Abstract
IMPORTANCE
Caloric restriction mimetic drugs have geroprotective effects that delay or reduce risks for a variety of age-associated systemic diseases, suggesting that such drugs might also have the potential to reduce risks of blinding ophthalmologic conditions for which age is a major risk factor.
OBJECTIVE
To determine whether the caloric restriction mimetic drug metformin hydrochloride is associated with reduced risk of open-angle glaucoma (OAG) in persons with diabetes mellitus.
DESIGN, SETTING, AND PATIENTS
Retrospective cohort study of patients aged 40 years or older with diabetes mellitus and no preexisting record of OAG in a large US managed care network from January 1, 2001, through December 31, 2010.
EXPOSURES
Quantity of metformin and other prescribed diabetes medications as captured from outpatient pharmacy records.
MAIN OUTCOMES AND MEASURES
Risk of developing OAG.
RESULTS
Of 150 016 patients with diabetes mellitus, 5893 (3.9%) developed OAG. After adjusting for confounding factors, those prescribed the highest quartile of metformin hydrochloride (> 1110 g in 2 years) had a 25% reduced OAG risk relative to those who took no metformin (hazard ratio = 0.75; 95% CI, 0.59–0.95; P = .02). Every 1-g increase in metformin hydrochloride use was associated with a 0.16% reduction in OAG risk (adjusted hazard ratio = 0.99984; 95% CI, 0.99969–0.99999; P = .04), which predicts that taking a standard dose of 2 g of metformin hydrochloride per day for 2 years would result in a 20.8% reduction in risk of OAG. After accounting for potential confounders, including metformin and diabetic medications, the risk of developing OAG was increased by 8% (hazard ratio = 1.08; 95% CI, 1.03–1.13; P = .003) for each unit of increase in glycated hemoglobin level.
CONCLUSIONS AND RELEVANCE
Metformin use is associated with reduction in risk of developing OAG, and risk is reduced even when accounting for glycemic control in the form of glycated hemoglobin level. Other diabetes medications did not confer a similar OAG risk reduction. This study suggests that metformin may be affecting OAG risk on multiple levels, some involving improved glycemic control and some involving mechanisms outside glycemic control such as neurogenesis, inflammatory systems, or longevity pathways targeted by caloric restriction mimetic drugs. If confirmed by prospective clinical trials, these findings could lead to novel treatments for this sight-threatening disease.
Long-term caloric restriction (CR) can lengthen life span and reduce the risk of some age-associated diseases such as cancer, diabetes mellitus, and cardiovascular disease.1–3 The geroprotective effects of CR and CR mimetic drugs such as rapamycin and metformin hydrochloride are accompanied by changes in the amounts of different gene products produced, so that CR or CR mimetic treatment of an older individual can back shift the expression profile to resemble the expression profile of a younger person who is untreated or on an unrestricted diet.4–7
It is not known whether use of CR mimetic drugs such as metformin affects risk of age-associated eye diseases of great public health interest such as macular degeneration, diabetic retinopathy, cataract, or glaucoma. It is important to understand factors that can alter the risk of primary open-angle glaucoma (OAG), which typically manifests in late middle age or late age,8 affects more than 60 million individuals worldwide,9 and has caused blindness in more than 4.5 million individuals.9
We hypothesized that use of CR mimetic drugs such as metformin would be associated with reduced risk of late-onset eye diseases such as OAG. To test our hypothesis, we used data from a large nationwide health care claims database containing detailed billing records for more than 150 000 older individuals with diabetes mellitus, some of whom were being prescribed metformin, to compare the risk of developing OAG among users vs nonusers of metformin and to determine whether a dose-response relationship exists such that those who consume more metformin show a greater OAG risk reduction.
Methods
Data Source
This study used 10 years of data from the Clinformatics Data-Mart Database (OptumInsight), previously referred to as the i3 InVision DataMart database, a health claims database of longitudinal data for 40 million patients enrolled in a large, geographically diverse US managed care network from January 1, 2001, through December 31, 2010. Data on each enrollee include medical claims (inpatient, outpatient, skilled nursing facility), demographic (age, sex, race/ethnicity) and socioeconomic ( education, household net worth) information, outpatient laboratory test results (Table 1), and outpatient medication prescriptions filled (Table 2). All enrollees were fully enrolled in the pharmaceutical plan. The 14 million–patient subset used for this analysis included patients with 1 or more International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 360 through 379.9, Current Procedural Terminology, Fourth Edition codes for eye-related visits, diagnostic procedures, or therapeutic procedures (65091–68899 or 92002–92499), or other ICD-9-CM or Current Procedural Terminology codes submitted by an ophthalmologist or optometrist (eTable 1 in the Supplement). This database has been previously used to investigate other ocular conditions and their risk factors.10 The University of Michigan Institutional Review Board approved this as a nonregulated study not requiring consent from study participants because the data have been anonymized through the removal of protected health information.
Table 1.
Descriptive Statistics of the Study Population
| Covariate | Diabetic Patients, No. (%) | ||
|---|---|---|---|
| Did Not Develop OAG (n = 144123) |
Developed OAG (n = 5893) |
Total (N = 150016) |
|
| Age at plan enrollment, y | |||
| 40 to <45 | 9752 (6.8) | 227 (3.9) | 9979 (6.7) |
| 45 to <50 | 15069 (10.5) | 489 (8.3) | 15558 (10.4) |
| 50 to <55 | 21510 (14.9) | 858 (14.6) | 22 368 (14.9) |
| 55 to <60 | 27252 (18.9) | 1125 (19.1) | 28377 (18.9) |
| 60 to <65 | 24620 (17.1) | 999 (17.0) | 25 619 (17.1) |
| ≥65 | 45920 (31.9) | 2195 (37.3) | 48115 (32.1) |
| Sex | |||
| Male | 70263 (48.8) | 2854 (48.4) | 73117 (48.7) |
| Female | 73860 (51.3) | 3039 (51.6) | 76899 (51.3) |
| Race/ethnicity | |||
| European ancestry | 106876 (82.5) | 4008 (74.6) | 110884 (82.2) |
| African ancestry | 9885 (7.6) | 716 (13.3) | 10601 (7.9) |
| Latino | 8130 (6.3) | 415 (7.7) | 8545 (6.3) |
| Asian American | 3356 (2.6) | 181 (3.4) | 3537 (2.6) |
| Other | 1253 (1.0) | 55 (1.0) | 1308 (1.0) |
| Education | |||
| <High school | 2417 (1.8) | 135 (2.4) | 2552 (1.8) |
| High school diploma | 56675 (41.6) | 2408 (42.8) | 59083 (41.7) |
| Some college | 51987 (38.2) | 2130 (37.8) | 54117 (38.2) |
| College diploma | 24894 (18.3) | 950 (16.9) | 25844 (18.2) |
| Advanced degree | 196 (0.1) | 7 (0.1) | 203 (0.1) |
| Geographic region of residence | |||
| Northeast | 21552 (15.0) | 943 (16.0) | 22495 (15.0) |
| Southeast | 60714 (42.2) | 2538 (43.1) | 63252 (42.2) |
| Midwest | 47769 (33.2) | 1742 (29.6) | 49511 (33.0) |
| West | 13981 (9.7) | 665 (11.3) | 14646 (9.8) |
| Net worth, $ | |||
| 0 to <25000 | 10847 (8.3) | 497 (9.2) | 11344 (8.3) |
| 25000 to <75000 | 9186 (7.0) | 382 (7.1) | 9568 (7.0) |
| 75000 to <150000 | 18177 (13.8) | 824 (15.2) | 19001 (13.9) |
| 150000 to < 500000 | 62335 (47.5) | 2471 (45.6) | 64806 (47.4) |
| ≥500000 | 30765 (23.4) | 1247 (23.0) | 32012 (23.4) |
| Ocular comorbiditiesa | |||
| Nonexudative AMD | 14653 (10.2) | 788 (13.4) | 15441 (10.3) |
| Exudative AMD | 2839 (2.0) | 197 (3.3) | 3036 (2.0) |
| Cataract | 78299 (54.3) | 3768 (63.9) | 82067 (54.7) |
| Pseudophakia or aphakia | 23024 (16.0) | 1372 (23.3) | 24396 (16.3) |
| NPDR | 39313 (27.3) | 1950 (33.1) | 41263 (27.5) |
| PDR | 12430 (8.6) | 800 (13.6) | 13230 (8.8) |
| Cataract surgery | 27752 (19.3) | 1706 (29.0) | 29458 (19.6) |
| Retina surgery | 16194 (11.2) | 1093 (18.6) | 17287 (11.5) |
| Other comorbiditiesa | |||
| Hyperlipidemia | 131020 (90.9) | 5415 (91.9) | 136435 (91.0) |
| Obesity | 31888 (22.1) | 1416 (24.0) | 33304 (22.2) |
| Dementia | 3508 (2.4) | 176 (3.0) | 3684 (2.5) |
| Depression | 11104 (7.7) | 419 (7.1) | 11523 (7.7) |
| Hypertension | 128920 (89.5) | 5484 (93.1) | 134404 (89.6) |
| Type 1 diabetesb | 53078 (36.8) | 2437 (41.4) | 55515 (37.0) |
| Adapted CCI score, mean (SD)c | 0.9 (1.5) | 0.8 (1.4) | 0.9 (1.5) |
| Other covariates, mean (SD) | |||
| Eye-related visits, No. | 1.6 (1.2) | 3.1 (2.4) | 1.6 (0.5) |
| HbA1c level, % of total hemoglobin | 7.2 (1.5) | 7.3 (1.7) | 7.2 (1.5) |
Abbreviations: AMD, age-related macular degeneration; CCI, Charlson comorbidity index; HbA1c, glycated hemoglobin; NPDR, nonproliferative diabetic retinopathy; OAG, open-angle glaucoma; PDR, proliferative diabetic retinopathy.
SI conversion factor: To convert HbA1c to proportion of total hemoglobin, multiply by 0.01.
Some individuals may have more than 1 comorbidity, so percentages for comorbidities do not total 100%.
Characteristics of patients assigned this code suggest that this code may also be used for some patients with type 2 diabetes who are using insulin.
The adapted CCI score is an index of general health, with diabetes and dementia removed so they are not counted twice during analysis.
Table 2.
Study Population Use of Diabetes Medicationsa
| Diabetes Medication Type | Diabetic Patients, No. (%) |
P Value Based on t Test |
All Diabetic Patients, No. (%) |
|
|---|---|---|---|---|
| Did Not Develop OAG | Developed OAG | |||
| Sulfonylureas | 44 659 (31.0) | 1846 (31.3) | .58 | 46 505 (31.0) |
| Metformin hydrochloride | 57 993 (40.2) | 2221 (37.7) | <.001 | 60 214 (40.1) |
| Thiazolidinediones | 34 265 (23.8) | 1442 (24.5) | .22 | 35 707 (23.8) |
| Meglitinides | 3510 (2.4) | 153 (2.6) | .43 | 3663 (2.4) |
| Insulin | 32 704 (22.7) | 1244 (21.1) | .005 | 33 948 (22.6) |
| Othersb | 10 536 (7.3) | 283 (4.8) | <.001 | 10 819 (7.2) |
Abbreviation: OAG, open-angle glaucoma.
Some enrollees were prescribed more than 1 medication class.
Exenatide, sitagliptin, or pramlintide.
Inclusion and Exclusion Criteria
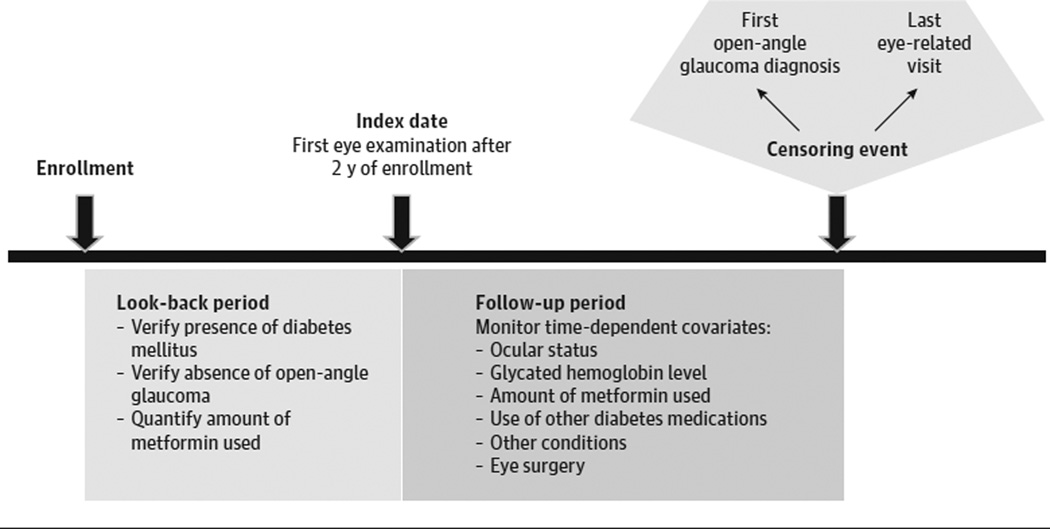
We identified all patients aged 40 years or older enrolled in the plan continuously for more than 2 consecutive years and diagnosed as having diabetes during their first 2 years in the plan (Figure 1 and eFigure in the Supplement). Diagnosis of diabetes was based on ICD-9-CM diabetes diagnosis codes 250. xx or 362.01–362.07 (eTable 1 in the Supplement). Patients selected had at least 1 eye examination during this 2-year period to exclude those with preexisting OAG (codes 365.1, 365.10–365.12, and 365.15) and at least 1 eye examination after this period to identify incident OAG. Patients with incomplete, missing, or duplicate data or discontinuous enrollment were excluded. Patients were followed up from the index date (ie, the date corresponding to their first eye examination on or after the 2-year look-back period) until incident OAG or their last eye examination, whichever came first.
Figure 1. Study Design.
A 2-year look-back period identified individuals meeting study inclusion criteria. Multivariable Cox regression modeling with delayed entry assessed the relationship between metformin hydrochloride use and time to development of open-angle glaucoma, adjusting for confounding factors.
Quantifying Metformin and Other Diabetes Medications
Use of metformin and other medications for diabetes came from a review of outpatient medication prescriptions filled. For these analyses, we used prescriptions filled as a surrogate for medication consumption, although we acknowledge it is not a direct measure of actual consumption.
Statistical Analysis
Statistical analysis used Stata version 13.1 statistical software (StataCorp LP). Patient characteristics were summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Survival analysis using Cox proportional hazards modeling assessed the effect of metformin exposure on the risk of developing OAG. Four regression models were created. All models generated hazard ratios (HRs) with 95% confidence intervals.
Model 1
Cumulative amount of metformin hydrochloride use based on prescriptions filled during a 2-year moving time window was stratified into 4 quartiles: 1 to 315 g (first quartile), 316 to 660 g (second quartile), 661 to 1110 g (third quartile), and more than 1110 g (fourth quartile). We compared risk of developing OAG for persons with each of the 4 dosage quartiles against persons with no prescriptions for metformin (Table 3).
Table 3.
Univariate and Multivariate Cox Proportional Regression Models Assessing Risk of Developing Open-Angle Glaucoma
| Covariates | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age at plan enrollment, y | ||||
| 40 to <45 | 1 [Reference] | … | 1 [Reference] | … |
| 45 to <50 | 1.34 (1.10–1.64) | .004 | 1.24 (0.92–1.67) | .16 |
| 50 to <55 | 1.69 (1.40–2.04) | <.001 | 1.74 (1.33–2.26) | <.001 |
| 55 to <60 | 1.88 (1.57–2.26) | <.001 | 1.88 (1.43–2.46) | <.001 |
| 60 to <65 | 2.07 (1.72–2.49) | <.001 | 2.23 (1.67–2.98) | <.001 |
| ≥65 | 2.24 (1.88–2.67) | <.001 | 2.98 (2.20–4.02) | <.001 |
| Sex | ||||
| Male | 1 [Reference] | … | 1 [Reference] | … |
| Female | 1.03 (0.95–1.12) | .47 | 0.96 (0.84–1.10) | .56 |
| Race/ethnicity | ||||
| European ancestry | 1 [Reference] | … | 1 [Reference] | … |
| African ancestry | 2.18 (1.94–2.46) | <.001 | 1.95 (1.61–2.35) | <.001 |
| Latino | 1.21 (1.04–1.42) | .02 | 1.26 (1.03–1.55) | .03 |
| Asian American | 1.46 (1.16–1.82) | .001 | 1.53 (1.11–2.10) | .008 |
| Other | 0.88 (0.63–1.25) | .48 | 1.43 (0.89–2.30) | .13 |
| Education | ||||
| <High school | 1 [Reference] | … | 1 [Reference] | … |
| High school diploma | 0.86 (0.71–1.03) | .10 | 0.94 (0.72–1.24) | .68 |
| Some college | 0.83 (0.69–1.00) | .06 | 0.93 (0.70–1.25) | .64 |
| College diploma | 0.74 (0.60–0.90) | .003 | 0.72 (0.51–1.02) | .06 |
| Advanced degree | 1.11 (0.46–2.69) | .81 | 1.11 (0.19–6.37) | .90 |
| Geographic region of residence | ||||
| Northeast | 1 [Reference] | … | 1 [Reference] | … |
| Southeast | 1.06 (0.96–1.18) | .24 | 1.15 (0.95–1.38) | .16 |
| Midwest | 0.84 (0.75–0.94) | .003 | 1.01 (0.82–1.25) | .93 |
| West | 1.13 (0.98–1.31) | .10 | 1.25 (0.90–1.73) | .18 |
| Net worth, $ | ||||
| 0 to <25 000 | 1 [Reference] | … | 1 [Reference] | … |
| 25 000 to <75 000 | 0.83 (0.68–1.02) | .08 | 1.10 (0.80–1.51) | .58 |
| 75 000 to <150 000 | 1.06 (0.91–1.24) | .46 | 1.02 (0.79–1.31) | .88 |
| 150 000 to <500 000 | 0.87 (0.77–0.99) | .03 | 0.88 (0.71–1.11) | .28 |
| ≥500 000 | 0.87 (0.75–1.00) | .04 | 0.92 (0.69–1.23) | .57 |
| Ocular comorbiditiesa | ||||
| Nonexudative AMD | 1.12 (1.00–1.26) | .04 | 0.87 (0.69–1.11) | .26 |
| Exudative AMD | 1.19 (0.97–1.46) | .10 | 0.58 (0.33–1.02) | .06 |
| Cataract | 1.09 (0.99–1.19) | .07 | 0.91 (0.78–1.06) | .21 |
| Pseudophakia or aphakia | 1.27 (1.16–1.39) | <.001 | 0.91 (0.73–1.14) | .42 |
| NPDR | 1.04 (0.95–1.13) | .39 | 0.89 (0.75–1.05) | .16 |
| PDR | 1.37 (1.22–1.53) | <.001 | 0.93 (0.74–1.18) | .55 |
| Cataract surgery | 1.26 (1.15–1.39) | <.001 | 1.20 (0.96–1.50) | .11 |
| Retina surgery | 1.53 (1.37–1.71) | <.001 | 1.10 (0.87–1.40) | .43 |
| Other comorbiditiesa | ||||
| Hyperlipidemia | 0.86 (0.73–1.00) | .049 | 0.82 (0.56–1.22) | .34 |
| Obesity | 0.94 (0.85–1.03) | .16 | 0.94 (0.80–1.09) | .40 |
| Dementia | 1.02 (0.82–1.27) | .87 | 0.87 (0.50–1.52) | .63 |
| Depression | 0.80 (0.69–0.94) | .006 | 0.89 (0.68–1.16) | .39 |
| Hypertension | 1.20 (1.00–1.44) | .05 | 1.37 (1.04–1.81) | .03 |
| Type 1 diabetesb | 0.97 (0.89–1.05) | .44 | 0.84 (0.72–0.97) | .02 |
| Adapted CCI scorec | 0.92 (0.89–0.94) | <.001 | 0.86 (0.81–0.92) | <.001 |
| Other covariates | ||||
| Eye-related visits, No. | 1.06 (1.06–1.07) | <.001 | 1.07 (1.06–1.08) | <.001 |
| HbA1c level, % of total hemoglobin | 1.03 (0.98–1.08) | .20 | 1.08 (1.03–1.13) | .003 |
| Metformin use, g | ||||
| Never used | 1 [Reference] | … | 1 [Reference] | … |
| 0–315 | 0.89 (0.78–1.01) | .08 | 0.85 (0.69–1.04) | .11 |
| 316–660 | 0.92 (0.81–1.04) | .19 | 0.89 (0.72–1.09) | .25 |
| 661–1110 | 0.92 (0.80–1.05) | .22 | 1.03 (0.84–1.27) | .78 |
| >1110 | 0.84 (0.74–0.96) | .01 | 0.75 (0.59–0.95) | .02 |
| Use of other diabetes medications | ||||
| Sulfonylureas | 1.01 (0.91–1.11) | .88 | 1.03 (0.88–1.22) | .68 |
| Thiazolidinediones | 1.01 (0.91–1.12) | .88 | 1.09 (0.91–1.31) | .34 |
| Meglitinides | 0.88 (0.63–1.23) | .47 | 0.85 (0.56–1.28) | .43 |
| Insulin | 0.92 (0.81–1.03) | .14 | 0.86 (0.71–1.03) | .10 |
| Others | 1.07 (0.85–1.35) | .56 | 1.11 (0.84–1.47) | .47 |
Abbreviations: AMD, age-related macular degeneration; CCI, Charlson comorbidity index; HbA1c, glycated hemoglobin; HR, hazard ratio; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; ellipses, not applicable.
SI conversion factor: To convert HbA1c to proportion of total hemoglobin, multiply by 0.01.
Some individuals may have more than 1 comorbidity, so percentages for comorbidities do not total 100%.
Characteristics of patients assigned this code suggest that this code may also be used for some patients with type 2 diabetes who are using insulin.
The adapted CCI score is an index of general health, with diabetes and dementia removed so they are not counted twice during analysis.
The regression models were adjusted for a number of potential confounding factors. Covariates for the model were selected based on a combination of previously reported associations of covariates with OAG11 and univariate results from analysis of our data (Table 3). Time-constant covariates included demographic factors (age at plan enrollment, sex, race), socioeconomic factors, geographic region of residence within the United States, comorbid ocular diseases (exudative or nonexudative age-related macular degeneration, cataract, proliferative diabetic retinopathy, nonproliferative diabetic retinopathy, and pseudophakia or aphakia), comorbid medical conditions (hyperlipidemia, obesity, dementia, depression, and hypertension), type of diabetes, and general overall health as captured using the Charlson comorbidity index12 (Table 3). Time-dependent covariates in the models included cataract surgery, retina surgery, and exposure to each of the other common diabetes medication classes (sulfonylureas, thiazolidinediones, meglitinides, insulin, and others).
The level of diabetic control captured by glycated hemoglobin (HbA1c) levels was also incorporated into the model as a time-dependent covariate. Not all enrollees with diabetes had records of HbA1c levels. We were concerned that patients missing HbA1c data may differ from others who had HbA1c data; for example, persons without HbA1c data may be seeking medical care less often than those with HbA1c data. To address this concern, we used the inverse probability weighting method of logistic regression to identify the covariates that systematically correlated with patients missing HbA1c data, then used the inverse (reciprocal) of the predicted probabilities as the frequency weights for our survival analysis. This inverse probability weighting method has been widely used in social science research to deal with systematic differences between groups being compared.13, 14
Model 2
Metformin was treated as a binary variable: more than 1110 g of metformin hydrochloride within a 2-year moving window vs 1110 g or less (including no use). We used the same inverse probability weighting method to deal with missing HbA1c results and the same set of covariates as in model 1 to adjust for confounding factors.
Model 3
Cumulative amount of metformin use during the 2-year moving time window was treated as a continuous variable. We used the same inverse probability weighting method to deal with missing HbA1c results and the same set of covariates as in model 1 to adjust for confounding factors.
Model 4
We analyzed absolute OAG risk among patients with diabetes who used different amounts of metformin and had different levels of diabetes control. First, we determined each patient’s inherent risk of OAG using logistic regression modeling considering the same time-constant covariates listed earlier. Based on these covariates, we assigned an inherent OAG risk score to each patient. Those whose scores were in the top quartile were classified as high risk and those in the bottom quartile were classified as low risk. Second, we performed separate Cox proportional hazards regression modeling for the low- and high-risk groups and controlled for the time-dependent covariates from the relative risk models to obtain baseline survival functions for each group. Third, we compared the absolute risk reduction of patients with 3 levels of blood glucose control (HbA1c levels of 7%, 10%, and 13% of total hemoglobin [to convert to proportion of total hemoglobin, multiply by 0.01]), 3 levels of metformin exposure (no use, 1–1110 g, and >1110 g of metformin hydrochloride use within the 2-year moving window), and 2 levels of inherent OAG risk (high and low).
Results
Of 150 016 individuals with diabetes who met the inclusion criteria, 5893 (3.9%) developed incident OAG (Table 1). The population included 73 117 men (48.7%), 110 884 individuals (82.2%) of European ancestry, 10 601 individuals (7.9%) of African ancestry, 8545 individuals (6.3%) of Latino ancestry, and 3537 individuals (2.6%) of Asian ancestry. Open-angle glaucoma developed in individuals of African (6.7%) and Latino (4.6%) ancestry at a higher rate than in populations of European (3.6%) and Asian (3.7%) ancestry. For patients who did not develop OAG, the mean (SD) duration from the first eye examination until the last eye examination without incident OAG was 52.8 (20.9) months; for those who developed OAG, the mean (SD) duration from the first eye examination until initial OAG diagnosis was 63.3 (23.4) months.
The mean (SD) HbA1c level was 7.2% (1.5%), indicating that, on average, the blood glucose levels were relatively well controlled. Throughout the study period, 60 214 patients (40.1%) filled at least 1 metformin prescription, 46 505 (31.0%) filled at least 1 sulfonylurea prescription, 35 707 (23.8%) filled at least 1 thiazolidinedione prescription, 3663 (2.4%) filled at least 1 meglitinide prescription, and 33 948 (22.6%) filled at least 1 insulin prescription. Some patients filled prescriptions for multiple medication classes (Table 2).
In Cox regression model 1, covariates known from previous work to be associated with OAG showed association with OAG in this data set (Table 3). For example, patients aged 65 years or older were approximately 3 times more likely to be diagnosed as having OAG compared with patients aged 40 to 45 years (HR = 2.98; 95% CI, 2.20–4.02; P < .001). Patients of African ancestry had a 95% higher risk of developing OAG than patients of European ancestry (HR = 1.95; 95% CI, 1.61–2.35; P < .001). For every additional visit to an eye care professional, there was a 7% increase in the risk of being diagnosed as having OAG (HR = 1.07; 95% CI, 1.06–1.08; P < .001), which is expected because eye diseases are more likely to be detected in individuals who are being examined more often.
After adjusting for time-dependent and time-constant covariates, taking more than 1110 g of metformin hydrochloride cumulatively over 2 years (>75th percentile among users of this medication) was associated with a 25% reduced risk of developing OAG compared with those with no metformin use (HR = 0.75; 95% CI, 0.59–0.95; P = .02) (Table 3).
A confident reduction in risk of developing OAG during that same period could not be identified for persons prescribed lesser quantities of metformin compared with nonusers (P ≥ .11 for all comparisons) (Table 3). When we stratified metformin use into 2 dosage categories (model 2), those who were prescribed more than 1110 g of metformin hydrochloride had a 22% reduced risk of OAG compared with those who were prescribed 1110 g or less (including no use) (HR = 0.78; 95% CI, 0.63–0.97; P = .02).
Next, we evaluated the relative risk of developing OAG using the metformin dosage as a continuous variable (model 3). Using cumulative metformin dosage during a 2-year window, every 1-g increase in metformin hydrochloride use was associated with a 0.16% reduction in OAG risk (adjusted HR = 0.99984; 95% CI, 0.99969–0.99999; P = .04), which predicts that taking a standard dose of 2 g of metformin hydrochloride per day for 2 years would result in a 20.8% reduction in risk of OAG. A confident effect of other classes of diabetes medications used to control blood glucose levels on the risk of developing OAG could not be identified (P ≥ .10 for all comparisons). Because this analysis showed that metformin is associated with reduced OAG risk but other diabetes medications are not, it was important to also assess whether OAG risk is associated with HbA1c level, which reflects blood glucose levels during the previous 3 months. After accounting for diabetic medications prescribed and other potential confounders, OAG risk was increased by 8% (HR = 1.08; 95% CI, 1.03–1.13; P = .003) for each unit of increase in HbA1c level (Table 3).
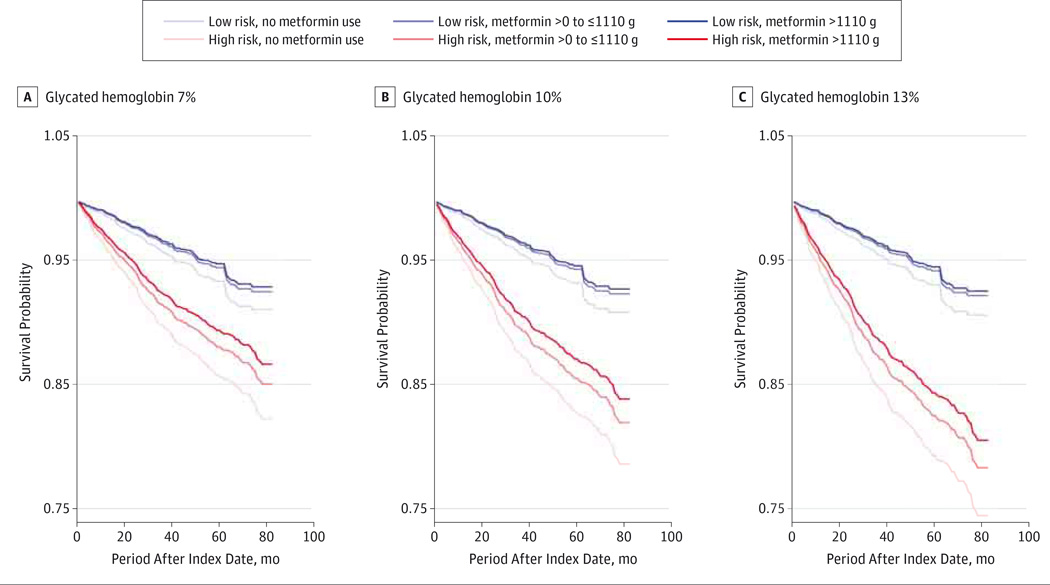
We then built a model of absolute OAG risk (model 4). With patients stratified by inherent OAG risk and HbA1c level and with cumulative metformin exposure as a continuous variable, the largest risk reduction was seen for persons taking more than 1110 g of metformin hydrochloride cumulatively during a 2-year period in those who had the highest inherent OAG risk and the worst glycemic control (Figure 2).
Figure 2. Absolute Risk of Open-Angle Glaucoma.
Patients were stratified by inherent glaucoma risk (high or low), glycated hemoglobin levels of 7%(A), 10% (B), or 13%(C) of total hemoglobin (to convert to proportion of total hemoglobin, multiply by 0.01), and amount of metformin hydrochloride prescriptions filled during a 2-year moving time window.
Discussion
This study suggests that metformin is associated with reduced risk of developing OAG in people with diabetes. Every 1 g of metformin hydrochloride conferred a 0.16% reduction in OAG risk (model 3), so that those taking a standard dose (2 g/d, or 1460 g over 2 years) experienced a 20.8% OAG risk reduction. In model 2, those taking more than 1110 g over 2 years showed a 22% OAG risk reduction compared with those taking 1110 g or less over 2 years. Similarly, in model 1, those taking the highest quartile dosage (>1110 g over 2 years) experienced a 25% OAG risk reduction compared with nonusers. Our finding that greater metformin quantity is associated with a greater reduction in OAG risk is consistent with previous findings that metformin has dose-dependent effects on cellular processes in studies of other diseases.15, 16
Our data suggest that metformin may be affecting OAG risk on multiple levels, some involving improved glycemic control and some involving other mechanisms. After controlling for diabetic medication use, worse glycemic control as indicated by increased HbA1c levels is associated with increased OAG risk; because metformin can lower HbA1c levels through control of glucose metabolism, we cannot rule out the possibility that some aspects of metformin’s influence on OAG risk may happen through improving glycemic control. However, after controlling for HbA1c levels, a confident effect of other diabetes medications on reduction in risk of developing OAG could not be identified, where as metformin appears to reduce risk of OAG even when we take HbA1c levels into account in our models. Thus, our findings raise questions about whether metformin may be acting to reduce OAG risk via mechanisms beyond glycemic control. While all diabetes medications target glycemic control,17–20 metformin not only affects gluconeogenesis in the liver18 but also has complex effects on other pathways and organs.6, 7, 15, 16, 18, 21, 22 Of special interest, metformin acts via known CR and CR mimetic mechanisms involving inflammation, neurogenesis, and longevity pathways.6, 21
Another interesting finding from this study is the perspective on previous contradictory findings regarding whether diabetes mellitus itself is associated with OAG. Of 14 cross-sectional population-based studies that we reviewed, 9 reported an association of diabetes with OAG, while the other 5 did not (eTable 2 in the Supplement).23–35 We identified 3 prospective longitudinal cohort studies reporting that diabetes is associated with OAG and 4 studies indicating otherwise (eTable 3 in the Supplement).11, 36–41 Many of these studies did not consider HbA1c level or diabetes medications as potential confounding variables in the analysis, and when medications for diabetes were considered, the studies did not account for medication class or quantity (eTable 2 and eTable 3 in the Supplement). For instance, Tan et al35 found no association of diabetes with OAG when diabetic treatment (no treatment, dietary treatment, or medication) was taken in to account, while Pasquale et al40 found an association of diabetes with OAG when diabetes medications (hypoglycemics vs no hypoglycemics) were taken into account. A recent meta-analysis of 47 studies concluded that diabetes, disease duration, and fasting glucose levels were associated with an increase in glaucoma risk.42 Our findings suggest that studies of the association of diabetes with other traits, especially late-onset traits that might be influenced by metformin effects outside glycemic control, may benefit from separately accounting for the dosage of metformin and other diabetes medications as well as HbA1c levels.
Advantages of using this kind of large administrative database include large sample sizes providing adequate power to address the questions posed and adequate numbers of racial minorities known to be disproportionately affected by vision loss from OAG. A limitation is that diagnoses based on ICD-9-CM billing codes captured in these analyses could not be confirmed from medical records; however, in a recent study, confirmation from medical records showed that 97% of OAG encounters were assigned the correct ICD-9-CM code.43 In this study, the population is so large that any 1 misdiagnosis or miscoding is expected to have a negligible effect on the outcome of the analysis, and there is no reason that the use of metformin or other diabetes medications would alter the probability of correctly diagnosing or coding for OAG. The results of our study will be relevant to a broad range of people, but the insured population in this study may not correctly reflect what is happening in uninsured populations because the number of eye examinations, level of glycemic control, and use of diabetes medications are associated with the probability of being diagnosed as having OAG but may differ for insured and uninsured populations. Finally, this study population is limited to individuals with diabetes. Future clinical studies will also be needed to evaluate effects of metformin on nondiabetic populations and on important glaucoma endophenotypes not available in this database such as intraocular pressure, central corneal thickness, angle grade, cup-disc ratio, or visual field test results.
Conclusions
We originally raised the questions addressed in this study because of the known role that CR and CR mimetic drugs play in life span and in risk of some later-onset traits.2, 7, 21 Our findings suggest that future efforts to understand risk factors for late-onset traits will benefit from inclusion of dosage information on metformin or other CR mimetic therapies being used in the study population. Although the impact of metformin on risk is known for some traits such as cardiovascular disease, diabetes, and some specific cancers,1, 2, 7, 21 this study points out the importance of understanding the potential impact of CR mimetic drugs on the risk of developing other medical conditions that affect older persons. It will also be important to elucidate the mechanisms of metformin action, at both the molecular and the clinical level, in the ocular tissues involved in OAG pathology.
Our study adds OAG to a growing list of traits that suggest reduced risk in individuals or organisms using metformin. Should these findings be confirmed in other populations in a prospective clinical trial, they may lead to novel treatments for this sight-threatening disease and offer new opportunities to reduce other risks of aging.
Supplementary Material
At a Glance.
Caloric restriction mimetic drugs such as metformin can reduce the risk of certain late-onset diseases. To evaluate whether use of such drugs is associated with incident open-angle glaucoma, a disease with older age at onset, this study analyzed longitudinal data from 150 016 patients with diabetes mellitus.
Use of metformin, but not other diabetes medications, is associated with a reduced risk of open-angle glaucoma, after adjustment for diabetes control (glycated hemoglobin level) and other variables.
The reduced risk of open-angle glaucoma may be explained partly by patients𒀙 improved glycemic control with metformin therapy.
Metformin may also be acting through nonglycemic mechanisms, perhaps involving the drug’s effects on neurogenesis, inflammatory systems, or longevity pathways targeted by caloric restriction mimetic drugs.
Acknowledgments
Funding/Support: This work was supported by exploratory/developmental grant R21-EY021000 (Dr Richards) and K23 Mentored Clinician Scientist Awards 1K23EY019511 (Dr Stein) and K12EY022299 (Dr Newman-Casey) from the National Eye Institute, a fellowship from the Heed Foundation (Dr Newman-Casey), and a physician-scientist award (Dr Stein) and unrestricted grant from Research to Prevent Blindness.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamaophthalmology.com
Author Contributions: Drs Lin and Stein had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lin, Stein, Nan, Childers, Thompson, Richards.
Acquisition, analysis, or interpretation of data: Lin, Stein, Nan, Newman-Casey, Richards.
Drafting of the manuscript: Lin, Stein, Richards.
Critical revision of the manuscript for important intellectual content: Lin, Nan, Childers, Newman-Casey, Thompson, Richards.
Statistical analysis: Lin, Nan, Childers.
Obtained funding: Stein, Richards.
Administrative, technical, or material support: Newman-Casey.
Study supervision: Stein, Newman-Casey, Richards.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
REFERENCES
- 1.Fontana L, Partridge L, Longo VD. Extending healthy life span: from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37(1):47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth LW, Polotsky AJ. Can we live longer by eating less? a review of caloric restriction and longevity. Maturitas. 2012;71(4):315–319. doi: 10.1016/j.maturitas.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Mercken EM, Crosby SD, Lamming DW, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12(4):645–651. doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox LS, Mattison JA. Increasing longevity through caloric restriction or rapamycin feeding in mammals: common mechanisms for common outcomes? Aging Cell. 2009;8(5):607–613. doi: 10.1111/j.1474-9726.2009.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005;23(3):343–350. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]
- 7.Rösen P, Wiernsperger NF. Metformin delays the manifestation of diabetes and vascular dysfunction in Goto-Kakizaki rats by reduction of mitochondrial oxidative stress. Diabetes Metab Res Rev. 2006;22(4):323–330. doi: 10.1002/dmrr.623. [DOI] [PubMed] [Google Scholar]
- 8.Guedes G, Tsai JC, Loewen NA. Glaucoma and aging. Curr Aging Sci. 2011;4(2):110–117. doi: 10.2174/1874609811104020110. [DOI] [PubMed] [Google Scholar]
- 9.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein JD, Newman-Casey PA, Talwar N, Nan B, Richards JE, Musch DC. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012;119(10):2074–2081. doi: 10.1016/j.ophtha.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118(7):1318–1326. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Hirano K, Imbens GW, Ridder G. Efficient estimation of average treatment effects using the estimated propensity score. Econometrica. 2003;71(4):1161–1189. [Google Scholar]
- 14.DiNardo J, Fortin NM, Lemieux T. Labor market institutions and the distribution of wages, 1973–1992: a semiparametric approach. Econometrica. 1996;64(5):1001–1044. [Google Scholar]
- 15.Marini C, Salani B, Massollo M, et al. Direct inhibition of hexokinase activity by metformin at least partially impairs glucose metabolism and tumor growth in experimental breast cancer. Cell Cycle. 2013;12(22):3490–3499. doi: 10.4161/cc.26461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 17.Aquilante CL. Sulfonylurea pharmacogenomics in type 2 diabetes: the influence of drug target and diabetes risk polymorphisms. Expert Rev Cardiovasc Ther. 2010;8(3):359–372. doi: 10.1586/erc.09.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20(6):953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Pastromas S, Koulouris S. Thiazolidinediones: antidiabetic drugs with cardiovascular effects. Hellenic J Cardiol. 2006;47(6):352–360. [PubMed] [Google Scholar]
- 21.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32(3):159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Del Barco S, Vazquez-Martin A, Cufí S, et al. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2(12):896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn HA, Milton RC. Alternative definitions of open-angle glaucoma: effect on prevalence and associations in the Framingham Eye Study. Arch Ophthalmol. 1980;98(12):2172–2177. doi: 10.1001/archopht.1980.01020041024003. [DOI] [PubMed] [Google Scholar]
- 24.Klein BE, Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes: the Beaver Dam Eye Study. Ophthalmology. 1994;101(7):1173–1177. doi: 10.1016/s0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- 25.Wormald RP, Basauri E, Wright LA, Evans JR. The African Caribbean Eye Survey: risk factors for glaucoma in a sample of African Caribbean people living in London. Eye (Lond) 1994;8(pt 3):315–320. doi: 10.1038/eye.1994.64. [DOI] [PubMed] [Google Scholar]
- 26.Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP. Risk factors for open-angle glaucoma: the Barbados Eye Study. Arch Ophthalmol. 1995;113(7):918–924. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 27.Tielsch JM, Katz J, Quigley HA, Javitt JC, Sommer A. Diabetes, intraocular pressure, and primary open-angle glaucoma in the Baltimore Eye Survey. Ophthalmology. 1995;102(1):48–53. doi: 10.1016/s0161-6420(95)31055-x. [DOI] [PubMed] [Google Scholar]
- 28.Dielemans I, de Jong PT, Stolk R, Vingerling JR, Grobbee DE, Hofman A. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population: the Rotterdam Study. Ophthalmology. 1996;103(8):1271–1275. doi: 10.1016/s0161-6420(96)30511-3. [DOI] [PubMed] [Google Scholar]
- 29.Kaimbo Wa Kaimbo D, Missotten L. Risk factors for open-angle glaucoma in 260 black subjects in Congo. Bull Soc Belge Ophtalmol. 1997;267:29–34. [PubMed] [Google Scholar]
- 30.Mitchell P, Smith W, Chey T, Healey PR. Open-angle glaucoma and diabetes: the Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104(4):712–718. doi: 10.1016/s0161-6420(97)30247-4. [DOI] [PubMed] [Google Scholar]
- 31.Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119(12):1819–1826. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 32.Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP Los Angeles Latino Eye Study Group. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study. Ophthalmology. 2008;115(2):227–232.e1. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vijaya L, George R, Paul PG, et al. Prevalence of open-angle glaucoma in a rural south Indian population. Invest Ophthalmol Vis Sci. 2005;46(12):4461–4467. doi: 10.1167/iovs.04-1529. [DOI] [PubMed] [Google Scholar]
- 34.Xu L, Xie XW, Wang YX, Jonas JB. Ocular and systemic factors associated with diabetes mellitus in the adult population in rural and urban China: the Beijing Eye Study. Eye (Lond) 2009;23(3):676–682. doi: 10.1038/sj.eye.6703104. [DOI] [PubMed] [Google Scholar]
- 35.Tan GS, Wong TY, Fong CW, Aung T Singapore Malay Eye Study. Diabetes, metabolic abnormalities, and glaucoma. Arch Ophthalmol. 2009;127(10):1354–1361. doi: 10.1001/archophthalmol.2009.268. [DOI] [PubMed] [Google Scholar]
- 36.Ellis JD, Evans JM, Ruta DA, et al DARTS/MEMO Collaboration. Glaucoma incidence in an unselected cohort of diabetic patients: is diabetes mellitus a risk factor for glaucoma? Br J Ophthalmol. 2000;84(11):1218–1224. doi: 10.1136/bjo.84.11.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 38.Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44(9):3783–3789. doi: 10.1167/iovs.03-0077. [DOI] [PubMed] [Google Scholar]
- 39.de Voogd S, Ikram MK, Wolfs RC, et al. Is diabetes mellitus a risk factor for open-angle glaucoma? the Rotterdam Study. Ophthalmology. 2006;113(10):1827–1831. doi: 10.1016/j.ophtha.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 40.Pasquale LR, Kang JH, Manson JE, Willett WC, Rosner BA, Hankinson SE. Prospective study of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology. 2006;113(7):1081–1086. doi: 10.1016/j.ophtha.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 41.Wise LA, Rosenberg L, Radin RG, et al. A prospective study of diabetes, lifestyle factors, and glaucoma among African-American women. Ann Epidemiol. 2011;21(6):430–439. doi: 10.1016/j.annepidem.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao D, Cho J, Kim MH, Friedman DS, Guallar E. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015;122(1):72–78. doi: 10.1016/j.ophtha.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 43.Muir KW, Gupta C, Gill P, Stein JD. Accuracy of International Classification of Diseases, Ninth Revision, Clinical Modification billing codes for common ophthalmic conditions. JAMA Ophthalmol. 2013;131(1):119–120. doi: 10.1001/jamaophthalmol.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.