Abstract
Context: The independent or interactive effects of vitamin D and calcium on adiposity remain inconclusive. Objective: The objective of this systematic review and meta-analysis was to assess whether vitamin D and calcium supplements cause changes in adiposity. Data Sources: MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials databases were searched for literature published from 1966 to March 2014. Study Selection: A systematic search was conducted for randomized clinical trials with ≥50 participants aged ≥18 years at baseline who had received at least 12 weeks of treatment. Among the inclusion criteria were supplementation with vitamin D with or without calcium and measurement of adiposity (weight, body mass index [BMI], and/or fat mass). Data Extraction: The primary endpoints assessed were changes in weight, BMI, or fat mass. Data Synthesis: Of 953 trials identified, 26 randomized clinical trials (n = 12, vitamin D alone; n = 10, vitamin D plus calcium versus calcium control; n = 4, vitamin D plus calcium versus placebo) with a total of 42 430 participants (median duration, 12 months) met the inclusion criteria. When compared with placebo, vitamin D supplementation had no significant effect on BMI (weighted mean difference [WMD], −0.06 kg/m2; 95% confidence interval [95%CI], −0.14 to 0.03), weight (WMD, −0.05 kg; 95%CI, −0.32 to 0.23), or fat mass (WMD, −0.43 kg; 95%CI, −1.69 to 0.84). Likewise, no significant reduction in BMI (WMD, 0.02 kg/m2; 95%CI, −0.11 to 0.14), weight (WMD, 0.12 kg; 95%CI, −0.24 to 0.49), or fat mass (WMD, 0.12 kg; 95%CI, −0.22 to 0.45) was observed in participants who received vitamin D plus calcium compared with those who received calcium control. Conclusions: Supplementation with vitamin D showed no effect on adiposity measures in adults.
Keywords: adiposity, obesity, supplementation, vitamin D
INTRODUCTION
Obesity is one of the greatest causes of preventable morbidity and mortality worldwide1 and often coexists with vitamin D insufficiency.2 Given the increasing rates of obesity1 across many populations worldwide, finding strategies to curb this epidemic is an urgent public health issue. Obesity augments risk of cardiovascular disease, type 2 diabetes, and many other chronic diseases. Vitamin D is an essential fat-soluble vitamin that is stored in adipose tissue,3,4 and its role in the pathogenesis of obesity and chronic diseases is an area of tremendous importance to clinical nutrition and public health.
A bidirectional relationship exists between obesity and vitamin D metabolism and storage.3 Observational studies have reported an increased risk of vitamin D deficiency in obese individuals, but the direction of causality and the underlying mechanisms are unclear.4 The greater storage capacity for vitamin D in obese individuals by fat sequestration3 or volumetric dilution5 may result in lower plasma vitamin D. Furthermore, there has been recent debate about what constitutes vitamin D deficiency and sufficiency.6 The most recent compilation of data suggests that a 25-hydroxyvitamin D [25(OH)D] level of 50 nmol/L (20 ng/mL) is adequate for the population. However, a 2011 Institute of Medicine report concluded that, currently, the available evidence is sufficient to provide health guidelines only for skeletal health and that more data are needed on nonskeletal outcomes and to identify the threshold effects for other health outcomes.7,8
A clearer understanding of the inverse relationship between vitamin D and measures of body fat is essential. By reverse causation, prevention of obesity may improve vitamin D status.5 Possible anti-obesity mechanisms of calcium and vitamin D include the control of adipocyte death, the regulation of adipogenesis, and the improvement of lipid metabolism.9 Observational studies have suggested that sufficient vitamin D status [25(OH)D ≥50 nmol/L] is associated with a reduced risk of diseases that cluster with obesity, such as cardiovascular disease, diabetes, and certain cancers.10,11 Vitamin D supplements may interact with calcium and parathyroid hormone to affect adiposity.2 Elevated parathyroid hormone levels in the presence of low serum 25(OH)D concentrations [25(OH)D <50 nmol/L] might affect calcium influx into adipose cells and promote weight gain.12 The active vitamin D metabolite 1,25 dihydroxyvitamin D3 might also modulate adipogenesis independently of parathyroid hormone.13 A recent study reported that weight gain in mice fed a high-fat diet with calcium and vitamin D was lower than that in mice fed the high-fat diet alone.14 Animal studies on vitamin-D-receptor null mice suggest a role for vitamin D in energy regulation.15
Since the 1980s, observations in humans of lower levels of 25(OH)D in obese than in nonobese individuals highlight a possible inverse relation between vitamin D and obesity.16 Cross-sectional studies have shown an inverse association between 25(OH)D levels and adiposity assessed by various measures.18,19 This significant association has not been shown in all studies.17,18
Similarly, conflicting results about the association between directly measured total fat and 25(OH)D levels compared with other anthropometric measures have been reported.19 For example, Moschonis and Manios.20 observed significant associations between vitamin D levels and body composition indices as measured by dual-energy X-ray absorptiometry (DXA), but no significant associations between anthropometric indices of body mass and vitamin D levels.20 Another study suggests that anthropometric measures and total fat directly measured by DXA were inversely associated with 25(OH)D levels.19
These observational results suggest that improving vitamin D status may be an effective intervention for prevention and management of obesity. Few intervention trials were specifically designed to evaluate the direct effects of vitamin D supplementation on adiposity measures, and existing trials with adiposity as a secondary outcome have produced conflicting results. Some trials showed no association of vitamin D supplementation with weight loss,21,22 while others showed potential benefits that may be dependent on adjunctive calcium supplementation.23,24 The choice of adiposity measures may be important when evaluating relationships between vitamin D supplementation and adiposity.25 Only a few trials of vitamin D have assessed changes in body composition, visceral fat, or other fat depots, as directly measured by DXA,21,25,26 magnetic resonance imaging, or computed tomography.27,28 DXA provides measures of overall adiposity, lean tissue, and regional distributions, with good reproducibility and minimal radiation exposure.29,30 Adequately powered randomized controlled trials (RCTs) with direct assessments of adiposity, such as DXA, are warranted to clarify the direct effect of vitamin D with or without calcium on adiposity.
A recent meta-analysis assessing the effect of vitamin D supplementation alone on adiposity measures reported null results, but an effect by vitamin D dose was not evaluated.31 Therefore, the aim of this systematic review was to conduct a meta-analysis of RCTs to quantitatively assess the dose effects of vitamin D supplementation alone or in combination with calcium on changes in three widely used adiposity measures: body weight, body mass index (BMI), and fat mass. The systematic review and meta-analysis were performed using PRISMA guidelines (see Appendix S1 in the Supporting Information for the article online).
METHODS
Data sources and literature search
On the basis of the hypothesis that vitamin D supplementation alone or with calcium alters adiposity measures, a standard search protocol for this systematic literature review and meta-analysis was developed and followed (Figure 1). The PICOS criteria are listed in Table 1. The MEDLINE, Embase, and Cochrane Central Register of Controlled Trials databases were searched for literature published from 1966 to March 2014. The search terms were selected to capture generic and specific words relevant to the exposure and outcome on the basis of Medical Subject Heading (MeSH) terms and text keywords from articles identified a priori. Terms selected for vitamin D included vitamin D intake, vitamin D supplement, calcidiol, calcitriol, cholecalciferol (vitamin D3), and ergocalciferol (vitamin D2). Terms for adiposity included overweight, weight loss, BMI, adipose tissue, fat mass, or body fat distribution. The search was restricted to articles published in English and studies of human subjects aged 18 years or older. The same search strategy was applied to each database. Reference lists of retrieved articles were also searched for additional studies. Details of the literature search are provided in Appendix S2 in the Supporting Information for this article online. All vitamin D data were converted, as necessary, to international units (IU) per day for intake (except for the study by Ljunghall et al.,32 as it used alphacalcidiol, which does not have an IU conversion or a dose approximation for vitamin D3 or vitamin D2), and nanomole per liter for 25(OH)D status.
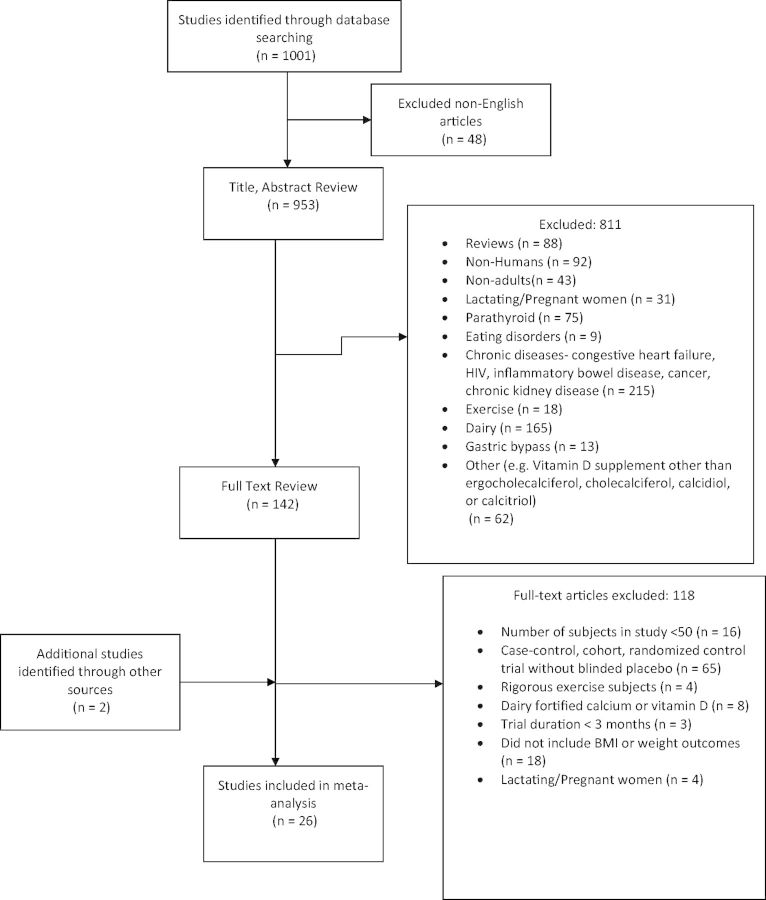
Figure 1.
Flow chart of the study selection
Table 1.
Summary of PICOS criteria used in the meta-analysis to address the research question: Does the use of vitamin D supplementation alone or with calcium change adiposity measures?
| Parameter | Description |
|---|---|
| Population | General adult population |
| Interventions | Supplementation with vitamin D alone or supplementation with vitamin D plus calcium |
| Comparators | Placebo or calcium alone |
| Outcome | Change in adiposity measures (BMI, fat mass, or weight) |
| Setting | Randomized, double-blind, controlled trials with a minimum of 50 participants, a minimum duration of intervention of 3 mo, and measurement of BMI, fat mass, or weight |
Abbreviation: BMI, body mass index (kg/m2).
Study selection
Two independent investigators (P.D.C. and X.Z.) assessed each abstract and article according to the inclusion criteria and critically evaluated the methodological quality. Study selection was limited to randomized, double-blind, controlled trials that had a minimum of 50 participants, a minimum duration of intervention of 3 months, and a measurement of BMI, body weight, or fat mass (Table 1). Fifty is generally accepted as the minimum number of participants required for adequate power in correlation or regression models.33 An intervention period of at least 3 months allows for adequate time to assess changes in adiposity measures.34 Maximum weight loss from pharmacologic35,36 and behavioral interventions37,38 usually peaks around 6 months. Short-term efficacy is a suboptimal endpoint because recidivism is common when anti-obesity medications are stopped.34 One-third to two-thirds of weight loss is typically regained within 1 year and almost all is regained within 5 years.38,39 Body weight, BMI, and fat mass were the measures of adiposity analyzed because they are commonly reported outcome measures. Waist circumference was not used as an adiposity outcome measure because the number of studies included in this meta-analysis with available information21,25,40,41 was not large enough for a meaningful analysis. The primary method used to measure fat mass in the included studies was DXA, but other fat mass values were also reported, including truncal fat, whole-body fat, and body fat percentage. Bioelectrical impedance analysis was used in 1 study.41 Caloric restriction and changes in background diet were a parallel focus in 3 studies.23,41,42 If baseline and end-of-study values were not reported for BMI, weight, or fat mass, the authors were contacted for additional information. Studies of children and adolescents and studies that did not assess use of vitamin D supplements, with or without calcium, were excluded on the basis of the abstract review. Articles that passed abstract screening for a full-text review were retrieved, and studies involving patients with chronic diseases such as cancer, end-stage renal disease, and inflammatory bowel disease, which may have induced pathologic changes in adiposity, were further excluded.
Assessment of methodological quality
Two investigators (P.D.C. and X.Z.) reviewed and extracted data on study design, participant characteristics, interventions, and outcomes. The methodological quality of each included trial was assessed using the Jadad score.43 The domains used in the present meta-analysis pertained to randomization and allocation concealment (selection bias), blinding (performance and detection bias), and loss to follow-up and adherence to the intention-to-treat principle (attrition bias). All studies are presented and were assigned a summary score for study quality as assessed across studies. Two measures were used to estimate fat mass: DXA, and bioelectrical impedance.
Statistical analyses
Studies that compared vitamin D supplementation alone with placebo, vitamin D plus calcium supplementation with calcium control (which is a test of vitamin D), and vitamin D plus calcium supplementation with placebo were analyzed. Most of these studies reported more than one outcome measure of adiposity. To investigate the dose–response effect, subgroup analyses stratified by vitamin D dose were performed. In each of these subgroup analyses, each study contributed only one dose category, except for the study of Gallagher et al.26
The DerSimonian–Laird random-effects model was used to examine the effects of vitamin D with or without calcium supplements on adiposity measurements. The weighted mean differences (95% confidence intervals) were calculated on the basis of the random-effects model. Heterogeneity among trials was assessed using the chi-square statistic with the significance level set at P < 0.05. The extent of heterogeneity was also quantified with the I2 value, where the percentages of I2, i.e., 25%–50%, 50%–75%, and >75%, indicate low, medium, and high heterogeneity, respectively.44 To examine whether the summary estimates were robust to the results from individual studies, prespecified sensitivity analyses were employed by repeating the analysis after the study with the largest effect was removed. In a meta-analysis, the heterogeneous nature of the pooled meta-analysis results may present a challenge for validation and interpretation of any quantitative synthesis.45 To understand major sources of heterogeneity, sensitivity analyses with and without the major source of heterogeneity (identified as the study with the largest effect) were performed to assess the robustness of the pooled estimates. Analyses were conducted using Stata SE 13 software (StataCorp, College Station, TX, USA). All P values were 2-tailed, and P < 0.05 was considered to indicate a significant difference.
Results
From the literature search, a total of 953 studies were identified through an electronic database search and 2 through manual searches. Figure 1 summarizes the results from the literature search and study selection. Twenty-six RCTs met the inclusion criteria, providing data on 42 430 participants with a median treatment duration of 12 months. Study summary data for 10 studies were obtained directly from the authors.26,46–53 All studies reported adequate randomization and blinding of study data to data collectors and outcome assessors. Studies had a Jadad score of 3–5. Of the 26 studies included in this meta-analysis, 24 reported BMI as an outcome, 13 reported fat mass as an outcome, and 21 reported weight as an outcome. Overall, the median (interquartile) duration of treatment was 12 (interquartile range, 6–36) months, the baseline BMI was 29.3 (interquartile range, 27.5–32.1) kg/m2, and the baseline age was 60.6 (interquartile range, 48.8–68.0) years. All studies used vitamin D3, except for 1 that used vitamin D254 and another that used alphacalcidiol.32
Table 2 21,23–26,32,40–42,46–62 provides an overview of the number of participants, the methodological quality, and the baseline and end-of-intervention values of weight, BMI, or fat mass in each included trial. Twenty-five studies reported no significant effect of vitamin D alone or of vitamin D plus calcium supplementation on weight, BMI, or fat mass. Seven studies examined change in weight, BMI, or fat mass as the primary outcome.
Table 2.
Characteristics of the randomized controlled trials selected for the meta-analysis
| Reference | Country/region (Jadad scorea) | Treatment group | No. of subjects | Duration (months) | Mean age (SD) in years | No. (%) of men | Baseline 25(OH)D level, nmol/Lb | VitaminD (IU/d)/calcium (mg/d)c | Adiposity measure | Baseline value | End-of-study valued | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin D alone | ||||||||||||
| Salehpour et al. (2012)25 | Iran (4) | D3 | 42 | 3 | 38 (7) | 0 | 36.8 (30) | 1000/– | BMI | 30.1 (3.9) | 30 (4) | Adiposity |
| Wt | 73.9 (10.2) | 75.1 (11.9) | ||||||||||
| FM | 30.2 (6.9) | 28.2 (7.5) | ||||||||||
| Placebo | 43 | 37 (8) | 0 | 46.9 (32) | BMI | 29.5 (4.4) | 29.5 (4.6) | |||||
| Wt | 73.5 (10.4) | 75 (12.3) | ||||||||||
| FM | 29.0 (8.7) | 28.6(8.9) | ||||||||||
| Wamberg et al. (2013)40 | Denmark (5) | D3 | 26 | 6.5 | 39.5 (8.0) | 8 (30.8) | 34.5 (10.8) | 7000/– | BMI | 36.3 (3.5) | 36.4 (3.5) | Adiposity |
| Wt | 105 (14) | 105 (13) | ||||||||||
| FM | 40.2 (8.1) | 41.8 (7.6) | ||||||||||
| Placebo | 26 | 41.2 (6.8) | 7 (26.9) | 34.6 (10.3) | BMI | 34.8 (3.3) | 34.6 (3.0) | |||||
| Wt | 100 (13) | 100 (14) | ||||||||||
| FM | 37.4 (6.5) | 37.7 (6.6) | ||||||||||
| Zittermann et al. (2009)41 | Germany (3) | D3 | 100 | 12 | 47.4 (10.3) | 31 (37.8) | 30.0 (17.5) | 3332/– | BMI | 33.7 (4.1) | 31.8 (4.8) | Adiposity in a weight-loss program |
| Wt | 101.9 (16.1) | 96.1 (15.0) | ||||||||||
| FM | 40.1 (10.2) | 35.9 (11.1) | ||||||||||
| Placebo | 100 | 48.8 (10.1) | 23 (27.7) | 30.3 (20.1) | BMI | 33.0 (4.3) | 30.9 (4.6) | |||||
| Wt | 96.2 (17.4) | 89.7 (14.5) | ||||||||||
| FM | 38.5 (9.9) | 33.5 (9.5) | ||||||||||
| Mason et al. (2014)42 | USA (5) | D3 | 109 | 12 | 60.3 (5.3) | 0 | 53.3 (15.2) | 2000/– | BMI | 32.3 (5.5) | 29.5 (5.6) | Adiposity in a weight-loss program |
| Wt | 87.4 (15.5) | 80.2 (15.6) | ||||||||||
| FM | 23.7 (6.2) | 19.6 (6.6) | ||||||||||
| Placebo | 109 | 59.0 (4.7) | 0 | 53.3 (15.4) | BMI | 32.5 (6.1) | 29.7 (6.1) | |||||
| Wt | 88.1 (17.1) | 80.7 (17.6) | ||||||||||
| FM | 24.2 (6.0) | 20.5 (6.9) | ||||||||||
| Ljunghall et al. (1987)32 | Scandinavia (3) | Vitamin D (alphacalcidiol) | 33 | 3 | 61–65e | 100 | 92.4 (23.5) | 0.75 μg/– | BMI | 27.5 (3.4) | 27.2 (3.2) | Insulin secretion |
| Wt | 85.7 (12.8) | 84.6 (12.4) | ||||||||||
| Placebo | 32 | 61–65e | 100 | 97.3 (72.4) | BMI | 28.2 (4.0) | 28.1 (3.9) | |||||
| Wt | 87.6 (13.7) | 87.5 (13.5) | ||||||||||
| von Hurst et al. (2010)55 | New Zealand (3) | D3 | 42 | 6 | 41.8 (10.1) | 0 | 21 [11, 40] | 4000/– | BMI | 27.5 (5.0) | N/Ad | Insulin resistance |
| Placebo | 39 | 41.5 (9.1) | 0 | 19 [13, 29] | BMI | 27.4 (3.7) | N/Ad | |||||
| Grimnes et al. (2011)56 | Norway (5) | D3 | 51 | 6 | 51.5 (8.8) | 27 (55) | 42.2 (13.9) | 5714/– | BMI | 27.2 (3.1) | 26.8 (3.2) | Insulin sensitivity |
| Placebo | 53 | 52.7 (9.7) | 22 (49) | 39.2 (12.1) | BMI | 26.3 (2.9) | 26.2 (2.8) | |||||
| Wood et al. (2012)51 | UK (5) | D3 | 102 | 12 | 63.5 (1.9) | 0 | 32.7 (12.9) | 400/– | BMI | 26.6 (4.2) | 26.4 (3.9) | Serum lipid profile, insulin resistance, inflammatory biomarkers |
| Wt | 68.6 (12.7) | 68.1 (12.1) | ||||||||||
| FM | 27.9 (8.1) | 27.6(8.2) | ||||||||||
| D3 | 101 | 64.1 (2.3) | 0 | 32.4 (13.8) | 1000/– | BMI | 26.8 (4.2) | 26.8 (4.2) | ||||
| Wt | 69.6 (11.9) | 69.8 (12.0) | ||||||||||
| FM | 27.9 (8.1) | 28.3 (8.5) | ||||||||||
| Placebo | 102 | 63.9 (2.3) | 0 | 36.2 (17.1) | BMI | 26.6 (4.4) | 26.9 (4.4) | |||||
| Wt | 69.3 (12.5) | 70.3 (12.3) | ||||||||||
| FM | 27.7 (8.3) | 28.5 (8.7) | ||||||||||
| Davidson et al. (2013)48 | USA (4) | D3 | 56 | 12 | 52.3 (8.0) | 20 (36) | 54.9 (11.2) | 12 695/– | BMI | 32.1 (4.7) | 32.6 (4.5) | Insulin secretion and sensitivity |
| Placebo | 53 | 52.5 (7.0) | 15 (29) | 54.9 (11.9) | BMI | 32.9 (4.3) | 31.6 (4.7) | |||||
| Harris et al. (2011)52 | USA (3) | D3 | 23 | 4 | 29 (2) | 9 (41) | 34.3 (2.2) | 2000/– | BMI | 30.4 (8.6) | 30.5 (8.7) | Flow-mediated dilation |
| Wt | 87.2 (24.3) | 85.5 (20.8) | ||||||||||
| Placebo | 21 | 31 (2) | 12 (52) | 38.2 (3.0) | BMI | 29.1 (7.4) | 28.9 (7.9) | |||||
| Wt | 87.1 (24.0) | 84.9 (21.5) | ||||||||||
| Kjaergaard et al. (2012)57 | Norway (4) | D3 | 120 | 6 | 53.4 (10.3) | 54 (45) | 47.4 (15.8) | 5714/– | BMI | 27.5 (4.0) | 28.0 (4.2) | Depressive symptoms |
| Placebo | 110 | 53.3 (10.1) | 47 (42.7) | 47.7 (15.5) | BMI | 27.5 (4.0) | 28.0 (4.3) | |||||
| Glendenning et al. (2012)47 | Australia (5) | D3 | 353 | 9 | 76.9 (4.0) | 0 | 65.0 (17.8) | 5000/– | BMI | 27.41 (4.7) | 27.39 (4.91) | Falls, muscle strength, and mobility |
| Wt | 70.61 (13.0) | 70.19 (13.4) | ||||||||||
| Placebo | 333 | 76.5 (4.0) | 0 | 66.5 (27.1) | BMI | 27.24 (4.8) | 27.19 (4.69) | |||||
| Wt | 70.48 (12.89) | 70.10 (12.66) | ||||||||||
| Vitamin D and calcium vs calcium control | ||||||||||||
| Sneve et al. (2008)21 | Norway (3) | D3/calcium | 153 | 12 | 46.4 (11.3) | 57 (37.2) | 54.5 (16.7) | 5714/500 | BMI | 35.0 (4.1) | N/A | Adiposity |
| Wt | 101.0 (14.5) | 100.3 (14.9) | ||||||||||
| FM | 42.9 (7.9) | N/A | ||||||||||
| D3/calcium | 143 | 47.6 (11.9) | 51 (35.7) | 51.4 (18.4) | 2857/500 | BMI | 34.4 (3.9) | N/A | ||||
| Wt | 98.6 (14.3) | 97.8 (13.3) | ||||||||||
| FM | 42.9 (7.6) | N/A | ||||||||||
| Calcium | 149 | 48.9 (11.0) | 51 (34.2) | 53.2 (15.4) | –/500 | BMI | 35.1 (3.8) | N/A | ||||
| Wt | 100.6 (13.9) | 101.2 (14.6) | ||||||||||
| FM | 43.1 (6.9) | N/A | ||||||||||
| Zhou et al. (2010)24 | USA (4) | D3/calcium | 336 | 48 | 66.5 (7.5) | 0 | 73.1 (18.8) | 1100/1400–1500 | BMI | 28.7 (5.2) | 28.2 (5.2) | Adiposity |
| Wt | 75.5 (14.3) | 73.4 (14.5) | ||||||||||
| FM | 29.8 (8.7) | 29.2 (9.3) | ||||||||||
| Placebo | 206 | 65.2 (6.5) | 0 | 73.6 (20.7) | BMI | 28.8 (5.3) | 28.7 (5.5) | |||||
| Wt | 76.4 (14.2) | 75.8 (14.3) | ||||||||||
| FM | 30.1 (9) | 30.5 (9.2) | ||||||||||
| Placebo/calcium | 328 | 66.0 (6.6) | 0 | 73.0 (20.4) | –/1400–1500 | BMI | 28.9 (5.4) | 28.7 (5.4) | ||||
| Wt | 76.6 (14.8) | 74.4 (14.4) | ||||||||||
| FM | 30.7 (9.2) | 29.7 (9.0) | ||||||||||
| Jorde et al. (2010)58 | Norway (3) | D3/calcium | 150 | 12 | 46.3 (11.3) | 56 (37.3) | 58.7 (21.2) | 5714/500 | BMI | 34.8 (4.0) | N/Ad | Lipids and blood pressure |
| D3/calcium | 139 | 47.3 (11.9) | 50 (35.9) | 56.7 (21.2) | 2857/500 | BMI | 34.4 (3.8) | N/Ad | ||||
| Calcium | 149 | 48.9 (11.0) | 51 (34.2) | 58.8 (21.0) | –/500 | BMI | 35.1 (3.8) | N/Ad | ||||
| Heikkinen et al. (1997)59 | Finland (2) | D3/calcium | 83 | 36 | 52.8 (0.47) | 0 | N/A | 300/500 | BMI | 26.8 (0.47) | N/A | Lipids |
| FM | 71.5 (1.24) | 72.8 (1.38) | ||||||||||
| Calcium | 95 | 52.5 (0.22) | 0 | N/A | –/500 | BMI | 26.4 (0.42) | N/A | ||||
| FM | 67.6 (1.07) | 69.8 (1.26) | ||||||||||
| Chandler et al. (2013)60 | USA (5) | D3/calcium | 81 | 3 | 51.9 (11.6) | 22 (27.2) | 43.2 (22.3) | 1000/200 | BMI | 32.5 (7.4) | 32.5 (7.4) | Plasma inflammatory markers |
| Wt | 89.4 (19.6) | 89.9 (19.9) | ||||||||||
| D3/calcium | 83 | 51.3 (11.6) | 28 (33.7) | 40.1 (22.1) | 2000/200 | BMI | 33.0 (8.2) | 33.0 (8.2) | ||||
| Wt | 92.7 (23.4) | 92.7 (23.4) | ||||||||||
| D3/calcium | 83 | 51.5 (11.6) | 29 (34.9) | 44.3 (22.2) | 4000/200 | BMI | 32.2 (7.2) | 32.3 (7.3) | ||||
| Wt | 89.5 (21.5) | 89.9 (21.9) | ||||||||||
| Placebo/calcium | 81 | 51.1 (11.1) | 27 (33.3) | 42.5 (23.0) | –/200 | BMI | 31.9 (7.7) | 32.0 (7.9) | ||||
| Wt | 89.6 (20.8) | 90.0 (21.3) | ||||||||||
| Aloia et al. (2005)49 | USA (3) | D3/calcium | 104 | 36 | 59.9 (6.2) | 0 | 48.1 (20.8) | 800–2000/1200–1500 | BMI | 29 (4.0) | 29.1 (4.8) | Bone mineral density |
| Wt | 78.0 (13.6) | 76.9 (13.0) | ||||||||||
| Calcium | 104 | 61.2 (6.3) | 0 | 42.9 (16.6) | –/1200–1500 | BMI | 30 (4.0) | 30.6 (4.4) | ||||
| Wt | 79.2 (12.6) | 80.2 (12.6) | ||||||||||
| Dawson-Hughes et al. (1991)53 | USA (3) | D3/calcium | 124 | 12 | 61.4 (0.5) | 0 | N/A | 400/377 | Wt | 61.4 (0.5) | N/Ad | Seasonal bone loss |
| Placebo/calcium | 125 | 61.9 (0.5) | 0 | N/A | –/377 | Wt | 61.9 (0.5) | N/Ad | ||||
| Gallagher et al. (2012)26 | USA (5) | D3/calcium | 20 | 12 | 68 (8.6) | 0 | 37.8 (10.8) | 400/– | BMI | 30.3 (5.4) | N/A | Serum 25(OH)D and PTH |
| Wt | 77.8 (13.4) | N/A | ||||||||||
| FM | 31.3 (8.7) | 32.3 (7.1) | ||||||||||
| D3/calcium | 21 | 68 (8.1) | 0 | 39.0 (9.5) | 800/– | BMI | 28.2 (6.1) | N/A | ||||
| Wt | 74.3 (16.6) | N/A | ||||||||||
| FM | 29.1 (10.7) | 29.9 (12.1) | ||||||||||
| D3/calcium | 20 | 66 (7.4) | 0 | 37.4 (10.2) | 1600/– | BMI | 30.0 (5.4) | N/A | ||||
| Wt | 76.4 (14.5) | N/A | ||||||||||
| FM | 30.4 (8.8) | 30.8 (9.5) | ||||||||||
| D3/calcium | 21 | 66 (6.3) | 0 | 38.2 (10.1) | 2400/– | BMI | 30.4 (5.4) | N/A | ||||
| Wt | 78.0 (13.0) | N/A | ||||||||||
| FM | 32.0 (8.0) | 33.1 (8.6) | ||||||||||
| D3/calcium | 20 | 69 (7.7) | 0 | 39.8 (8.2) | 3200/– | BMI | 30.2 (5.7) | N/A | ||||
| Wt | 78.6 (15.8) | N/A | ||||||||||
| FM | 32.2 (9.9) | 32.4 (10.2) | ||||||||||
| D3/calcium | 20 | 66 (7.1) | 0 | 37.2 (9.2) | 4000/– | BMI | 29.7 (6.4) | N/A | ||||
| Wt | 76.2 (16.2) | N/A | ||||||||||
| FM | 30.1 (10.6) | 31.2 (10.7) | ||||||||||
| D3/calcium | 20 | 65 (6.1) | 0 | 38.6 (9.1) | 4800/– | BMI | 32.1 (6.2) | N/A | ||||
| Wt | 83.4 (17.9) | N/A | ||||||||||
| FM | 34.7 (11.1) | 34.6 (10.8) | ||||||||||
| Placebo/calcium | 21 | 66 (6.5) | 0 | 37.7 (9.1) | BMI | 31.1 (5.3) | N/A | |||||
| Wt | 81.3 (16.3) | N/A | ||||||||||
| FM | 33.6 (11.7) | 33.7 (11.6) | ||||||||||
| Prince et al. (2008)54 | Australia (5) | D2/calcium | 151 | 12 | 77.0 (4.2) | 0 | 45.2 (12.5) | 1000/1000 | BMI | 28.34 (4.92) | 28.37 (4.94) | Risk of falls |
| Wt | 71.99 (12.83) | 71.75 (12.79) | ||||||||||
| FM | 28.2 (7.8) | 28.1 (8.1) | ||||||||||
| Placebo/calcium | 151 | 77.4 (5.0) | 0 | 44.2 (12.7) | –/1000 | BMI | 29.55 (5.42) | 29.47 (5.45) | ||||
| Wt | 73.94 (14.14) | 73.44 (13.99) | ||||||||||
| FM | 28.1 (8.3) | 30 (8.2) | ||||||||||
| Zhu et al. (2008)46 | Australia (5) | D3/calcium | 39 | 60 | 75.4 (2.7) | 0 | 70.2 (25.6) | 1000/1200 | BMI | 27.6 (5.1) | 27.8 (5.5) | BMD, biomarkers of bone turnover |
| Wt | 67.2 (12.2) | 66.4 (12.7) | ||||||||||
| FM | 24.4 (7.6) | 23.5 (7.6) | ||||||||||
| Placebo | 41 | 74.8 (2.8) | 0 | 67.3 (34.2) | BMI | 28.0 (6.0) | 28.6 (6.3) | |||||
| Wt | 71.2 (15.2) | 71.5(15.8) | ||||||||||
| FM | 26.3 (8) | 24.3(7.5) | ||||||||||
| Placebo/calcium | 40 | 74.1 (2.0) | 0 | 66.6 (25.9) | BMI | 27.9 (6.0) | 28.6 (6.3) | |||||
| Wt | 71.2 (15.2) | 71.5 (15.8) | ||||||||||
| FM | 28.2 (7.7) | 26.2 (7.6) | ||||||||||
| Vitamin D and calcium vs placebo | ||||||||||||
| Caan et al. (2007)23 | USA (5) | D3/calcium | 18 129 | 84 | 50–79f | 0 | N/A | 400/1000 | BMI | 28.9 (6.0) | N/A | Adiposity |
| Wt | 76.0 (16.9) | N/Ad | ||||||||||
| Placebo | 18 055 | 50–79f | 0 | N/A | BMI | 28.8 (6.0) | N/A | |||||
| Wt | 75.9 (17.1) | N/Ad | ||||||||||
| Major et al. (2007)61 | Canada (4) | D3/calcium | 30 | 3.75 | 43.6 (5.0) | 0 | N/A | 400/1200 | BMI | 31.4 (2.5) | 29.8 (2.8) | Blood pressure, plasma lipid and lipoprotein concentrations, and glucose and insulin concentrations |
| Wt | 81.5 (8.3) | 77.5 (9.0) | ||||||||||
| Placebo | 33 | 41.6 (6.1) | 0 | N/A | BMI | 32.3 (3.5) | 31.1 (3.7) | |||||
| Wt | 83.6 (11.1) | 80.6 (11.7) | ||||||||||
| Pittas et al. (2007)62 | USA (4) | |||||||||||
| NFG | D3/calcium | 108 | 36 | 70.6 (0.4) | 44 (40.7) | 81.4 (3.7) | 700/500 | BMI | 26.1 (0.3) | N/Ad | Insulin sensitivity, plasma C-reactive protein | |
| Wt | 71.6 (1.2) | N/A | ||||||||||
| Placebo | 114 | 71.7 (0.4) | 41 (35.9) | 70.6 (2.8) | BMI | 26.2 (0.3) | N/Ad | |||||
| IFG | D3/calcium | 45 | 71.1 (0.7) | 22 (48.9) | 71.2 (5.2) | 700/500 | Wt | 71.1 (1.2) | N/A | |||
| Placebo | 47 | 71.3 (0.8) | 26 (55.3) | 81.2 (4.7) | BMI | 28.1 (0.7) | N/Ad | |||||
| Wt | 80.0 (2.3) | N/Ad | ||||||||||
| Dawson-Hughes et al. (1997)50 | USA (3) | |||||||||||
| Men | D3/calcium | 86 | 36 | 70 (4) | 100 | 82.2 (40.6) | 700/500 | Wt | 82.4 (11.3) | N/Ad | BMD and fractures | |
| Placebo | 90 | 71 (5) | 100 | 83.7 (31.6) | Wt | 81.5 (12.8) | N/Ad | |||||
| Women | D3/calcium | 101 | 71 (4) | 0 | 71.5 (33.1) | 700/500 | Wt | 67.6 (12.1) | N/Ad | |||
| Placebo | 112 | 72 (5) | 0 | 61.0 (25.7) | Wt | 68.1 (12.4) | N/Ad | |||||
aThe 5-point Jadad score is based on the description of randomization, double-blinding, and withdrawals.
bIn study of von Hurst et al,55 baseline 25(OH)D reported as median [quartile 1, quartile 3].
cVitamin D given in micrograms per day, since alphacalcidiol does not have a conversion for IU or an estimation of equivalent dose to vitamin D2 or vitamin D3.
dNet difference between treatment and placebo group is reported, but end-of-study values are not available.
eMean (SD) for age not reported.
fMean age was 62.4 (SD 6.9) years for entire cohort.
Abbreviations: BMD, bone mineral density; BMI, body mass index (kg/m2); EOS, end of study; FM, fat mass; FPG, fasting plasma glucose; IFG, impaired fasting glucose; N/A, not available; NFG, normal fasting glucose; PTH, parathyroid hormone; SD, standard deviation; 25(OH)D, 25-hydroxyvitamin D (nmol/L); Wt, weight.
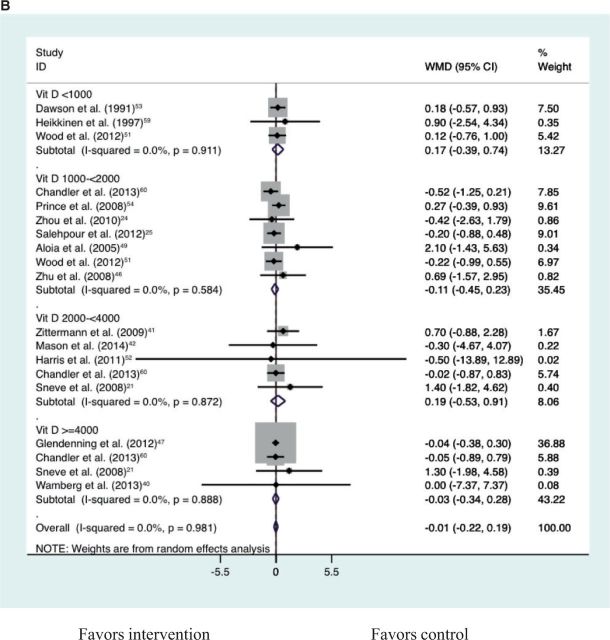
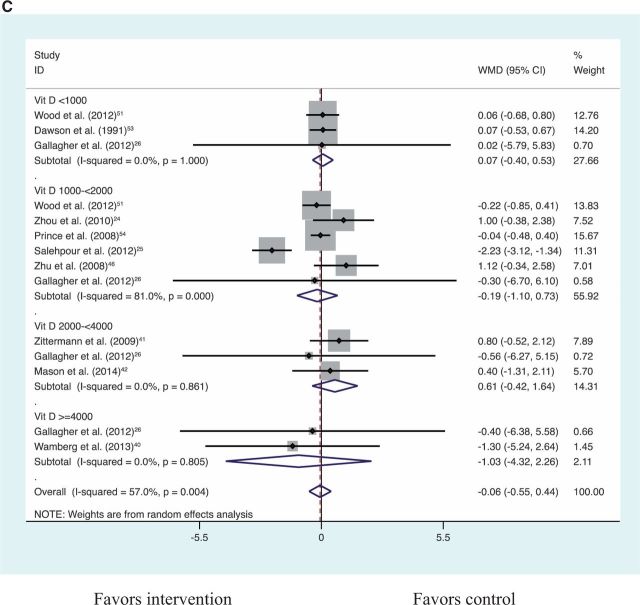
Vitamin D supplementation alone compared with placebo resulted in no significant change in BMI, weight, or fat mass (Table 3). Vitamin D plus calcium supplementation compared with calcium control also showed no significant reduction in BMI, weight, or fat mass (Table 3). Together, vitamin D alone compared with placebo and vitamin D plus calcium compared with calcium control showed no significant reduction in BMI, weight, or fat mass (Table 3; Figures S1–S321,24,26,32,40–42,46–49,51–60 in the Supporting Information online). An analysis for a dose–response effect by vitamin D3 doses of <1000 IU/d, 1000 to <2000 IU/d, 2000 to <4000 IU/d, and >4000 IU/d (Figures 2A–2C21,24,26,32,40–42,46–49,51–60) revealed no significant effect of vitamin D in any of the dose groups on any of the adiposity outcomes (all P > 0.05). The highest daily dose in the studies included in this meta-analysis was vitamin D3, 12 695 IU/day.48 With a limited number of eligible trials, vitamin D plus calcium compared with placebo showed no significant reduction in BMI or fat mass but a significant reduction in body weight (Table 3). In the sensitivity analysis, the significant result for weight was largely driven by the inclusion of a single trial for which only the weight estimate was available, i.e., the Women’s Health Initiative Calcium/Vitamin D Supplemental Trial23 (Table 3). This trial reported the largest and most significant effect on weight. Weight change was not significantly different for vitamin D plus calcium compared with placebo after excluding this Women’s Health Initiative trial (Table 3; Figures S4–S723,50,61,62 in the Supporting Information online).
Table 3.
Weighted mean differences of 3 adiposity measurements by supplementation with vitamin D alone compared with placebo or vitamin D plus calcium compared with control groups (placebo or calcium)
| Supplement | No. of studies (treatment/control) | Mean difference (95%CI) | P value for mean difference | P value for heterogeneity, chi-squared | I2 (%) |
|---|---|---|---|---|---|
| Vitamin D alone vs placebo | |||||
| BMI (kg/m2) | 11 (1123/991) | −0.06 (−0.14 to 0.03) | 0.20 | 0.79 | 0 |
| Weight (kg) | 8 (889/766) | −0.05 (−0.32 to 0.23) | 0.74 | 0.99 | 0 |
| Fat mass (kg) | 5 (479/378) | −0.43 (−1.69 to 0.84) | 0.51 | <0.001 | 81.2 |
| Vitamin D plus calcium vs calcium | |||||
| BMI (kg/m2) | 7 (1290/978) | 0.02 (−0.11 to 0.14) | 0.80 | 0.88 | 0 |
| Weight (kg) | 8 (1380/1073) | 0.12 (−0.24 to 0.49) | 0.51 | 0.80 | 0 |
| Fat mass (kg) | 5 (790/665) | 0.12 (−0.22 to 0.45) | 0.50 | 0.42 | 0 |
| Vitamin D alone vs placebo and vitamin D plus calcium vs calcium | |||||
| BMI (kg/m2) | 18 (2413/1969) | −0.03 (−0.10 to 0.04) | 0.36 | 0.92 | 0 |
| Weight (kg) | 16 (2269/1839) | 0.01 (−0.21 to 0.23) | 0.90 | 0.99 | 0 |
| Fat mass (kg) | 10 (1251/1043) | −0.03 (−0.63 to 0.57) | 0.92 | <0.001 | 69.9 |
| Vitamin D plus calcium vs placebo | |||||
| BMI (kg/m2) | 6 (744/643) | −0.03 (−0.13 to 0.08) | 0.65 | 0.02 | 64 |
| Weight (kg) with WHIa | 5 (18 732/18 623) | −0.13 (−0.21 to −0.05) | 0.001 | 0.85 | 0 |
| Weight (kg) without WHIa | 4 (591/482) | −0.19 (−0.88 to 0.49) | 0.58 | 0.71 | 0 |
| Fat mass (kg) | 3 (405/280) | 0.67 (−0.33 to 1.66) | 0.19 | 0.83 | 0 |
aCaan et al. (2007),23 the Women’s Health Initiative Calcium/Vitamin D Supplemental Trial, 36 184 participants, 7 years, 400 IU vitamin D3 plus 1000 mg of calcium daily.
Figure 2.
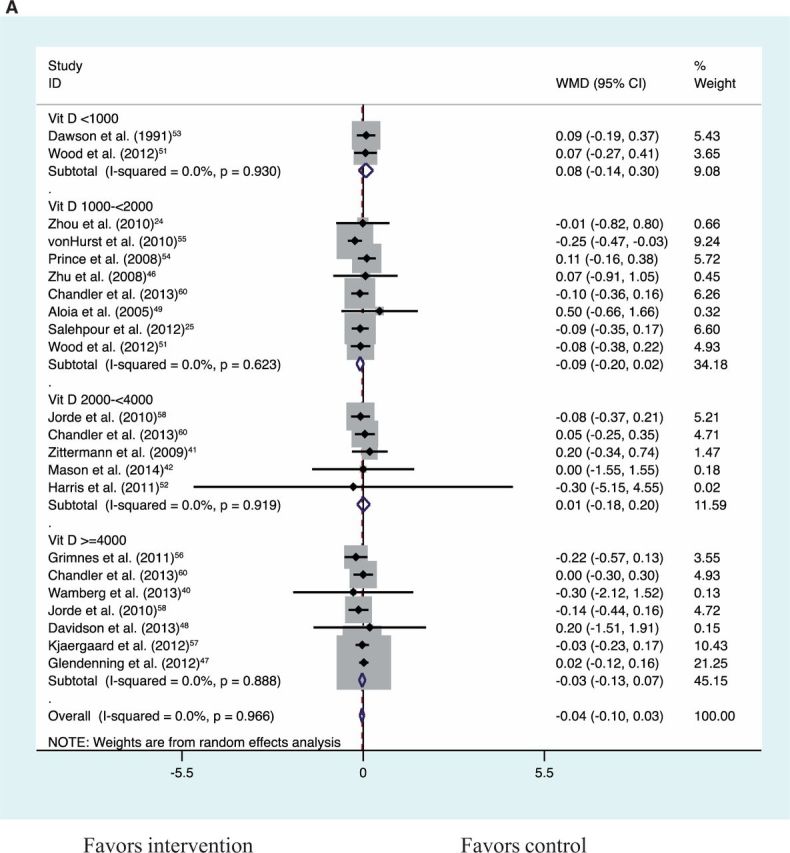
Dose–effect comparisons for vitamin D supplementation alone compared with placebo and for vitamin D plus calcium supplementation compared with calcium control for BMI (A), weight (B), and fat mass (C)
Neither Begg’s test nor Egger’s test was significant for publication bias with regard to the major effects of vitamin D with or without calcium supplements on BMI, weight, or fat mass.
Discussion
The possible role of vitamin D in the pathogenesis of obesity is an area of importance to public health and clinical nutrition worldwide given the suboptimal vitamin D status across many populations63 and the soaring global prevalence of obesity.1 However, there is a lack of knowledge to guide dietary recommendations for vitamin D intake as it relates to adiposity. The Institute of Medicine’s definition of vitamin D inadequacy (25[OH]D<50 nmol/L) is derived predominantly from bone health outcomes and is still evolving.7,8 How vitamin D levels vary by BMI and total and regional fat (e.g., abdominal) is understudied, with little known about the dynamics of vitamin D storage and reentry into the circulation.64
The conventional explanation for low vitamin D status in obese individuals is that the volume of distribution for this fat-soluble vitamin is larger in patients with excess fat.3 It was postulated that obesity is a result of low vitamin D. The results of this meta-analysis of 26 RCTs showed no overall evidence for significant effects of vitamin D or vitamin D plus calcium supplementation on BMI, weight, or fat mass. There was no evidence of a dose–response effect from the analyses stratified by vitamin D dosages, edven though the baseline BMI (median, 29.3 kg/m2) of the overall study population was nearly at the threshold for obesity. This analysis was conducted in adults and may not be generalizable to other groups.
The trials of vitamin D alone compared with placebo and of vitamin D plus calcium compared with calcium control are clear tests of vitamin D, whereas the comparison of vitamin D plus calcium with placebo assesses the combined effect of vitamin D and calcium. The pooled result of vitamin D plus calcium compared with placebo was not robust to the inclusion and exclusion of the Women’s Health Initiative Calcium/Vitamin D Supplemental Trial, arguing against the possibility of a genuine effect.23 The Women’s Health Initiative Calcium/Vitamin D Trial was embedded in the other Women’s Health Initiative RCTs, including a diet modification trial that may have led to weight loss, thus influencing the effects of combined vitamin D/calcium supplementation.
In any meta-analysis, the heterogeneous nature of the pooled results of the meta-analysis presents a challenge for interpretation of any quantitative synthesis. In the present study, the prespecified inclusion/exclusion criteria were applied when combining the results from all eligible studies, including the Women’s Health Initiative trial. However, the results of the Women’s Health Initiative trial contributed predominantly to between-study heterogeneity. To understand such a major source of heterogeneity, sensitivity analyses with and without the results of the Women’s Health Initiative trial were performed to assess the robustness of the pooled estimates.
The number of subjects included in this meta-analysis is small compared with the number of subjects who have participated in clinical trials of vitamin D because the study selection criteria included only randomized, double-blind, placebo-controlled clinical trials in which the duration of treatment was at least 3 months. This criterion for selection resulted in a median duration of treatment of 12 months for a robust assessment of weight loss.34,35
The observed significant heterogeneity for fat mass outcomes may have been influenced by the variety of fat mass measures reported or the age of study participants. Although all studies in this meta-analysis used DXA, with the exception of 1 study that used bioelectrical impedance,41 fat mass measures included truncal fat, whole-body fat, and body fat percentage. Fat mass heterogeneity may also have been influenced by age, since the study with the greatest reduction in fat mass for the vitamin-D-only group was Salehpour et al.25 (mean age, 38 y), where as the other studies had older participants. Fat mass increases with age.65–69
The results highlight the need for intervention studies of sufficient size to help clarify the relationship between vitamin D and adiposity, as affirmed in the Institute of Medicine report.7,8 Objective assessments of adiposity that include measures of fat mass (total and regional) evaluated with gold standard methods, such as DXA, are warranted. DXA provides measures of total body weight, overall adiposity and regional fat distribution, and non-fat-containing tissues (lean and bone mass), with good reproducibility and minimal radiation exposure.29,30 The VITamin D and OmegA-3 TriaL (VITAL) is a double-blind, placebo-controlled trial assessing the role of the interventions (vitamin D3, 2000 IU/d, and omega-3 fatty acid, 1 g/d) in reducing the risks of cancer and cardiovascular disease among men and women in the United States. An ancillary VITAL study will comprehensively test the effects of supplemental vitamin D and/or omega-3 on skeletal health by using DXA scans to assess changes in bone and body composition.70 Results from VITAL will help clarify the relationship between supplemental vitamin D and adiposity outcomes and will inform clinical care and public health guidelines on the use of supplemental vitamin D in obese individuals.
Observational studies show lower levels of 25(OH)D in obese than in nonobese individuals, suggesting a possible beneficial effect of vitamin D on obesity.16 A study of community-dwelling participants suggested that almost all the variability in serum 25(OH)D concentrations was attributable to obesity.5 Once serum 25(OH)D concentrations were adjusted by body size, there was no longer a difference between obese and nonobese participants.5 Intervention trials specifically designed to evaluate the direct effects of vitamin D on adiposity measures have produced conflicting results. Results of some RCTs suggested beneficial effects of vitamin D supplementation23,71 on body weight regulation, but others did not.21,24 A systematic review2 of 5 RCTs21,41,55,72,73 found that vitamin D supplementation did not promote weight or fat loss. A recent meta-analysis of 12 studies found no significant effect of vitamin D supplementation alone on adiposity measures, but an effect by vitamin D dose was not evaluated.31 A bidirectional genetic study suggested that higher BMI results in lower 25(OH)D, but the effects of lower 25(OH)D on BMI are likely to be small.74 In addition, despite plausible mechanisms and in vitro evidence14,16 supporting a role for vitamin D in weight reduction, it remains difficult to determine whether the effects are due to vitamin D itself or are related to the calcium that is usually consumed in combination with vitamin D.
The overall results may be explained by multiple reasons. First, it is possible that there is no biological effect of supplementation with vitamin D, with or without calcium, on adiposity. Second, clinical trials that evaluated the effect of vitamin D and calcium on measures of adiposity varied by study design. The published studies differ substantially in terms of methodology, including participant recruitment and intervention, making it difficult to pool the findings. For example, some studies that showed no effect of vitamin D supplementation on weight recruited participants who were vitamin D replete. The results may consequently not apply to individuals who were vitamin D insufficient. There has been recent debate about what constitutes vitamin D deficiency and sufficiency,6 and thresholds have recently shifted. The most recent compilation of data suggests 25(OH)D levels of 50 nmol/L (20 ng/mL) are adequate for the population. However, the 2011 Institute of Medicine report concluded that, currently, the evidence is sufficient to provide health guidelines only for skeletal health and that more data are needed on nonskeletal outcomes and to identify the threshold effects for other health outcomes.7,8
Third, the choice of adiposity measure75 is important when evaluating the relationship between vitamin D supplementation and adiposity. A limitation of anthropometric measures such as BMI or weight is that they do not separate fat from lean mass and are unable to characterize the type and distribution of fat deposits (e.g., intramyocellular, subcutaneous, or visceral).76 Lipids stored in other tissue, such as liver and muscle, also contribute to the adipose compartment.77,78 Anthropometric measurements such as subscapular and triceps skinfold thickness, waist circumference, and waist-to-hip ratio allow for indirect assessment of fat distribution.79 Similarly, DXA, a noninvasive method for measuring regional fat mass, cannot differentiate between visceral, subcutaneous, and intramyocellular fat.80 In contrast, computed tomography and magnetic resonance imaging allow precise quantification of visceral adipose tissue and subcutaneous adipose tissue.76 Similarly, magnetic resonance spectroscopy can measure fat in other tissues such as muscle and liver.81 Thus, this analysis omits a variety of measures of adiposity.
Due to limited data, the present analysis did not examine regional adipose tissue distribution or waist circumference. Cross-sectional and observational studies provide evidence of an inverse association between 25(OH)D levels and obesity and, in some instances, fat mass, fat distribution, and anthropomorphic measures.3,16,19,82–89 This relationship, however, was not evident in all studies.90,91
Fourth, the older age of the participants (median age, 60.6 y) may have influenced the findings. At the same body weight, fat mass distribution differs by age, sex, and fitness.65 Changes in body composition associated with aging include increase in fat mass in mid to early old age65–69 and loss of fat-free mass,67,92 including muscle and bone.68,69,92,93 Furthermore, vitamin D insufficiency may not have been corrected with vitamin D supplementation, since markedly higher proportions of insufficient vitamin D levels have been reported among elderly adults.94–99
Finally, the quality of the studies in this analysis was limited by the small sample size and short duration of some trials. The literature search identified only 8 studies with small sample sizes that evaluated the effect of vitamin D alone or of vitamin D plus calcium (with calcium control) on fat mass, and the meta-analysis showed no effect of vitamin D supplementation. In all but 2 studies, body composition was measured by DXA, a high-quality method. Yet, a recent review of 15 RCTs evaluated the potential role of calcium and vitamin D in the regulation of body weight or body fat and also found no overall effect of vitamin D and calcium on body weight or body fat.100
It is possible that vitamin D and calcium deficiency may have important latent effects. The inadequate intake of nutrients contributes to many chronic diseases that take years to manifest. Thus, calcium and vitamin D may have short- and long-term effects on the development of obesity.101 The vitamin D and calcium intakes required to prevent the long-latency chronic disorders may be higher than those required to prevent developmental problems such as rickets. However, whether these actions of vitamin D are important enough to result in obesity in D-deficient individuals is doubtful. The negative result of this study suggests that vitamin D supplementation will not be helpful in reducing obesity.
Some limitations of this analysis deserve consideration, including the inability to conduct robust subgroup analyses based on duration of intervention, baseline 25(OH)D concentration, baseline BMI, or baseline waist circumference. Whether different formulations of vitamin D, such as vitamin D2, have different effects on adiposity measures could not be evaluated because most of the studies used vitamin D3. Furthermore, the influence of seasonality on vitamin D response to supplementation was not evaluated. Importantly, the results were stratified by vitamin D dose, but no evidence of a dose-responsive trend for an effect of vitamin D on BMI or weight was observed. Several important factors, including behavioral factors, may confound a relationship between vitamin D status and obesity. For example, obese people may be less likely to expose themselves to sunshine.
In patients who are truly vitamin D deficient, supplementation with vitamin D improves the bone density, which in turn will increase the lean tissue mass. This could mask a beneficial effect on fat mass if body weight is the only outcome measure.
CONCLUSION
This meta-analysis of RCTs showed no overall evidence of direct effects of vitamin D on 3 measures of adiposity: BMI, body weight, and fat mass. Fat mass and fat distribution are more meaningful measures of adiposity than body weight and BMI. BMI and body weight, however, were reported as outcomes in only a minority of the RCTs included. The robust findings are relevant to public health and clinical nutrition and corroborate and support the need for further research on the relationship between vitamin D and obesity. There is a clear need for adequately powered RCTs that assess baseline 25(OH)D levels and include objective measures of obesity evaluated with gold standard methods such as DXA.
Supplementary Material
Acknowledgments
The authors thank Cara Marcus, MSLIS, AHIP, Director of Library Services, Brigham and Women’s Faulkner Hospital, Boston, Massachusetts, for facilitating access to reference articles; Carol Mita, MS, Reference and Education Services Librarian, Countway Library of Medicine, Boston, Massachusetts, for helping to prepare the search strategy; Chunying Li, MPH, Division of Preventive Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, for providing statistical support; and Helen Akinboule for reviewing articles.
Author contributions. P.D.C., J.E.M., L.W., H.D.S., Y.S., J.S.D., and M.S.L. designed the research. P.D.C., J.E.M., O.B., X.Z., Y.S., and M.V.M. analyzed data or performed statistical analysis. All authors wrote the manuscript. P.D.C. and L.W. had primary responsibility for the final content. All authors read and approved the final manuscript.
Funding. Dr Chandler received support from grant 3U01CA138962-05S1 from the National Cancer Institute. Dr Wang was supported by grant R00-HL095649 from the National Heart, Lung, and Blood Institutes. Dr Song is supported by grants R01-DK088078 and R01-HL113056 from the National Institutes of Health. Dr Danik receives support from grant 11980009 from the American Heart Association. The salary of Dr Lewis is supported by a Raine Medical Research Foundation Priming Grant. Dr Manson receives support from the National Institutes of Health for the VITamin D and OmegA-3 TriaL (UO1CA138962). These funding sources had no role in the conception or conduct of the study, took no part in the data collection or analysis, and had no role in the drafting, review, or approval of the article.
Declaration of interest. The authors have no relevant interests to declare.
SUPPORTING INFORMATION
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1. Completed PRISMA checklist
Appendix S2. Search strategy
Figure S1. Overall effect of vitamin D alone (versus placebo) and vitamin D plus calcium supplementation (versus calcium control) on change in BMI
Figure S2. Overall effect of vitamin D alone (versus placebo) and vitamin D plus calcium supplementation (versus calcium control) on change in weight
Figure S3. Overall effect of vitamin D alone (versus placebo) and vitamin D plus calcium supplementation (versus calcium control) on change in weight
Figure S4. Effect of vitamin D plus calcium supplementation (versus placebo) on change in BMI
Figure S5. Effect of vitamin D plus calcium supplementation (versus placebo) on change in weight with Women’s Health Initiative (WHI) study
Figure S6. Effect of vitamin D plus calcium supplementation (versus placebo) on change in weight without Women’s Health Initiative (WHI) study
Figure S7. Effect of vitamin D plus calcium supplementation (versus placebo) on change in fat mass
REFERENCES
- 1.Caballero B. The global epidemic of obesity: an overview. Epidem Rev. 2007;29:1–5. [DOI] [PubMed] [Google Scholar]
- 2.Soares MJ, Chan She Ping-Delfos W, Ghanbari MH. Calcium and vitamin D for obesity: a review of randomized controlled trials. Eur J Clin Nutr. 2011;65:994–1004. [DOI] [PubMed] [Google Scholar]
- 3.Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. [DOI] [PubMed] [Google Scholar]
- 4.Jain M, Nilsson R, Sharma S, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drincic AT, Armas LA, Van Diest EE, et al. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20:1444–1448. [DOI] [PubMed] [Google Scholar]
- 6.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. [DOI] [PubMed] [Google Scholar]
- 7.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 9.Renzaho AM, Halliday JA, Nowson C. Vitamin D, obesity, and obesity-related chronic disease among ethnic minorities: a systematic review. Nutrition. 2011;27:868–879. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. [DOI] [PubMed] [Google Scholar]
- 11.Parker J, Hashmi O, Dutton D, et al. Levels of vitamin D and cardiometabolic disorders: systematic review and meta-analysis. Maturitas. 2010;65:225–236. [DOI] [PubMed] [Google Scholar]
- 12.Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: mechanisms and implications. J Am College Nutr. 2002;21:146S–151S. [DOI] [PubMed] [Google Scholar]
- 13.Duque G, Macoritto M, Kremer R. Vitamin D treatment of senescence accelerated mice (SAM-P/6) induces several regulators of stromal cell plasticity. Biogerontology. 2004;5:421–429. [DOI] [PubMed] [Google Scholar]
- 14.Sergeev IN, Song Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol Nutr Food Res. 2014;58:1342–1348. [DOI] [PubMed] [Google Scholar]
- 15.Wong KE, Szeto FL, Zhang W, et al. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metabol. 2009;296:E820–E828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell NH, Epstein S, Greene A, et al. Evidence for alteration of the vitamin D–endocrine system in obese subjects. J Clin Invest. 1985;76:370–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamia C, Lagiou P, Buckland G, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol. 2013;28:317–328. [DOI] [PubMed] [Google Scholar]
- 18.Forsythe LK, Livingstone MB, Barnes MS, et al. Effect of adiposity on vitamin D status and the 25-hydroxycholecalciferol response to supplementation in healthy young and older Irish adults. Br J Nutr. 2012;107:126–134. [DOI] [PubMed] [Google Scholar]
- 19.Snijder MB, van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metabol. 2005;90:4119–4123. [DOI] [PubMed] [Google Scholar]
- 20.Moschonis G, Manios Y. Skeletal site-dependent response of bone mineral density and quantitative ultrasound parameters following a 12-month dietary intervention using dairy products fortified with calcium and vitamin D: the Postmenopausal Health Study. Br J Nutr. 2006;96:1140–1148. [DOI] [PubMed] [Google Scholar]
- 21.Sneve M, Figenschau Y, Jorde R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur J Endocrinol. 2008;159:675–684. [DOI] [PubMed] [Google Scholar]
- 22.Holecki M, Zahorska-Markiewicz B, Wiecek A, et al. Influence of calcium and vitamin D supplementation on weight and fat loss in obese women. Obes Facts. 2008;1:274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caan B, Neuhouser M, Aragaki A, et al. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch Intern Med. 2007;167:893–902. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Zhao LJ, Watson P, et al. The effect of calcium and vitamin D supplementation on obesity in postmenopausal women: secondary analysis for a large-scale, placebo controlled, double-blind, 4-year longitudinal clinical trial. Nutr Metab. 2010;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salehpour A, Hosseinpanah F, Shidfar F, et al. A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher JC, Sai A, Templin T, II, et al. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156:425–437. [DOI] [PubMed] [Google Scholar]
- 27.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 28.Piché ME, Lemieux S, Corneau L, et al. Measuring insulin sensitivity in postmenopausal women covering a range of glucose tolerance: comparison of indices derived from the oral glucose tolerance test with the euglycemic-hyperinsulinemic clamp. Metabolism. 2007;56:1159–1166. [DOI] [PubMed] [Google Scholar]
- 29.Haarbo J, Gotfredsen A, Hassager C, et al. Validation of body composition by dual energy X-ray absorptiometry (DEXA). Clin Physiol. 1991;11:331–341. [DOI] [PubMed] [Google Scholar]
- 30.Byrne TA, Morrissey TB, Gatzen C, et al. Anabolic therapy with growth hormone accelerates protein gain in surgical patients requiring nutritional rehabilitation. Ann Surg. 1993;218:400–416; discussion 416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ljunghall S, Lind L, Lithell H, et al. Treatment with one-alpha-hydroxycholecalciferol in middle-aged men with impaired glucose tolerance – a prospective randomized double-blind study. Acta Med Scand. 1987;222:361–367. [DOI] [PubMed] [Google Scholar]
- 33.Cohen SB. A modified approach to small area estimation. NIDA Res Monogr. 1979;24:98–134. [PubMed] [Google Scholar]
- 34.Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2003(4):CD004094. [DOI] [PubMed] [Google Scholar]
- 35.Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8:iii–iv, 1–182. [DOI] [PubMed] [Google Scholar]
- 36.Andersen T, Astrup A, Quaade F. Dexfenfluramine as adjuvant to a low-calorie formula diet in the treatment of obesity: a randomized clinical trial. Int J Obes Relat Metab Disord. 1992;16:35–40. [PubMed] [Google Scholar]
- 37.Dombrowski SU, Avenell A, Sniehott FF. Behavioural interventions for obese adults with additional risk factors for morbidity: systematic review of effects on behaviour, weight and disease risk factors. Obes Facts. 2010;3:377–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wadden TA. Treatment of obesity by moderate and severe caloric restriction. Results of clinical research trials. Ann Intern Med. 1993;119(7 Pt 2):688–693. [DOI] [PubMed] [Google Scholar]
- 39.Technology Assessment Conference Panel. Methods for voluntary weight loss and control: Technology Assessment Conference statement. Ann Intern Med. 1992;116:942–949. [DOI] [PubMed] [Google Scholar]
- 40.Wamberg L, Kampmann U, Stodkilde-Jorgensen H, et al. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels – results from a randomized trial. Eur J Intern Med. 2013;24:644–649. [DOI] [PubMed] [Google Scholar]
- 41.Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–1327. [DOI] [PubMed] [Google Scholar]
- 42.Mason C, Xiao L, Imayama I, et al. Vitamin D3 supplementation during weight loss: a double-blind randomized controlled trial. Am J Clin Nutr. 2014;99:1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 44.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickersin K, Berlin JA. Meta-analysis: state-of-the-science. Epidemiol Rev. 1992;14:154–176. [DOI] [PubMed] [Google Scholar]
- 46.Zhu K, Devine A, Dick IM, et al. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93:743–749. [DOI] [PubMed] [Google Scholar]
- 47.Glendenning P, Zhu K, Inderjeeth C, et al. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: a randomized controlled trial. J Bone Miner Res. 2012;27:170–176. [DOI] [PubMed] [Google Scholar]
- 48.Davidson MB, Duran P, Lee ML, et al. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36:260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aloia JF, Talwar SA, Pollack S, et al. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165:1618–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dawson-Hughes B, Harris SS, Krall EA, et al. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. New Engl J Med. 1997;337:670–676. [DOI] [PubMed] [Google Scholar]
- 51.Wood AD, Secombes KR, Thies F, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: a parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97:3557–3568. [DOI] [PubMed] [Google Scholar]
- 52.Harris RA, Pedersen-White J, Guo DH, et al. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011;24:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dawson-Hughes B, Dallal GE, Krall EA, et al. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med. 1991;115:505–512. [DOI] [PubMed] [Google Scholar]
- 54.Prince RL, Austin N, Devine A, et al. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med. 2008;168:103–108. [DOI] [PubMed] [Google Scholar]
- 55.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient - a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. [DOI] [PubMed] [Google Scholar]
- 56.Grimnes G, Figenschau Y, Almas B, et al. Vitamin D, insulin secretion, sensitivity, and lipids: results from a case-control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes. 2011;60:2748–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kjaergaard M, Waterloo K, Wang CE, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201:360–368. [DOI] [PubMed] [Google Scholar]
- 58.Jorde R, Sneve M, Torjesen P, et al. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010;267:462–472. [DOI] [PubMed] [Google Scholar]
- 59.Heikkinen AM, Tuppurainen MT, Niskanen L, et al. Long-term vitamin D3 supplementation may have adverse effects on serum lipids during postmenopausal hormone replacement therapy. Eur J Endocrinol. 1997;137:495–502. [DOI] [PubMed] [Google Scholar]
- 60.Chandler PD, Scott JB, Drake BF, et al. Impact of vitamin D supplementation on inflammatory markers in African-Americans: results of a four-arm, randomized, placebo-controlled trial. Cancer Prevent Res. 2013;7:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Major GC, Alarie F, Dore J, et al. Supplementation with calcium + vitamin D enhances the beneficial effect of weight loss on plasma lipid and lipoprotein concentrations. Am J Clin Nutr. 2007;85:54–59. [DOI] [PubMed] [Google Scholar]
- 62.Pittas AG, Harris SS, Stark PC, et al. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30:980–986. [DOI] [PubMed] [Google Scholar]
- 63.van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab. 2011;25:671–680. [DOI] [PubMed] [Google Scholar]
- 64.Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am. 2010;39:109–124. [DOI] [PubMed] [Google Scholar]
- 65.Siervo M, Oggioni C, Lara J, et al. Age-related changes in resting energy expenditure in normal weight, overweight and obese men and women. Maturitas. 2015;80:406–413. [DOI] [PubMed] [Google Scholar]
- 66.Mott JW, Wang J, Thornton JC, et al. Relation between body fat and age in 4 ethnic groups. Am J Clin Nutr. 1999;69:1007–1013. [DOI] [PubMed] [Google Scholar]
- 67.Guo SS, Zeller C, Chumlea WC, et al. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr. 1999;70:405–411. [DOI] [PubMed] [Google Scholar]
- 68.Going S, Williams D, Lohman T. Aging and body composition: biological changes and methodological issues. Exerc Sport Sci Rev. 1995;23:411–458. [PubMed] [Google Scholar]
- 69.Karlsson MK, Obrant KJ, Nilsson BE, et al. Changes in bone mineral, lean body mass and fat content as measured by dual energy X-ray absorptiometry: a longitudinal study. Calcif Tissue Int. 2000;66:97–99. [DOI] [PubMed] [Google Scholar]
- 70.LeBoff MS, Yue AY, Copeland T, et al. VITAL-Bone Health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega-3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials. 2015;41:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Major GC, Alarie FP, Dore J, et al. Calcium plus vitamin D supplementation and fat mass loss in female very low-calcium consumers: potential link with a calcium-specific appetite control. Br J Nutr. 2009;101:659–663. [DOI] [PubMed] [Google Scholar]
- 72.Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;48:349–354. [DOI] [PubMed] [Google Scholar]
- 73.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. [DOI] [PubMed] [Google Scholar]
- 74.Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raja GK, Sarzynski MA, Katzmarzyk PT, et al. Commonality versus specificity among adiposity traits in normal-weight and moderately overweight adults. Int J Obes. 2014;38:719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ludescher B, Machann J, Eschweiler GW, et al. Correlation of fat distribution in whole body MRI with generally used anthropometric data. Invest Radiol. 2009;44:712–719. [DOI] [PubMed] [Google Scholar]
- 77.Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. [DOI] [PubMed] [Google Scholar]
- 78.Frank-Wilson AW, Johnston JD, Olszynski WP, et al. Measurement of muscle and fat in postmenopausal women: precision of previously reported pQCT imaging methods. Bone. 2015;75:49–54. [DOI] [PubMed] [Google Scholar]
- 79.Megnien JL, Denarie N, Cocaul M, et al. Predictive value of waist-to-hip ratio on cardiovascular risk events. Int J Obes Relat Metabol Disord. 1999;23:90–97. [DOI] [PubMed] [Google Scholar]
- 80.Pineau JC, Frey A. Comparison of skinfold thickness models with DEXA: impact of visceral adipose tissue. J Sports Med Phys Fitness. 2015. [published online ahead of print February 13, 2015]. [PubMed] [Google Scholar]
- 81.Lindeboom L, Nabuurs CI, Hesselink MK, et al. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am J Clin Nutr. 2015;101:65–71. [DOI] [PubMed] [Google Scholar]
- 82.Chiu KC, Chu A, Go VL, et al. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. [DOI] [PubMed] [Google Scholar]
- 83.Ding C, Parameswaran V, Blizzard L, et al. Not a simple fat-soluble vitamin: changes in serum 25-(OH)D levels are predicted by adiposity and adipocytokines in older adults. J Intern Med. 2010;268:501–510. [DOI] [PubMed] [Google Scholar]
- 84.Gilsanz V, Kremer A, Mo AO, et al. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metabol. 2010;95:1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heaney RP, Davies KM, Barger-Lux MJ. Calcium and weight: clinical studies. J Am Coll Nutr. 2002;21:152S–155S. [DOI] [PubMed] [Google Scholar]
- 86.Liel Y, Ulmer E, Shary J, et al. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43:199–201. [DOI] [PubMed] [Google Scholar]
- 87.Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89:1196–1199. [DOI] [PubMed] [Google Scholar]
- 88.Valina-Toth AL, Lai Z, Yoo W, et al. Relationship of vitamin D and parathyroid hormone with obesity and body composition in African Americans. Clin Endocrinol. 2010;72:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young KA, Engelman CD, Langefeld CD, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94:3306–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arunabh S, Pollack S, Yeh J, et al. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–161. [DOI] [PubMed] [Google Scholar]
- 91.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr. 1995;49:640–646. [PubMed] [Google Scholar]
- 92.Coin A, Sergi G, Minicuci N, et al. Fat-free mass and fat mass reference values by dual-energy X-ray absorptiometry (DEXA) in a 20-80 year-old Italian population. Clin Nutr. 2008;27:87–94. [DOI] [PubMed] [Google Scholar]
- 93.Visser M, Pahor M, Tylavsky F, et al. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol. 2003;94:2368–2374. [DOI] [PubMed] [Google Scholar]
- 94.Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637–1642. [DOI] [PubMed] [Google Scholar]
- 96.Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13:257–264. [DOI] [PubMed] [Google Scholar]
- 97.LeBoff MS, Hawkes WG, Glowacki J, et al. Vitamin D-deficiency and post-fracture changes in lower extremity function and falls in women with hip fractures. Osteoporos Int. 2008;19:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.LeBoff MS, Kohlmeier L, Hurwitz S, et al. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA. 1999;281:1505–1511. [DOI] [PubMed] [Google Scholar]
- 99.Pieper CF, Colon-Emeric C, Caminis J, et al. Distribution and correlates of serum 25-hydroxyvitamin D levels in a sample of patients with hip fracture. Am J Geriatr Pharmacother. 2007;5:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soares MJ, Murhadi LL, Kurpad AV, et al. Mechanistic roles for calcium and vitamin D in the regulation of body weight. Obes Rev. 2012;13:592–605. [DOI] [PubMed] [Google Scholar]
- 101.Heaney RP. Long-latency deficiency disease: insights from calcium and vitamin D. Am J Clin Nutr. 2003;78:912–919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.