Several immune-related toxic effects have been reported with ipilimumab therapy for cutaneous melanoma. We describe a novel reaction, to our knowledge, involving the choroidal vasculature and resulting in bilateral serous retinal detachments without overt inflammatory signs.
Report of a Case
A woman in her early 70s with acral lentiginous melanoma on her heel was initially treated with wide excision and sentinel lymph node resection followed by ipilimumab (3 mg/kg) adjuvant therapy per protocol. Prior to cycle 4 of ipilimumab therapy, she was found to have nodal recurrence. She underwent resection and cycle 4 was held per protocol as she was recovering from surgery. She was deemed to have no evidence of disease and received her first maintenance dose of ipilimumab per protocol during week 24.
Four weeks after her maintenance dose, the patient developed decreased vision, mild photophobia, and ocular tenderness on palpation in each eye. The review of systems revealed nausea, itchiness, and weight loss. The only new medication she had received was ipilimumab. Her visual acuity was 20/40 OU and she had bilateral multifocal serous retinal detachments without signs of inflammation (Figure, A and B). Spectral-domain optical coherence tomography showed subretinal fluid (Figure, C). Fluorescein angiography findings were unremarkable (Figure, D). Ultrasonography showed relatively high signal posteriorly with possible thickening of the choroid in each eye. Findings on magnetic resonance imaging of the orbits were normal. Serum protein electrophoresis, rapid plasma reagin, fluorescent treponemal antibody absorption, antineutrophil cytoplasmic antibodies, myeloperoxidase, QuantiFERON-TB Gold, Toxoplasma gondii IgG, antiproteinase 3, angiotensin-converting enzyme, and lysozyme test results were unremarkable. Owing to development of ocular adverse effects and progressive disease, ipilimumab therapy was permanently discontinued and treatment with temozolomide and topical prednisolone was initiated.
Figure. Serous Detachments and Choroidopathy After Ipilimumab Therapy.
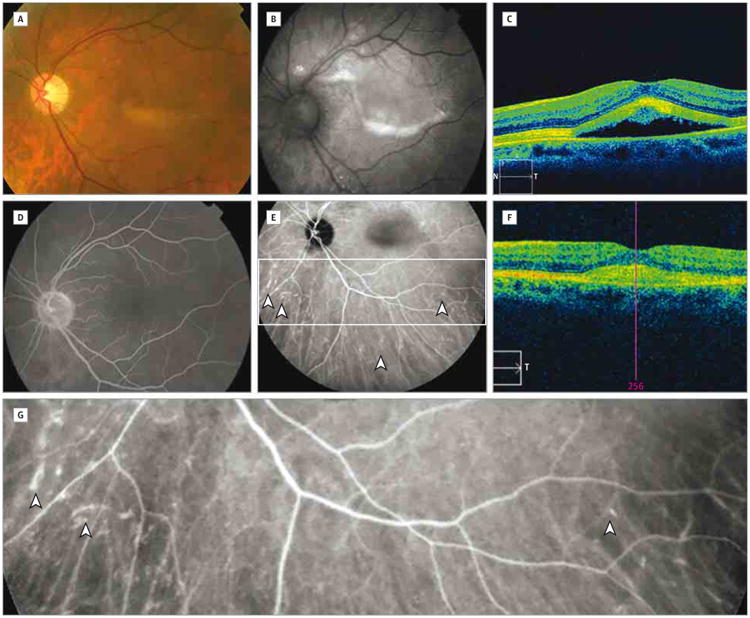
A-E, Color fundus photograph (A), autofluorescence photograph (B), spectral-domain optical coherence tomographic image (N indicates nasal; S, superior, and T, temporal) (C), fluorescein angiographic image (D), and indocyanine green angiographic image (E) before dexamethasone treatment. D, The fluorescein lacks abnormal hyperfluorescence. E, Arrowheads indicate staining of small and midsized choroidal vessels; box, area shown in G. F, Spectral-domain optical coherence tomographic image following dexamethasone treatment (T indicates temporal; pink line, foveal center). G, Magnified inset from E showing indocyanine green staining of small and midsized choroidal vessels (arrowheads).
After 1 month, the fundus was unchanged except for accumulation of yellowish subretinal material with increased autofluorescence (Figure, B). Indocyanine green angiography revealed late, moderate staining of small and midsized choroidal vessels in 2 quadrants of the right eye and 3 quadrants of the left eye (Figure, E and G). The angiographic score1 of vasculopathy was 2 in the right eye and 3 in the left eye. The patient began treatment with oral dexamethasone, 4 mg daily. After 6 weeks, the serous retinal detachments had resolved with residual hyperreflective subretinal material visible on spectral-domain optical coherence tomography (Figure, F), and indocyanine green angiography showed reduction in choroidal vessel staining. At 6 months, repeated indocyanine green angiography findings were negative for abnormal hyperfluorescence and visual acuity recovered to 20/25 OU.
Discussion
Ipilimumab's common adverse effects are inflammatory in nature.2 The choroidal findings in our patient may share the same pathophysiology as ipilimumab-related vasculopathies reported elsewhere in the body, including the nervous system.3 To our knowledge, this is the first case of ipilimumab-associated bilateral serous retinal detachments due to choroidal vascular injury. Our case has similarities to a case of ipilimumab-induced Vogt-Koyanagi-Harada syndrome with serous retinal detachments.4 However, our patient had significantly less intraocular inflammation documented, no symptoms of Vogt-Koyanagi-Harada syndrome, and no hyperfluorescence on fluorescein angiography. Indocyanine green angiography was helpful in revealing occult abnormal choroidal vascular hyperfluorescence. While the pathophysiology of this vascular injury is unclear, we hypothesize that it is due to an autoimmune or ischemic mechanism.
There is no consistent treatment duration associated with the development of retinal pathology. In 3 reports that we could identify, a granulomatous panuveitic Vogt-Koyanagi-Harada syndrome–like response developed 2 weeks after the first dose of ipilimumab,4 bilateral multifocal choroidal neovascularization developed in a patient receiving ipilimumab for 1 year,5 and a case of melanoma-associated retinopathy developed after the fourth cycle of ipilimumab.6 These cases were presumed to receive ipilimumab doses of 3 mg/kg.
Importantly in this patient, discontinuation of ipilimumab therapy and treatment with dexamethasone were associated with resolution of serous retinal detachments and anomalous findings on indocyanine green angiography, with restoration of vision.
Acknowledgments
Funding/Support: This work was supported by grant K08EY022672 from the National Eye Institute and by the Ohio Lions Eye Research Foundation and the Patti Blow Fund. ECOG 1609 provided drug and support for the clinical trial.
Role of the Funder/Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Contributor Information
Dimosthenis Mantopoulos, Havener Eye Institute, Department of Ophthalmology and Visual Science, The Ohio State University, Wexner Medical Center, Columbus.
Kari L. Kendra, Department of Internal Medicine, Division of Medical Oncology, The Ohio State University, Wexner Medical Center, Columbus.
Alan D. Letson, Havener Eye Institute, Department of Ophthalmology and Visual Science, The Ohio State University, Wexner Medical Center, Columbus.
Colleen M. Cebulla, Havener Eye Institute, Department of Ophthalmology and Visual Science, The Ohio State University, Wexner Medical Center, Columbus.
References
- 1.Tugal-Tutkun I, Herbort CP, Khairallah M Angiography Scoring for Uveitis Working Group. Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis) Int Ophthalmol. 2010;30(5):539–552. doi: 10.1007/s10792-008-9263-x. [DOI] [PubMed] [Google Scholar]
- 2.Voskens CJ, Goldinger SM, Loquai C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manousakis G, Koch J, Sommerville RB, et al. Multifocal radiculoneuropathy during ipilimumab treatment of melanoma. Muscle Nerve. 2013;48(3):440–444. doi: 10.1002/mus.23830. [DOI] [PubMed] [Google Scholar]
- 4.Wong RK, Lee JK, Huang JJ. Bilateral drug (ipilimumab)-induced vitritis, choroiditis, and serous retinal detachments suggestive of Vogt-Koyanagi-Harada syndrome. Retin Cases Brief Rep. 2012;6(4):423–426. doi: 10.1097/ICB.0b013e31824f7130. [DOI] [PubMed] [Google Scholar]
- 5.Modjtahedi BS, Maibach H, Park S. Multifocal bilateral choroidal neovascularization in a patient on ipilimumab for metastatic melanoma. Cutan Ocul Toxicol. 2013;32(4):341–343. doi: 10.3109/15569527.2013.781618. [DOI] [PubMed] [Google Scholar]
- 6.Audemard A, de Raucourt S, Miocque S, et al. Melanoma-associated retinopathy treated with ipilimumab therapy. Dermatology. 2013;227(2):146–149. doi: 10.1159/000353408. [DOI] [PubMed] [Google Scholar]


