Abstract
Alteration of glutamatergic-neurotransmission is a hallmark of alcohol abuse. We have previously reported that chronic ethanol-drinking downregulated glutamate transporter 1 (GLT-1) in nucleus accumbens (NAc) in male P rats in a manner that was reversed by ceftriaxone treatment. However, the effect of ceftriaxone on extracellular glutamate concentrations in NAc after chronic ethanol-drinking has not yet been studied. In the present study, male P rats were treated with ceftriaxone (100 mg/kg/day, i.p.) for five consecutive days following five-weeks of free choice ethanol (15% and 30%) drinking. In vivo microdialysis was performed to measure the extracellular glutamate concentrations in NAc and the effect of blockade of GLT-1 with dihydrokainic acid (DHK) on extracellular glutamate in NAc of ceftriaxone-treated rats was determined. Ceftriaxone treatment attenuated ethanol intake as well as ethanol preference. Extracellular glutamate was significantly higher in NAc after five-weeks of ethanol drinking in saline-treated compared to water control rats. Ceftriaxone treatment blocked the increase extracellular glutamate produced by ethanol intake. Blockade of GLT-1 by DHK reversed the effects of ceftriaxone on glutamate and implicated the role of GLT-1 in the normalization of extracellular glutamate by ceftriaxone. In addition, GLT-1 protein was decreased in ethanol exposed animals and ceftriaxone treatment reversed this deficit. Ceftriaxone treatment also increased glutamine synthetase activity in NAc but not in PFC as compared to ethanol drinking saline-treated rats. Our present study demonstrates that ceftriaxone treatment prevents ethanol drinking in part through normalization of extracellular glutamate concentrations in NAc of male P rats via GLT-1.
Keywords: Alcohol abuse, GLT-1, no-net-flux microdialysis, ceftriaxone, dihydrokainic acid
1. Introduction
Alcoholism is a common glutamate-related neuropsychiatric disorder (Tsai et al., 1995). An alteration of cortico-striatal glutamatergic neurotransmission is a hallmark of alcohol abuse. Furthermore, the changes in cortico-striatal glutamatergic-neurotransmission produced by ethanol include: 1) decreased levels of glutamate transporter 1 (GLT-1, its human homolog termed excitatory amino acid transporter 2, EAAT2) (Sari et al., 2013b) and cystine/glutamate exchanger (xCT) (Alhaddad et al., 2014) in nucleus accumbens (NAc) following chronic ethanol drinking, 2) increased extracellular glutamate concentrations in the NAc following acute ethanol administration (Dahchour et al., 2000, Melendez et al., 2005), 3) decreased glutamate uptake in the NAc following repeated ethanol exposure (Melendez et al., 2005), and 4) increased extracellular glutamate concentrations in NAc shell following chronic ethanol drinking (Ding et al., 2013).
Extracellular glutamate is regulated by several glutamate transporters (Gegelashvili and Schousboe, 1997, Seal and Amara, 1999, Anderson and Swanson, 2000), however GLT-1 regulates the majority of extracellular glutamate (Rothstein, 1995, Danbolt, 2001, Mitani and Tanaka, 2003). Ceftriaxone (CEF), a β-lactam antibiotic, is known to cross the blood-brain barrier (BBB) (Lucht et al., 1990, Prasil et al., 2010) and has been shown to upregulate GLT-1 (Rothstein et al., 2005, Lee et al., 2008, Ramos et al., 2010, Sari et al., 2010), xCT levels (Lewerenz et al., 2009, Knackstedt et al., 2010, Alhaddad et al., 2014) and produce sustained reductions of extracellular glutamate in the NAc of rats (Rasmussen et al., 2011b). Furthermore, CEF is known to have neuroprotective effects (Mimura et al., 2011) and attenuated cocaine-seeking (Sari et al., 2009, Knackstedt et al., 2010, Sondheimer and Knackstedt, 2011), cannabinoid tolerance (Gunduz et al., 2011), amphetamine-induced hyperactivity and behavioral sensitization (Rasmussen et al., 2011a), and morphine-evoked hyperthermia (Rawls et al., 2007). CEF has also been reported to attenuate both chronic and relapse-like ethanol drinking (Sari et al., 2011, Qrunfleh et al., 2013, Sari et al., 2013a, Sari et al., 2013b, Alhaddad et al., 2014, Rao and Sari, 2014), and ethanol-withdrawal manifestations (Abulseoud et al., 2014).
Drugs of abuse, including ethanol, activate dopaminergic projections from ventral tegmental area (VTA) to PFC and NAc. NAc receives glutamatergic inputs from PFC, hippocampus (Hipp) and amygdala (Amy) (Kalivas and O’Brien, 2008). The PFC-NAc glutamate projection commences adaptive behavior and Hipp/Amy-NAc glutamate projections help to retrieve previously experienced emotional and circumstantial information (Moussawi and Kalivas, 2010). Thus, NAc becomes a key brain region modulating addiction and has been focused in this study. Less is known about the role of extracellular glutamate in NAc following chronic ethanol consumption. Although CEF treatment produced sustained reductions of basal extracellular glutamate in NAc (Rasmussen et al., 2011b), it is unknown whether CEF modulates extracellular glutamate in NAc following chronic ethanol drinking. In this study, P rats voluntarily drank ethanol using a three-bottle choice paradigm for five weeks as a model of chronic alcoholism. We tested the hypothesis that chronic voluntary ethanol-drinking would increase the extracellular glutamate concentrations in NAc of P rats and CEF treatment would reverse the increases in extracellular glutamate after chronic voluntary ethanol drinking. We used in-vivo microdialysis with no-net-flux to measure extracellular concentrations of glutamate in NAc of P rats. Western blot analysis was used to examine the relative expression level of GLT-1. Furthermore, dihydrokainic acid, a GLT-1 blocker, was locally perfused into the NAc of CEF-treated P rats to investigate the contribution of GLT-1 to the modulation of extracellular glutamate by CEF. Since glutamatergic neurotransmission is regulated by the glutamate-glutamine cycle and glutamine synthetase (GS) (Tani et al., 2014) , we also investigated the effect of chronic ethanol-drinking and CEF treatment on GS activity in PFC and NAc of P rats.
2. Materials and methods
2.1. Subjects
Male P rats were shipped at their age of 3-4 weeks from Indiana University School of Medicine, Indianapolis; IN. At the age of 80-85 days, P rats were single housed with 21°C temperature and 50% humidity in a 12h light/dark cycle. Food and water were available unrestricted throughout the experiments; and all experimental procedures were conducted in light cycle. All housing and experimental procedures are approved by the Institutional Animal Care and Use Committee of The University of Toledo in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission of Life sciences, 1996).
2.2. Ethanol Drinking Procedures
At the age of 90 days, male P rats had continuous and free access to ethanol (15% and 30%, v/v, concurrently), and/ or water for five weeks. Ethanol and water intake were measured (as g/kg/day) three times per week during the last two weeks and served as baseline intake. During week six of ethanol drinking, rats were randomly divided into two groups: ethanol-drinking saline-treated (EtOH-Sal) rats and ethanol-drinking CEF-treated (EtOH-CEF) rats. The Water-drinking saline-treated (WD-Sal) group had free access to water and food only throughout the experiment. The EtOH-CEF group received CEF at a dose of 100 mg/kg (i.p.) once daily for five consecutive days during week six. Both WD-Sal and EtOH-Sal groups received saline (i.p.) once daily for five consecutive days during week six. The stereotaxic surgery for implantation of microdialysis probes was performed on day six and in-vivo reverse microdialysis with no-net-flux was performed on day seven of week six. Another group of CEF-treated rats was perfused with DHK (500 μM) during the microdialysis procedure and termed as EtOH-CEF+DHK group.
CEF 100 mg/kg/day was used because it was reported to significantly increase the level of GLT-1 in both PFC and NAc of P rats (Sari et al., 2011, Sari et al., 2013b). This dose also significantly reduced the baseline extracellular glutamate concentrations in the NAc of rats (Rasmussen et al., 2011b).
2.3. In-vivo reverse microdialysis (no-net-flux microdialysis)
Probes for reverse microdialysis were constructed as previously described (Halpin et al., 2014). Briefly, probes were made of PE 20 tubing (Becton Dickinson), silica tubing (OD, 150 μm; Polymicro Technologies), 26 ga stainless steel hypodermic tubing (Small parts), Hollow Fiber Microdialysis Membrane ( active membrane, 1.7 mm; 13,000 molecular cutoff; 216 μm outer diameter; Spectrum Laboratories), 2 ton waterproof epoxy and tygon microbore tubing. Surgeries for the probe placement were performed a day before the microdialysis experiment. The microdialysis procedure on next day of probe placement has been shown to provide stable baseline glutamate concentration (Halpin et al., 2014). The probes were stereotaxically placed into the NAc (AP+1.8, ML+1.5, DV-6.5/-7mm). Xylazine (5 mg/kg) and ketamine (75 mg/kg) were used as anesthetics.
Artificial cerebrospinal fluid (aCSF) was perfused at a flow rate of 1.5 μl/min through the probes during next day. The flow rate was fixed at 1.5 μl/min throughout the microdialysis procedure. Following a 1 h equilibration period, baseline samples were collected every 30 min for 3 h after which the aCSF was switched to aCSF containing 2.5, 5, and 10 μM glutamate. Two samples were collected at 30 min interval for each concentration. For EtOH-CEF+DHK group, DHK (500 μM) was perfused for 30 min at flow rate of 1.5 μl/min prior to baseline samples collection and each concentration switch. Only rats with probe placement in NAc (core or shell) were included in the study. A concentration of 500 μM DHK was used based on previous literature showing effective blockade of GLT-1 (Fletcher and Johnston, 1991, Fischer et al., 2013).
2.4. Quantification of glutamate
Glutamate content of the dialysates was analyzed using an HPLC system (ESA, Inc) with electrochemical detection as previously described (Breier et al., 2006). Dialysate samples were derivatized with O-phthalaldehyde (OPA) and sodium sulfite with an ESA Model 540 autosampler before injecting onto a C18 column (3.0×50 mm, 2.5 μm particle size, Waters). The elution was performed with a mobile phase containing 0.1M Na2HPO4, 0.1mM EDTA, and 7.5% Methanol (pH 3.0). Glutamate was detected by CoulArray coulometric detector (model 5600A, ESA, Inc.), and the data were recorded using CoulArray software. Glutamate concentration in each dialysate was analyzed by peak height and compared with an external standard.
2.5. Brain Tissue Harvesting
After the microdialysis procedure, P rats were euthanized with CO2 inhalation and rapidly decapitated with a guillotine. Brains were removed and instantly frozen with dry ice and stored at −70°C.
2.6. Western Blot
After probe placement verification, the whole NAc in the hemisphere contralateral to the probe placement was dissected from cryostat sections through the NAc (Paxinos and Watson, 1998). The whole NAc was dissected since CEF increased the levels of GLT-1 in both the core and shell of NAc (Sari et al., 2013b). The cytoplasmic fraction of the dissected NAc was used to determine the level of GLT-1. Cytoplasmic and nuclear fractions were separated as described previously (Ahmed et al., 2006). Equal amounts of protein were loaded and separated by 10-20% tris-glycine gel (Life Technologies, Grand Island, NY). Proteins were then transferred onto PVDF membrane and incubated at room temperature with blocking buffer for 30 min. The membranes were incubated overnight at 4°C with guinea pig-anti GLT-1 (1: 5000, Millipore, 60 kDa). The membranes were then washed with TBST, incubated with HRP-linked secondary antibody (1:5000) at room temperature for 90 minutes, and developed using HRP chemiluminescent kit (SuperSignal West Pico, Pierce Inc.). Furthermore, membranes were exposed to Kodak BioMax MR films (Fisher Inc.), and the films were developed by SRX-101A machine. The bands were quantified using MCID software and normalized to a β-tubulin (50 kDa) internal loading control.
2.7. Glutamine Synthetase (GS) Activity Assay
Cytoplasmic fractions of PFC and NAc were used to determine glutamine synthetase (GS) activity. GS activity was measured by γ-glutamyl transfer assay as previously described (Miller et al., 1978, van der Vos et al., 2012). Briefly, equal volume of cytoplasmic fractions of PFC and NAc were mixed with assay mixture (50 mM imidazole chloride (pH 7.4), 50 mM L-glutamine, 25 mM hydroxylamine, 25 mM Na-arsenate, 2 mM MnCl2 and 0.16 mM ADP) and incubated in a 96-well plate at 37°C for 30 min. The reaction was terminated by the addition of GS stop solution (2.42% FeCl3, 1.45% trichloroacetic acid and 1.82 N HCl). The absorbance of the formed γ-glutamyl hydroxamate was measured at 570 nm and converted into nanomoles by a calibration curve using authentic γ-glutamyl hydroxamate. Parallel incubations of cytoplasmic fractions with assay mixture lacking Na-arsenate and ADP served as blank.
2.8. Statistical Analysis
Differences in ethanol intake, ethanol preference (amount of ethanol consumed/total amount of fluid consumed × 100) and body weight between EtOH-Sal and EtOH-CEF groups were analyzed with two-way (repeated measure) ANOVA followed by Bonferroni’s multiple comparison test. No-net-flux microdialysis data were analyzed with linear regression analysis where the x-intercept and slope represented extracellular glutamate concentrations and probe recovery, respectively. Differences in extracellular glutamate concentration and probe recovery were further analyzed with one-way ANOVA followed by Tukey’s post hoc test. Western blot and GS activity assay data were analyzed with one-way ANOVA followed by Tukey’s post hoc test. All statistical analyses were performed using GraphPad Prism. All data are presented as mean ± SEM.
2.9. Drugs
CEF was purchased from Apotex Corp (USA) and dissolved in saline solution (0.9% NaCl). Dihydrokainic acid (DHK) was purchased from Tocris Bioscience (Ellisville, MO, USA) and dissolved in dialysis buffer.
3. Results
3.1. Attenuation of Ethanol Intake and Ethanol Preference by Ceftriaxone Treatment
We determined the effects of CEF treatment on ethanol intake and ethanol preference as well as body weights. Two-way (repeated measure) ANOVA showed a significant effect of day on ethanol intake [F (5, 35) = 9.84, p<0.0001], a significant effect of treatment [F (1, 7) = 83.77, p<0.0001], and a significant day × treatment interaction [F (5, 35) = 14.25, p<0.0001]. Bonferroni’s multiple comparison test revealed a significant reduction in ethanol intake in EtOH-CEF group compared to EtOH-Sal group (p<0.0001 for day 1 through day 5) (Fig. 1A).
Figure 1.
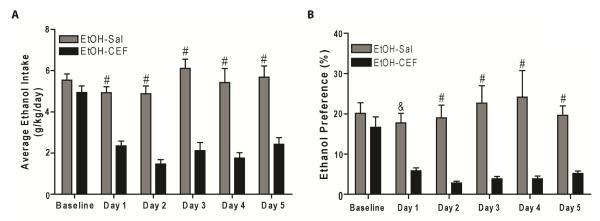
Effect of CEF treatment on ethanol intake and ethanol preference in male P rats. P rats had free access to ethanol (15% and 30%, concurrently) and/or water for five weeks. P rats were treated with CEF (100 mg/kg/day, i.p.) or saline (i.p.) for five consecutive days during week six. Ethanol and water intake were measured during the last two weeks and served as baseline intake. A. CEF treatment significantly reduced ethanol intake in EtOH-CEF group during treatment period (day 1 through day 5) compared to the EtOH-Sal group (#, p<0.0001). B. CEF treatment significantly decreased ethanol preference in EtOH-CEF group during treatment period (day 1 through day 5) compared to the EtOH-Sal group (&, p<0.001; #, p<0.0001). All data are expressed as mean ± SEM. n=8/ group. EtOH, ethanol; Sal, Saline; CEF, ceftriaxone.
A two-way (repeated measure) ANOVA conducted on ethanol preference revealed a significant effect of day [F (5, 35) = 3.33, p=0.01], a significant effect of treatment [F (1, 7) = 18.66, p=0.003] and a significant interaction between day and treatment [F (5, 35) = 6.04, p=0.0004]. Bonferroni’s multiple comparison test revealed that CEF treatment significantly decreased ethanol preference in EtOH-CEF group compared to EtOH-Sal group (p<0.001 for day 1 and p<0.0001 for day 2 through day 5). Baseline ethanol preference between EtOH-Sal and EtOH-CEF groups was not significantly different (p>0.99) (Fig. 1B).
3.2. Ceftriaxone Treatment Normalizes Extracellular Glutamate Concentration in NAc
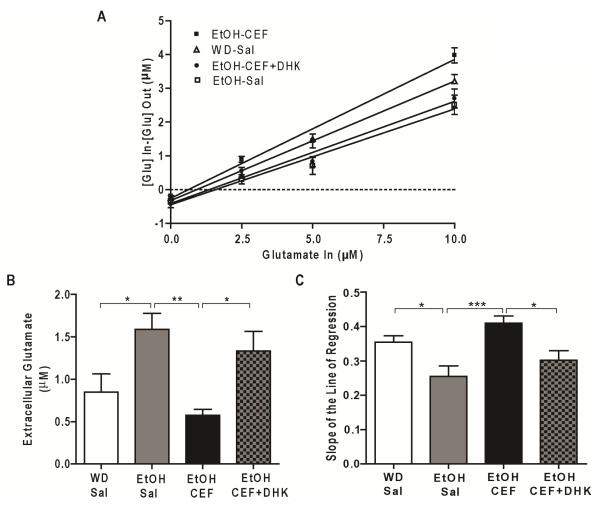
Figure 2 shows microdialysis probe placements in NAc. We conducted a one-way ANOVA on basal glutamate concentrations (x-intercept of linear regression curve when y=0) and on the slopes of the linear regression analysis. A one-way ANOVA conducted on basal glutamate data revealed a significant main effect among WD-Sal, EtOH-Sal, EtOH-CEF and EtOH-CEF+DHK groups [F (3, 24) =6.48, p=0.002] (Fig 3A). Tukey’s multiple comparison test revealed that the extracellular glutamate level in NAc is significantly higher in EtOH-Sal group compared to WD-Sal group (p=0.03) (Fig. 3B). CEF treatment for five consecutive days significantly reduced extracellular glutamate level in NAc in EtOH-CEF group compared to EtOH-Sal group (p=0.002). Extracellular glutamate concentrations in NAc in EtOH-CEF group were not significantly different from WD-Sal group (p=0.71). Importantly, blockade of GLT-1 by perfusion of DHK (500 μM) significantly increased extracellular glutamate concentrations in NAc in EtOH-CEF+DHK group compared to EtOH-CEF group (p=0.04). Extracellular glutamate in NAc in EtOH-CEF+DHK group was not significantly different from EtOH-Sal group (p=0.76) (Fig. 3B).
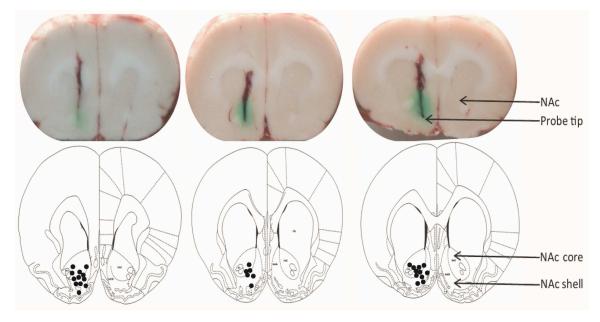
Figure 2.
Coronal slices of rat brains showing microdialysis probe tips in NAc. The dots in bottom pictures [adapted from Paxinos G and Watson C. (1998)] represent the microdialysis probe tips of rats included in the study.
Figure 3.
Effect of chronic ethanol-drinking and CEF treatment on extracellular glutamate concentration in NAc of P rats. A. CEF (100 mg/kg, i.p., every 24h×5) treatment restored extracellular glutamate concentration in NAc, and the effect of CEF was reversed by intra-NAc infusion of DHK (500 μM). B. Extracellular glutamate concentration in EtOH-Sal was significantly increased in NAc following five-week of voluntary ethanol drinking as compared to the water drinking saline-treated (WD-Sal) group. CEF treatment restored glutamate concentration in NAc. In addition, intra-NAc infusion of DHK (500 μM) significantly increased extracellular glutamate concentration in EtOH-CEF group. C. EtOH-Sal group displayed reduced slope of the line of regression compared to WD-Sal animals, and CEF-treated animals increased slope of the line of regression compared to the EtOH-Sal group. Intra-NAc infusion of DHK reversed slope of the line of regression in EtOH-CEF animals. *p<0.05; **p<0.01; and ***p<0.001. n= 6-8/group. WD, water drinking; Sal, saline; EtOH, ethanol; CEF, ceftriaxone; DHK, dihydrokainate.
A one-way ANOVA conducted on slope of regression analysis data revealed a significant main effect among groups [F (3, 24) =7.37, p=0.001]. The slope of linear regression line (probe recovery) was significantly lower in EtOH-Sal group compared to WD-Sal group (p=0.03). CEF treatment normalized the slope of linear regression line in EtOH-CEF group compared to the EtOH-Sal group (p=0.0008). In addition, the blockade of GLT-1 significantly lowered the slope of regression line in EtOH-CEF+DHK group compared to EtOH-CEF group (p=0.03). The slope of regression line in EtOH-Sal group was not significantly different from EtOH-CEF+DHK group (p=0.57) (Fig. 3C).
3.3. Chronic Ethanol Consumption Caused Downregulation of GLT-1 Level and Ceftriaxone Treatment Normalized GLT-1 Level
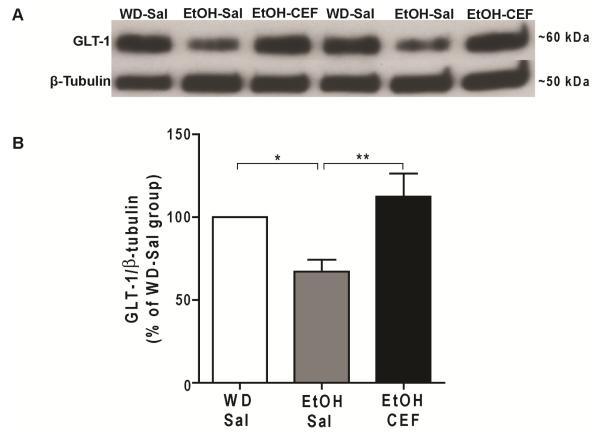
We used western blot analysis to test the effect of voluntary ethanol-drinking and CEF treatment on expression level of GLT-1 in NAc (Fig. 4A). One-way ANOVA, followed by Tukey’s post hoc, revealed a significant main effect of ethanol-drinking and CEF treatment on GLT-1 level [F (2, 21) = 6.85, p= 0.005]. Tukey’s multiple comparison test revealed a significantly lower level of GLT-1 in EtOH-Sal group compared to WD-Sal group (p=0.04), and significantly higher level of GLT-1 in EtOH-CEF group compared to EtOH-Sal group (p=0.004). There was no significant difference in GLT-1 level between WD-Sal and EtOH-CEF groups (Fig. 4B).
Figure 4.
Effect of voluntary ethanol drinking and CEF treatment on GLT-1 expression level in NAc. A. Representative Western blots for GLT-1 and β-tubulin as loading control. B. GLT-1 level was significantly downregulated in NAc of EtOH-Sal group compared to WD-Sal group. CEF treatment restored GLT-1 level in NAc of EtOH-CEF group compared to EtOH-Sal group. *p<0.05, **p<0.01 (n=8/group). WD, water drinking; Sal, saline; EtOH, ethanol; CEF, ceftriaxone.
3.4. Increase in GS Activity by CEF Treatment in PFC and NAc of P rats
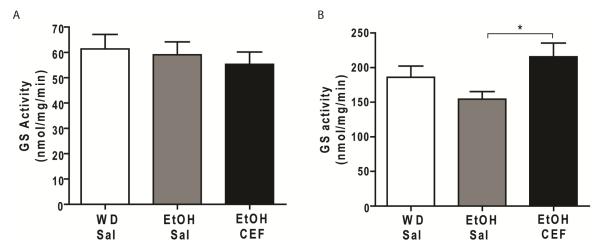
We further tested the effect of voluntary ethanol-drinking on GS activity in PFC and NAc of P rats. In PFC, one-way ANOVA revealed no significant main effect among WD-Sal, EtOH-Sal and EtOH-CEF groups [F (2, 18) = 0.34, p= 0.71] (Fig. 5A). Tukey’s multiple comparison test revealed no significant difference among WD-Sal, EtOH-Sal and EtOH-CEF groups. In NAc, One-way ANOVA, followed by Tukey’s post hoc, revealed a significant main effect among WD-Sal, EtOH-Sal and EtOH-CEF groups [F (2, 20) = 3.623, p= 0.04]. Tukey’s multiple comparison tests revealed that GS activity was not significantly different between EtOH-Sal and WD-Sal groups following five-week of voluntary ethanol drinking (p=0.34) (Fig. 5B). We further tested whether CEF treatment had any effect on GS activity in NAc. CEF treatment indeed significantly increased GS activity of NAc in EtOH-CEF group compared to EtOH-Sal group (p=0.03). GS activity in NAc was not significantly different between WD-Sal and EtOH-CEF groups (p=0.40) (Fig. 5B).
Figure 5.
Effect of chronic ethanol-drinking and CEF treatment on glutamine synthetase (GS) activity. A. GS activity was not significantly different among WD-Sal, EtOH-Sal and EtOH-CEF groups in PFC. B. GS activity was not significantly altered following five weeks of voluntary ethanol drinking in NAc of EtOH-Sal group compared to WD-Sal group. However, CEF increased GS activity in NAc of EtOH-CEF group compared to the EtOH-Sal group. *p<0.05 (n=7-8/group). WD, water drinking; Sal, saline; EtOH, ethanol; CEF, ceftriaxone.
4. Discussion
Our current study is unique in several aspects. First, we used reverse microdialysis (no-net-flux microdialysis) to evaluate the effect of CEF treatment on extracellular glutamate level in NAc of P rats following chronic ethanol drinking. Second, we blocked GLT-1 through perfusion of DHK to investigate the involvement of GLT-1 in CEF-modulated glutamate level in NAc of P rats. Third, we also investigated the effect of CEF treatment on GS activity in NAc of P rats. The present findings showed that CEF treatment significantly attenuated voluntary ethanol drinking in male P rats using three-bottle choice (15% ethanol, 30% ethanol and water) drinking paradigm. The attenuated ethanol-drinking was associated with a significant decrease in ethanol preference during the five days of CEF treatment. However, CEF treatment didn’t cause any significant changes on the body weight of male P rats (data not shown). These results are in accordance with previous findings that showed an attenuation of both chronic (Sari et al., 2011, Alhaddad et al., 2014), and relapse-like (Qrunfleh et al., 2013) ethanol drinking in P rats, and decreased ethanol preference in mice (Lee et al., 2013) treated with CEF. Here, we used a three-bottle choice drinking model because P rats were found to drink more than 4 g/kg/day with this paradigm (Sari et al., 2011, Sari et al., 2013b, Alhaddad et al., 2014). The voluntary ethanol drinking paradigm is also known to produce physical dependence on ethanol (Kampov-Polevoy et al., 2000) and produce pharmacologically relevant blood alcohol concentrations (BACs) of 50-200 mg% (Murphy et al., 2002, McBride et al., 2013).
In the present study, in-vivo microdialysis revealed that a high amount of voluntary ethanol drinking (≥4 g/kg/day) was associated with significantly higher extracellular glutamate level in NAc of ethanol-drinking saline (EtOH-Sal) rats compared to water-drinking saline-treated (WD-Sal) rats. These are in accordance with previous findings, which reported increased glutamate levels and decreased glutamate uptake in NAc following ethanol exposure (Melendez et al., 2005, Ding et al., 2013, Griffin Iii et al., 2014). The increased glutamate has also been reported during the ethanol-withdrawal period (Rossetti et al., 1999). It is very important to note that our P rats were not in withdrawal during the microdialysis procedure since P rats have been reported to show onset of withdrawal symptoms only after 20 hours of cessation of severe ethanol-intoxication only (Chefer et al., 2011, Abulseoud et al., 2014).
Importantly, we revealed that CEF normalized the extracellular glutamate concentrations in NAc as compared to EtOH-Sal rats. This effect is in accordance with previous reports showing normalization of accumbal glutamatergic neurotransmission by CEF treatment (Rasmussen et al., 2011b, Trantham-Davidson et al., 2012). To investigate the mechanism by which CEF normalizes glutamate in NAc, we blocked GLT-1 though perfusion of DHK in NAc of CEF-treated P rats. Interestingly, blockade of GLT-1 in CEF-treated P rats by perfusion of DHK significantly increased the glutamate concentrations in NAc. The reversal of CEF effect by DHK suggests that CEF normalized extracellular glutamate through modulation of GLT-1. Since DHK was perfused through microdialysis probe, there is possibility of presence of DHK in dialysate samples. Hence, we verified that DHK was not co-eluted with glutamate in HPLC system. This confirms that the increased glutamate concentration in DHK-perfused rats was due to the pharmacological action of DHK, but not for co-elution of DHK with glutamate in HPLC system.
The observed changes in glutamate in EtOH-Sal rats indicate decreased clearance of glutamate (Bungay et al., 2003, Trantham-Davidson et al., 2012). The decreased glutamate clearance might arise from dysregulation of Na+-dependent glutamate transporters such as EAATs. Among all five subtypes of EAATs, GLAST (EAAT1) and GLT-1 (EAAT2) account for the majority of glutamate transport in CNS. GLT-1 is the major glutamate transporter in forebrain and striatum (Rothstein, 1995, Danbolt, 2001, Mitani and Tanaka, 2003, Abulseoud et al., 2014) and consequently plays a key role in maintaining striatal glutamate homeostasis (Rothstein, 1995, Danbolt, 2001, Mitani and Tanaka, 2003, Melendez et al., 2005). Thus, the decrease in GLT-1 following chronic voluntary ethanol drinking may be related to increased extracellular glutamate concentrations in NAc. This finding is consistent with previous reports showing downregulation of GLT-1 in NAc following chronic voluntary ethanol drinking (Sari and Sreemantula, 2012, Sari et al., 2013b).
The decreased GLT-1 immunoreactive protein found in our present study differs from other studies showing no change of GLT-1 level following ethanol administration (Melendez et al., 2005, Ding et al., 2013). This disparity might be due to five-week of voluntary ethanol drinking in contrast to acute systemic ethanol administration or differential chronic ethanol exposure used in previous studies. It remains to be examined if the change in extracellular glutamate after ethanol exposure is mediated primarily by the downregulation of GLT-1 protein or the impaired transport function of GLT-1. Regardless, CEF treatment normalized GLT-1 protein levels in NAc and is consistent with earlier reports showing GLT-1 upregulation in NAc by CEF (Rothstein et al., 2005, Sari et al., 2010, Sari et al., 2011, Qrunfleh et al., 2013, Sari et al., 2013a, Sari et al., 2013b, Abulseoud et al., 2014, Rao and Sari, 2014).
Furthermore, we report here that CEF treatment did not show any significant effect on GS activity in PFC of P rats. But CEF treatment significantly increased GS activity in NAc of EtOH-CEF treated rats as compared to ethanol exposed rats. GS is present in astrocytes and converts imported glutamate (via EAATs) into glutamine (Miguel-Hidalgo, 2006). Although, GS activity is decreased in astrocytes by ethanol treatment (Davies and Vernadakis, 1984), we did not observe any significant difference in GS activity in NAc between WD-Sal and EtOH-Sal P rats. This discrepancy might be due to neuroadaptative changes which occurred during the five-week of voluntary ethanol drinking. Our finding is consistent with another report that showed no significant difference in packing density of GS-immunoreactive astrocytes between water drinking and ethanol drinking P rats for 2 months (Miguel-Hidalgo, 2006). It has been shown that activation of EAATs stimulates the release of astrocytic glutamine, a converted product of glutamate by GS (Uwechue et al., 2012). Therefore, the increased GS activity in NAc with CEF treatment might be due to normalization of glutamate inflow through GLT-1 within astrocytes. We previously showed that GLT-1 expression was not significantly altered in PFC of P rats following five weeks of ethanol drinking (Sari and Sreemantula, 2012). Thus the unaltered GS activity in PFC might be correlated with unaltered GLT-1 level in PFC. Further studies are required to investigate the underlying direct mechanisms of action of CEF on GS activity in NAc of P rats.
In summary, consecutive five-day CEF treatment attenuated voluntary ethanol drinking as well as ethanol preference in male P rats. Five-week voluntary ethanol drinking was associated with the increase in extracellular glutamate concentrations in NAc that was reversed by CEF treatment. Blockade of GLT-1 by DHK reversed the effect of CEF treatment in extracellular glutamate suggesting the involvement of GLT-1 in the mechanism of CEF. Furthermore, ethanol-induced higher extracellular glutamate was associated, in part, with decreased expression of GLT-1 in NAc in a manner reversed by CEF treatment. In addition, CEF treatment increased GS activity in NAc of EtOH-CEF rats compared to EtOH-Sal rats. Overall, our present study suggests that CEF prevented ethanol drinking through normalization of extracellular glutamate concentrations in NAc via restoration of GLT-1 levels.
Highlights.
Ceftriaxone treatment blocked the increase of extracellular glutamate. > Blockade of GLT-1 by DHK reversed the effects of ceftriaxone. > Ceftriaxone treatment increased glutamine synthetase activity in NAc.
Acknowledgements
This work was supported by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism (NIAAA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health. The authors would like to thank Kaila Mugford and Yusuf Althobaiti for their contribution to microdialysis experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statements
The authors declare no conflict of interest.
References
- Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi DS. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1674–1684. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Pakozdi A, Koch AE. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis and rheumatism. 2006;54:2393–2401. doi: 10.1002/art.22023. [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014;231:4049–4057. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Breier JM, Bankson MG, Yamamoto BK. L-tyrosine contributes to (+)-3,4-methylenedioxymethamphetamine-induced serotonin depletions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:290–299. doi: 10.1523/JNEUROSCI.3353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. Journal of neurochemistry. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer V, Meis J, Wang G, Kuzmin A, Bakalkin G, Shippenberg T. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addiction biology. 2011;16:229–237. doi: 10.1111/j.1369-1600.2010.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, Hoffman A, Deitrich R, de Witte P. Effects of ethanol on extracellular amino acid levels in high-and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol. 2000;35:548–553. doi: 10.1093/alcalc/35.6.548. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Davies DL, Vernadakis A. Effects of ethanol on cultured glial cells: proliferation and glutamine synthetase activity. Brain research. 1984;318:27–35. doi: 10.1016/0165-3806(84)90059-2. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addiction biology. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:9319–9327. doi: 10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EJ, Johnston GA. Regional heterogeneity of L-glutamate and L-aspartate high-affinity uptake systems in the rat CNS. Journal of neurochemistry. 1991;57:911–914. doi: 10.1111/j.1471-4159.1991.tb08237.x. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Griffin Iii WC, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz O, Oltulu C, Ulugol A. Role of GLT-1 transporter activation in prevention of cannabinoid tolerance by the beta-lactam antibiotic, ceftriaxone, in mice. Pharmacology, biochemistry, and behavior. 2011;99:100–103. doi: 10.1016/j.pbb.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Halpin LE, Northrop NA, Yamamoto BK. Ammonia mediates methamphetamine-induced increases in glutamate and excitotoxicity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1031–1038. doi: 10.1038/npp.2013.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcoholism, clinical and experimental research. 2000;24:278–284. [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Ruby CL, Hinton DJ, Choi S, Adams CA, Young Kang N, Choi DS. Striatal adenosine signaling regulates EAAT2 and astrocytic AQP4 expression and alcohol drinking in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:437–445. doi: 10.1038/npp.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. The Journal of biological chemistry. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, Wiedau-Pazos M, Schubert D, Maher P, Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. Journal of neurochemistry. 2009;111:332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Lucht F, Dorche G, Aubert G, Boissier C, Bertrand AM, Brunon J. The penetration of ceftriaxone into human brain tissue. The Journal of antimicrobial chemotherapy. 1990;26:81–86. doi: 10.1093/jac/26.1.81. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li TK. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats - Animal models of alcoholism. Alcohol. 2013 doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism, clinical and experimental research. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol Alcohol. 2006;41:379–385. doi: 10.1093/alcalc/agl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RE, Hackenberg R, Gershman H. Regulation of glutamine synthetase in cultured 3T3-L1 cells by insulin, hydrocortisone, and dibutyryl cyclic AMP. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:1418–1422. doi: 10.1073/pnas.75.3.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura K, Tomimatsu T, Minato K, Jugder O, Kinugasa-Taniguchi Y, Kanagawa T, Nozaki M, Yanagihara I, Kimura T. Ceftriaxone preconditioning confers neuroprotection in neonatal rats through glutamate transporter 1 upregulation. Reprod Sci. 2011;18:1193–1201. doi: 10.1177/1933719111410710. [DOI] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. European journal of pharmacology. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behavior genetics. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sixth Edition Academic Press; New York: 1998. [Google Scholar]
- Prasil P, Buchta V, Paterova P, Hanovcova I. [Penetration of ceftriaxone into the cerebrospinal fluid and its relationship to inflammatory markers during bacterial meningitis] Klinicka mikrobiologie a infekcni lekarstvi. 2010;16:64–72. [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J Psychopharmacol. 2013;27:541–549. doi: 10.1177/0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos KM, Lewis MT, Morgan KN, Crysdale NY, Kroll JL, Taylor FR, Harrison JA, Sloane EM, Maier SF, Watkins LR. Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone: therapeutic efficacy in a range of experimental nervous system disorders. Neuroscience. 2010;169:1888–1900. doi: 10.1016/j.neuroscience.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Sari Y. Effects of Ceftriaxone on Chronic Ethanol Consumption: a Potential Role for xCT and GLT1 Modulation of Glutamate Levels in Male P Rats. Journal of molecular neuroscience : MN. 2014;54:71–77. doi: 10.1007/s12031-014-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B, Unterwald EM, Rawls SM. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug and alcohol dependence. 2011a;118:484–488. doi: 10.1016/j.drugalcdep.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BA, Baron DA, Kim JK, Unterwald EM, Rawls SM. beta-Lactam antibiotic produces a sustained reduction in extracellular glutamate in the nucleus accumbens of rats. Amino acids. 2011b;40:761–764. doi: 10.1007/s00726-010-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Tallarida R, Robinson W, Amin M. The beta-lactam antibiotic, ceftriaxone, attenuates morphine-evoked hyperthermia in rats. British journal of pharmacology. 2007;151:1095–1102. doi: 10.1038/sj.bjp.0707309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93:1135–1140. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- Rothstein JD. Excitotoxicity and neurodegeneration in amyotrophic lateral sclerosis. Clin Neurosci. 1995;3:348–359. [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sari Y, Franklin KM, Alazizi A, Rao PS, Bell RL. Effects of ceftriaxone on the acquisition and maintenance of ethanol drinking in peri-adolescent and adult female alcohol-preferring (P) rats. Neuroscience. 2013a;241:229–238. doi: 10.1016/j.neuroscience.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Prieto AL, Barton SJ, Miller BR, Rebec GV. Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. Journal of biomedical science. 2010;17:62. doi: 10.1186/1423-0127-17-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–335. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee MR, Choi DS. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. Journal of molecular neuroscience : MN. 2013b;51:779–787. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behavioural brain research. 2011;225:252–258. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, Reimer RJ. A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron. 2014;81:888–900. doi: 10.1016/j.neuron.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. The American journal of psychiatry. 1995;152:332–340. doi: 10.1176/ajp.152.3.332. [DOI] [PubMed] [Google Scholar]
- Uwechue NM, Marx MC, Chevy Q, Billups B. Activation of glutamate transport evokes rapid glutamine release from perisynaptic astrocytes. The Journal of physiology. 2012;590:2317–2331. doi: 10.1113/jphysiol.2011.226605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vos KE, Eliasson P, Proikas-Cezanne T, Vervoort SJ, van Boxtel R, Putker M, van Zutphen IJ, Mauthe M, Zellmer S, Pals C, Verhagen LP, Groot Koerkamp MJ, Braat AK, Dansen TB, Holstege FC, Gebhardt R, Burgering BM, Coffer PJ. Modulation of glutamine metabolism by the PI(3)K-PKB-FOXO network regulates autophagy. Nature cell biology. 2012;14:829–837. doi: 10.1038/ncb2536. [DOI] [PubMed] [Google Scholar]