behavior on the elevated plus-maze than socially isolated (SI n = 35) counterparts, as evidenced by more time spent in the open arms of the maze (a; p = 0.029) and more open arm entries (b; p = 0.008). GH animals also exhibited a greater number of closed arm entries, a measure of non-specific locomotor activity (c; p = 0.047). Additionally, SI rats exhibited greater locomotor activity in response to a novel environment in the open field test than GH counterparts (d,e; p = 0.026).
3.2. Social isolation disrupts extinction learning
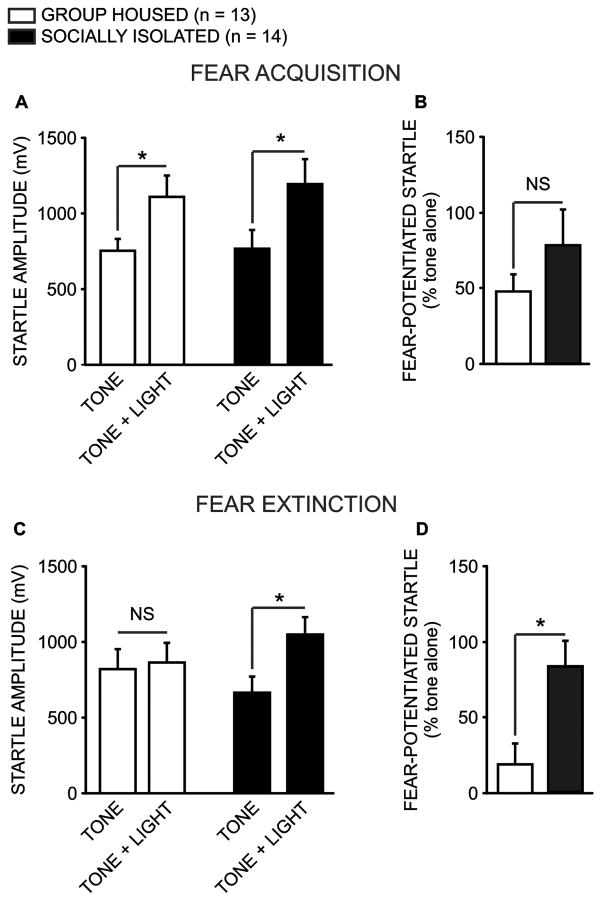
Both socially isolated and group housed animals acquired fear conditioning, as evidenced by exhibiting a potentiated startle response in the presence of a light that had been paired with a footshock 24 hours prior to testing (Fig. 3a; GH n = 13; SI n = 14). A two-way repeated measures ANOVA revealed a significant main effect of noise condition (noise alone vs CS-paired, Fig. 3c; F(1,25) = 31.246, p < 0.001), and post-hoc analyses confirmed that the average startle response amplitude exhibited in response to noise bursts presented in the presence of the light CS was greater than the average response to the noise alone (q = 7.905, p < 0.001). Importantly, there was no main effect of housing condition (F(1, 25) = 0.89, p = 0.354), and no significant interaction (F(1, 25) = 0.903, p = 0.351), indicating that the fear-potentiated startle response did not differ between groups. Furthermore, a direct comparison of the percent increase in startle responding in the presence of the CS reveled no difference in the magnitude of fear memory acquisition between groups (Fig 3b; t(25) = 0.837, p = 0.411).
Figure 3.
Social isolation disrupts extinction learning. Group housed (GH n = 13) and socially isolated (SI n = 14) animals acquired comparable fear conditioning, as evidenced by similarly potentiated responses to a startle-inducing noise burst in the presence of a light that had been paired with a footshock 24 hours prior to testing (a; p = 0.345). As such, there was no difference between groups in the magnitude of fear-potentiated startle to the noise burst in the presence of the light (b; p = 0.411). However, GH and SI animals differed significantly in their ability to acquire extinction learning; specifically, following three consecutive extinction sessions during which the footshock-paired light was presented alone repeatedly, GH animals no longer exhibited a potentiated startle to a noise burst when presented coincidently with the light (c; p = 0.343). Conversely, SI animals continued to exhibit a potentiated startle response to the light-paired noise burst, relative to the noise presented alone (p < 0.001), and as such the groups differed in the magnitude of their fear-potentiated startle response (d; p = 0.036).
In contrast, following three consecutive extinction training sessions, a two-way repeated measures ANOVA revealed a significant interaction between housing and noise condition (F(1,25) = 15.587, p < 0.001), and post-hoc analyses confirmed that socially isolated animals continued to exhibit a potentiated startle response in the presence of the CS (q = 6.627, p < 0.001), while response amplitude among group housed animals was equivalent whether the startle-inducing noise burst was presented alone or in the presence of the light CS (q = 1.368, p = 0.343). As such, on average the fear-potentiated startle response remained significantly elevated among socially isolated animals, relative to group housed controls (Fig. 3d; t(25) = 2.210, p = 0.036). Importantly, no differences in basal startle response (tone alone trials, t(25) = 0.800, p = 0.432) or response to the shock stimulus during training (t(25) = 0.484, p = 0.632) were found between the two groups (data not shown).
3.3. Social isolation increases intermittent ethanol intake
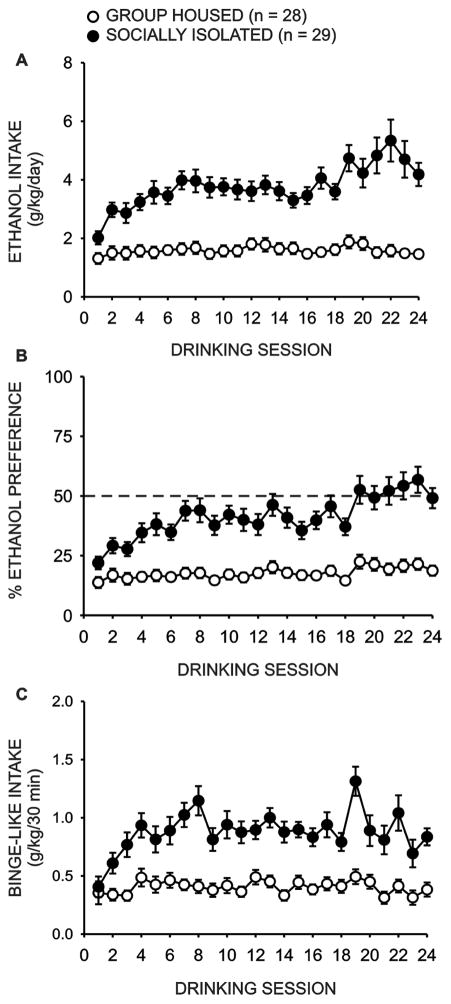
Analysis of 24 hour ethanol intake data (Fig. 4a) during intermittent access two-bottle choice drinking sessions revealed a main effect of housing condition (GH n = 28; SI n = 29; F(1,917) = 40.727, p < 0.001) and drinking session (F(17,917) = 5.901, p < 0.001), as well as a significant interaction between these factors (F(17,917) = 3.356, p < 0.001). Post-hoc analyses revealed no difference in ethanol intake during the first day of ethanol access (q = 2.665, p = 0.059); however, by the second ethanol access period group housed rats drank significantly less ethanol than socially isolated rats (q = 5.552, p < 0.001). This effect persisted throughout the remaining 22 drinking sessions (p < 0.001).
Figure 4.
Social isolation increases intermittent access two-bottle choice ethanol self-administration. Adolescent social isolation (SI n = 29) resulted in significantly increased daily ethanol intake (a; p < 0.001) and preference for ethanol over water (b; p < 0.001) relative to group housed (GH n = 28) animals. Additionally, SI animals drank significantly more ethanol in the first thirty minutes of daily access than GH conspecifics, a measure of binge-like intake (c; p < 0.001).
Similarly, socially isolated rats exhibited a greater preference for ethanol over water (Fig. 4b); analyses revealed a main effect of housing condition (F(1,917) = 29.729, p < 0.001) and drinking session (F(1,917) = 8.302, p < 0.001), as well as a significant interaction between these factors (F(1,917) = 4.082, p < 0.001). Post-hoc analyses revealed that although group housed animals did not show a reduced preference for ethanol relative to socially isolated conspecifics during the first day of ethanol availability (q = 2.512, p = 0.079), this difference was significant on the second drinking day (q = 3.778, p = 0.009) and remained significant on each subsequent drinking day (p < 0.05).
There was also an overall effect of housing condition (Fig. 4c; F(1,917) = 39.859, p < 0.001) and drinking session (F(1,917) = 4.138, p < 0.001) on 30 minute ethanol intake, with a significant interaction between these factors (F(1,917) = 2.091, p = 0.006). Drinking during the first 30 minutes of daily access using the intermittent model has been proposed to represent binge-like drinking, as rats tend to achieve BECs which meet the National Institute on Alcohol Abuse and Alcoholism (NIAAA) criterion for binge drinking during this period (Carnicella et al., 2014). Post-hoc analyses revealed that group housed and socially isolated rats exhibited similar intake patterns on day one of home-cage drinking (q = 1.414, p = 0.318). However, socially isolated rats drank significantly more ethanol on drinking day two during the first 30 minutes of daily access (q = 3.381, p = 0.017), and this difference remained significant for the duration of home-cage drinking (p < 0.001).
As a subset of animals from each housing condition underwent fear conditioning prior to ethanol access, we also ran a two-way ANOVA assessing the effect of fear conditioning/extinction and housing condition on average daily ethanol intake. Our results revealed a significant main effect of housing condition (F(1,52) = 46.692, p < 0.001), but no main effect of fear conditioning (F(1,52) = 2.503, p = 0.12). However, there was a significant interaction between these factors (F(1,52) = 5.184, p = 0.027), and follow-up post-hoc tests confirmed that exposure to the fear conditioning paradigm was associated with increased ethanol drinking among animals subjected to adolescent social isolation (q = 3.964, p = 0.007) but had no effect on drinking among animals group housed during adolescence (q = 0.677, p = 0.634). Importantly, socially isolated animals who were not fear conditioned still consumed more ethanol than non-shocked group housed conspecifics (q = 4.317, p = 0.004). Finally, as ethanol access was delayed among animals exposed to fear and extinction learning, we investigated via two-way ANOVA whether age at onset of ethanol access had an effect on average daily ethanol intake. Our results did not reveal a significant main effect of age on ethanol intake, indicating that the delayed onset of ethanol access had no observable effect on drinking behavior (F(1,52) = 0.153, p = 0.677).
3.4 BECs are elevated among socially isolated animals
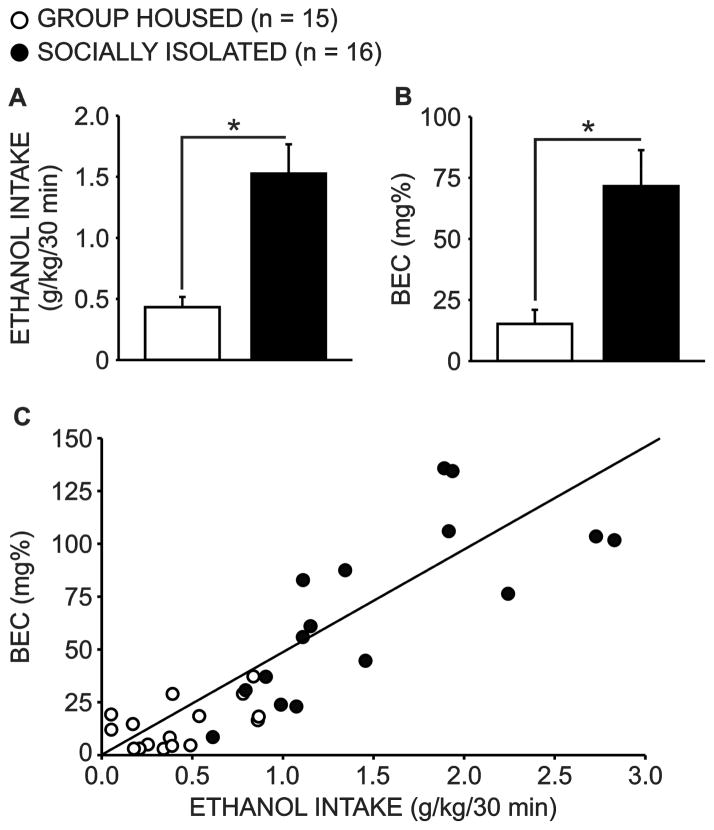
Blood ethanol determinations were made immediately following the first 30 minutes of ethanol drinking during week three of ethanol access. Socially isolated animals consumed more ethanol in the first 30 minutes of daily access (Fig. 5a; GH n = 15; SI n = 16; U(29) = 16, p < 0.001), and BECs were correspondingly elevated in this group (Fig. 5b; U(29) = 32, p < 0.001). Blood ethanol levels were strongly correlated with 30 minute ethanol intake (Fig. 5c; r = 0.848, p < 0.001). Further analysis of each group separately revealed that this relationship was driven by the socially isolated animals (r = 0.777, p < 0.001), as the correlation between BECs and ethanol intake was not significant in group housed subjects (r = 0.448, p = 0.094).
Figure 5.
Blood ethanol concentrations (BECs) are elevated after thirty minute access to ethanol in socially isolated animals. On the day that BECs were measured, socially isolated animals (SI n = 16) consumed more ethanol in the first thirty minutes of daily ethanol access than group housed (GH n = 15) animals (a; p < 0.001) and BEC’s were correspondingly elevated in this group (b; p < 0.001). Furthermore, BECs were positively correlated with 30 minute ethanol intake (c; p < 0.001).
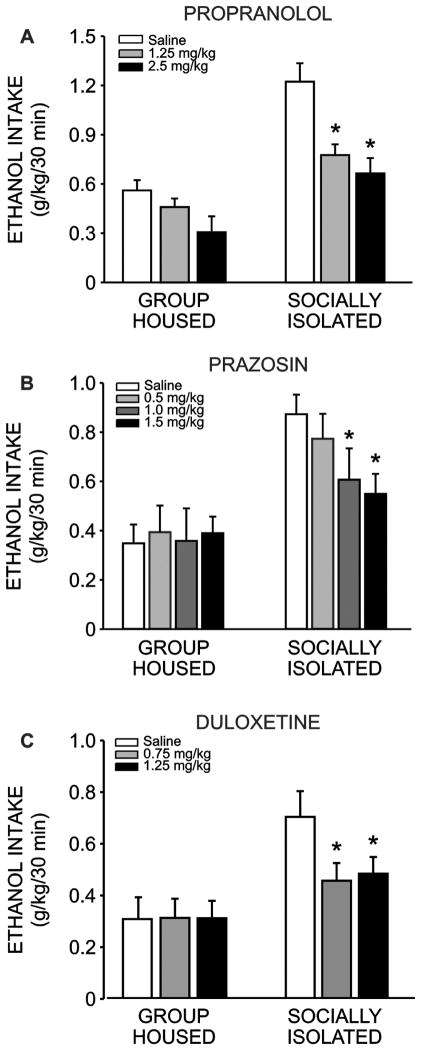
3.5 Prazosin, propranolol and duloxetine decrease binge-like ethanol intake
A two-way repeated measures ANOVA comparing the effectiveness of propranolol to decrease ethanol intake in the first 30 minutes of daily access in group housed and socially isolated animals revealed significant main effects of drug dose (F(2, 28) = 28.147, p < 0.001) and housing condition (F(1, 28) = 16.623, p < 0.001), and well as a significant interaction between these two factors (Fig. 6a; F(2, 28) = 5.555, p = 0.009). Post-hoc analyses revealed that propranolol decreased drinking relative to vehicle at both the low (1.25 mg/kg, q = 6.917, p < 0.001) and high (2.5 mg/kg, q = 2.882, p < 0.001) doses in animals that had been socially isolated throughout adolescence. However, no dose of propranolol tested significantly decreased ethanol intake among animals that had been group housed during this developmental period (p > 0.05). Interestingly, between-group analyses revealed that SI animals continued to consume more ethanol in the first 30 minutes of daily access, relative to GH animals, regardless of drug treatment (VEH: q = 8.652, p < 0.001; 1.25 mg/kg: q = 3.501, p = 0.018; 2.5 mg/kg: q = 3.958, p = 0.008).
Figure 6.
Propranolol, prazosin and duloxetine decrease binge-like ethanol intake in socially isolated animals. Propranolol dose-dependently decreased ethanol intake in the first thirty minutes of daily access among socially isolated animals (SI n = 8, a; p < 0.001), but had no effect on drinking among group housed animals (GH n = 8; p > 0.05). Similarly, prazosin significantly decreased binge-like intake in GH animals at the two highest doses administered (n = 8, b; p < 0.01), but had no effect on drinking among SI animals at any dose (n = 8; p > 0.05). Finally, duloxetine decreased binge-like intake in SI animals (n = 13, c; p < 0.001), but had no effect on drinking among GH animals (n = 12; p > 0.05).
Similarly, an analysis of prazosin administration on ethanol intake revealed a significant main effect of housing condition (F(1, 39) = 11.766, p = 0.004), but no main effect of drug dose (F(3,39) = 2.292, p = 0.093). However, the interaction of these two factors was significant (Fig. 6b; F(3,39) = 2.882, p = 0.048) and post-hoc analysis revealed that prazosin effectively decreased drinking among socially isolated animals at the middle (1.0 mg/kg, q = 4.483, p = 0.008) and high (1.5 mg/kg, q = 5.206, p = 0.004) doses, but not at the lowest dose (0.5 mg/kg, q = 2.409, p = 0.097). Prazosin did not decrease intake during the first 30 minutes of daily access at any dose among animals group housed during adolescence (p > 0.05). Regarding between-group differences, SI animals consumed more ethanol in the first 30 minutes of daily access following treatment with vehicle or the lowest dose of prazosin, as compared to GH conspecifics (q = 6.056, p < 0.001; q = 3.722, p = 0.012, respectively). However, administration of the medium (q = 2.441, p = 0.093) or high (q = 1.561, p = 0.227) doses of prazosin abolished this difference.
Finally, a two-way repeated measures ANOVA assessing the effectiveness of duloxetine to acutely decrease ethanol drinking among animals group housed or socially isolated during adolescence revealed main effects of housing condition (F(1,46) = 8.620, p = 0.007) and drug dose (F(2,46) = 3.827, p = 0.029), as well as a significant interaction of these factors (Fig. 6c; F(2,46) = 9.498, p < 0.001). Follow-up post-hoc analyses revealed that duloxetine decreased ethanol intake among socially isolated animals during the first 30 minutes of daily access at both the low (0.75 mg/kg, q = 6.015, p < 0.001) and high (1.25 mg/kg, q = 6.545, p < 0.001) doses, while having no discernible effect on drinking among group housed animals at any dose (p > 0.05). Furthermore, although SI animals consumed more ethanol than GH conspecifics following vehicle injection (q = 5.650, p < 0.001), this difference was no longer apparent following treatment with the low (q = 2.397, p = 0.097) or high (q = 2.016, p = 0.161) doses of duloxetine. No drug treatments affected 24 hour EtOH intake (p > 0.05, data not shown).
One potential shortcoming of our experimental design is that vehicle was always administered on Monday, and drug treatments were always administered on Wednesday; thus, some non-drug specific confound related to the day of the week could explain the decreased binge-like intake observed following drug delivery in socially isolated animals. To confirm that this was not the case, we ran a two way repeated measures ANOVA comparing average 30 minute intake as a function of housing condition and day of the week. As expected, this analysis revealed a significant main effect of housing condition on binge-like ethanol intake (F(1,108) = 32.864, p < 0.001). However, there was no main effect of day (F(2,108) = 0.957, p = 0.387), and no interaction between these factors (F(2,108) = 0.091, p = 0.912). Thus, we conclude that the effects of drug treatment on binge-like ethanol intake are not likely due to some non-specific effect of the day that drug treatment was administered.
4. Discussion
These studies replicate and extend our previous reports that early life stress in the form of adolescent social isolation increases anxiety-like behavior, locomotor response to a novel environment, ethanol intake, and blood ethanol levels in adult male Long-Evans rats. Specifically, we report that, relative to adolescent group housed animals, rats socially isolated during adolescence exhibit a significant increase in intermittent ethanol drinking that endures for at least two months. Notably, adolescent social isolation also engenders binge-like ethanol intake in adulthood, a phenotype not observed in subjects that were group housed during adolescence and then isolated in adulthood. These studies also demonstrate that adolescent social isolation significantly disrupts extinction of fear learning in adult male Long-Evans rats. Furthermore, fear conditioning prior to ethanol exposure significantly increases drinking in socially isolated (but not group housed) animals; this preliminary finding suggests that trauma exposure and chronic developmental stress may synergistically promote ethanol consumption in later life. Together, these findings support our hypothesis that adolescent social isolation produces behavioral outcomes reminiscent of those observed in established preclinical models of alcohol addiction, anxiety, and PTSD.
Further strengthening the potential utility of this model in investigating the neural maladaptations resulting from chronic developmental stress, we report that acute administration of anxiolytic adrenergic drugs dose-dependently decreases binge-like ethanol intake among animals exposed to adolescent social isolation, while having no discernable effect on drinking among animals group housed during adolesence. Specifically, systemic administration of the α1-AR antagonist prazosin, the β1/2-AR antagonist propranolol, or the SNRI duloxetine dose-dependently decreases ethanol intake in socially isolated animals during the first 30 minutes of ethanol availability, without effecting drinking among group-reared conspecifics. Furthermore, both duloxetine and prazosin appeared to “normalize” binge-like intake in animals socially isolated during adolescence. Interestingly, although propranolol decreased drinking relative to vehicle, propranolol-treated animals continued to drink more than adolescent group-housed controls. These findings are consistent with a recent report from our group that duloxetine and prazosin, but not propranolol, decrease ethanol intake and anxiety when administered chronically across four weeks (Skelly and Weiner, 2014). Taken together, these findings replicate the efficacy of noradrenergic therapeutics for the treatment of alcohol use disorder in some clinical populations (Boehnlein and Kinzie, 2007; Famularo et al., 1988; Fox et al., 2012; Lader, 1988; Petrakis et al., 2012; Simpson et al., 2009) and provide initial evidence that social isolation during adolescence may disrupt some of the same neurobiological substrates that contribute to alcoholism and/or anxiety and trauma- and stressor-related disorders.
Our lab has previously reported that adolescent social isolation results in increased ethanol intake across four weeks using an intermittent access home-cage drinking regimen (Chappell et al., 2013). Here we replicate these drinking effects, and further report that ethanol intake remains elevated for at least eight weeks among animals that were isolation reared during this critical developmental period. Socially isolated animals from all three cohorts tested consumed, on average, about 1 g/kg ethanol within the first 30 minutes of daily access, and the BECs achieved by these groups approached the NIAAA established criteria for a binge-drinking session (~80 mg/dl) (Carnicella et al., 2014). Furthermore, these animals exhibited increased 24 hour ethanol intake and preference for ethanol over water, as compared to group housed counterparts. In contrast, rats that were group housed during adolescence but then isolated throughout the adult drinking regimen never escalated their binge-like intake beyond an average of 0.5 g/kg, and 24 hour preference and total intake likewise remained low in these cohorts.
Thus, as we have reported previously, animals reared in group housed conditions do not display the increase in drinking typically seen with this intermittent access procedure (Chappell et al., 2013; Simms et al., 2008) even when this protocol is extended to eight weeks. The observation that adolescent group housed animals were isolated throughout the drinking study but did not show any signs of escalating their ethanol intake underscores the importance of adolescence as a developmental period during which chronic stress can engender increases in behavioral risk factors associated with addiction. This disparity is consistent with the hypothesis that protracted activation of the stress response system during adolescence leads to long-lasting neurobiological and behavioral perturbations (Heim and Nemeroff, 2001; Romeo et al., 2006; Spear, 2009). Taken together, our findings to date support extant evidence that chronic stress in adolescence may increase behaviors reminiscent of alcohol use and anxiety disorders in later life (Brady and Sinha, 2005; Enoch, 2011; Gillespie et al., 2009; Kaufman et al., 2007). However, it is important to note that exposure to chronic stress prior to the onset of puberty similarly increases anxiety and ethanol consumption (Huot et al., 2001), and these behavioral disruptions might also arise from stress-induced disruptions in central noradrenergic signaling (Smith and Aston-Jones, 2008). Future research is needed to determine the specific effects of chronic stress on neurodevelopment throughout childhood. Additionally, as animals reared in groups appear to be resilient to the effects of later-life isolation and trauma exposure (in the form of fear conditioning), as evidenced by decreased anxiety-like behavior and no excalation of ethanol intake in the two-bottle choice intermittent access paradigm, future work should be aimed at identifying the apparent neuroprotective effects of this rearing environment.
We also used the fear-potentiated startle paradigm, a highly translational measure of fear and extinction learning, to demonstrate that animals exposed to chronic developmental stress in the form of adolescent social isolation are significantly impaired in acquiring extinction learning. Difficulty extinguishing conditioned fear is a common symptom of PTSD, and the fear-potentiated startle paradigm can be used to model deficiencies in extinction learning in rodents (Barad, 2005). The inability of fear conditioned rodents to acquire extinction learning closely resembles the reduced ability of individuals with PTSD to recognize that stimuli previously associated with a traumatic event no longer predict impending danger (Goswami et al., 2013; Jovanovic et al., 2010; Milad et al., 2008). Importantly, a recent rodent study identified a link between chronic ethanol exposure and later PTSD-like impairments in extinction learning (Holmes et al., 2012). A similar effect has also been observed in mice exposed to adolescent isolation; these animals did eventually acquire extinction learning, but at a markedly slower rate than group housed conspecifics (Naert et al., 2011). Hence, disrupted (or at least delayed) acquisition of extinction learning may be an additional behavioral change associated with adolescent isolation in an animal model of voluntary anxiety-related ethanol intake. Interestingly, socially isolated animals that underwent fear conditioning went on to drink more, on average, than non-fear conditioned isolates, indicating that there may be some synergistic effect of chronic developmental stress and later-life trauma on ethanol drinking. This finding further strengthens the applicability of this model to identifying the neurological disruptions common to anxiety disorders, PTSD, and alcohol dependence among clinical populations.
Chronic social isolation during adolescence has been shown to increase ethanol intake among several species of rats, including rats selectively bred for high ethanol preference (Chappell et al., 2013; Ehlers et al., 2007; Hall et al., 1998a; Juarez and Vazquez-Cortes, 2003; McCool and Chappell, 2009). These results are consistent with the hypothesis that protracted stress during adolescence, a period of dynamic neurodevelopment and endocrine responsivity, might promote subsequent behavioral disruptions, including excessive ethanol intake (Becker et al., 2011; Romeo, 2010; Spear, 2009). Interestingly, our analysis revealed that socially isolated animals that were exposed to fear conditioning prior to ethanol access consumed more ethanol on average than isolation-reared animals that were not fear conditioned. This finding is consistent with previous work suggesting that footshock stress increases ethanol intake (Meyer et al., 2013; Vengeliene et al., 2003), although exposure to footshock stress has also been reported to decrease or have no effect on drinking (Becker et al., 2011). Notably, just as adolescent group housed animals did not exhibit an escalation in ethanol intake in the intermittent drinking regimen, they were also resilient to the enhancing effects of prior fear conditioning on subsequent ethanol drinking. These initial findings raise the intriguing possibility that early life stress increases vulnerability to the effects of other environmental stressors on ethanol intake. Future work in our lab will directly assess the apparent synergistic effect of adolescent isolation and fear conditioning on voluntary drinking behaviors.
Previous investigations aimed at identifying the neuroadaptations pertinent to these comorbidities have revealed a potential role of noradrenergic neurotransmission, which is an integral component of the stress response system. Brainstem noradrenergic afferents, such as those originating in the LC, fire in response to stress-induced HPA axis activation (Valentino et al., 1983), and chronic stress leads to persistent increases in LC excitability (Bingham et al., 2011; Pavcovich et al., 1990; Pavcovich and Ramirez, 1991). As chronic stress-induced alterations in LC output have been linked to elevated anxiety, this may be one mechanism whereby repeated stress during adolescence contributes to later life anxiety and alcohol use (Bingham et al., 2011; Spear, 2009). In fact, hyperactivity of the noradrenergic system has been observed among individuals with anxiety disorders (Ressler and Nemeroff, 2001; Yamamoto et al., 2014), and cyclic alcohol intake and withdrawal likewise lead to increased central noradrenergic signaling (Lanteri et al., 2008; Patkar et al., 2004; Rasmussen et al., 2006). Noradrenaline levels are also tonically elevated in individuals with PTSD, and central norepinephrine concentrations correlate positively with symptom severity in this population (Geracioti et al., 2001). Taken together, these findings point to noradrenergic dysregulation as a potential common mechanism driving the development and maintenance of stress-related anxiety, PTSD, and alcohol use disorder.
Consistent with this theory, pharmacological interventions presumed to decrease noradrenergic signaling have proven effective in attenuating anxiety and alcohol intake in both human alcoholics and rodent models of ethanol dependence. For example, prazosin decreases drinking and stress-induced alcohol craving in alcoholics (Fox et al., 2012; Simpson et al., 2009) and reduces voluntary ethanol consumption in rodent models of anxiety-related drinking (Rasmussen et al., 2009; Verplaetse et al., 2012; Walker et al., 2008). Similarly, β1/2-AR antagonists have been used clinically to treat anxiety associated with alcohol withdrawal (Bailly et al., 1992; Sellers et al., 1977), and decrease operant responding for ethanol in a rodent model of dependence (Gilpin and Koob, 2010). SNRIs have shown promise in reducing anxiety-related alcohol intake, reportedly lessening alcohol craving and anxiety following withdrawal in clinical populations (Kim et al., 2005; Liappas et al., 2005; Petrakis et al., 2012) and likewise attenuating anxiety-like behavior, ethanol drinking and the symptoms of acute withdrawal in rodents (Ji et al., 2008; Mochizuki et al., 2002; Saglam et al., 2004; Simon O’Brien et al., 2011). Furthermore, we recently reported that prazosin and duloxetine, when delivered chronically, decrease home-cage ethanol intake and anxiety-like behavior in rats displaying relatively high levels of antecedent anxiety (Skelly and Weiner, 2014). Our current findings corroborate these studies and further suggest that acute prazosin, propranolol, and duloxetine decrease voluntary ethanol consumption in adult rats expressing high levels of stress-induced anxiety-like behavior resulting from adolescent social isolation, without significantly affecting drinking in group housed conspecifics. A limitation of the current study is that drug doses were not counterbalanced; however the significant washout period between injections likely precluded any carryover effects. Thus, the effectiveness of various noradrenergic pharmacological agents in treating comorbid anxiety and alcohol dependence resulting from chronic adolescent stress appears promising.
Although we did not directly assess the mechanisms whereby prazosin, proproanolol, and duloxetine decrease binge-like intake among aminals isolated during adolescence, we hypothesize that these treatments decrease anxiety by blocking or reducing the excitatory effects of noradrenergic signaling in brain regions responsible for generating fear- and anxiety-related behavioral responses. In support of this, our group has recently shown that ethanol-induced noradrenaline release in the basolateral amygdala (BLA), a brain region important for coordinating the stress response, is increased following adolescent social isolation (Karkhanis et al., 2014). As stress-induced norepinephrine release promotes BLA excitability and fear- and anxiety-related behaviors in part via activation of β1/2-ARs, propranolol may reduce ethanol intake acutely by inhibiting the excitatory effects of noradrenaline in this region. Similarly, prazosin may be exerting its effects in part via antagonism of α1-AR in the bed nucleus of the stria terminalis (BNST), a nucleus of the extended amygdala thought to contribute to withdrawal-related anxiety (Aston-Jones and Harris, 2004; Delfs et al., 2000; Walker et al., 2008). Additionally, blockade of α1- or β1/2-ARs in the lateral septum, which is reciprocally connected to the amygdala, has been shown to reduce stress-related behaviors acutely (Bondi et al., 2007). Finally, duloxetine may decrease anxiety-related ethanol intake by attenuating LC output via activation of presynaptic α2-ARs, which act as autoreceptors in this region. In support of this, SNRIs have been shown to decrease LC output (Berrocoso and Mico, 2007), and α2-AR agonists are reported to reduce ethanol self-administration (Le et al., 2005). Further work is needed to determine how noradrenergic signaling is disrupted by chronic adolescent stress and subsequent ethanol intake, and where in the brain these pharmacotherapeutics are exerting their effects on stress-related voluntary ethanol consumption.
Beyond the previously observed connection between adolescent stress and both anxiety-like behavior and ethanol consumption, the results of the current set of studies suggest that the ability to extinguish conditioned fear is disrupted after adolescent social isolation; we suspect that alterations in adrenoreceptor signaling may contribute to these deleterious behavioral sequelae. The effects of the pharmacotheraputics used herein mimic their actions in clinical populations, and these drugs are markedly more effective in reducing drinking among socially isolated animals than their group housed counterparts. As such, our results suggest that adolescent social isolation model may prove useful in identifying the neuroadaptations resulting from chronic stress in adolescence which contribute to future psychiatric diseases, and assist in the development of new pharmacological interventions targeted at treating stress-associated behavioral maladaptations. Furthermore, the observed increases in anxiety measures and ethanol intake and deficiencies in fear extinction, which mimic elements of comorbid anxiety, PTSD, and alcohol use disorders, provide further evidence that this model of early life stress engenders behavioral and possibly neurobiological changes similar to those observed in clinical populations. Thus, these housing manipulations may represent a simple and effective way of modeling early life stress and related behavioral disruptions. Future studies will examine the effectiveness of propranolol, prazosin and duloxetine on the extinction deficiencies and anxiety-like behaviors observed in these preliminary experiments, and also investigate whether noradrenergic drugs continue to suppress ethanol intake and anxiety when administered chronically to adult animals following adolescent social isolation.
Highlights.
Adolescent social isolation increases anxiety-like behavior in adulthood
Extinction of conditioned fear is disrupted following adolescent social isolation
Adolescent social isolation increases later-life ethanol self-administration
Prazosin, propranolol and duloxetine acutely decrease isolation-induced drinking
Adolescent social isolation models some symptoms of anxiety, PTSD, and alcoholism
Acknowledgments
The authors would like to thank Tracy Butler, PhD for helpful comments and feedback on the manuscript. These studies were supported by R37 AA017531 (J.L.W.), P01 AA21099 (J.L.W.), R37 AA010422 (J.L.W.), and F31 AA022275 (M.J.S.)
Footnotes
Author contributions
M.J.S. and J.L.W. designed the research; M.J.S., A.E.C. and E.C. performed experiments; M.J.S. analyzed data and drafted the paper, and J.L.W. provided critical feedback which significantly shaped the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Bailly D, Servant D, Blandin N, Beuscart R, Parquet PJ. Effects of beta-blocking drugs in alcohol withdrawal: a double-blind comparative study with propranolol and diazepam. Biomed Pharmacother. 1992;46:419–424. doi: 10.1016/0753-3322(92)90047-b. [DOI] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res. 2012;34:448–458. doi: 10.35946/arcr.v34.4.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Berrocoso E, Mico JA. In vivo effect of venlafaxine on locus coeruleus neurons: Role of opioid, alpha(2)- adrenergic, and 5-hydroxytryptamine(1A) receptors. Journal of Pharmacology and Experimental Therapeutics. 2007;322:101–107. doi: 10.1124/jpet.107.120915. [DOI] [PubMed] [Google Scholar]
- Bingham B, McFadden K, Zhang X, Bhatnagar S, Beck S, Valentino R. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology. 2011;36:896–909. doi: 10.1038/npp.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13:72–78. doi: 10.1097/01.pra.0000265763.79753.c1. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Barrera G, Lapiz MD, Bedard T, Mahan A, Morilak DA. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:482–495. doi: 10.1016/j.pnpbp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Brawman-Mintzer O, Lydiard RB. Biological basis of generalized anxiety disorder. J Clin Psychiatry. 1997;58(Suppl 3):16–25. discussion 26. [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce SE, Yonkers KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, Shea MT, Keller MB. Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: a 12-year prospective study. Am J Psychiatry. 2005;162:1179–1187. doi: 10.1176/appi.ajp.162.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol. 2014;48:243–252. doi: 10.1016/j.alcohol.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E394–403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, da Planeta CS, Miczek KA. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl) 2008;201:459–468. doi: 10.1007/s00213-008-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TA, Jovanovic T, Norrholm SD, Glover EM, Swanson M, Spann S, Bradley B. Substance Use Attenuates Physiological Responses Associated With PTSD among Individuals with Co-Morbid PTSD and SUDs. J Psychol Psychother Suppl. 2013;7 doi: 10.4172/2161-0487.S7-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deehan GA, Cain ME, Kiefer SW. Differential rearing conditions alter operant responding for ethanol in outbred rats. Alcoholism-Clinical and Experimental Research. 2007;31:1692–1698. doi: 10.1111/j.1530-0277.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Driessen M, Meier S, Hill A, Wetterling T, Lange W, Junghanns K. The course of anxiety, depression and drinking behaviours after completed detoxification in alcoholics with and without comorbid anxiety and depressive disorders. Alcohol Alcohol. 2001;36:249–255. doi: 10.1093/alcalc/36.3.249. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Walker BM, Pian JP, Roth JL, Slawecki CJ. Increased alcohol drinking in isolate-housed alcohol-preferring rats. Behav Neurosci. 2007;121:111–119. doi: 10.1037/0735-7044.121.1.111. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famularo R, Kinscherff R, Fenton T. Propranolol treatment for childhood posttraumatic stress disorder, acute type. A pilot study. Am J Dis Child. 1988;142:1244–1247. doi: 10.1001/archpedi.1988.02150110122036. [DOI] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, Jr, Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 2010;212:431–439. doi: 10.1007/s00213-010-1967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Rodriguez-Sierra O, Cascardi M, Pare D. Animal models of post-traumatic stress disorder: face validity. Front Neurosci. 2013;7:89. doi: 10.3389/fnins.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology (Berl) 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998a;139:210–216. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998b;139:203–209. doi: 10.1007/s002130050705. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hellemans KGC, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Developmental Brain Research. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2011;152:629–638. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Finlay JM, Sved AF, Grace AA. Chronic cold exposure potentiates CRH-evoked increases in electrophysiologic activity of locus coeruleus neurons. Biol Psychiatry. 2001;49:351–359. doi: 10.1016/s0006-3223(00)01057-x. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behavioural Pharmacology. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL. Translational neuroscience measures of fear conditioning across development: applications to high-risk children and adolescents. Biol Mood Anxiety Disord. 2013;3:17. doi: 10.1186/2045-5380-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez J, Vazquez-Cortes C. Alcohol intake in social housing and in isolation before puberty and its effects on voluntary alcohol consumption in adulthood. Dev Psychobiol. 2003;43:200–207. doi: 10.1002/dev.10133. [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, McCool BA, Weiner JL, Jones SR. Social Isolation Stress Augments Dopamine and Norepinephrine Responses to Ethanol in the Nucleus Accumbens and the Basolateral Amygdala. Alcoholism-Clinical and Experimental Research. 2014;38:81a–81a. doi: 10.1111/acer.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter J. Genetic and environmental predictors of early alcohol use. Biol Psychiatry. 2007;61:1228–1234. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Kim W, Pae CU, Chae JH, Jun TY, Bahk WM. The effectiveness of mirtazapine in the treatment of post-traumatic stress disorder: a 24-week continuation therapy. Psychiatry Clin Neurosci. 2005;59:743–747. doi: 10.1111/j.1440-1819.2005.01447.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Lader M. Beta-adrenoceptor antagonists in neuropsychiatry: an update. J Clin Psychiatry. 1988;49:213–223. [PubMed] [Google Scholar]
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology. 2008;33:1724–1734. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Leeies M, Pagura J, Sareen J, Bolton JM. The use of alcohol and drugs to self-medicate symptoms of posttraumatic stress disorder. Depress Anxiety. 2010;27:731–736. doi: 10.1002/da.20677. [DOI] [PubMed] [Google Scholar]
- Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:55–60. doi: 10.1016/j.pnpbp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early Social Isolation in Male Long-Evans Rats Alters Both Appetitive and Consummatory Behaviors Expressed During Operant Ethanol Self-Administration. Alcoholism-Clinical and Experimental Research. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I. Stress Increases Voluntary Alcohol Intake, but Does not Alter Established Drinking Habits in a Rat Model of Posttraumatic Stress Disorder. Alcoholism-Clinical and Experimental Research. 2013;37:566–574. doi: 10.1111/acer.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki D, Tsujita R, Yamada S, Kawasaki K, Otsuka Y, Hashimoto S, Hattori T, Kitamura Y, Miki N. Neurochemical and behavioural characterization of milnacipran, a serotonin and noradrenaline reuptake inhibitor in rats. Psychopharmacology (Berl) 2002;162:323–332. doi: 10.1007/s00213-002-1111-5. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Naert A, Callaerts-Vegh Z, D’Hooge R. Nocturnal hyperactivity, increased social novelty preference and delayed extinction of fear responses in post-weaning socially isolated mice. Brain Res Bull. 2011;85:354–362. doi: 10.1016/j.brainresbull.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Marsden CA, Naik PC, Kendall DA, Gopalakrishnan R, Vergare MJ, Weinstein SP. Differences in peripheral noradrenergic function among actively drinking and abstinent alcohol-dependent individuals. Am J Addict. 2004;13:225–235. doi: 10.1080/10550490490459898. [DOI] [PubMed] [Google Scholar]
- Pavcovich LA, Cancela LM, Volosin M, Molina VA, Ramirez OA. Chronic stress-induced changes in locus coeruleus neuronal activity. Brain Res Bull. 1990;24:293–296. doi: 10.1016/0361-9230(90)90219-p. [DOI] [PubMed] [Google Scholar]
- Pavcovich LA, Ramirez OA. Time course effects of uncontrollable stress in locus coeruleus neuronal activity. Brain Res Bull. 1991;26:17–21. doi: 10.1016/0361-9230(91)90186-n. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Ralevski E, Desai N, Trevisan L, Gueorguieva R, Rounsaville B, Krystal JH. Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacology. 2012;37:996–1004. doi: 10.1038/npp.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during “abstinence”. Alcohol. 2006;38:173–177. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology of neuropsychiatric disorders. CNS Spectr. 2001;6:663–666. 670. doi: 10.1017/s1092852900001358. [DOI] [PubMed] [Google Scholar]
- Robinson J, Sareen J, Cox BJ, Bolton JM. Role of self-medication in the development of comorbid anxiety and substance use disorders: a longitudinal investigation. Arch Gen Psychiatry. 2011;68:800–807. doi: 10.1001/archgenpsychiatry.2011.75. [DOI] [PubMed] [Google Scholar]
- Roman E, Nylander I. The impact of emotional stress early in life on adult voluntary ethanol intake-results of maternal separation in rats. Stress. 2005;8:157–174. doi: 10.1080/10253890500188666. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Saglam E, Uzbay IT, Kayir H, Celik T, Beyazyurek M. Effects of venlafaxine on ethanol withdrawal syndrome in rats. Fundam Clin Pharmacol. 2004;18:693–698. doi: 10.1111/j.1472-8206.2004.00281.x. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Zilm DH, Degani NC. Comparative efficacy of propranolol and chlordiazepoxide in alcohol withdrawal. Journal of Studies on Alcohol. 1977;38:2096–2108. doi: 10.15288/jsa.1977.38.2096. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon O’Brien E, Legastelois R, Houchi H, Vilpoux C, Alaux-Cantin S, Pierrefiche O, Andre E, Naassila M. Fluoxetine, desipramine, and the dual antidepressant milnacipran reduce alcohol self-administration and/or relapse in dependent rats. Neuropsychopharmacology. 2011;36:1518–1530. doi: 10.1038/npp.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Weiner JL. Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain Behav. 2014;4:468–483. doi: 10.1002/brb3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Structure & Function. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: Effects of deprivation and stress phases on voluntary alcohol intake. Alcoholism-Clinical and Experimental Research. 2003;27:1048–1054. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL. Effects of prazosin, an alpha1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012;36:881–886. doi: 10.1111/j.1530-0277.2011.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: A pathophysiological view. Psychiatry Clin Neurosci. 2014;68:1–20. doi: 10.1111/pcn.12126. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013;37:1022–1031. doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]