Abstract
For the first time new treatments in melanoma have produced significant responses in advanced diseases, but 30–90% of melanoma patients do not respond or eventually relapse after the initial response to the current treatments. The resistance of these melanomas is likely due to tumor heterogeneity, which may be explained by models such as the stochastic, hierarchical, and phenotype-switching models. This review will discuss the recent advancements in targeting BCL-2 family members for cancer treatments, and how this approach can be applied as an alternative option to combat melanoma, and overcome melanoma relapse or resistance in current treatment regimens.
The need for developing alternative melanoma treatment options
Malignant melanoma is a devastating disease with historically poor prognosis. For the first time, several drugs provide a significant improvement in overall survival of these patients (Finn et al., 2012; Lo and Fisher, 2014; Menaa, 2013; Tronnier and Mitteldorf, 2014). These drugs fall into two categories: they either block the MAPK signaling pathway in BRAFV600E mutated melanoma, or they alter the patient’s own immune system to attack melanoma. Pathway-targeting drugs include BRAF inhibitors that specifically target the BRAFV600E mutation (vemurafenib and dabrafenib), or MEK inhibitors (trametinib and cobimetinib), either alone or in combination (Flaherty et al., 2012). Recent clinical trials with the combinations of both BRAF and MEK inhibitors showed improvements in progression-free survival compared to a single inhibitor in BRAFV600E melanoma patients, but also with increased side effects in some cases (Larkin et al., 2014; Long et al., 2014a; Long et al., 2014b). Although these treatments lead to a quick initial response, for instance ~ 50% of patients respond to vemurafenib, responses are not durable (Chapman et al., 2011). Most patients eventually relapse, and the intrinsic or acquired resistance mechanisms include reactivation of MAPK signaling through alternative pathways, or activation of other oncogenic pathways such as PI3K/AKT (Chapman, 2013; Fedorenko et al., 2011). In addition, patients without the BRAFV600E mutation do not benefit from these treatments.
For immunotherapy, the humanized monoclonal antibodies ipilimumab and pembrolizumab block the negative regulators of immune responses, thereby stimulating T-cell response against melanoma (Drake et al., 2014; Gyorki et al., 2013). Ipilimumab targets CTLA-4 (Hodi et al., 2010) and pembrolizumab targets PD-1 (Robert et al., 2014). Efficacy with ipilimumab is longer than with BRAF/MEK inhibitors, but response rate is poor at only 10–15% (Hodi et al., 2010). A distinct genetic landscape of neoantigens is reported to be present in melanomas with a strong response to ipilimumab (Snyder et al., 2014). Moreover, both antibodies lead to significant immune-mediated side effects (Finn et al., 2012), and relapse rate is not yet known for pembrolizumab. Therefore, many patients do not respond to current therapies, and there is still a pressing need to develop alternative treatment options for metastatic melanoma, especially for those with wild-type BRAF.
Human tumors are composed of heterogeneous cell populations. Tumor heterogeneity is implicated in tumor cells’ resistance to treatment, and may be explained by several models. The stochastic (clonal evolution) model states that every cell within a tumor has similar tumorigenic and self-renewing potential. These cells evolve and lead to heterogeneity, through clonal expansion with acquired genetic and epigenetic changes over time (Greaves and Maley, 2012; Nowell, 1976; Shackleton et al., 2009). This process is influenced by extrinsic factors including therapies. In contrast, the hierarchical model states that only a subpopulation of tumor cells, cancer stem cells (CSCs), possess self-renewal capacity and contribute to intra-tumoral heterogeneity by giving rise to non-CSCs within the same tumor (Clarke et al., 2006; Frank et al., 2010; Nguyen et al., 2012; Reya et al., 2001). This model suggests eliminating CSCs will help eradicating tumors. Cancer cell plasticity also contributes to tumor heterogeneity by mechanisms such as phenotype-switching. Cells may switch among proliferative, invasive and stem cell-like states in response to microenvironments (Cheli et al., 2011; Cheli et al., 2012; Hoek et al., 2008; Hoek and Goding, 2010). Moreover, a dynamic process mediated by a temporarily distinct subpopulation may also contribute to melanoma heterogeneity (Roesch et al., 2010). These models/mechanisms are not mutually exclusive. The CSC populations themselves are heterogeneous and dynamic, and may evolve through clonal evolution or display phenotype-switching (Lee et al., 2014; Nguyen et al., 2015). In melanoma, the high mutation load also likely contributes to heterogeneity. Interestingly, wild-type BRAF/NRAS melanomas seem to have a higher mutation load than mutant BRAF/NRAS tumors (Mar et al., 2013).
To prevent relapse, it is proposed that effective therapy needs to eradicate all subpopulations of tumor cells, killing the mass of cancer cells (de-bulking) as well as any resistant subpopulations such as CSCs (Han et al., 2013). One known cancer resistance mechanism is the dysregulation of BCL-2 family members, and recent studies indicate that this also contributes to the survival of CSCs. This review provides an overview of developing new melanoma treatments by targeting BCL-2 dysregulation with the purpose of de-bulking melanomas and eliminating drug-resistant subpopulations of CSCs in melanoma. We include the rationale, methods used, and current promising approaches.
The regulation of apoptosis by the BCL-2 family
The BCL-2 family of proteins plays a crucial role in regulating intrinsic or mitochondrial apoptosis (Czabotar et al., 2014). The members are divided into three groups based on their functions and structures: 1) anti-apoptotic proteins (also called inhibitors), which include BCL-2, BCL-XL, BCL-W, MCL-1, A1, and BCL-B; 2) multi-BH domain pro-apoptotic proteins (also called effectors), which include BAX and BAK; 3) and BH3-only pro-apoptotic proteins (also called activators or enhancers), which include NOXA, BIM, BID, BAD, BMF, BIK and others (Figure 1a). Upon activation, the effectors (BAX/BAK) oligomerize, triggering cytochome C release from the mitochondria and apoptosome activation, and finally result in caspase-9-dependent apoptosis (Czabotar et al., 2014). Anti-apoptotic proteins bind to BAX/BAK, preventing their activation. The balance of the interactions between different BCL-2 family members determines whether cells will initiate apoptosis (Chipuk et al., 2010; Mohana-Kumaran et al., 2014). Some BH3-only members only bind to one group of inhibitors, e.g., NOXA only binds to MCL-1/A1. Others can bind to multiple inhibitors, such as BIM, PUMA, and tBID.
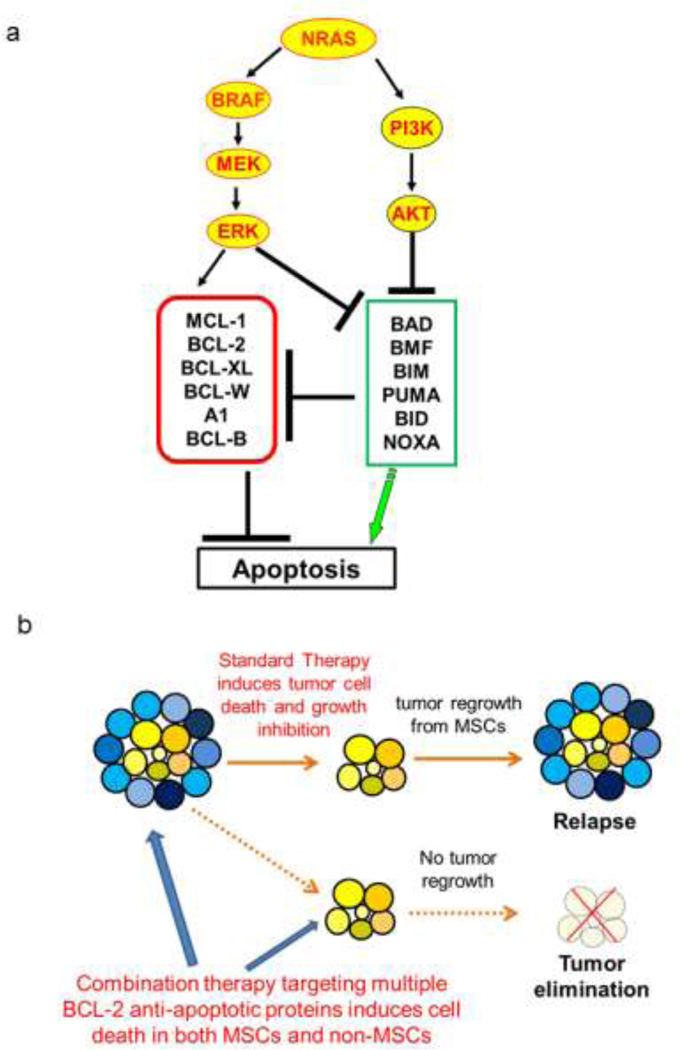
Figure 1. Targeting the BCL-2 family as a treatment option for melanoma.
(a) A simplified illustration for the rationale to target BCL-2 family members in melanoma. Green box represents the pro-apoptotic proteins, and red box represents the anti-apoptotic proteins. Multiple BCL-2 proteins are downstream of the RAS/BRAF/MAPK and PI3K/AKT signaling pathways, the commonly activated pathways in melanoma. The activation of these pathways leads to the dysregulated expression of multiple BCL-2 proteins, and likely contributes to resistance to cell death in melanoma. For instance, the activated RAS/BRAF/MAPK signal upregulates MCL-1 and blocks BIM and BAD (see text for details). Thus, targeting the BCL-2 family may provide an alternative way to combat melanoma regardless of its BRAF status, and to overcome melanoma relapse from current treatments. (b) Combination therapy debunks and kills the MSCs. The various blue cells represent heterogeneous non-MSCs populations, and the yellow cells represent the MSC populations. Standard therapy may be successful in debulking melanoma cells initially. However, it fails to kill MSCs, resulting in tumor relapse due to the self-renewal capacity of MSCs. The combination therapy targeting multiple BCL-2 anti-apoptotic members debulks and kills the MSCs, preventing future relapse of melanoma.
BAX/BAK oligomerization is necessary for activation of apoptosis, and the activation has been proposed to be direct or indirect (Czabotar et al., 2014). In the direct activation model, the BH3-only proteins directly bind to effectors (BAX/BAK) leading to activation. In the indirect model, binding of BH3-only proteins to the inhibitors releases the effectors BAX/BAK, allowing for activation. In the indirect model, apoptosis only occurs when all the anti-apoptotic members are neutralized. These two models are not mutually exclusive; one may be more dominant than the other, dependent on the circumstances. Nevertheless, in both models, the binding of BH3-only proteins to anti-apoptotic members can activate effectors and induce apoptosis (Czabotar et al., 2014). Thus, mimicking the pro-apoptotic BH3-only proteins by small molecules is a logical approach to develop cancer drugs (Billard, 2013; Mohana-Kumaran et al., 2014; Ni Chonghaile and Letai, 2008).
The rationale to target BCL-2 family members in melanoma
Dysregulation of BCL-2 family proteins occurs frequently, and plays a major role in conferring resistance to cell death in many cancers, including melanoma (Norris, 1995; Plati et al., 2011). Importantly, many BCL-2 proteins are downstream of the commonly activated signaling pathways RAS/BRAF/MAPK and PI3K/AKT (Figure 1a) (Haass and Schumacher, 2014; Kwong and Davies, 2013). For instance, oncogenic BRAFV600E suppresses apoptosis by upregulating anti-apoptotic MCL-1 (Haass and Schumacher, 2014; McKee et al., 2013). Also, through phosphorylation, constitutively activated RAS/BRAF/MAPK signals down-regulate or inactivate pro-apoptotic BAD (Eisenmann et al., 2003) or BIM (Goldstein et al., 2009). Thirdly, activated PI3K/AKT signals inhibit cell death by inactivating BAD’s ability to bind BCL-2 or BCL-XL (Datta et al., 1999; Steelman et al., 2011) or down-regulating transcription of pro-apoptotic members BIM and PUMA through sequestering the transcription factors FOXOs away from their promoters (Zhang et al., 2011). Activated AKT also prevents induction of pro-apoptotic BIM-EL and BMF upon BRAF inhibition, contributing to melanoma resistance to BRAF inhibitors (Shao and Aplin, 2010) (Figure 1a). Moreover, gene amplification results in up-regulation of anti-apoptotic BCL2A1 in some melanoma (Haq et al., 2013). Furthermore, the melanocyte-specific transcription factor (microphthalmia-associated transcription factor, MITF) is amplified in a subset of melanoma which can upregulate BCL-2 and contribute to resistance to cell death (Hartman and Czyz, 2015).
The effects of BRAF inhibitors in melanoma are dependent on the induction of BH3-only protein BIM and BMF (Shao and Aplin, 2010); BIM repression by epigenetic chromatin remodeling (Shao and Aplin, 2012) contributes to melanoma resistance to BRAF inhibition. Considering that some BCL-2 family members are regulated by MAPK or PI3K/AKT signaling and that activation of these pathways contributes to relapses from treatment of BRAF inhibitor, targeting downstream apoptotic proteins may be alternative options to overcome relapse. Indeed, the BAD-mimetic, ABT-737, has been shown to overcome AKT-mediated resistance to BRAF inhibition (Perna et al., 2015).
Taken together, multiple mechanisms lead to dysregulation of the BCL-2 family, and this likely contributes to melanoma development of resistance to current treatments (Figure 1a). Thus targeting the BCL-2 family is an alternative way to combat melanoma, independent of BRAF mutation status, and overcome melanoma relapse from current treatments.
De-bulking Cancer cells by targeting BCL-2 family members
Single Agents targeting BCL-2 or MCL-1
Early attempts at targeting BCL-2 family members included antisense, single-chain antibodies, ribozymes, BH3 peptides and hydrocarbon stapling. However, most failed, possibly due to poor delivery systems and the short-term stability of the compounds (see review (Thomas et al., 2013). Current more successful approaches focus on stable small molecule inhibitors (SMI), called “BH3 mimetics”, which mimic the pro-apoptotic BH3-only proteins to induce apoptosis in cancer cells (Table 1).
Table 1.
Examples of SMIs targeted BCL-2 family members in clinical trials or pre-clinical studies
| SMI | Target(s) | Clinical Trials or Pre-Clinical studies | ||
|---|---|---|---|---|
| Cancer Type | Combination Drug | Clinical Trials* | ||
| Gossypol | BCL-2 BCL-XL MCL-1 BCL-W BCL-B |
Adrenocortical carcinoma | N/A | II |
| Lymphoma | Paclitaxel, carboplatin | I | ||
| Advanced SCLC, solid tumors | Cisplatin, etoposide | I | ||
| Advanced prostate cancer | Bicalutamide | II | ||
| Obatoclax | BCL-2 MCL-1 BCL-XL BCL-W BCL-B BFL-1 |
Advanced SCLC | Carboplatin, etoposide | I/II |
| AML | N/A | II | ||
| CLL | N/A | I/II | ||
| Hodgkins lymphoma | N/A | II | ||
| MCL, Lymphoma | Bortezomib | I/II | ||
| NSCLC | Docetaxel | I/II | ||
| SCLC | Topotecan hydrochloride | I/II | ||
| ABT-263 (ABT-737) | BCL-2 BCL-XL BCL-W |
CLL, SCLC, Leukemia | N/A | II,I,II |
| Lymphoma, CLL, solid tumors | Ketoconaxole | I | ||
| CLL | Fludarabine, Cyclophosphomide, Rituximab | I | ||
| Solid tumors | Doxetaxel, Gemcitabine, Etoposide, Cisplatin, Paclitaxel | I | ||
| Lymphoma; CLL | Rifampin | I; II | ||
| ABT-199 | BCL-2 | SLL, NHL, CLL, AML | N/A | I; II |
| B-cell Lymphoma | Rituximab | IB | ||
| Maritoclax | MCL-1 | Melanoma | Single and with ABT-737; In vitro and In vivo | N/A |
| AML | Single; In vitro | |||
| Clitocine | MCL | hepatocellular carcinoma | Single; In vitro and In vivo | N/A |
| UMI-77 | MCL | Pancreatic cancer | Single; In vitro and In vivo | N/A |
The information for clinical trials is adapted from www.clinicaltrials.gov
Abbreviations: AML – acute myeloid leukemia, CLL – chronic lymphocytic leukemia, MCL – mantle cell lymphoma, NSCLC – non-small cell lung cancer, NHL – non-Hodgkins lymphoma, SCLC –small cell lung cancer, SLL – small lymphocytic leukemia, N/A–not available
Note: None of the MCL-1 inhibitors listed is in clinical trials yet, thus we provided the information on preclinical studies for these instead. More details can be found in reviews (Belmar and Fesik, 2014; Thomas et al., 2013).
Currently ABT-263 (navitoclax) and ABT-199 are in clinical trials, with encouraging preliminary results (Gandhi et al., 2011; Roberts et al., 2012; Wilson et al., 2010). ABT-263, an oral version of ABT-737, is a mimetic of BH3-only BAD that inhibits BCL-2, BCL-XL and BCL-W, but not MCL-1 (Oltersdorf et al., 2005; Tse et al., 2008; van Delft et al., 2006). ABT-199 was modified from ABT-263, with a sub-nano-molar affinity for BCL-2 but not BCL-XL (Davids and Letai, 2013). Unfortunately, single agent treatment of melanoma with ABT-737/ABT-263 is not effective, with MCL-1 as the main contributor of resistance (Chen et al., 2007; Lucas et al., 2012; Miller et al., 2009).
MCL-1 overexpression is linked to the pathogenesis of multiple cancers, including melanoma (Boisvert-Adamo et al., 2009; Khodadoust et al., 2009; McKee et al., 2013; Thomas et al., 2010). Many groups are developing SMIs that target MCL-1 with the aim of overcoming cancer resistance to ABT-737/263. Promising compounds include Maritoclax, WP1130, UMI-77, Clitocine and Compound 11, with some efficacy in animal studies (Belmar and Fesik, 2014; Quinn et al., 2011; Sun et al., 2014; Wei et al., 2012). However, clinical trials are still needed (Table 1).
Combination Therapy
As mentioned above, targeting a single BCL-2 family member is not sufficient to kill melanoma. In addition, therapeutics with single molecular targets often fails due to the heterogeneity and dynamic nature of cancer cells. Thus, utilizing combination therapies is an emerging strategy to treat cancer. Combination therapy increases the number of cells responding to treatment, decreases the possibility of drug resistance, kills heterogeneous populations of cells within the tumor, and may also lower side-effects.
Several combination strategies target BCL-2 family members, with promising pre-clinical evidence (Table 1). As mentioned earlier, MCL-1 is the main mediator of melanoma resistance to BCL-2 inhibitors such as ABT-737, and NOXA is a pro-apoptotic BH3-only protein that inhibits MCL-1. Therefore, inhibiting BCL-2, combined with either inhibition of MCL-1 or activation of NOXA, is effective at killing melanoma(Lucas et al., 2012; Miller et al., 2009; Mukherjee et al., 2015; Reuland et al., 2011; Reuland et al., 2012). Another strategy is sensitizing melanoma with BCL-2 SMIs, coupling with molecular-targeted agents such as BRAF inhibitors (Wroblewski et al., 2013). The last strategy combines BCL-2 SMIs with immune therapies, again using BH3 mimetics such as ABT-737, to sensitize tumor cells to immunotherapies (Begley et al., 2009; Karlsson et al., 2013). All these approaches provide treatment opportunities that may limit escape mechanisms in different patients and enhance the possibilities for personalized cancer medicine approach to treatment. Although the above strategies are promising, none explored the effectiveness of specifically eradicating resistant subpopulations, which is an important contributor for melanoma resistance.
Cancer Stem Cells (CSCs) in melanoma
CSCs are defined as a subpopulation of cancer cells which possess two essential stem-cell functions: self-renewal and differentiation (Clarke et al., 2006). CSCs enhance cancer initiation, progression, metastasis, and chemo-resistance (Clarke et al., 2006; Nguyen et al., 2012; Pattabiraman and Weinberg, 2014). CSCs also contribute to intra-tumoral heterogeneity, with their ability to give rise to non-CSCs within the same tumor (Frank et al., 2010; Pattabiraman and Weinberg, 2014). To prevent relapse, targeting CSCs should be an important part of treatment strategies.
The gold standard method to confirm the existence of CSCs is to detect their capacity to self-renew and differentiate, using in vivo serial xenotransplantation assays (Clarke et al., 2006). Recently, several lineage tracing studies with genetically engineered mouse models provided further evidence for the existence of CSCs (Pattabiraman and Weinberg, 2014). To date, CSCs have been identified in many cancers (Pattabiraman and Weinberg, 2014; Tabarestani and Ghafouri-Fard, 2012).
Quintana and colleagues reported a high frequency of tumor-initiating cells (TICs) in human melanoma using highly permissive conditions, raising questions on the existence of CSCs in human melanoma (Quintana et al., 2008). However, TICs are not the same as CSCs with self-renewal and differentiation capacity (Shakhova and Sommer, 2013), and the high frequency of TICs does not disprove the presence of CSCs (see reviews (Lang et al., 2013; Lee et al., 2014; Nguyen et al., 2015; Shakhova and Sommer, 2013)). Rather, Quintana’s papers simply suggest that TICs are influenced by many experimental factors. Indeed, when using the gold standard in vivo assays, numerous groups have detected subpopulations of melanoma cells that fulfill the criteria for CSCs (Lang et al., 2013; Lee et al., 2014; Nguyen et al., 2015; Shakhova and Sommer, 2013), and these cells are associated with tumor progression and chemoresistance.
Several melanoma stem cell (MSC) markers have been suggested in peer-reviewed literatures, and these include cell surface markers ABCB5 (Schatton et al., 2008) and CD271 (Boiko et al., 2010; Civenni et al., 2011; Redmer et al., 2014), and activity of ALDH (a detoxifying enzyme) (Luo et al., 2012). ABCB5 belongs to the ATP-binding cassette (ABC) transporter superfamily, implicated in multidrug resistance. CD271 is a nerve growth factor receptor, and is also a marker of mesenchymal stem cells (Watson et al., 2013) and hypopharyngeal CSCs (Imai et al., 2013). However, recent evidence suggests that not all CD271+ cells are the same, and only the slow growing CD271+ population displays a high tumorigenic potential and stemness (Cheli et al., 2014). In addition, knockdown of CD271 alone decreases, but does not prevent, the accumulation of the stress-induced drug resistant cell population (Menon et al., 2015). These recent data suggest that CD271 may be an imperfect MSC marker.
In sum, subpopulations of melanoma cells possess characteristics of CSCs (tumorigenesis with self-renewal and differentiation capacities), with enhanced ability for melanoma initiation, maintenance, and resistance to treatments. Although various markers can enrich MSCs, the most critical functional trait of these subpopulations is their self-renewal capacity for long-term growth of melanoma. Therefore self-renewability of these subpopulations should be targeted to prevent relapse after treatment.
Eradicating CSCs by targeting BCL-2 family members
Ideally, cancer treatments should eliminate both non-CSCs (the bulk of the tumors) and CSCs (Frank et al., 2010; Rameshwar, 2014). Utilizing combination therapy is likely the best strategy for targeting all subpopulations in heterogeneous tumors, and promising new drugs should test the efficacy of killing CSCs.
Potential strategies that focus on CSCs include differentiating CSCs into mature cells with no self-renewal capacity, eliminating CSCs by targeting essential pathways, or disrupting their niche. A successful example of differentiation therapy is the use of all-trans retinoic acid on acute promyelocytic leukemia patients to differentiate the cancer cells into mature granulocytes (Pattabiraman and Weinberg, 2014). Another approach to kill CSCs is through targeting their dependent signaling pathways, such as NOTCH, ABC cassettes, NF-κB, TGF-β, WNT, and JAK-STAT (Chen et al., 2013; Pattabiraman and Weinberg, 2014). In addition, tumor microenvironment may serve as CSC’s niche and can also be a drug target to eliminate CSCs. For example, angiogenesis is crucial in maintaining tumor niches, and the melanoma niche encompasses a network consisting of not only endothelial-lined blood vessels, but also “vasculogenic mimicry” channels formed by melanoma cells (Lai et al., 2012). Drugs targeting angiogenesis (such as VEGF inhibitors) can disrupt tumor niche and indirectly eradicate CSCs (Frank et al., 2011; Lee et al., 2014; Pattabiraman and Weinberg, 2014; Schatton et al., 2008).
Numerous recent studies show that CSCs are especially vulnerable to SMIs targeting the BCL-2 family. Lagadinou et al showed BCL-2 was overexpressed in quiescent leukemia stem cells (LSCs) with low levels of ROS, and BCL-2 inhibitor ABT-263 selectively eradicated the LSCs (Lagadinou et al., 2013). In bone-marrow-resident human LSCs, splice-isoform switching favored expression of multiple pro-survival BCL-2 members, and a pan-BCL-2 inhibitor sensitized them to tyrosine kinase inhibitors (Goff et al., 2013). Other CSCs, such as breast and colon CSCs, displayed upregulated BCL-2 (Fulda, 2013). BCL-XL overexpression contributed to survival of colon CSCs (Colak et al., 2014) and lung CSCs (Zeuner et al., 2014). When treated with ABT-737 but not ABT-199, these cells died (Zeuner et al., 2014) or were sensitized to chemotherapies (Colak et al., 2014). Furthermore, suppressing MCL-1 may overcome the tumor-plasticity-induced drug resistance in multiple myeloma cells (Dalva-Aydemir et al., 2014). These findings highlight that inhibition of pro-survival BCL-2 family proteins is a promising approach to target chemotherapy-resistant CSCs and plasticity of tumors.
To our knowledge, few studies explored the BCL-2 family members as targets for eradicating heterogeneous melanomas or MSCs. Recently we identified the combination of a BCL-2 Inhibitor with the retinoid derivative fenretinide as a promising treatment, for both wild-type and mutant BRAF cells, and for non-MSCs and MSCs (Mukherjee et al., 2015) (Figure 1b). The combination synergistically inhibited melanoma growth in vitro and in vivo, disrupted melanoma spheres, decreased the percentage of ALDHhigh cells and inhibited the self-renewal capacity of MSCs. These effects were observed in melanoma cells with mutations of either BRAF or NRAS. Interestingly, single drug treatments increased characteristics of MSCs for some melanoma samples, and only the combination treatment significantly reduced the self-renewal capacity of MSCs in all the samples tested. Proliferation stopped post-treatment, with no re-growth of tumor cells. The mechanism of action for the combination involves antagonizing multiple anti-apoptotic BCL-2 members at once (Mukherjee et al., 2015) (Figure 1b). These results support the idea that combination treatments are more potent to eliminate MSCs or other resistant subpopulation and targeting multiple pro-survival BCL-2 family members is a promising approach for melanoma (Figure 1b).
Summary
By killing heterogeneous tumors and eliminating drug-resistant subpopulations, SMIs, targeting multiple BCL-2 family members, provide an option for melanoma, especially the wild-type BRAF melanomas. This approach thus offers an alternative way to combat melanoma and may help achieve longer lasting treatment effects.
ACKNOWLEDGEMENTS
This work was supported in part by a Southwestern Skin Cancer SPORE Pilot project and NIH/NIAMS R03AR064555 to YGS; and by a Veterans Administration merit grant from the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development) to DAN. We apologize to all the colleagues whose important work is not cited because of space constrain. We thank Karoline Lambert for her help on editing the manuscript.
Abbreviations
- MSC
Melanoma Stem Cells
- CSC
Cancer Stem Cells
- BH
BCL-2 homolog
- MITF
Microphthalmia-associated transcription factor
- SMI
Small Molecule Inhibitors
- LSC
Leukemia Stem Cells
- TIC
Tumor Initiating Cells
Footnotes
CONFLICT OF INTEREST
The authors declared no conflicts of interest.
REFERENCES
- Begley J, Vo DD, Morris LF, et al. Immunosensitization with a Bcl-2 small molecule inhibitor. Cancer immunology, immunotherapy : CII. 2009;58:699–708. doi: 10.1007/s00262-008-0592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmar J, Fesik SW. Small molecule Mcl-1 inhibitors for the treatment of cancer. Pharmacology & therapeutics. 2014 doi: 10.1016/j.pharmthera.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard C. BH3 mimetics: status of the field and new developments. Molecular cancer therapeutics. 2013;12:1691–1700. doi: 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- Boiko AD, Razorenova OV, van de Rijn M, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert-Adamo K, Longmate W, Abel EV, et al. Mcl-1 is required for melanoma cell resistance to anoikis. Molecular Cancer Research. 2009;7:549–556. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PB. Mechanisms of resistance to RAF inhibition in melanomas harboring a BRAF mutation. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2013 doi: 10.14694/EdBook_AM.2013.33.e80. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli Y, Bonnazi VF, Jacquel A, et al. CD271 is an imperfect marker for melanoma initiating cells. Oncotarget. 2014;5:5272–5283. doi: 10.18632/oncotarget.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli Y, Giuliano S, Botton T, et al. Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene. 2011;30:2307–2318. doi: 10.1038/onc.2010.598. [DOI] [PubMed] [Google Scholar]
- Cheli Y, Giuliano S, Fenouille N, et al. Hypoxia and MITF control metastatic behaviour in mouse and human melanoma cells. Oncogene. 2012;31:2461–2470. doi: 10.1038/onc.2011.425. [DOI] [PubMed] [Google Scholar]
- Chen K-F, Su J-C, Liu C-Y, et al. A novel obatoclax derivative, SC-2001, induces apoptosis in hepatocellular carcinoma cells through SHP-1-dependent STAT3 inactivation. Cancer letters. 2012;321:27–35. doi: 10.1016/j.canlet.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta pharmacologica Sinica. 2013;34:732–740. doi: 10.1038/aps.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Dai Y, Harada H, et al. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer research. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, et al. The BCL-2 family reunion. Molecular cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civenni G, Walter A, Kobert N, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Colak S, Zimberlin CD, Fessler E, et al. Decreased mitochondrial priming determines chemoresistance of colon cancer stem cells. Cell death and differentiation. 2014;21:1170–1177. doi: 10.1038/cdd.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, et al. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews Molecular cell biology. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Dalva-Aydemir S, Bajpai R, Martinez M, et al. Targeting the Metabolic Plasticity of Multiple Myeloma with FDA Approved Ritonavir and Metformin. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-14-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes & development. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Davids MS, Letai A. ABT-199: taking dead aim at BCL-2. Cancer cell. 2013;23:139–141. doi: 10.1016/j.ccr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nature reviews Clinical oncology. 2014;11:24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann KM, VanBrocklin MW, Staffend NA, et al. Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. Cancer Res. 2003;63:8330–8337. [PubMed] [Google Scholar]
- Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochemical pharmacology. 2011;82:201–209. doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn L, Markovic SN, Joseph RW. Therapy for metastatic melanoma: the past, present, and future. BMC medicine. 2012;10:23. doi: 10.1186/1741-7015-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. The New England journal of medicine. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. The Journal of clinical investigation. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank NY, Schatton T, Kim S, et al. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer research. 2011;71:1474–1485. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S. Regulation of apoptosis pathways in cancer stem cells. Cancer Lett. 2013;338:168–173. doi: 10.1016/j.canlet.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Gandhi L, Camidge DR, de Oliveira MR, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. Journal of clinical oncology. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DJ, Court Recart A, Sadarangani A, et al. A Pan-BCL2 inhibitor renders bone-marrow-resident human leukemia stem cells sensitive to tyrosine kinase inhibition. Cell Stem Cell. 2013;12:316–328. doi: 10.1016/j.stem.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein NB, Johannes WU, Gadeliya AV, et al. Active N-Ras and B-Raf inhibit anoikis by downregulating Bim expression in melanocytic cells. J Invest Dermatol. 2009;129:432–437. doi: 10.1038/jid.2008.227. [DOI] [PubMed] [Google Scholar]
- Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorki D, Callahan M, Wolchok JD, et al. The delicate balance of melanoma immunotherapy. Clinical & Translational Immunology. 2013;2:e5. doi: 10.1038/cti.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass NK, Schumacher U. Melanoma never says die. Experimental dermatology. 2014;23:471–472. doi: 10.1111/exd.12400. [DOI] [PubMed] [Google Scholar]
- Han L, Shi S, Gong T, et al. Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharmaceutica Sinica B. 2013;3:65–75. [Google Scholar]
- Haq R, Yokoyama S, Hawryluk EB, et al. BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4321–4326. doi: 10.1073/pnas.1205575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ML, Czyz M. Pro-survival role of MITF in melanoma. J Invest Dermatol. 2015;135:352–358. doi: 10.1038/jid.2014.319. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek KS, Eichhoff OM, Schlegel NC, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer research. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- Hoek KS, Goding CR. Cancer stem cells versus phenotype-switching in melanoma. Pigment cell & melanoma research. 2010;23:746–759. doi: 10.1111/j.1755-148X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- Imai T, Tamai K, Oizumi S, et al. CD271 defines a stem cell-like population in hypopharyngeal cancer. PLoS One. 2013;8:e62002. doi: 10.1371/journal.pone.0062002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H, Lindqvist AC, Fransson M, et al. Combining CAR T cells and the Bcl-2 family apoptosis inhibitor ABT-737 for treating B-cell malignancy. Cancer gene therapy. 2013;20:386–393. doi: 10.1038/cgt.2013.35. [DOI] [PubMed] [Google Scholar]
- Khodadoust MS, Verhaegen M, Kappes F, et al. Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer research. 2009;69:6405–6413. doi: 10.1158/0008-5472.CAN-09-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong LN, Davies MA. Navigating the therapeutic complexity of PI3K pathway inhibition in melanoma. Clin Cancer Res. 2013;19:5310–5319. doi: 10.1158/1078-0432.CCR-13-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Schwartz BE, Hsu M. CD133+ Melanoma Subpopulations Contribute to Perivascular Niche Morphogenesis and Tumorigenicity through Vasculogenic Mimicry. Cancer research. 2012;72:5111–5118. doi: 10.1158/0008-5472.CAN-12-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D, Mascarenhas JB, Shea CR. Melanocytes, melanocyte stem cells, and melanoma stem cells. Clinics in dermatology. 2013;31:166–178. doi: 10.1016/j.clindermatol.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. The New England journal of medicine. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- Lee N, Barthel SR, Schatton T. Melanoma stem cells and metastasis: mimicking hematopoietic cell trafficking? Laboratory investigation; a journal of technical methods and pathology. 2014;94:13–30. doi: 10.1038/labinvest.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JA, Fisher DE. The melanoma revolution: from UV carcinogenesis to a new era in therapeutics. Science. 2014;346:945–949. doi: 10.1126/science.1253735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. The New England journal of medicine. 2014a;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- Long GV, Stroyakovsky DL, Gogas H, et al. COMBI-d: A randomized, double-blinded, Phase III study comparing the combination of dabrafenib and trametinib to dabrafenib and trametinib placebo as first-line therapy in patients (pts) with unresectable or metastatic BRAFV600E/Kmutation-positive cutaneous melanoma. Journal Of Clinical Oncology; 2014 ASCO Annual Meeting; 2014b. abstr 9011. [Google Scholar]
- Lucas KM, Mohana-Kumaran N, Lau D, et al. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clinical Cancer Research. 2012;18:783–795. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]
- Luo Y, Dallaglio K, Chen Y, et al. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30:2100–2113. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar VJ, Wong SQ, Li J, et al. BRAF/NRAS wild-type melanomas have a high mutation load correlating with histologic and molecular signatures of UV damage. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4589–4598. doi: 10.1158/1078-0432.CCR-13-0398. [DOI] [PubMed] [Google Scholar]
- McKee DSH, CP R, et al. Oncogenic BRAF signalling increases Mcl-1 expression in cutaneous metastatic melanoma. Experimental dermatology. 2013;22:767–769. doi: 10.1111/exd.12254. [DOI] [PubMed] [Google Scholar]
- Menaa F. Latest approved therapies for metastatic melanoma: what comes next? Journal of skin cancer. 2013;2013:735282. doi: 10.1155/2013/735282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon DR, Das S, Krepler C, et al. A stress-induced early innate response causes multidrug tolerance in melanoma. Oncogene. 2015 doi: 10.1038/onc.2014.432. [DOI] [PubMed] [Google Scholar]
- Miller LA, Goldstein NB, Johannes WU, et al. BH3 mimetic ABT-737 and a proteasome inhibitor synergistically kill melanomas through Noxa-dependent apoptosis. J Invest Dermatol. 2009;129:964–971. doi: 10.1038/jid.2008.327. [DOI] [PubMed] [Google Scholar]
- Mohana-Kumaran N, Hill DS, Allen JD, et al. Targeting the intrinsic apoptosis pathway as a strategy for melanoma therapy. Pigment cell & melanoma research. 2014;27:525–539. doi: 10.1111/pcmr.12242. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Reuland SN, Lu Y, et al. Combining a BCL2 Inhibitor with the Retinoid Derivative Fenretinide Targets Melanoma Cells Including Melanoma Initiating Cells. J Invest Dermatol. 2015;135:842–850. doi: 10.1038/jid.2014.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LV, Vanner R, Dirks P, et al. Cancer stem cells: an evolving concept. Nature reviews Cancer. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Couts KL, Luo Y, et al. Understanding melanoma stem cells. Melanoma Management. 2015 doi: 10.2217/mmt.15.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Chonghaile T, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27(Suppl 1):S149–S157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DA. Differential control of cell death in the skin. Archives of dermatology. 1995;131:945–948. [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nature reviews Drug discovery. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna D, Karreth FA, Rust AG, et al. BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. Proceedings of the National Academy of Sciences of the United States of America. 2015 doi: 10.1073/pnas.1418163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integrative biology : quantitative biosciences from nano to macro. 2011;3:279–296. doi: 10.1039/c0ib00144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn BA, Dash R, Azab B, et al. Targeting Mcl-1 for the therapy of cancer. Expert opinion on investigational drugs. 2011;20:1397–1411. doi: 10.1517/13543784.2011.609167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshwar P. Future Challenges to Target Cancer Stem Cells. Enliven: Challenges Cancer Detect Ther. 2014;1:001. [Google Scholar]
- Redmer T, Welte Y, Behrens D, et al. The nerve growth factor receptor CD271 is crucial to maintain tumorigenicity and stem-like properties of melanoma cells. PLoS One. 2014;9:e92596. doi: 10.1371/journal.pone.0092596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuland SN, Goldstein NB, Partyka KA, et al. The combination of BH3-mimetic ABT-737 with the alkylating agent temozolomide induces strong synergistic killing of melanoma cells independent of p53. PLoS One. 2011;6:e24294. doi: 10.1371/journal.pone.0024294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuland SN, Goldstein NB, Partyka KA, et al. ABT-737 synergizes with Bortezomib to kill melanoma cells. Biol Open. 2012;1:92–100. doi: 10.1242/bio.2011035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. Journal of Clinical Oncology. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Quintana E, Fearon ER, et al. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Shakhova O, Sommer L. Testing the cancer stem cell hypothesis in melanoma: the clinics will tell. Cancer Lett. 2013;338:74–81. doi: 10.1016/j.canlet.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670–6681. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Aplin AE. BH3-only protein silencing contributes to acquired resistance to PLX4720 in human melanoma. Cell death and differentiation. 2012;19:2029–2039. doi: 10.1038/cdd.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. The New England journal of medicine. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman LS, Chappell WH, Abrams SL, et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JG, Li H, Li X, et al. Clitocine targets Mcl-1 to induce drug-resistant human cancer cell apoptosis in vitro and tumor growth inhibition in vivo. Apoptosis : an international journal on programmed cell death. 2014;19:871–882. doi: 10.1007/s10495-014-0969-0. [DOI] [PubMed] [Google Scholar]
- Tabarestani S, Ghafouri-Fard S. Cancer stem cells and response to therapy. Asian Pacific journal of cancer prevention : APJCP. 2012;13:5951–5958. [PubMed] [Google Scholar]
- Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS letters. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- Thomas S, Quinn BA, Das SK, et al. Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther Targets. 2013;17:61–75. doi: 10.1517/14728222.2013.733001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronnier M, Mitteldorf C. Treating advanced melanoma: current insights and opportunities. Cancer management and research. 2014;6:349–356. doi: 10.2147/CMAR.S49494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer research. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JT, Foo T, Wu J, et al. CD271 as a marker for mesenchymal stem cells in bone marrow versus umbilical cord blood. Cells, tissues, organs. 2013;197:496–504. doi: 10.1159/000348794. [DOI] [PubMed] [Google Scholar]
- Wei G, Margolin AA, Haery L, et al. Chemical genomics identifies small-molecule MCL1 repressors and BCL-xL as a predictor of MCL1 dependency. Cancer Cell. 2012;21:547–562. doi: 10.1016/j.ccr.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH, O'Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. The lancet oncology. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski D, Mijatov B, Mohana-Kumaran N, et al. The BH3-mimetic ABT-737 sensitizes human melanoma cells to apoptosis induced by selective BRAF inhibitors but does not reverse acquired resistance. Carcinogenesis. 2013;34:237–247. doi: 10.1093/carcin/bgs330. [DOI] [PubMed] [Google Scholar]
- Zeuner A, Francescangeli F, Contavalli P, et al. Elimination of quiescent/slow-proliferating cancer stem cells by Bcl-XL inhibition in non-small cell lung cancer. Cell death and differentiation. 2014;21:1877–1888. doi: 10.1038/cdd.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tang N, Hadden TJ, et al. Akt, FoxO and regulation of apoptosis. Biochimica et biophysica acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]