Abstract
Since superoxide dismutase (SOD) knockout enhances arteriolar remodeling and contractility, we hypothesized that remodeling enhances contractility. In the isolated and perfused renal afferent arterioles from SOD wild type (+/+) and gene-deleted mice, contractility were assessed from reductions in luminal diameter with perfusion pressure from 40 to 80 mmHg (myogenic responses) or angiotensin II (ANG II; 10−6 mol·l−1), remodeling from media:lumen area ratio, superoxide (O2·−) and hydrogen peroxide (H2O2) from fluorescence microscopy and wall stress from wall tension/wall thickness. Compared to +/+ strains, arterioles from SOD1 −/−, SOD2 +/− and SOD 3−/− mice developed significantly (P<0.05) more O2·− with perfusion pressure and ANG II and significantly increased myogenic responses (SOD1 −/−: −20.7±2.2 vs 12.7±1.6%; SOD2 +/−: −7.4 ±1.3 vs 12.6 ±1.4% and SOD3 −/−: −9.1 ±1.9 vs −15.8 ±2.2%) and ANG II contractions and ~2-fold increased media:lumen ratios. media:lumen ratios correlated with myogenic responses (r2 =0.23, P<0.01), ANG II contractions (r2=0.57, P<0.0001), and active wall tension (r2 =0.19, P<0.01) but not with active wall stress (r2=0.08; NS). Differences in myogenic responses among SOD3 mice were abolished by bath addition of SOD and were increased 3 days after inducing SOD3 knockout (26.9 ± 1.7% vs −20.1 ± 0.7%; P<0.05), despite unchanged M:L ratios (2.01 ± 0.09 vs 2.02 ± 0.03, NS). We conclude that cytosolic, mitochondrial or extracellular O2·− enhance afferent arteriolar contractility and remodeling. Although remodeling does not enhance contractility, it does prevent the potentially damaging effects of increased wall stress.
Keywords: Reactive oxygen species, oxidative stress, hypertension, renal autoregulation, vascular remodeling, myogenic response
Introduction
Blood vessels in hypertensive humans 1–3 or animal models 4, 5 characteristically have evidence of remodeling, endothelial dysfunction 2, 6 and increased reactive oxygen species (ROS) 7. Although these features predict subsequent cardiovascular disease (CVD) 8–10, the effects of the remodeling on microvascular function are unclear 4, 11, 12. Folkow and colleagues proposed that repeated episodes of blood pressure elevation elicited vascular remodeling that enhanced vasoconstriction and sustained hypertension 11, 13. Later studies identified a specific role for structural remodeling of the renal afferent arteriole in human and experimental hypertension 14, 15, 16. Thus, an autopsy study of kidneys from 1,377 subjects reported that renal afferent arteriopathy, which entailed medial thickening and luminal narrowing, occurred in 97% of subjects known to be hypertensive yet in less than 12% of those known to be normotensive whereas remodeling was not prominent in arterioles from other sites 17. Moreover, Mulvany and colleagues reported that young spontaneously hypertensive rats whose renal afferent arterioles from nephrectomy specimens had a lesser diameter developed a higher blood pressure when adult 18. Indeed, a reduction in luminal diameter of renal afferent arterioles should increase renal vascular resistance (RVR) and limit the glomerular filtration rate (GFR), thereby contributing to hypertension.
To gain further insight into the consequences of remodeling of renal afferent arterioles, arteriolar media:lumen area ratios from mice with gene deletions for superoxide dismutase (SOD) isoforms were related to the generation of ROS and to contractile responses to perfusion pressure (myogenic response) or to angiotensin II (ANG II). The SOD −/− mouse model provides prolonged increases in afferent arteriolar ROS, contractility and remodeling without renal damage 7, 19, 20.
Myogenic responses were assessed from reductions in luminal diameter of mouse isolated and perfused afferent arterioles during increases in perfusion pressure. Active wall tension (AWT) was calculated from the difference between wall tension developed in physiological solution (WTphysiol) and passive tension in Ca2+-free solution with ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) (WTpassive). Myogenic and ANG II responses were related to remodeling and to the generation of arteriolar O2·− but these were dissociated by incubation of arterioles with pegalated (PEG) SOD or PEG-catalase (to metabolize ROS without changing remodeling) or from short-term induction of SOD-3 knockout (to increase ROS without remodeling). Active wall stress (AWS = AWT/wall thickness) normally increases with contractility and has been proposed to contribute to microvascular damage 21, 22. Therefore, we related contractility to active wall tension and active wall stress to determine their roles in the functional adaption to a prolonged increase in afferent arteriolar ROS.
Methods
Animal models
The first series used male littermate SOD1, SOD2 and SOD3 +/+, +/− and SOD1 and SOD3 −/− mice (SOD2 −/− is lethal). The second series used male mice three days after induction of SOD3 knockout with tamoxifen (3 mg·20 g−1 body weight, i.p) compared to littermates given vehicle 23. Mice were aged 3 to 4 months and weighed 25–30 g. All procedures conformed to the Guide for Care and Use of Laboratory Animals prepared by The Institute for Laboratory Animal Research. Studies were approved by the Georgetown University Animal Care and Use Committee.
Isolation and perfusion of mouse afferent arterioles and measurements of superoxide (O2·−) and hydrogen peroxide (H2O2)
As described previously 7, 24–26 and in Supplemental Methods, O2·− generation was assessed by fluorescence microscopy from PEG-SOD inhibitable ethidium:dihydroethidium (E:DHE) fluorescence ratio (f/f0) 27 and H2O2 from PEG-catalase inhibitable 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (C-H2DCFDA) fluorescence, as described 28.
Renal SOD protein expression
This was assessed by Western analysis of lysates of renal cortex as described 29 (see Supplemental Methods).
Determination of passive wall compliance
This was assessed from passive wall stress/strain relationships as described 30 (see Supplemental Methods).
Statistics
Data were obtained from 5 to 7 mice per group and analyzed by ANOVA (with repeated measures for ANG II) to assess individual effects of genotype, intervention (perfusion pressure or ANG II) and interaction (effects of genotype on the response to the intervention). Where appropriate, a post-hoc Bonferroni t test was used to test differences between groups. Results (mean ± SEM values) are considered significant at P<0.05.
Results
The SOD protein expression was studied in renal cortical lysates to determine whether genetic deletion of one SOD isoform caused adaptive changes in renal expression in other SOD isoforms. Compared to littermate +/+ mice, the expression of the corresponding SOD isoforms were almost absent in SOD1 or SOD3 −/− mice, and were approximately one half in +/− mice (Supplemental figures S1 and S2). There were no apparent compensations by one SOD isoform for the loss of another.
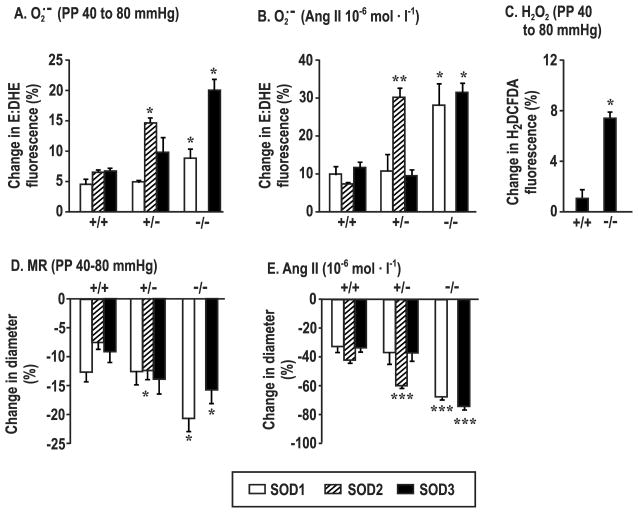
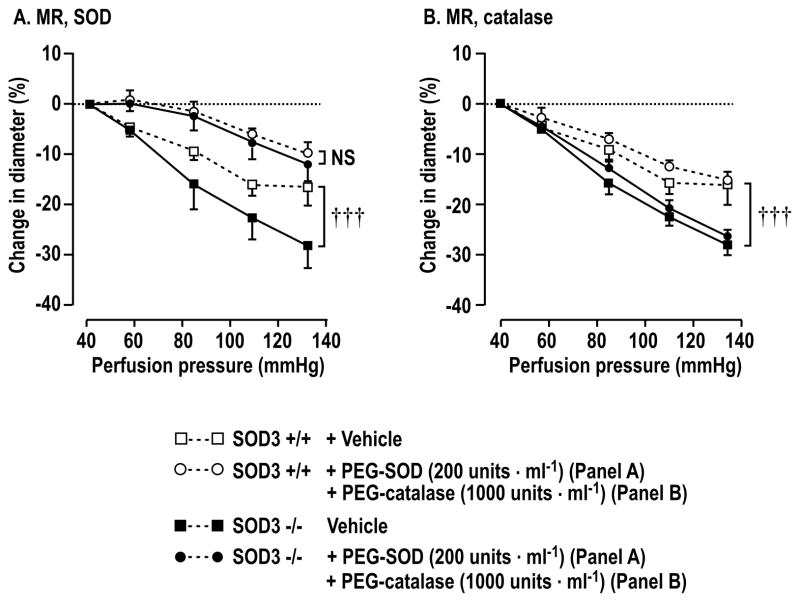
PEG-SOD (200 units·ml−1) blocked > 80% of the E:DHE fluorescence and PEG-catalase (1,000 units·ml−1) blocked > 80% of the C-H2DCFDA fluorescence in SOD3 mice (Supplemental figure S3A and S3B). Compared to arterioles from littermate +/+ mice, the increases in afferent arteriolar O2·− with perfusion pressure from 40 to 80 mmHg or with 10−6 mol·l−1 ANG II were enhanced significantly in arterioles from SOD1 −/−, SOD2 +/− or SOD3 −/− mice (Figure 1A and 1B). The increases in O2·− in arterioles from SOD2 +/− mice were generally similar to those in SOD1 −/− and SOD3 −/− but there were no differences between SOD1 or SOD3 +/+ and +/− mice. An increase in perfusion pressure did not increase H2O2 significantly in afferent arterioles from SOD3 +/+ mice but did increase it in SOD3 −/− mice (Figure 1C). A strictly similar increase in H2O2 with perfusion pressure was also seen in arterioles from SOD1 −/− mice compared to SOD3 −/− mice (7.8±1.5 versus 7.4±0.5%). Compared to arterioles from littermate +/+ mice, contractility was increased significantly in response to perfusion pressure (Figure 1D) or ANG II (Figure 1E) in arterioles from SOD1 −/−, SOD3 −/− and SOD2 +/− mice. Bath addition of PEG-SOD prevented the enhanced contractions of SOD3 −/− mouse arterioles to perfusion pressure (Figure 2A) (without changing remodeling), whereas PEG-catalase was ineffective (Figure 2B). Arteriolar O2·− generation was correlated with contractions to both perfusion pressure (r2 =0.39, P<0.05) and ANG II (r2 =0.85, P<0.0001) (Supplemental Fig S4A and S4B). This analysis used group mean data since ROS and myogenic responses could not be measured in the same arterioles.
Figure 1.
Deletion of SOD genes enhances afferent arteriolar generation of reactive oxygen species and contractility. Changes in superoxide with perfusion pressure (Panel A) or angiotensin II (Panel B) or hydrogen peroxide with perfusion pressure (Panel C) or contraction with perfusion pressure (Panel D) or angiotensin II (Panel E) in arterioles from SOD-1 (open boxes), SOD-2 (cross-hatched boxes) or SOD-3 (solid boxes) gene manipulated mice (mean ± SEM; n = 5–7 per group). Compared to corresponding +/+ mouse vessels; *, P<0.05; **, P<0.01; ***, P<0.005.
Figure 2.
Changes in diameter of afferent arterioles with perfusion pressure in the presence of pegalated agents. Comparing vessels from SOD3 +/+ (open symbols) with SOD3 −/− (solid symbols) mice incubated with vehicle (square symbols) or pegalated agents (circular symbols). Compared to SOD3 +/+, †††, P<0.005.
We had shown previously that blockade of nitric oxide synthase (NOS) with L-NAME increased the sensitivity and responsiveness of afferent arterioles to ANG II in SOD1 +/+ but not SOD1 −/− mice and abolished differences between genotypes 7, 19. Thus, ANG II responses were not repeated. Myogenic contractility also was enhanced by NOS inhibition in SOD1 +/+ but an enhanced myogenic contractility in vessels from SOD-1 −/− mice persisted during L-NAME (Supplemental figure S5).
Compared to arterioles from littermate +/+ mice, those from SOD2 +/− and SOD1 −/− and SOD3 −/− mice had an increased media wall area, and/or a reduced vessel lumen area that resulted in striking and uniform increases by >2-fold in media:lumen ratios (Figure 3A, 3B and 3C). These structural changes persisted in Ca2+-free solution which abolished vascular tone. The basal arteriolar lumen diameters are shown in Supplemental Table S1.
Figure 3.
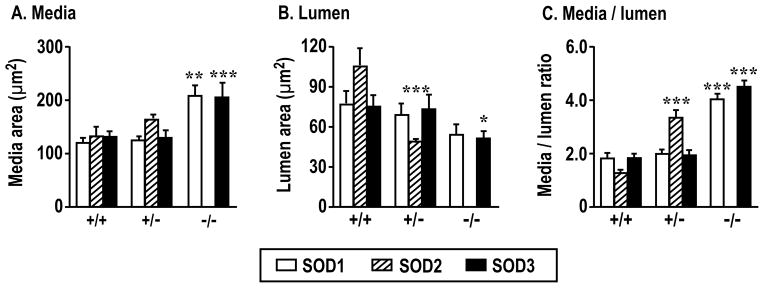
Deletion of SOD genes enhances afferent arteriolar remodeling in SOD-1 (open boxes), SOD-2 (cross hatched boxes) or SOD-3 (solid boxes) gene manipulated mice. Compared to corresponding +/+ mouse vessels: *, P<0.05; **, P<0.01; ***, P<0.005.
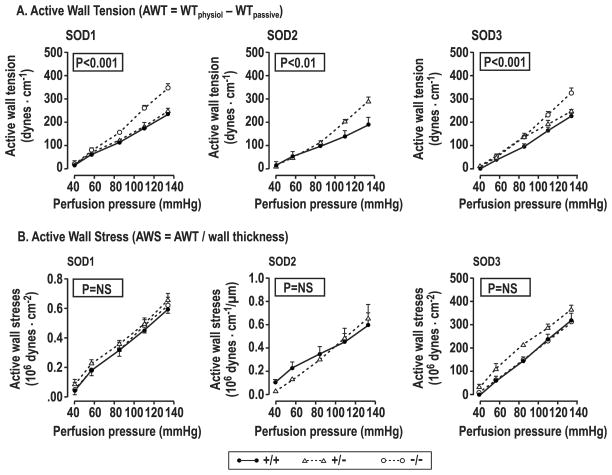
Active wall tension (AWT; dynes·cm−1) increased lineally with perfusion pressure as in prior studies 24–26. Compared to littermate +/+ mice, the slope of the regression of AWT on perfusion pressure 26 (to quantitate myogenic responses) was increased significantly in arterioles from SOD1 −/−, SOD2 +/− and SOD3 −/− mice (SOD1: 3.49±0.30 vs 2.30±0.27; P< 0.05; SOD2: 3.00±0.19 vs 1.77±0.22; P<0.05; SOD3: 3.51±0.20 vs 2.43±0.15; P<0.01) (Figure 4A and Supplemental Table S2). Active wall stress (AWS; dynes·cm−2) also increased with perfusion pressure but, unlike AWT, there were no differences between littermate and SOD gene-deleted mice (Figure 4B and Supplemental Table S3).
Figure 4.
Active wall tension and active wall stress increase with perfusion pressure in SOD +/+ (solid circles and continuous lines), +/− (open triangles and dashed lines) or −/− (open squares and dotted lines) mice. P values refer to ANOVA with repeated measures for differences between genotypes.
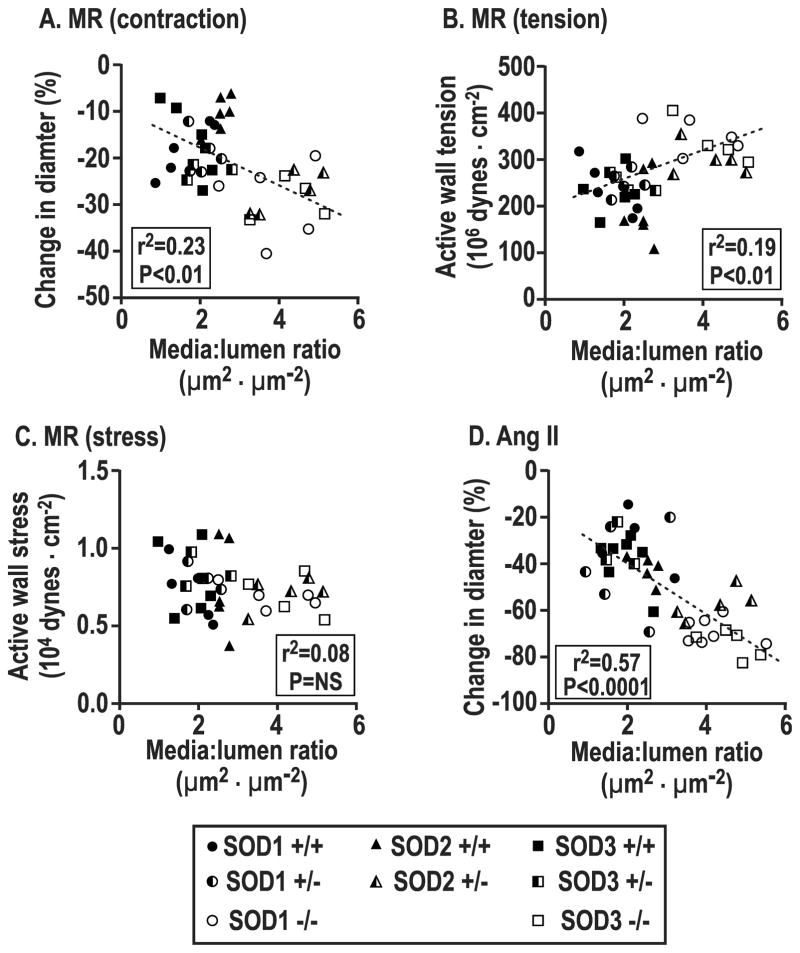
After pooling individual data points across genotypes, changes in arteriolar diameter with perfusion pressure or ANG II were positively correlated with afferent arteriolar media:lumen ratio (perfusion pressure, r2 =0.23, P<0.001, Figure 5A; ANGII, r2=0.57, P<0.0001, Figure 5D). During increased perfusion pressure, AWT depended significantly on media:lumen ratio (r2 =0.19, P<0.01, Figure 5B) whereas active wall stress was independent (r2 = 0.08, NS, Figure 5C).
Figure 5.
Correlation of individual afferent arteriolar values for changes in diameter (Panel A), active wall tension (Panel B) or active wall stress (Panel C) with perfusion pressure or angiotensin II (Panel D) related to vessel media to lumen area ratio.
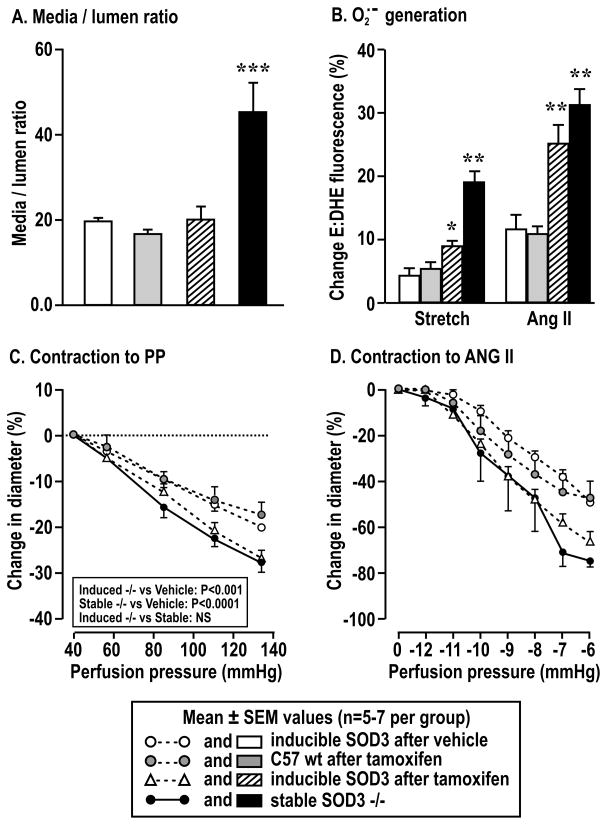
Compared to arterioles from littermate mice with the same genotype give vehicle, the afferent arterioles three days after induction of SOD3 knockout with tamoxifen were not remodeled (Figure 6A) but developed a similarly enhanced generation of O2·− with perfusion pressure or ANG II (Figure 6B) and similarly increased contractions with perfusion pressure (Figure 6C) or ANG II (Figure 6D) as arterioles from lifetime SOD3 −/− mice.
Figure 6.
Structural change, superoxide generation and contraction of afferent arterioles from mice with tamoxifen inducible SOD-3 −/− after vehicle (open boxes and open circles), inducible SOD-3 after tamoxifen (cross hatched boxes and open triangles) or stable SOD-3 −/− (solid boxes and solid circles). Compared to arterioles from inducible SOD-3 −/− after vehicle: *, P<0.05; **, P<0.01; ***, P<0.005.
The passive arteriolar wall compliance, which was assessed from passive wall stress/strain relationships, was unaffected by SOD genotype (Supplemental figure S6).
Discussion
These studies confirm that SOD1 −/− mice have afferent arteriolar remodeling and increases in O2·− generation and contractile responses to ANG II 7. The main new findings are that there were no compensatory upregulations in renal expression of other SOD isoforms in SOD1, SOD2 or SOD3 gene deleted mice. There were similar increases in afferent arteriolar remodeling, O2·− generation and contractile responses to perfusion pressure and ANG II among afferent arterioles from SOD1 −/−, SOD2 +/− and SOD3 −/− mice. The sharp increase in O2·− with perfusion pressure in arterioles from SOD3 −/− deleted mice accounted for their enhanced contractility since metabolism of O2·− by PEG-SOD normalized the myogenic responses (without a change in media:lumen ratio) whereas the increase in H2O2 was not sufficient to impact myogenic responses significantly since they were unaffected by metabolizing H2O2 with PEG-catalase. The myogenic responses of arterioles from SOD3 −/− mice remained stronger than SOD3 +/+ mice during NOS blockade. Myogenic responses increased with O2·− but active wall stress was maintained since increases in tension in gene deleted mice were balanced by equivalent increases in wall thickness.
Afferent arterioles from mice 3 days after inducing SOD3 knockout had similar increases in O2·− and contractions with PP and ANG II as those from lifelong SOD3 −/− mice but lacked any change in arteriolar media:lumen ratio. This, and the finding of similar myogenic responses in SOD3 +/+ and −/− mice during metabolism of O2·− despite a maintained enhanced media:lumen ratio in the knockout mice, dissociates the increases in contractile responses in SOD3 −/− mice from vessel remodeling. Finally, passive vessel wall compliance was preserved in SOD1 −/−, SOD2 +/− and SOD3 −/− mice. Thus, both short and long term gene deletions of SODs increase afferent arteriolar O2·− generation which is implicated in an increased contractility to perfusion pressure or ANG II. The remodeling of the vessel wall that develops in the long term does not account for the increased contractility but does permit an increase in contractility without an increase in active wall stress. An increase in vascular wall stress is a stimulus for cell growth and can be provoked by an increase in myogenic tone31. However, the medial hypertrophy does not appear to cause the enhanced myogenic tone and apparently does not make a dominant contribution to hypertension either since SOD1, SOD2 and SOD3 gene deleted mice all had prominent vascular remodeling yet have generally been reported to be normotensive 19.
A strong afferent arteriolar contraction is a key component of renal autoregulation 7, 32, 33 which maintains a constant renal blood flow, GFR and glomerular capillary pressure during changes in blood pressure 34. These adjustments of preglomerular vascular tone prevent transmission of the hypertension into the glomeruli 34 thereby damaging podocytes 35 and the renal parenchyma 16, 36.
The SOD3 −/− mouse has increased excretion of 8-isoprostane F2α and increased RVR 29, consistent with the increased O2·− and tone in isolated afferent arterioles in this study. ANG II generates both arteriolar NO that blunts the contraction 37 and O2·− that bioinactivates NO 38–40. The enhanced ANG II contractions in afferent arterioles from SOD1 −/− mice were ascribed to the enhanced NO bioinactivation since they were normalized during NOS inhibition 7. In contrast, a stronger myogenic response persisted in arterioles from SOD1 −/− mice during NOS blockade. Thus, all of the enhanced ANG II contractility, but only about one half of the enhanced myogenic responses, in arterioles from SOD1 −/− mice can be ascribed to NO bioinactivation. This is consistent with strong modulation of afferent arteriolar contractions to ANG II 38, thromboxane 41 or endothelin-1 39 by NO but a minor role of endothelial NOS in modulation of myogenic responses 42 and no effect of eNOS deletion 25.
Although arterioles from SOD3−/− mice generated more H2O2 with perfusion pressure, the enhanced contractility likely relates primarily to the enhanced generation of O2·− since deletion of p47phox, which is a prominent source of O2·−, prevents myogenic responses 24 and PEG-SOD, but not PEG-catalase, prevented the exaggerated myogenic responses in arterioles from SOD3−/− mice. The enhanced generation of H2O2 in arterioles from SOD3 −/− mice may reflect metabolism of increased O2·− by other SOD isoforms. On the other hand, aortic vascular remodeling is reduced in NOX2 gene deleted mice 43 and is prevented in vascular smooth muscle cell catalase transgenic mice 44. Thus, enhanced vascular contractility during oxidative stress is dependent on O2·− whereas remodeling can be dependent on H2O2, thereby further dissociating contractility from remodeling.
In conclusion, afferent arterioles from SOD gene deleted mice generate more O2·− with increased perfusion pressure and ANG II which accounts for enhanced contractions. Their vessels are prominently remodeled but the enhanced contractions can be ascribed to O2·− whereas the remodeling permits an enhanced active wall tension during increased perfusion pressure without the potentially damaging effects of increased wall stress.
Supplementary Material
Perspectives.
Results from this study do not support the hypothesis that remodeling of resistance vessels sustains hypertension 11, 13. Indeed, careful studies in SOD gene deleted mice generally report consistent remodeling 7 yet normal basal levels of BP 19 although the early increase in blood pressure with ANG II is usually enhanced, consistent with the increased ANG II contractions of their afferent arterioles in this study 7, 19. Sustained hypertension requires renal Na+ and fluid retention to prevent the corrective effects of a pressure natriuresis 45, 46. Whereas ROS enhance Na+ reabsorption in the loop of Henle 47, they impair reabsorption in the proximal tubule 48. Thus, the absence of hypertension in SOD gene deleted mice, despite enhanced arteriolar contractility, may relate to a preserved pressure natriuresis (not yet tested), or perhaps to confounding effects of renal H2O2. On the other hand, an increase in afferent arteriolar wall thickness in SOD gene-deleted mice permits an increase in active wall tension with PP during generation of O2·−, as required for enhanced myogenic responses, without an increase in active wall stress. The renal, cerebral, and epicardial resistance arterioles are exposed directly to high levels of pressure and are designated “strain vessels” 21, 22. The finding that afferent arteriolar wall tension can be dissociated from active wall stress suggests that microvascular remodeling may be an adaptive response to prevent the potentially damaging vascular effects of wall stress while permitting the beneficial effects of enhanced wall tension that are required for strong myogenic responses to maintain autoregulation and prevent the damaging effects of hypertension on the renal parenchyma. This scenario postulates that hypertensive remodeling may convey some cryptic adaptive advantage and could account for its remarkably close association with human hypertension 17.
Novelty and significance.
What Is New?
Our studies of the afferent arteriolar myogenic and angiotensin responses in SOD gene deleted mice have dissociated the enhanced contractility from the remodeling and shown rather that the enhanced contractility was due to the enhanced superoxide.
What Is Relevant?
Since the remodeling of resistance arterioles in this study did not enhance contractility, microvascular remodeling cannot be essential for increased vascular resistance in hypertension. However, remodeling did prevent the enhanced contractility from enhancing wall stress and thereby may exert a cryptic adaptive advantage in limiting vascular damage during oxidative stress.
Summary
This provider a novel view of microvascular remodeling as an adaptation to hypertension or oxidative stress that permits enhanced contractility without the potentially damaging effects of prolonged increases in microvascular active wall stress.
Acknowledgments
Sources of Funding
This work was supported by research grants to En Yin Lai from the National Nature Science Foundation of China (31471100) and Zhejiang Province Natural Science Foundation (LY13H070002); and to CSW from the NIDDK (DK-49870 and DK-36079) and the NHLBI (HL-68686) and from the George E. Schreiner Chair of Nephrology, the Smith/Kogod Family Foundation and the Georgetown University Hypertension, Kidney and Vascular Research Center. ZL was supported by a NIH training grant (DK-59274). We thank David Harrison, MD (University of Vanderbilt, TN) for a generous gift of inducible SOD3 −/− mice and Kathryn Windels for preparing the manuscript.
Footnotes
Disclosures section
NONE
References
- 1.Xu S, Touyz RM. Reactive oxygen species and vascular remodelling in hypertension: still alive. The Canadian journal of cardiology. 2006;22:947–951. doi: 10.1016/s0828-282x(06)70314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiffrin EL. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension. 2012;59:367–374. doi: 10.1161/HYPERTENSIONAHA.111.187021. [DOI] [PubMed] [Google Scholar]
- 3.Schiffrin EL, Deng LY, Larochelle P. Effects of a beta-blocker or a converting enzyme inhibitor on resistance arteries in essential hypertension. Hypertension. 1994;23:83–91. doi: 10.1161/01.hyp.23.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circulation research. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 5.Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. Journal of hypertension. 2001;19:921–930. doi: 10.1097/00004872-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 7.Carlstrom M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertension. 2010;56:907–913. doi: 10.1161/HYPERTENSIONAHA.110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacology & therapeutics. 2010;126:119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 10.Martin BJ, Anderson TJ. Risk prediction in cardiovascular disease: the prognostic significance of endothelial dysfunction. The Canadian journal of cardiology. 2009;25(Suppl A):15A–20A. doi: 10.1016/s0828-282x(09)71049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkow B. “Structural factor” in primary and secondary hypertension. Hypertension. 1990;16:89–101. doi: 10.1161/01.hyp.16.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Mulvany MJ. Resistance vessel growth and remodelling: cause or consequence in cardiovascular disease. Journal of human hypertension. 1995;9:479–485. [PubMed] [Google Scholar]
- 13.Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 14.Skov K, Mulvany MJ. Structure of renal afferent arterioles in the pathogenesis of hypertension. Acta physiologica Scandinavica. 2004;181:397–405. doi: 10.1111/j.1365-201X.2004.01311.x. [DOI] [PubMed] [Google Scholar]
- 15.Skov K, Fenger-Gron J, Mulvany MJ. Effects of an angiotensin-converting enzyme inhibitor, a calcium antagonist, and an endothelin receptor antagonist on renal afferent arteriolar structure. Hypertension. 1996;28:464–471. doi: 10.1161/01.hyp.28.3.464. [DOI] [PubMed] [Google Scholar]
- 16.Skov K, Hamet P, Nyengaard JR, Mulvany MJ. Morphology of renal afferent arterioles and glomeruli, heart weight, and blood pressure in primates. Am J Hypertens. 2001;14:331–337. doi: 10.1016/s0895-7061(00)01295-4. [DOI] [PubMed] [Google Scholar]
- 17.Moritz AR, Oldt MR. Arteriolar Sclerosis in Hypertensive and Non-Hypertensive Individuals. The American journal of pathology. 1937;13:679–728. [PMC free article] [PubMed] [Google Scholar]
- 18.Norrelund H, Christensen KL, Samani NJ, Kimber P, Mulvany MJ, Korsgaard N. Early narrowed afferent arteriole is a contributor to the development of hypertension. Hypertension. 1994;24:301–308. doi: 10.1161/01.hyp.24.3.301. [DOI] [PubMed] [Google Scholar]
- 19.Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal. 2014;20:74–101. doi: 10.1089/ars.2013.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circulation research. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 21.Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertension research: official journal of the Japanese Society of Hypertension. 2009;32:115–121. doi: 10.1038/hr.2008.27. [DOI] [PubMed] [Google Scholar]
- 22.Nagasawa T, Mori T, Ohsaki Y, Yoneki Y, Guo Q, Sato E, Oba I, Ito S. Albuminuria indicates the pressure-associated injury of juxtamedullary nephrons and cerebral strain vessels in spontaneously hypertensive stroke-prone rats. Hypertension research: official journal of the Japanese Society of Hypertension. 2012;35:1024–1031. doi: 10.1038/hr.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gongora MC, Lob HE, Landmesser U, Guzik TJ, Martin WD, Ozumi K, Wall SM, Wilson DS, Murthy N, Gravanis M, Fukai T, Harrison DG. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. The American journal of pathology. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai EY, Solis G, Luo Z, Carlstrom M, Sandberg K, Holland S, Wellstein A, Welch WJ, Wilcox CS. p47(phox) is required for afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension. 2012;59:415–420. doi: 10.1161/HYPERTENSIONAHA.111.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai EY, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension. 2011;58:650–656. doi: 10.1161/HYPERTENSIONAHA.111.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai EY, Onozato ML, Solis G, Aslam S, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertension. 2010;55:983–989. doi: 10.1161/HYPERTENSIONAHA.109.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Jose P, Wilcox CS. beta(1) Receptors protect the renal afferent arteriole of angiotensin-infused rabbits from norepinephrine-induced oxidative stress. Journal of the American Society of Nephrology: JASN. 2006;17:3347–3354. doi: 10.1681/ASN.2006030212. [DOI] [PubMed] [Google Scholar]
- 28.Gabriela Soares D, Goncalves Basso F, Hebling J, de Souza Costa CA. Effect of hydrogen-peroxide-mediated oxidative stress on human dental pulp cells. Journal of dentistry. 2014 Dec 17; doi: 10.1016/j.jdent.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill PS, Aslam S, Wang X, Ji H, Sandberg K, Jose P, Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertension. 2006;48:934–941. doi: 10.1161/01.HYP.0000242928.57344.92. [DOI] [PubMed] [Google Scholar]
- 30.Leibovitz E, Ebrahimian T, Paradis P, Schiffrin EL. Aldosterone induces arterial stiffness in absence of oxidative stress and endothelial dysfunction. Journal of hypertension. 2009;27:2192–2200. doi: 10.1097/HJH.0b013e328330a963. [DOI] [PubMed] [Google Scholar]
- 31.Laurent S, Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circulation research. 2015;116:1007–1021. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 32.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 33.Just A. Mechanisms of renal blood flow autoregulation: dynamics and contributions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1–17. doi: 10.1152/ajpregu.00332.2006. [DOI] [PubMed] [Google Scholar]
- 34.Pelayo JC, Westcott JY. Impaired autoregulation of glomerular capillary hydrostatic pressure in the rat remnant nephron. The Journal of clinical investigation. 1991;88:101–105. doi: 10.1172/JCI115264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriz W, Lemley KV. A Potential Role for Mechanical Forces in the Detachment of Podocytes and the Progression of CKD. Journal of the American Society of Nephrology: JASN. 2015;26:258–269. doi: 10.1681/ASN.2014030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Rhinehart K, Solis G, Pittner J, Lee-Kwon W, Welch WJ, Wilcox CS, Pallone TL. Chronic ANG II infusion increases NO generation by rat descending vasa recta. American journal of physiology Heart and circulatory physiology. 2005;288:H29–36. doi: 10.1152/ajpheart.00623.2004. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Chen Y, Chabrashvili T, Aslam S, Borrego Conde LJ, Umans JG, Wilcox CS. Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin II of afferent arterioles from rabbits infused with angiotensin II. Journal of the American Society of Nephrology: JASN. 2003;14:2783–2789. doi: 10.1097/01.asn.0000090747.59919.d2. [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circulation research. 2004;94:1436–1442. doi: 10.1161/01.RES.0000129578.76799.75. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Luo Z, Wang X, Jose PA, Falck JR, Welch WJ, Aslam S, Teerlink T, Wilcox CS. Impaired endothelial function and microvascular asymmetrical dimethylarginine in angiotensin II-infused rats: effects of tempol. Hypertension. 2010;56:950–955. doi: 10.1161/HYPERTENSIONAHA.110.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnackenberg CG, Welch WJ, Wilcox CS. TP receptor-mediated vasoconstriction in microperfused afferent arterioles: roles of O2·− and NO. American journal of physiology Renal physiology. 2000;279:F302–308. doi: 10.1152/ajprenal.2000.279.2.F302. [DOI] [PubMed] [Google Scholar]
- 42.Juncos LA, Garvin J, Carretero OA, Ito S. Flow modulates myogenic responses in isolated microperfused rabbit afferent arterioles via endothelium-derived nitric oxide. The Journal of clinical investigation. 1995;95:2741–2748. doi: 10.1172/JCI117977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan SL, Baumbach GL. Deficiency of Nox2 prevents angiotensin II-induced inward remodeling in cerebral arterioles. Front Physiol. 2013;4:133. doi: 10.3389/fphys.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Griendling KK, Dikalova A, Owens GK, Taylor WR. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension. 2005;46:732–737. doi: 10.1161/01.HYP.0000182660.74266.6d. [DOI] [PubMed] [Google Scholar]
- 45.Carlstrom M, Wilcox CS, Arendshorst W. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. Hypertension: physiology and pathophysiology. Comprehensive Physiology. 2012;2:2393–2442. doi: 10.1002/cphy.c110058. [DOI] [PubMed] [Google Scholar]
- 47.Garvin JL, Ortiz PA. The role of reactive oxygen species in the regulation of tubular function. Acta physiologica Scandinavica. 2003;179:225–232. doi: 10.1046/j.0001-6772.2003.01203.x. [DOI] [PubMed] [Google Scholar]
- 48.Panico C, Luo Z, Damiano S, Artigiano F, Gill P, Welch WJ. Renal proximal tubular reabsorption is reduced in adult spontaneously hypertensive rats: roles of superoxide and Na+/H+ exchanger 3. Hypertension. 2009;54:1291–1297. doi: 10.1161/HYPERTENSIONAHA.109.134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.