Abstract
Neanderthals are thought to have disappeared in Europe ~39,000–41,000 years ago but they have contributed one to three percent of the DNA of present-day people in Eurasia1. Here, we analyze DNA from a 37,000–42,000-year-old2 modern human from Peştera cu Oase, Romania. Although the specimen contains small amounts of human DNA, we use an enrichment strategy to isolate sites that are informative about its relationship to Neanderthals and present-day humans. We find that on the order of six to nine percent of the genome of the Oase individual is derived from Neanderthals, more than any other modern human sequenced to date. Three chromosomal segments of Neanderthal ancestry are over 50 centimorgans in size, indicating that this individual had a Neanderthal ancestor as recently as four to six generations back. However, the Oase individual does not share more alleles with later Europeans than with East Asians, suggesting that the Oase population did not contribute substantially to later humans in Europe.
Between 45,000 and 35,000 years ago, anatomically modern humans spread across Europe, while the Neanderthals, present since before 300,000 years ago, disappeared. How this process occurred has long been debated1,3,4,5. Comparisons between the Neanderthal genome and the genomes of present-day humans have shown that Neanderthals contributed approximately one to three percent of the genomes of all people living today outside sub-Saharan Africa6,7 suggesting that human populations ancestral to all non-Africans mixed with Neanderthals. The size of segments of Neanderthal ancestry in present-day humans suggests that this occurred between 37,000 and 86,000 years ago8. However, where and how often this occurred is not understood. For example, Neanderthals share more alleles with East Asians and Native Americans than with Europeans, which may reflect additional interbreeding in the ancestors of eastern non-African9–12. Surprisingly, analyses of present-day genomes have not yielded any evidence that Neanderthals mixed with modern humans in Europe, despite the fact that Neanderthals were numerous there and cultural interactions between the two groups have been proposed13,14.
More direct insight into the interactions between modern and archaic humans can be obtained by studying genomes from modern humans who lived at a time when they could have met Neanderthals. Recent analyses of genomes from a ~43,000–47,000-year-old modern human from western Siberia15 and a ~36,000–39,000-year-old modern human from eastern Europe16 showed that Neanderthal gene flow into modern humans occurred before these individuals lived. The Siberian individual’s genome contained some segments of Neanderthal ancestry as large as 6 million base pairs, suggesting that some Neanderthal gene flow could have occurred a few thousand years prior to his death15.
We report genome-wide data from a modern human mandible, Oase 1, found in 2002 in the Peştera cu Oase, Romania. The age of this specimen has been estimated to ~37,000–42,000 years by direct radiocarbon dating2,17,18. Oase 1 is therefore one of the earliest modern humans in Europe. Its morphology is generally modern but some aspects are consistent with Neanderthal ancestry19–21. Subsequent excavations uncovered a cranium from another, probably contemporaneous individual, Oase 2, which also carries morphological traits that could reflect admixture with Neanderthals17.
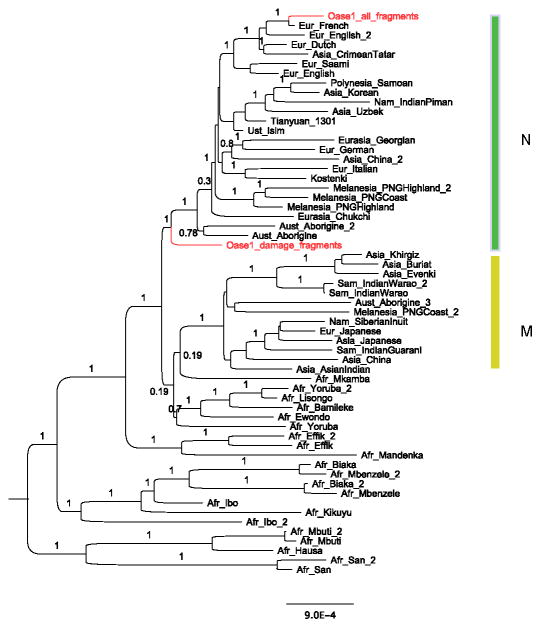
We prepared two DNA extracts from 25 and 10 mg of bone powder removed from the inferior right ramus of Oase 1. We treated an aliquot of each of these extracts with E. coli uracil-DNA-glycosylase (UDG), an enzyme that removes uracils from the interior parts of DNA molecules, but leaves a proportion of uracils at the ends of the molecules unaffected. Uracil residues occur in DNA molecules as a result of deamination of cytosine residues, and are particularly prevalent at the ends of ancient DNA molecules9,22. Among the DNA fragments sequenced from these two extracts, 0.18% and 0.06%, respectively, could be mapped to the human reference genome. We prepared three additional DNA libraries from the extract containing 0.18% human-like molecules, but omitted the UDG treatment to increase the number of molecules where terminal C to T substitutions could be seen and used to identify putatively ancient fragments. Because the fraction of endogenous DNA is so small, we used hybridization to DNA probes to isolate human DNA fragments from the libraries23. Applying this strategy to the mitochondrial (mt) genome allowed the mtDNA from the five libraries to be sequenced to an average coverage of 803-fold (Supplementary Information section 1). At the 3′-ends of the DNA fragments, cytosine residues (C) appeared as thymine residues (T) relative to the human mtDNA reference in 21% of fragments, reflecting appreciable levels of cytosine deamination. This suggests that at least some of the human mtDNA is of ancient origin. We determined mtDNA consensus sequences in two ways: using all mtDNA fragments, and using only deaminated fragments that carry C to T substitutions at either end relative to the consensus mtDNA sequence based on these sequences, an approach known to enrich for endogenous DNA9,24–26. The mtDNA sequence based on all fragments clusters with present-day Europeans (Extended Data Figure 1) (Supplementary Information section 1). In contrast, the mtDNA sequence based on deaminated fragments is related to a large group of present-day Eurasian mtDNAs (haplogroup N) but diverges from these before they diverged from each other. This Oase 1 mtDNA carries a few private mutations based on which its age can be estimated to 36,330 years before present (14,520–56,450; 95% confidence interval). Using six positions where the mtDNA sequence differs from at least 99% of 311 present-day humans, we estimate the contamination rate among all mtDNA fragments to 67% (95% confidence interval (CI) 65%–69%). When we restrict to mtDNA fragments that carry terminal C to T substitutions, the contamination estimate is 4% (95% CI of 2%–9%) (Supplementary Information section 1).
To isolate nuclear DNA from Oase 1, we used three sets of oligonucleotide probes that cover about two million sites that are single nucleotide polymorphisms (SNPs) in present-day humans and captured DNA molecules from the five libraries. Of the SNPs targeted, 51% (n=1,038,619) were covered by at least one DNA fragment, and 13% (n=271,326) were covered by at least one fragment with a terminal C to T substitution. To estimate nuclear DNA contamination, we tested whether Oase 1 DNA fragments with or without evidence of deamination share more alleles with present-day Europeans or with East Asians. We find that significantly more Oase 1 fragments without deamination match Europeans, implying European contamination of 17% to 30% (Supplementary Information section 1). Based on these findings and those from mitochondrial DNA, we restricted all subsequent analyses to DNA fragments that carry terminal C to T substitutions. After doing this, the fractions of SNPs from the X and Y chromosomes are similar, indicating that Oase 1 carried both an X and a Y chromosome and thus that he was male. The Y chromosome alleles belong to the F haplogroup, which is carried by most males in Eurasia today (Supplementary Information section 2).
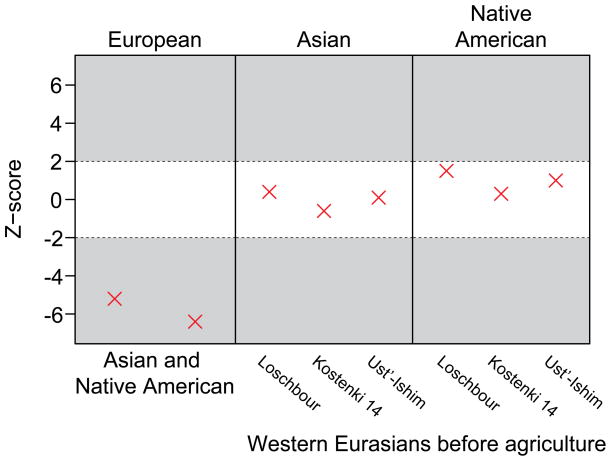
To determine the relationship of the Oase 1 individual to present-day populations, we first tested whether he shared more alleles with particular present-day individuals from different populations using D-statistics, which provides a robust estimate of admixture almost regardless of how SNPs for analysis are chosen27. We find that Oase 1 shared more alleles with present-day East Asians and Native Americans than with present-day Europeans, counter to what might naively be expected for an ancient individual from Europe (Fig. 1) (5.2≤|Z|≤6.4; Extended Data Table 1). However, it has been suggested that Europeans after the introduction of agriculture derive a part of their ancestry from a ‘basal Eurasian’ population that separated from the initial settlers of Europe and Asia before they split from each other28. Therefore, we replaced present-day Europeans with Paleolithic and Mesolithic European individuals in these analyses. We then find that the Oase 1 individual shares equally many alleles with these early Europeans as with present-day East Asians and Native Americans (Fig. 1) (|Z|≤1.5 in Extended Data Table 1). Restricting this analysis to transversion polymorphisms, which are not susceptible to errors induced by cytosine deamination, does not influence this result (Extended Data Table 2) (Supplementary Information section 3). This suggests that the Oase 1 individual belonged to a population that did not contribute much, or not at all, to later Europeans. This contrasts, for example, with the ~36,000–39,000-year-old Kostenki 14 individual from Western Russia, who was more closely related to later Europeans than to East Asians (1.9≤|Z|≤13.7; Extended Data Table 1)16.
Figure 1. Allele sharing between the Oase 1 individual and other genomes.
Each point indicates the extent to which the Oase 1 genome shares alleles with one or the other of a pair of genomes from different populations indicated above and below (see Extended Data Table 1 for numbers). Z-scores larger and smaller than |2| indicate an excess of allele sharing (grey).
To assess whether the ancestors of the Oase 1 individual mixed with Neanderthals, we tested whether the Altai Neanderthal genome shares more alleles with the Oase 1 genome than with sub-Saharan Africans. We find this to be the case (|Z|=7.7; Supplementary Information section 4). We then asked if the amount of Neanderthal ancestry in the Oase 1 genome is similar to that in present-day non-Africans. Surprisingly, the Neanderthal genome shares more alleles with the Oase 1 individual than it does with any present-day people in Eurasia that we tested indicating that he carries more Neanderthal-like DNA than present-day people (5.0≤|Z|≤8.2; Extended Data Table 3). We also observe more Neanderthal-like alleles in the Oase 1 individual when we compare him to four early modern humans: an 8,000-year-old individual from Luxembourg, and three individuals from Russia who vary in age between 24,000 and 45,000 years (3.6≤|Z|≤6.8; Extended Data Table 3). Thus, the Oase 1 individual appears to have carried more Neanderthal-like DNA than any other modern human analyzed to date. This observation cannot be explained by residual present-day human contamination among the DNA fragments that carry terminal C to T substitutions, because all modern humans studied to date carry less Neanderthal ancestry than the Oase 1 genome, and thus contamination would lower, rather than increase, the apparent Neanderthal ancestry.
We estimated the proportion of Neanderthal DNA in the Oase 1 genome using three different statistics7,29 (Supplementary Information section 4). Although the results differ, they all yield point estimates between 6.0% and 9.4% (Table 1). For one of the statistics, none of the 90% confidence intervals for Neanderthal ancestry in the other modern human samples overlap with the confidence interval in Oase 1. When we restrict analysis to transversion SNPs, the point estimates of Neanderthal ancestry are even higher (range of 8.4% to 11.3%) (Extended Data Table 4).
Table 1.
Estimated fraction of the Oase 1 genome that derives from Neanderthals.
| Sample | Statistic 1 | Statistic 2 | Statistic 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Prop. | S.E. | 90% CI | Prop. | S.E. | 90% CI | Prop. | S.E. | 90% CI | |||
| Oase 1 | 8.1% | 2.0% | 4.8%–11.3% | 9.4% | 1.1% | 7.5%–11.3% | 6.0% | 2.0% | 2.8%–9.3% | ||
| Ust’-Ishim | 3.6% | 0.9% | 2.2%–5.0% | 5.5% | 0.7% | 4.3%–6.6% | 0.4% | 1.2% | 0.0%–2.5% | ||
| Kostenki 14 | 3.8% | 1.0% | 2.1%–5.5% | 2.9% | 0.8% | 1.6%–4.2% | 1.7% | 1.3% | 0.0%–3.9% | ||
| MA1 | 1.2% | 1.1% | 0.0%–3.0% | 3.5% | 0.8% | 2.2%–4.8% | 2.3% | 1.3% | 0.1%–4.5% | ||
| Loschbour | 1.3% | 0.9% | 0.0%–2.8% | 3.9% | 0.7% | 2.7%–5.1% | 0.5% | 1.2% | 0.0%–2.6% | ||
| LaBrana | 3.1% | 1.0% | 1.4%–4.7% | 1.9% | 0.7% | 0.7%–3.1% | 1.4% | 1.2% | 0.0%–3.4% | ||
| Stuttgart | 3.0% | 0.9% | 1.5%–4.4% | 2.5% | 0.7% | 1.3%–3.7% | 0.4% | 1.2% | 0.0–2.4% | ||
| Han | 2.2% | 0.9% | 0.6%–3.7% | 2.2% | 0.8% | 1.0%–3.5% | 1.0% | 1.2% | 0.0%–3.1% | ||
| Dai | 2.6% | 0.9% | 1.1%–4.0% | 1.0% | 0.8% | 0.0%–2.3% | 0.7% | 1.2% | 0.0%–2.6% | ||
| French | 3.0% | 0.9% | 1.6%–4.5% | 3.0% | 0.7% | 1.8%–4.2% | 0.2% | 1.2% | 0.0%–2.2% | ||
| Sardinian | 2.0% | 0.8% | 0.6%–3.4% | 2.7% | 0.7% | 1.5%–3.9% | −0.3% | 1.2% | 0.0%–1.8% | ||
To study the spatial distribution of Neanderthal DNA across the Oase 1 genome, we designed capture probes for around 1.7 million nucleotide positions where nearly all individuals in a sub-Saharan African population carry one allele while Neanderthal genomes carry another allele. We used these probes to isolate DNA fragments from the Oase 1 individual. A total of 78,055 sites were covered by deaminated DNA fragments from the Oase 1 individual as well as from the ~36,000- to 39,000-year-old Kostenki 14 individual from western Russia16, the ~43,000- to 47,000-year-old individual from Ust’-Ishim in Siberia15, and three present-day human genomes from China, France and Sudan (Supplementary Information section 5). Because the Dinka from Sudan are thought to have little or no Neanderthal ancestry7, we subtracted the number of alleles that match the Neanderthals in the Dinka individual (485) from the number in the other genomes to estimate the number of alleles attributable to Neanderthal ancestry. The resulting numbers of putative Neanderthal alleles are 3,746 in the Oase 1 individual, 1,586 and 1,121 in the Ust’-Ishim and Kostenki 14 individuals, respectively, and 1,322 and 1,033 in the Chinese and the European individuals (Extended Data Table 5). Thus, the Neanderthal contribution to the Oase 1 genome appears to be between 2.3- and 3.6-fold larger than to the other genomes analyzed. Assuming that the Neanderthal contribution to the European individual is 2%7, this suggests that 7.3% of the Oase 1 genome is of Neanderthal origin. When the numbers of alleles matching the Neanderthal genome are compared per chromosome (Extended Data Table 5), the highest numbers are always observed for the Oase 1 genome, except in the case of chromosome 21, where the Ust’-Ishim individual carries a large segment of likely Neanderthal ancestry.
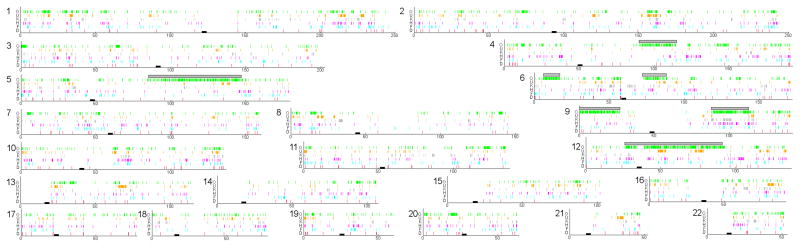
We plotted the positions of Neanderthal-like alleles across the Oase 1 genome (Fig. 2). We detect three segments that are over 50 cM in size, suggesting that the Neanderthal contribution to the Oase 1 individual occurred so recently in his family tree that chromosomal segments of Neanderthal origin had little time to break up due to recombination. To estimate the date of the most recent Neanderthal contribution to the Oase 1 genome, we studied the size spans of seven segments of the genome that we could clearly identify as being recently derived from Neanderthals. Their genetic lengths suggest that the Oase 1 individual had a Neanderthal ancestor as a 4th, 5th, or 6th degree relative (Supplementary Information section 5). This would predict that an average of 1.6% to 12.5% of the Oase 1 genome derived from this Neanderthal ancestor, which is in the range of our Neanderthal ancestry estimates (Extended Data Table 5). Visual inspection of the Oase 1 genome suggests that in addition to these seven segments, other smaller segments also carry Neanderthal-like alleles (Fig. 2). When we remove the seven longest segments, the estimate of Neanderthal ancestry in Oase 1 drops from 7.3% to 4.8%, which is still around twice the 2.0%–2.9% estimated for the French, Han, Kostenki and Ust’-Ishim individuals in this remaining part of the genome. This additional Neanderthal ancestry could reflect an older Neanderthal admixture into the ancestors of Oase 1, or that we failed to find all segments of recent Neanderthal ancestry.
Figure 2. Spatial distribution of alleles matching Neanderthals in modern humans.
Colored vertical lines indicate alleles shared with Neanderthals and no color indicates alleles shared with the great majority of West Africans. (O)ase 1, (K)ostenki 14, (U)st’-Ishim, (F)rench, (H)an, and (D)inka. The seven yellow bars indicate segments of putative recent Neanderthal ancestry. This analysis is based on 78,055 sites.
The Oase 1 genome shows that mixture between modern humans and Neanderthals was not limited to the first ancestors of present-day people to leave Africa, nor to the Near East; it occurred later as well and probably in Europe. The fact that the Oase 1 individual had a Neanderthal ancestor removed by only four to six generations allows this Neanderthal admixture to be dated to less than 200 years before the time he lived. However, the absence of a clear relationship of the Oase 1 individual to later modern humans in Europe suggests that he may have been a member of an initial early modern human population that interbred with Neanderthals but did not contribute much to later European populations. To better understand the interactions between early modern and Neanderthal populations, it will be important to study other specimens that, like Oase 1, have been suggested to carry morphological traits suggestive of admixture with Neanderthals30.
ONLINE METHODS
DNA extraction and library preparation
A dentistry drill was used to remove two samples of bone powder from an area where a larger sample had previously been removed for carbon dating2. Two extracts (E1406, E1843) were prepared from 25 mg and 10 mg of bone powder, respectively, as described31. Five libraries were produced from the two extracts using a single-stranded library protocol9,32 (Extended Data Table 6). One library from each extract (A5227, A5252) was treated with E. coli uracil-DNA-glycosylase (UDG) and endonuclease (Endo VIII) in order to remove deaminated cytosine residues from the interior parts of molecules33. All libraries were amplified by PCR for 35 cycles using AccuPrime Pfx DNA polymerase (Life Technologies)34 and primers carrying library-specific indexes35. Library concentrations were determined using a NanoDrop 2000 spectrophotometer.
Sequencing and DNA capture
The UDG-treated libraries A5252 and A5227 were shotgun sequenced and found to contain 0.06% and 0.18% human DNA, respectively. We used hybridization to oligonucleotide probes to enriched the libraries for subsets of the nuclear genome containing panels of known SNPs as described23 except that each SNP was targeted by four 52-nucleotide probes: two immediately flanking the SNP on both sides, and two centered on the SNP containing one or the other alternate allele, respectively. Four panels of probes were used:
Panel 1 “390k”: 394,577 SNPs, about 90% of which are on the Affymetrix Human Origins array27. See ref. 36 for SNPs and probes.
Panel 2 “840k”: 842,630 SNPs constituting the rest of the SNPs on the Human Origins array, all SNPs on the Illumina 610-Quad array, all SNPs on the Affymetrix 50k array, and smaller numbers of SNPs chosen for other purposes. See Supplementary Data 1.
Panel 3 “1000k”: 997,780 SNPs comprising all transversion polymorphisms seen in two Yoruba from Nigeria sequenced to high coverage and transversion polymorphisms seen in the Altai Neanderthal genome. The design was restricted to SNPs that passed strict quality filters in the Neanderthal genome (Map35_99%)7, had chimpanzee alleles available, and probes were designed from chimpanzee flanking sequences. See Supplementary Data 2.
Panel 4 “Archaic”: This panel contains SNPs that are highly informative about archaic ancestry ascertained such that West-African Yoruba carry a high frequency of one allele while at least one archaic sample has an alternative allele.
At each in the genome, we examined data from all Yoruba individuals from the 1000 Genomes Project37 covered by at least three reads passing filters. At these sites we called majority alleles (drawing a random allele in the case of equal numbers of reads supporting both alleles). We furthermore restricted the analysis to sites where ≥24 Yoruba individuals as well as the Altai Neanderthal and Denisovan had allele calls (Map35_50% filter7). We then selected sites where at most one alternative allele is seen among the Yoruba while at least one of four archaic genomes (Denisovan; Altai, Vindija and Mezmaiskaya Neanderthals) carry the alternative allele. The ancestral states were taken from the inferred ancestor of humans and chimpanzees (Ensembl Compara v64) 38,39.
The following classes of sites were used:
Class 1: 297,894 SNPs where Yoruba is derived and at least one ancestral allele is seen in the Altai, Vindija, Mezmaiskaya or Denisovan genomes.
-
Class 2: Sites where Yoruba alleles are all or nearly all ancestral and derived alleles are seen in archaic genomes. Since such derived alleles often arise due to errors in an archaic genome we restricted this class to the following three cases:
1,321,774 SNPs where the high-coverage Altai Neandertal and/or Denisovan genomes are homozygous derived.
523,041 SNPs where the Altai and/or Denisovan genomes are heterozygous but are not C→T or G→A substitutions relative to the ancestral allele.
30,735 SNPs that are homozygous ancestral in Altai and/or Denisovan and at least one copy of the derived allele is observed in the Mezmaiskaya or Vindija Neanderthal genomes, and the derived allele represents a transversion and is also seen in the Simons Genome Diversity Panel (https://www.simonsfoundation.org/life-sciences/simons-genome-diversity-project/).
After eliminating SNPs where capture probes covered ambiguous bases in the human (hg19) and chimpanzee (pantro2) genomes or overlapped for less than 35 nucleotides with mapable regions (Map35_50%7), this left us with a set of 1,749,385 SNPs. See Supplementary Data 3.
Sequencing of capture products and data processing
Capture products were sequenced using 2×75bp reads on an Illumina HiSeq2500 or an Illumina NextSeq500. We de-multiplexed the reads allowing one mismatch in each of the two indices (Extended Data Table 6), and merged paired reads into sequenced fragments requiring an overlap of at least 15 bp (allowing one mismatch) using a modified form of SeqPrep (https://github.com/jstjohn/SeqPrep), using the bases with the higher quality (and score) to represent the overlap region. After removing adapters, merged fragments were mapped to hg19 using BWA (v0.6.1) using the samse command. Duplicated fragments were identified based on sharing the same orientation and end positions, in which case the fragment with the highest quality was kept (Extended Data Table 7).
To focus on putatively deaminated fragments we used fragments with C→T substitutions relative to the hg19 human genome reference sequence in the first 5′ or last two 3′ bases for the UDG-treated libraries, and to fragments with C→T substitutions relative to hg19 in the terminal three bases at either end of fragment from non-UDG-treated libraries (Supplementary Information section 1, Extended Data Table 8).
Merging the Oase 1 data with genome sequences
At each SNP covered at least once in Oase 1, we selected the majority allele (in case of a tie, we picked a random allele). We then merged the Oase 1 data with 25 genomes of present-day humans sequenced to 24–42× coverage7, the Altai Neanderthal7, the Siberian Denisovan9, a ~45,000-year-old modern human from Ust’-Ishim in Siberia15, an ~8,000-year-old Mesolithic individual from Loschbour Cave, Luxembourg28 and a ~7,000-year-old early farmer from Stuttgart, Germany28 (Extended Data Table 9). All the calls for the five deeply sequenced ancient genomes were performed in the same way. We restricted analyses to sites with a minimum root-mean-square mapping quality of 30 in the 30 genomes. We added lower coverage shotgun data from the ~36,000-year-old Kostenki 14 from Russia16, the ~24,000-year-old Mal’ta Siberian individual from Russia40, an 8,000-year-old Mesolithic individual from La Brana Cave, Spain41, a Neanderthal from Mezmaiskaya in Russia7, and a pool of three Neanderthals from Vindija Cave in Croatia6, in these cases restricting to fragments with MAPQ≥37 to match the filter for the low coverage Oase 1 data (Extended Data Table 9).
Population genetic analyses
To determine the relationship of Oase 1 to other modern humans, we used D-statistics to evaluate whether sets of four tested samples are consistent with being related to one another according to an unrooted tree27 (Supplementary Information section 3). We used D-statistics and f4-statistic ratios27 to test both whether there is excess archaic ancestry in Oase 1 compared with other modern humans, and to estimate proportions of Neanderthal ancestry27 (Supplementary Information section 4). We studied the genomic distribution of alleles that are likely to derive from Neanderthals in the sense of being shared with Neanderthal but either absent or at very low frequency in West Africans. We used the spatial distribution of these sites to identify stretches of likely Neanderthal ancestry in several individuals including Oase 1. We also used these data to estimate the number of generations since the most recent Neanderthal ancestor of Oase 1 (Supplementary Information section 5).
Extended Data
Extended Data Figure 1. Mitochondrial DNA tree for Oase 1 and other modern humans.
The consensus sequences for all Oase 1 fragments and for deaminated fragments are shown. The tree is rooted with a Neanderthal mtDNA (Vindija33.25) as an outgroup.
Extended Data Table 1. Allele sharing between early modern humans and other humans.
We compute D(Non-African1, Non-African2; Early Modern Human, African) to test whether an early modern human (Oase 1, Ust’-Ishim, or Kostenki 14) shares more alleles with Non-African1 (in which case the statistic is positive) or Non-African2 (negative). We use a pool of six sub-Saharan African genomes (2 Mbuti, 2 Yoruba, 2 Dinka) as an outgroup; a pool of four genomes (2 French, 2 Sardinians) to represent Europeans; a pool of four genomes (2 Han, 2 Dai) to represent East Asians; and a pool of three genomes (2 Karitiana, 1 Mixe) to represent Native Americans. Results are based on 242,122 transition and transversion SNPs covered by at least one deaminated fragment in Oase 1, and covered in all other samples with the possible exception of MA1 (for MA1, 176,569 SNPs are used).
| Non-African1 | Non-African2 | Oase 1 | Ust’-Ishim | Kostenki 14 | |||
|---|---|---|---|---|---|---|---|
| D | Z | D | Z | D | Z | ||
| Oase 1 | Ust’-Ishim | −0.0033 | −3.8 | ||||
| Oase 1 | Kostenki 14 | −0.0037 | −4.1 | ||||
| Oase 1 | MA1 | −0.0032 | −3.5 | −0.0092 | −9.8 | ||
| Oase 1 | Loschbour | −0.0032 | −3.9 | −0.0101 | −12.2 | ||
| Oase 1 | East Asian | −0.0027 | −3.8 | −0.0011 | −1.6 | ||
| Oase 1 | Native American | −0.0030 | −4.1 | −0.0039 | −5.5 | ||
| Ust’-Ishim | Kostenki 14 | −0.0005 | −0.6 | ||||
| Ust’-Ishim | MA1 | −0.0007 | −0.8 | −0.0059 | −6.4 | ||
| Ust’-Ishim | Loschbour | 0.0002 | 0.3 | −0.0068 | −8.5 | ||
| Ust’-Ishim | East Asian | 0.0000 | −0.1 | 0.0022 | 3.3 | ||
| Ust’-Ishim | Native American | −0.0007 | −1.0 | −0.0006 | −0.8 | ||
| Kostenki 14 | MA1 | −0.0004 | −0.6 | 0.0003 | 0.4 | ||
| Kostenki 14 | Loschbour | 0.0007 | 1.0 | 0.0006 | 0.8 | ||
| Kostenki 14 | East Asian | 0.0004 | 0.6 | 0.0011 | 1.6 | ||
| Kostenki 14 | Native American | −0.0002 | −0.3 | 0.0008 | 1.1 | ||
| MA1 | Loschbour | 0.0012 | 1.7 | 0.0005 | 0.7 | −0.0012 | −1.5 |
| MA1 | East Asian | 0.0008 | 1.2 | 0.0007 | 1.1 | 0.0079 | 10.6 |
| MA1 | Native American | 0.0001 | 0.1 | 0.0004 | 0.6 | 0.0051 | 7.0 |
| Loschbour | East Asian | −0.0002 | −0.4 | 0.0005 | 0.9 | 0.0090 | 13.7 |
| Loschbour | Native American | −0.0009 | −1.5 | 0.0002 | 0.3 | 0.0062 | 9.0 |
| East Asian | Native American | −0.0006 | −1.6 | −0.0003 | −0.8 | −0.0028 | −6.6 |
|
| |||||||
| European | Oase 1 | 0.0004 | 0.6 | 0.0049 | 7.3 | ||
| European | Ust’-Ishim | −0.0023 | −3.5 | 0.0016 | 2.4 | ||
| European | Kostenki 14 | −0.0028 | −4.7 | −0.0033 | −5.1 | ||
| European | MA1 | −0.0033 | −5.4 | −0.0031 | −5.1 | −0.0041 | −6.0 |
| European | Loschbour | −0.0021 | −4.5 | −0.0027 | −5.7 | −0.0052 | −9.1 |
| European | East Asian | −0.0024 | −5.2 | −0.0022 | −5.3 | 0.0039 | 9.2 |
| European | Native American | −0.0030 | −6.4 | −0.0025 | −5.9 | 0.0010 | 2.2 |
| European | Stuttgart | −0.0007 | −1.5 | −0.0001 | −0.2 | −0.0002 | −0.3 |
| Stuttgart | Oase 1 | 0.0005 | 0.6 | 0.0051 | 6.7 | ||
| Stuttgart | Ust’-Ishim | −0.0017 | −2.3 | 0.0018 | 2.3 | ||
| Stuttgart | Kostenki 14 | −0.0021 | −3.2 | −0.0032 | −4.6 | ||
| Stuttgart | MA1 | −0.0027 | −3.9 | −0.0029 | −4.2 | −0.0041 | −5.0 |
| Stuttgart | Loschbour | −0.0015 | −2.4 | −0.0027 | −4.6 | −0.0050 | −7.5 |
| Stuttgart | East Asian | −0.0017 | −2.9 | −0.0022 | −3.8 | 0.0040 | 6.8 |
| Stuttgart | Native American | −0.0024 | −3.9 | −0.0025 | −4.4 | 0.0012 | 1.9 |
Extended Data Table 2. Allele sharing between early modern humans and other humans (transversions only).
We compute D(Non-African1, Non-African2; Early Modern Human, African), to test whether an early modern human (Oase 1, Ust’-Ishim, or Kostenki 14) shares more alleles with Non-African1 (in which case the statistic is positive) or Non-African2 (negative). We use a pool of six sub-Saharan African genomes (2 Mbuti, 2 Yoruba, 2 Dinka) as an outgroup; a pool of four genomes (2 French, 2 Sardinians) to represent Europeans; a pool of four genomes (2 Han, 2 Dai) to represent East Asians; and a pool of three genomes (2 Karitiana, 1 Mixe) to represent Native Americans. Statistics are as in Extended Data Table 1 but are based on 106,004 transversion SNPs covered by at least one deaminated fragment in Oase 1 and that also have coverage for all other samples although not necessarily MA1. For analyses involving MA1, a subset of 76,715 transversion SNPs is analyzed.
| Non-African1 | Non-African2 | Oase 1 | Ust’-Ishim | Kostenki 14 | |||
|---|---|---|---|---|---|---|---|
| D | Z | D | Z | D | Z | ||
| Oase 1 | Ust’-Ishim | −0.0019 | −2.1 | ||||
| Oase 1 | Kostenki 14 | −0.0031 | −3.3 | ||||
| Oase 1 | MA1 | −0.0026 | −2.9 | −0.0071 | −6.5 | ||
| Oase 1 | Loschbour | −0.0023 | −2.6 | −0.0081 | −8.8 | ||
| Oase 1 | East Asian | −0.0013 | −1.9 | 0.0007 | 1.0 | ||
| Oase 1 | Native American | −0.0019 | −2.7 | −0.0018 | −2.3 | ||
| Ust’-Ishim | Kostenki 14 | −0.0012 | −1.4 | ||||
| Ust’-Ishim | MA1 | −0.0006 | −0.7 | −0.0050 | −5.1 | ||
| Ust’-Ishim | Loschbour | 0.0003 | 0.4 | −0.0062 | −7.1 | ||
| Ust’-Ishim | East Asian | 0.0005 | 0.7 | 0.0026 | 3.8 | ||
| Ust’-Ishim | Native American | −0.0003 | −0.4 | 0.0001 | 0.1 | ||
| Kostenki 14 | MA1 | 0.0001 | 0.1 | 0.0002 | 0.3 | ||
| Kostenki 14 | Loschbour | 0.0015 | 2.0 | 0.0008 | 1.1 | ||
| Kostenki 14 | East Asian | 0.0017 | 2.3 | 0.0017 | 2.5 | ||
| Kostenki 14 | Native American | 0.0009 | 1.2 | 0.0012 | 1.6 | ||
| MA1 | Loschbour | 0.0019 | 2.2 | 0.0010 | 1.3 | −0.0013 | −1.3 |
| MA1 | East Asian | 0.0011 | 1.4 | 0.0013 | 1.9 | 0.0075 | 8.5 |
| MA1 | Native American | 0.0006 | 0.7 | 0.0007 | 1.1 | 0.0051 | 6.0 |
| Loschbour | East Asian | 0.0001 | 0.2 | 0.0009 | 1.5 | 0.0088 | 12.3 |
| Loschbour | Native American | −0.0006 | −0.9 | 0.0004 | 0.6 | 0.0063 | 8.4 |
| East Asian | Native American | −0.0008 | −1.7 | −0.0006 | −1.3 | −0.0025 | −5.3 |
|
| |||||||
| European | Oase 1 | −0.0005 | −0.7 | 0.0029 | 3.9 | ||
| European | Ust’-Ishim | −0.0023 | −3.3 | 0.0010 | 1.4 | ||
| European | Kostenki 14 | −0.0035 | −5.1 | −0.0035 | −5.2 | ||
| European | MA1 | −0.0033 | −4.5 | −0.0033 | −5.2 | −0.0038 | −4.8 |
| European | Loschbour | −0.0020 | −3.6 | −0.0027 | −5.1 | −0.0052 | −8.4 |
| European | East Asian | −0.0018 | −3.6 | −0.0018 | −4.0 | 0.0036 | 7.8 |
| European | Native American | −0.0026 | −4.8 | −0.0023 | −5.2 | 0.0011 | 2.1 |
| European | Stuttgart | −0.0009 | −1.7 | −0.0010 | −2.2 | −0.0012 | −2.3 |
| Stuttgart | Oase 1 | 0.0005 | 0.7 | 0.0041 | 4.7 | ||
| Stuttgart | Ust’-Ishim | −0.0014 | −1.8 | 0.0022 | 2.6 | ||
| Stuttgart | Kostenki 14 | −0.0026 | −3.3 | −0.0025 | −3.5 | ||
| Stuttgart | MA1 | −0.0026 | −3.1 | −0.0023 | −3.2 | −0.0031 | −3.4 |
| Stuttgart | Loschbour | −0.0011 | −1.6 | −0.0017 | −2.8 | −0.0040 | −5.2 |
| Stuttgart | East Asian | −0.0010 | −1.4 | −0.0008 | −1.3 | 0.0048 | 7.2 |
| Stuttgart | Native American | −0.0017 | −2.4 | −0.0013 | −2.2 | 0.0023 | 3.4 |
Extended Data Table 3. Testing whether archaic genomes share more alleles with Oase 1 than with other modern humans.
The statistic D(Test, Oase 1; Archaic, Outgroup) is negative if the archaic genomes share more alleles with Oase 1 than with a test sample. The outgroups are either chimpanzee or a sub-Saharan African (Mbuti).
| Test | Sites | Archaic = Altai | Archaic = Denisovan | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chimp | Mbuti | Chimp | Mbuti | ||||||
| D | Z | D | Z | D | Z | D | Z | ||
| Han | 115,300 | −0.0036 | −5.1 | −0.0071 | −7.6 | −0.0014 | −2.2 | −0.0049 | −6.3 |
| Dai | 115,300 | −0.0035 | −5.0 | −0.0077 | −8.2 | −0.0013 | −2.1 | −0.0056 | −7.0 |
| Karitiana | 115,300 | −0.0032 | −4.3 | −0.0063 | −6.9 | −0.0008 | −1.3 | −0.0040 | −5.3 |
| French | 115,300 | −0.0049 | −6.9 | −0.0074 | −8.2 | −0.0021 | −3.4 | −0.0047 | −6.2 |
| Sardinian | 115,300 | −0.0038 | −5.1 | −0.0071 | −7.8 | −0.0016 | −2.5 | −0.0050 | −6.5 |
| Papuan | 115,300 | −0.0026 | −3.6 | −0.0051 | −5.4 | 0.0009 | 1.5 | −0.0016 | −2.1 |
| Ust'-Ishim | 115,100 | −0.0026 | −3.6 | −0.0052 | −5.5 | −0.0009 | −1.5 | −0.0035 | −4.4 |
| Kostenki14 | 108,100 | −0.0032 | −4.1 | −0.0059 | −6.0 | −0.0017 | −2.4 | −0.0044 | −5.3 |
| MA1 | 83,200 | −0.0031 | −3.6 | −0.0050 | −4.7 | −0.0007 | −0.9 | −0.0028 | −2.8 |
| Loschbour | 114,300 | −0.0043 | −5.7 | −0.0066 | −6.8 | −0.0019 | −2.9 | −0.0043 | −5.3 |
| LaBrana | 111,000 | −0.0033 | −4.2 | −0.0072 | −7.3 | −0.0008 | −1.2 | −0.0047 | −5.4 |
| Stuttgart | 114,000 | −0.0037 | −5.1 | −0.0066 | −7.1 | −0.0013 | −2.1 | −0.0042 | −5.6 |
Extended Data Table 4. Estimated fraction of the Oase 1 genome that derives from Neanderthals.
Estimates are as in Table 1 but restrict to transversions. Modern human samples are from Panel B.
| Sample |
|
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prop. | S.E. | 90% CI | Prop. | S.E. | 90% CI | Prop. | S.E. | 90% CI | ||||
| Oase 1 | 11.3% | 2.8% | 6.7%–16% | 10.9% | 1.6% | 8.3%–13.6% | 8.4% | 2.7% | 4.0%–12.9% | |||
| Ust'-Ishim | 2.9% | 1.2% | 1.0%–4.9% | 6.0% | 0.8% | 4.7%–7.4% | 4.2% | 1.5% | 1.8%–6.6% | |||
| Kostenki 14 | 3.0% | 1.4% | 0.7%–5.3% | 3.0% | 0.9% | 1.6%–4.5% | 6.2% | 1.6% | 3.6%–8.7% | |||
| MA1 | 1.5% | 1.5% | 0.0%–4.0% | 3.6% | 1.0% | 1.9%–5.2% | 5.5% | 1.6% | 2.8%–8.2% | |||
| Loschbour | 1.1% | 1.2% | 0.0%–3.1% | 4.8% | 0.9% | 3.3%–6.2% | 3.6% | 1.5% | 1.2%–6.1% | |||
| LaBrana | 3.7% | 1.3% | 1.4%–5.9% | 2.4% | 0.9% | 0.9%–3.8% | 4.8% | 1.5% | 2.4%–7.2% | |||
| Stuttgart | 2.8% | 1.2% | 0.8%–4.8% | 3.4% | 0.9% | 2.0%–4.9% | 3.8% | 1.5% | 1.4%–6.2% | |||
| Han | 1.0% | 1.3% | 0.0%–3.1% | 2.8% | 0.9% | 1.3%–4.2% | 3.6% | 1.5% | 1.2%–6.1% | |||
| Dai | 2.1% | 1.2% | 0.2%–4.0% | 1.3% | 0.9% | 0.0%–2.8% | 3.8% | 1.5% | 1.4%–6.2% | |||
| French | 1.6% | 1.2% | 0.0%–3.5% | 3.3% | 0.9% | 1.9%–4.7% | 2.7% | 1.5% | 0.3%–5.2% | |||
| Sardinian | 2.7% | 1.2% | 0.8%–4.7% | 2.3% | 0.9% | 0.8%–3.7% | 3.7% | 1.4% | 1.3%–6.1% | |||
Extended Data Table 5. Number of alleles in selected modern humans that are present in at least one Neanderthal genome but are rare or absent in West Africans.
The analysis is based on 78,055 sites covered by at least one deaminated fragment in Oase 1. To convert the counts to estimates of ancestry, we subtract the Dinka count as an estimate of the false positive rate and divide by the number of sites covered (as indicated for the whole genome on the bottom). This gives the rate of alleles per screened site on this chromosome for this individual. We then multiply this quantity by 2%/1.32% to recalibrate the 1.32% seen genome-wide in the French to an assumed 2% genome-wide Neanderthal ancestry in the French.
| Chr | Sites | Neanderthal allele counts | Neanderthal ancestry | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oase 1 | Ust’-Ishim | Kostenki 14 | Han | French | Dinka | Oase 1 | Ust’-Ishim | Kostenki 14 | Han | French | ||
| 1 | 6740 | 323 | 196 | 148 | 129 | 117 | 25 | 6.70% | 3.84% | 2.77% | 2.34% | 2.07% |
| 2 | 7112 | 294 | 145 | 121 | 188 | 199 | 29 | 5.65% | 2.47% | 1.96% | 3.39% | 3.62% |
| 3 | 5417 | 177 | 102 | 96 | 74 | 98 | 28 | 4.17% | 2.07% | 1.90% | 1.29% | 1.96% |
| 4 | 4495 | 359 | 86 | 63 | 141 | 96 | 42 | 10.69% | 1.48% | 0.71% | 3.34% | 1.82% |
| 5 | 4330 | 446 | 108 | 66 | 103 | 95 | 23 | 14.80% | 2.97% | 1.50% | 2.80% | 2.52% |
| 6 | 4549 | 324 | 155 | 167 | 142 | 138 | 73 | 8.36% | 2.73% | 3.13% | 2.30% | 2.16% |
| 7 | 4422 | 147 | 68 | 65 | 102 | 72 | 34 | 3.87% | 1.16% | 1.06% | 2.33% | 1.30% |
| 8 | 4322 | 131 | 132 | 72 | 35 | 38 | 14 | 4.10% | 4.14% | 2.03% | 0.74% | 0.84% |
| 9 | 3107 | 500 | 69 | 120 | 118 | 49 | 15 | 23.65% | 2.63% | 5.12% | 5.02% | 1.66% |
| 10 | 4009 | 147 | 139 | 67 | 131 | 86 | 22 | 4.72% | 4.42% | 1.70% | 4.12% | 2.42% |
| 11 | 4193 | 153 | 93 | 88 | 81 | 73 | 26 | 4.59% | 2.42% | 2.24% | 1.99% | 1.70% |
| 12 | 3456 | 456 | 160 | 54 | 125 | 93 | 10 | 19.55% | 6.58% | 1.93% | 5.04% | 3.64% |
| 13 | 2457 | 96 | 81 | 33 | 54 | 30 | 18 | 4.81% | 3.89% | 0.93% | 2.22% | 0.74% |
| 14 | 2390 | 85 | 27 | 52 | 50 | 52 | 13 | 4.56% | 0.89% | 2.47% | 2.35% | 2.47% |
| 15 | 2327 | 73 | 78 | 47 | 38 | 32 | 5 | 4.43% | 4.75% | 2.73% | 2.15% | 1.76% |
| 16 | 3139 | 90 | 121 | 68 | 43 | 39 | 8 | 3.96% | 5.45% | 2.90% | 1.69% | 1.50% |
| 17 | 2543 | 72 | 89 | 37 | 85 | 75 | 56 | 0.95% | 1.97% | −1.13% | 1.73% | 1.13% |
| 18 | 2305 | 57 | 58 | 59 | 27 | 29 | 5 | 3.42% | 3.48% | 3.55% | 1.45% | 1.58% |
| 19 | 1769 | 79 | 49 | 33 | 43 | 35 | 12 | 5.74% | 3.17% | 1.80% | 2.66% | 1.97% |
| 20 | 2492 | 107 | 29 | 62 | 56 | 43 | 12 | 5.78% | 1.03% | 3.04% | 2.68% | 1.88% |
| 21 | 1026 | 36 | 53 | 22 | 8 | 11 | 10 | 3.84% | 6.35% | 1.77% | −0.30% | 0.15% |
| 22 | 1455 | 79 | 33 | 66 | 34 | 18 | 5 | 7.71% | 2.92% | 6.35% | 3.02% | 1.35% |
|
| ||||||||||||
| All | 78055 | 4231 | 2071 | 1606 | 1807 | 1518 | 485 | 7.27% | 3.08% | 2.18% | 2.57% | “2%” |
| Subtract | Dinka | 3746 | 1586 | 1121 | 1322 | 1033 | 0 | |||||
Extended Data Table 6. Ancient DNA libraries made from the Oase 1 mandible.
| Metainformation | Sequencing results | All fragments | Deaminated fragments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Library | Extract | UDG treatment | Index 1 | Index 2 | Extract used (μ1) | Sequences going into alignment | Sequences ≥35bp mapped | After dup. removal | Coverage | % C→T 5′end | % C→T 3′end | Coverage | % C→T 5′ end | % C→T 3′end |
| A5227 | E1406 | Yes | ACTTGCG | AACTCCG | 8 | 206,982 | 118,976 | 34,486 | 112 | 8 | 19 | 5 | 19 | 36 |
| A5252 | E1843 | Yes | GTAAGCC | TTGAAGT | 40 | 74,384 | 46,394 | 31,368 | 114 | 7 | 25 | 5 | 18 | 55 |
| A9032 | E1406 | No | ATAACGT | ACTATCA | 6 | 9,321,903 | 5,904,210 | 51,810 | 178 | 20 | 21 | 12 | 31 | 39 |
| A9033 | E1406 | No | AATAGGA | ACCAACT | 6 | 7,932,271 | 4,816,314 | 55,878 | 193 | 21 | 20 | 13 | 36 | 38 |
| A9034 | E1406 | No | ATCACGA | AACTCCG | 6 | 10,422,467 | 6,861,634 | 59,883 | 207 | 20 | 20 | 14 | 35 | 38 |
|
| ||||||||||||||
| All | Both | Mix | 27,958,007 | 17,747,528 | 233,425 | 803 | 17 | 21 | 49 | 30 | 39 | |||
Contamination estimate based on the direct comparison of C→T substitutions of all fragments and deaminated fragments only.
Extended Data Table 7. Sequencing metrics on all five libraries for each of four capture probe panels.
| Library | Panel | No. target SNPs | Fragments going into alignment | Fragments mapped to genome | Fragments on target after dup. removal and MAPQ37 filter | % SNPs hit at least once | Average coverage on SNPs |
|---|---|---|---|---|---|---|---|
| A9032 | 390k | 393,577 | 10,849,144 | 2,235,955 | 133,564 | 26.5% | 0.34 |
| A9033 | 390k | 393,577 | 17,159,085 | 2,808,704 | 73,824 | 15.9% | 0.19 |
| A9034 | 390k | 393,577 | 16,902,935 | 3,256,438 | 142,520 | 27.7% | 0.36 |
| A5227 | 390k | 393,577 | 63,441,719 | 22,124,247 | 195,161 | 36.0% | 0.5 |
| A5252 | 390k | 393,577 | 60,181,844 | 14,278,978 | 180,626 | 33.3% | 0.46 |
| All 5 | 390k | 393,577 | 168,534,727 | 44,704,322 | 724,653 | 73.0% | 1.84 |
|
| |||||||
| A9032 | 840k | 842,630 | 25,105,625 | 3,801,435 | 178,015 | 17.6% | 0.21 |
| A9033 | 840k | 842,630 | 29,196,969 | 4,655,434 | 183,093 | 17.9% | 0.22 |
| A9034 | 840k | 842,630 | 35,780,652 | 5,968,851 | 200,767 | 19.3% | 0.24 |
| A5227 | 840k | 842,630 | 28,209,496 | 4,276,439 | 152,411 | 15.3% | 0.18 |
| A5252 | 840k | 842,630 | 20,286,540 | 1,630,343 | 106,943 | 11.2% | 0.13 |
| All 5 | 840k | 842,630 | 138,579,282 | 20,332,502 | 818,648 | 51.7% | 0.97 |
|
| |||||||
| A9032 | 1000k | 997,780 | 26,088,835 | 2,964,094 | 159,162 | 13.5% | 0.16 |
| A9033 | 1000k | 997,780 | 26,641,358 | 4,490,372 | 158,614 | 13.3% | 0.16 |
| A9034 | 1000k | 997,780 | 28,795,043 | 4,985,140 | 154,177 | 13.0% | 0.15 |
| A5227 | 1000k | 997,780 | 25,848,311 | 4,395,413 | 71,537 | 6.4% | 0.07 |
| A5252 | 1000k | 997,780 | 25,691,323 | 2,254,636 | 53,932 | 5.0% | 0.05 |
| All 5 | 1000k | 997,780 | 133,064,870 | 19,089,655 | 596,107 | 36.1% | 0.6 |
|
| |||||||
| A9032 | Archaic | 1,749,385 | 19,329,832 | 2,086,208 | 205,095 | 10.0% | 0.12 |
| A9033 | Archaic | 1,749,385 | 24,629,023 | 2,768,355 | 237,818 | 11.4% | 0.14 |
| A9034 | Archaic | 1,749,385 | 31,200,466 | 3,783,805 | 257,351 | 12.2% | 0.15 |
| A5227 | Archaic | 1,749,385 | 27,659,125 | 3,606,375 | 195,356 | 9.6% | 0.11 |
| A5252 | Archaic | 1,749,385 | 31,472,143 | 2,435,080 | 136,637 | 6.8% | 0.08 |
| All 5 | Archaic | 1,749,385 | 134,290,589 | 14,679,823 | 1,022,046 | 34.6% | 0.58 |
|
| |||||||
| A9032 | Combined | 3,801,245 | 81,373,436 | 11,087,692 | 719,146 | 15.5% | 0.19 |
| A9033 | Combined | 3,801,245 | 97,626,435 | 14,722,865 | 698,890 | 15.1% | 0.18 |
| A9034 | Combined | 3,801,245 | 112,679,096 | 17,994,234 | 806,589 | 17.0% | 0.21 |
| A5227 | Combined | 3,801,245 | 145,158,651 | 34,402,474 | 666,195 | 14.2% | 0.18 |
| A5252 | Combined | 3,801,245 | 137,631,850 | 20,599,037 | 531,873 | 11.4% | 0.14 |
| All 5 | Combined | 3,801,245 | 574,469,468 | 98,806,302 | 3,406,685 | 45.5% | 0.90 |
Extended Data Table 8. Effect of filtering on amount of nuclear data available for analysis.
| All fragments | Deaminated fragments only | ||||||
|---|---|---|---|---|---|---|---|
| Panel | Target SNPs | No. SNPs hit ≥1× | % SNPs hit ≥1× | Average coverage | No. SNPs hit ≥1× | % SNPs hit ≥1× | Average coverage |
| Panels 1–3 | 2,051,902 | 1,038,619 | 50.6% | 1.03 | 271,326 | 13.2% | 0.16 |
| Panel 4 subset* | 954,849 | 361,681 | 37.9% | 0.69 | 87,803 | 9.2% | 0.11 |
| Panels 1–4 | 3,801,245 | 1,685,891 | 44.4% | 0.85 | 426,027 | 11.2% | 0.13 |
The Panel 4 subset excludes the sites where only Denisova differs from the African panel. Note: Numbers differ from Extended Data Table 7 because only sites with base quality ≥20 were used.
Extended Data Table 9. Genomes merged with the Oase 1 data.
| Sample ID | Human | Data type | Mean | UDG-treated |
|---|---|---|---|---|
| Oasel | Modern | Low coverage | Capture | Mix of library types |
|
| ||||
| Vindija | Archaic | Low coverage | 1.3 | No |
| Mezmaiskaya | Archaic | Low coverage | 0.5 | Yes |
| Altai | Archaic | High coverage | 52 | Yes |
| Denisova | Archaic | High coverage | 31 | Yes |
| Kostenki14 | Modern | Low coverage | 2.4 | Mix of library types |
| MA1 | Modern | Low coverage | 1 | No |
| LaBrana | Modern | Low coverage | 3.4 | No |
| Loschbour | Modern | High coverage | 22 | Yes |
| Stuttgart | Modern | High coverage | 19 | Yes |
| Ust’-Ishim | Modern | High coverage | 42 | Yes |
|
| ||||
| DinkaA | Modern | High coverage | 28 | .. |
| FrenchA | Modern | High coverage | 27 | .. |
| PapuanA | Modern | High coverage | 26 | .. |
| SardinianA | Modern | High coverage | 25 | .. |
| HanA | Modern | High coverage | 28 | .. |
| YorubaA | Modern | High coverage | 32 | .. |
| KaritianaA | Modern | High coverage | 26 | .. |
| SanA | Modern | High coverage | 33 | .. |
| MandenkaA | Modern | High coverage | 25 | .. |
| DaiA | Modern | High coverage | 28 | .. |
| MbutiA | Modern | High coverage | 24 | .. |
|
| ||||
| DaiB | Modern | High coverage | 37 | .. |
| FrenchB | Modern | High coverage | 42 | .. |
| HanB | Modern | High coverage | 35 | .. |
| MandenkaB | Modern | High coverage | 37 | .. |
| MbutiB | Modern | High coverage | 37 | .. |
| PapuanB | Modern | High coverage | 42 | .. |
| SanB | Modern | High coverage | 38 | .. |
| SardinianB | Modern | High coverage | 38 | .. |
| YorubaB | Modern | High coverage | 39 | .. |
| KaritianaB | Modern | High coverage | 35 | .. |
| MixeB | Modern | High coverage | 42 | .. |
| AustralianB1 | Modern | High coverage | 42 | .. |
| AustralianB2 | Modern | High coverage | 37 | .. |
| DinkaB | Modern | High coverage | 35 | .. |
Note: For the 25 present-day humans, samples ending with a subscript “A” are from “Panel A” of 11 individuals and samples ending with a subscript “B” are from “Panel B” of 14 individuals. Unless otherwise specified, in the analyses in this study we used Panel B individuals to represent specified present-day human populations.
Supplementary Material
Acknowledgments
We thank Erik Trinkaus for help making the sampling of Oase 1 possible, Johannes Krause for help with sampling and initial DNA analyses. Ayinuer Aximu for help with DNA hybridization captures and library preparation and Erik Trinkhaus and Linda Vigilant for critical reading of the manuscript. Q.F. is funded in part by Chinese Academy of Sciences (XDA05130202) and the Special Foundation of the President of the Chinese Academy of Sciences (2015–2016). O.T.M. and S.C. are supported by the Romanian National Research Council through project 31/2010 (Karst Climate Archives). D.R. is supported by US National Science Foundation HOMINID grant BCS-1032255, US National Institutes of Health grant GM100233, and the Howard Hughes Medical Institute. The laboratory work was funded by the Presidential Innovation Fund of the Max Planck Society.
Footnotes
Supplementary Information is available in the online version of the paper.
Author contributions. NP, KP, MM, JK, DR and SP supervised the study. SC and OTM collected and analyzed archaeological material. QF, MH and BN performed laboratory work. QF, MH, SM, PS, NP, NR, IL, BV, KP, JK and DR analyzed data. QF, SM, MM and DR designed capture probes. DR and SP wrote the manuscript with the help of all co-authors.
The aligned sequences are available through the European Nucleotide Archive under accession number PRJEB8987 (http://www.ebi.ac.uk/ena/data/view/PRJEB8987).
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
References
- 1.Higham T, et al. The timing and spatiotemporal patterning of Neanderthal disappearance. Nature. 2014;512:306–309. doi: 10.1038/nature13621. [DOI] [PubMed] [Google Scholar]
- 2.Trinkaus E, et al. An early modern human from the Pestera cu Oase, Romania. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11231–11236. doi: 10.1073/pnas.2035108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith FH, et al. The Assimilation Model, Modern Human Origins in Europe, and the Extinction of Neandertals. Quaternary International. 2005;137:7–19. [Google Scholar]
- 4.Zilhao J. Neandertals and Moderns Mixed, and It Matters. Evolutionary Anthropology. 2006;15:183–195. [Google Scholar]
- 5.Hublin J-J. The modern human colonization of western Eurasia: when and where? Quaternary Science Reviews. 2014 [Google Scholar]
- 6.Green RE, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prufer K, et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 2014;505:43–49. doi: 10.1038/nature12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sankararaman S, et al. The Date of Interbreeding between Neandertals and Modern Humans. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer M, et al. A high-coverage genome sequence from an archaic Denisovan individual. Science. 2012;338:222–226. doi: 10.1126/science.1224344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wall J, et al. Higher Levels of Neanderthal Ancestry in East Asians than in Europeans. Genetics. 2013;194:199–209. doi: 10.1534/genetics.112.148213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BY, Lohmueller KE. Selection and Reduced Population Size Cannot Explain Higher Amounts of Neandertal Ancestry in East Asian than in European Human Populations. American journal of human genetics. 2015 doi: 10.1016/j.ajhg.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernot B, Akey JM. Complex History of Admixture between Modern Humans and Neandertals. American journal of human genetics. 2015 doi: 10.1016/j.ajhg.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.d’Errico F, et al. Neanderthal Acculturation in Western Europe?: A Critical Review of the Evidence and Its Interpretation. Current Anthropology. 1998;39(Supplement):S1–S44. [Google Scholar]
- 14.Mellars P. The Impossible Coincidence. A Single-species Model for the Origins of Modern Human Behavior in Europe. Evolutionary Anthropology. 2005;14:12–27. [Google Scholar]
- 15.Fu Q, et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–449. doi: 10.1038/nature13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seguin-Orlando A, et al. Paleogenomics. Genomic structure in Europeans dating back at least 36,200 years. Science. 2014;346:1113–1118. doi: 10.1126/science.aaa0114. [DOI] [PubMed] [Google Scholar]
- 17.Rougier H, et al. Pestera cu Oase 2 and the cranial morphology of early modern Europeans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1165–1170. doi: 10.1073/pnas.0610538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constantin S, et al. Reconstructing the evolution of cave systems as a key to understanding the taphonomy of fossil accumulations: The case of Urşilor Cave (Western Carpathians, Romania) Quaternary International. 2014;339–340:25–40. [Google Scholar]
- 19.Lacy MK, et al. The 83rd Annual Meeting of the American Association of Physical Anthropologists.. [Google Scholar]
- 20.Frayer D. In: Continuity or Replacement? Controversies in Homo sapiens Evolution. Bräuer G, Smith FH, editors. A.A. Balkema Publishers; 1992. [Google Scholar]
- 21.Trinkaus E, et al. Life and Death at the Peştera cu Oase. A Setting for Modern Human Emergence in Europe. Oxford University Press; 2013. [Google Scholar]
- 22.Briggs AW, et al. Removal of deaminated cytosines and detection of in vivo methylation in ancient DNA. Nucleic acids research. 2010;38:e87. doi: 10.1093/nar/gkp1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Q, et al. DNA analysis of an early modern human from Tianyuan Cave, China. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2223–2227. doi: 10.1073/pnas.1221359110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawyer S, et al. Temporal patterns of nucleotide misincorporations and DNA fragmentation in ancient DNA. PLoS One. 2012;7:e34131. doi: 10.1371/journal.pone.0034131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer M, et al. A mitochondrial genome sequence of a hominin from Sima de los Huesos. Nature. 2014;505:403–406. doi: 10.1038/nature12788. [DOI] [PubMed] [Google Scholar]
- 26.Skoglund P, et al. Separating endogenous ancient DNA from modern day contamination in a Siberian Neandertal. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2229–2234. doi: 10.1073/pnas.1318934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patterson N, et al. Ancient admixture in human history. Genetics. 2012;192:1065–1093. doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazaridis I, et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature. 2014;513:409–413. doi: 10.1038/nature13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reich D, et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature. 2010;468:1053–1060. doi: 10.1038/nature09710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinkaus E. European early modern humans and the fate of the Neandertals. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7367–7372. doi: 10.1073/pnas.0702214104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dabney J, et al. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15758–15763. doi: 10.1073/pnas.1314445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gansauge MT, Meyer M. Single-stranded DNA library preparation for the sequencing of ancient or damaged DNA. Nat Protoc. 2013;8:737–748. doi: 10.1038/nprot.2013.038. [DOI] [PubMed] [Google Scholar]
- 33.Briggs AW, et al. Patterns of damage in genomic DNA sequences from a Neandertal. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14616–14621. doi: 10.1073/pnas.0704665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabney J, Meyer M. Length and GC-biases during sequencing library amplification: a comparison of various polymerase-buffer systems with ancient and modern DNA sequencing libraries. Biotechniques. 2012;52:87–94. doi: 10.2144/000113809. [DOI] [PubMed] [Google Scholar]
- 35.Kircher M, Sawyer S, Meyer M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic acids research. 2012;40:e3. doi: 10.1093/nar/gkr771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haak W, et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015 doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genomes Project C et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paten B, Herrero J, Beal K, Fitzgerald S, Birney E. Enredo and Pecan: genome-wide mammalian consistency-based multiple alignment with paralogs. Genome Res. 2008;18:1814–1828. doi: 10.1101/gr.076554.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paten B, et al. Genome-wide nucleotide-level mammalian ancestor reconstruction. Genome Res. 2008;18:1829–1843. doi: 10.1101/gr.076521.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raghavan M, et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature. 2013 doi: 10.1038/nature12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olalde I, et al. Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature. 2014;507:225–228. doi: 10.1038/nature12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.