Abstract
The sympathetic and pressor responses to exercise are exaggerated in hypertension. Evidence suggests that an overactive exercise pressor reflex (EPR) contributes to this abnormal responsiveness. The mechanisms underlying this EPR overactivity are poorly understood. An increasing body of evidence suggests aldosterone as well as excessive salt intake play a role in regulating resting sympathetic activity and blood pressure in hypertension. Therefore, each is a good candidate for the generation of EPR dysfunction in this disease. The purpose of this study was to examine whether excessive salt intake and/or chronic administration of aldosterone potentiate EPR function. Changes in mean arterial pressure (MAP) and renal sympathetic nerve activity (RSNA) induced by EPR stimulation were examined in vehicle and aldosterone treated (4 weeks via osmotic mini-pump) Sprague-Dawley rats given either water or saline (elevated salt load) to drink. Compared to vehicle/water-treated rats, stimulation of the EPR by muscle contraction evoked significantly greater increases in MAP in vehicle/saline, aldosterone/water and aldosterone/saline-treated animals (14±3, 29±3, 37±6, and 44±7mm Hg kg−1, respectively, P<0.01). A similar RSNA response profile was likewise produced (39±11, 87±15, 110±20, and 151±25 % kg−1, respectively, P<0.01). The pressor and sympathetic responses to the individual activation of the mechanically and chemically-sensitive components of the EPR were also augmented by both saline and aldosterone. These data provide the first direct evidence that both aldosterone and high salt intake elicit EPR overactivity. As such, each represents a potential mechanism by which sympathetic activity and blood pressure are augmented during exercise in hypertension.
Keywords: exercise pressor reflex, blood pressure, sympathetic nerve activity, renin angiotensin system
INTRODUCTION
The cardiovascular response to exercise is abnormally exaggerated in hypertensive patients and characterized by augmented increases in arterial blood pressure (BP), heart rate (HR), and sympathetic nerve activity (SNA) 1. Since such responses have been shown to be associated with elevated risks for myocardial ischemia, myocardial infarction, cardiac arrest and/or stroke during and after physical activity, elucidating the cause of the cardiovascular hyper-excitability is clinically as well as physiologically important 2.
Afferent signals from working skeletal muscle are an important source of neural input to the brain stem during exercise and contribute significantly to the regulation of sympathetic outflow as well as the cardiovascular system during physical activity 3. These contraction-induced signals, which comprise the skeletal muscle exercise pressor reflex (EPR), are generated by stimulation of group III (predominantly mechanically-sensitive A-δ fibers associated with the muscle mechanoreflex) and IV (primarily chemically-sensitive C fibers associated with the muscle metaboreflex) skeletal muscle afferents 3, 4. It has been extensively demonstrated in a number of animal models of human hypertension that the EPR is overactive in the disease contributing significantly to the exaggerated increases in SNA and BP that manifest during exercise 5–7. That being stated, the mechanisms underlying the pathogenesis of EPR dysfunction in hypertension have not been fully established.
Aldosterone is well known to contribute to the development of hypertension. Circulating aldosterone penetrates the blood-brain barrier at concentrations paralleling those found in plasma 8, 9. Aldosterone has been shown to act centrally stimulating the sympathetic nervous system 10, 11. Earlier studies demonstrated that direct infusion of aldosterone into the cerebral ventricles causes a sustained increase in BP and renal SNA (RSNA) in rats and dogs 12–16. As such, aldosterone represents a potential mechanistic candidate for the generation of EPR overactivity.
Like aldosterone, high salt intake has also been shown to activate the sympathetic nervous system by increasing sodium concentrations in cerebrospinal fluid and neural tissue 17, 18. Moreover, a recent study suggests that increased salt intake augments EPR function in rats 19 establishing this mechanism as an additional potential candidate for the generation of muscle reflex overactivity in hypertension. Importantly, it has been suggested that the central pressor action of aldosterone is observed only in the presence of sodium excess or in salt-sensitive animals 15, 20. As such, if aldosterone does indeed augment EPR activity its action may be amplified by the presence of increased sodium.
Therefore, this study was designed to test the hypotheses that i) chronic systemic administration of aldosterone potentiates EPR function and ii) aldosterone-induced EPR overactivity is exacerbated by concomitant salt-loading. In addition, studies were performed to confirm and support reports that increased sodium intake alone elicits EPR dysfunction. To test these hypotheses, we examined cardiovascular and sympathetic responses to activation of the EPR, as well as its individual mechanically and chemically-sensitive components, in vehicle and aldosterone treated Sprague-Dawley rats given either water or saline to drink.
METHODS
For a complete description of the Materials and Methods see the online-only Data Supplement.
Animal models
Experiments were performed on 46 male Sprague-Dawley rats (11–12 wk, 310–350 g) Under isoflurane anesthesia, osmotic minipumps (2ML4, ALZET) were implanted subcutaneously for 28 days to deliver 250 μg kg−1 day−1 doses of aldosterone (n = 23) or vehicle (n = 23). The animals in each group were assigned to drink normal water or 0.9% NaCl saline (vehicle/water: n = 11; vehicle/saline: n = 12; aldosterone/water: n =12; and aldosterone/saline: n =11). The procedures outlined were approved by the Institutional Animal Care and Use Committee. All studies were conducted in accordance with the US Department of Health and Human Services NIH Guide for the Care and Use of Laboratory Animals.
Experimental Protocols
MAP, HR, and RSNA were continuously measured at rest and during stimulation of either the EPR, the muscle mechanoreflex or muscle metaboreflex.
Muscle Reflex Stimulation
The EPR was stimulated by contracting the triceps surae muscles of the right hindlimb for 30s via electrical stimulation of isolated L4 and L5 ventral roots. Constant current stimulation was used at a 3 times motor threshold (i.e. the minimum current required to produce a muscle twitch) with a pulse duration of 0.1 ms at 40 Hz. Activation of the EPR in this manner stimulates mechanically and chemically-sensitive skeletal muscle afferent fibers concomitantly. To preferentially activate mechanically sensitive afferent neurons, the triceps surae muscles were passively stretched. Isolated activation of chemically sensitive fibers was achieved by administering graded concentrations of capsaicin into the arterial supply of the hindlimb.
Statistical Analyses
Data were analyzed using two-way ANOVA (aldosterone × saline) and three-way ANOVA (aldosterone × saline × administration period, aldosterone × saline × capsaicin concentration). When appropriate, a post hoc Fisher’s PLSD test was used to identify differences between specific group means. The significance level was set at P < 0.05. Results are presented as means ± S.E.M.
RESULTS
Morphometric characteristics and plasma aldosterone concentrations for each experimental are presented in Table 1. Body weight was lower in the aldosterone/saline treated animals compared to all other groups. Heart weight to body weight ratios and heart weight to tibial length ratios were significantly higher in aldosterone treated groups than vehicle treated animals. Plasma aldosterone concentrations in aldosterone/water as well as aldosterone/saline treated groups were markedly larger after 4 weeks of aldosterone administration compared to vehicle treated animals.
Table 1.
Morphometric characteristics and plasma aldosterone.
| Variables | Water | Saline | ||
|---|---|---|---|---|
| Vehicle | Aldosterone | Vehicle | Aldosterone | |
| n | 11 | 12 | 12 | 11 |
| Body weight (g) | 416 ± 4 | 429 ± 15 | 412 ± 5 | 366 ± 8 *†‡ |
| Heart weight/body weight (mg g−1) | 2.62 ± 0.04 | 2.88 ± 0.07*† | 2.64 ± 0.07 | 3.23 ± 0.11 *†‡ |
| Heart weight/tibial length (mg mm−1) | 27.4 ± 0.5 | 31.2 ± 0.8*† | 27.5 ± 0.7 | 29.5 ± 0.8 *† |
| Lung weight/body weight (mg g−1) | 5.66 ± 0.34 | 6.35 ± 0.79 | 5.96 ± 0.26 | 6.53 ± 0.48 |
| Plasma aldosterone concentration (ng 100 mL−1) | 10 ± 1 | 736 ± 503*† | 8 ± 4 | 444 ± 117*† |
Values are means ± S.E.M.
P < 0.05 compared to vehicle/water.
P < 0.05 compared to vehicle/saline.
P < 0.05 compared to aldosterone/water. Saline: 0.9% NaCl.
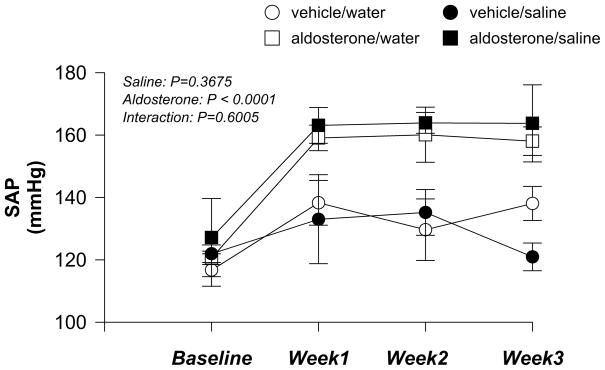
Four weeks of aldosterone administration, but not saline intake, significantly increased conscious resting systolic arterial pressure (SAP) measured by tail cuff (Fig. 1). Concomitant high salt intake did not further augment the increases in baseline SAP induced by aldosterone administration (aldosterone × saline: P = 0.6005).
Figure 1.
Conscious systolic arterial pressure (SAP) measured by tail cuff in vehicle/water, vehicle/saline, aldosterone/water and aldosterone/saline rats before and 3 weeks after mini-pump implantation (n = 5 in each group). Saline: 0.9% NaCl.
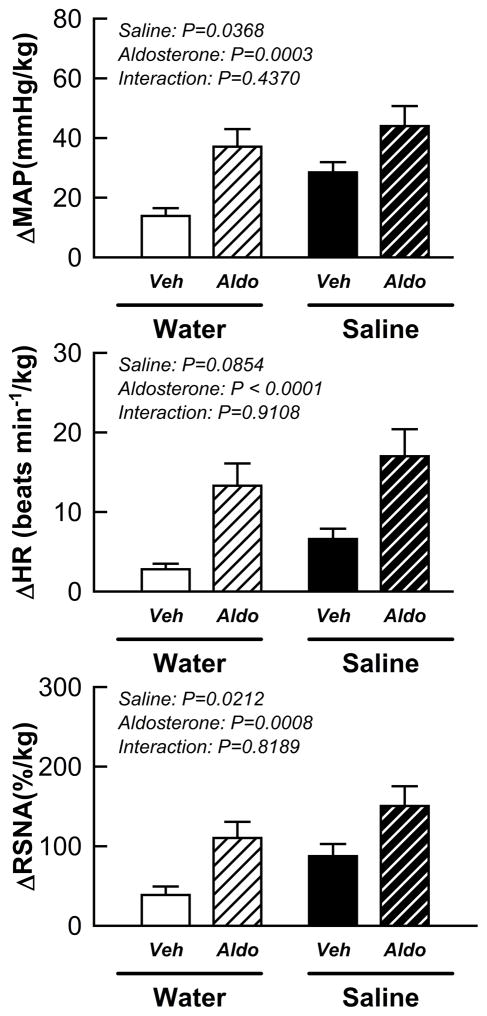
The pressor, tachycardic and sympathetic responses to stimulation of the EPR during static muscle contraction were augmented by salt intake alone as well as aldosterone administration alone compared to control animals (Fig. 2; aldosterone effect: P < 0.01 and saline effect: P < 0.05 for MAP and RSNA). Importantly, the effects of aldosterone on the EPR-induced rise in MAP, HR, and RSNA were not altered by sodium intake (all interaction p value > 0.1).
Figure 2.
Cardiovascular and sympathetic responses to activation of the EPR during static muscle contraction in vehicle/water, vehicle/saline, aldosterone/water and aldosterone/saline rats. Veh: vehicle; Aldo: aldosterone. Saline: 0.9% NaCl.
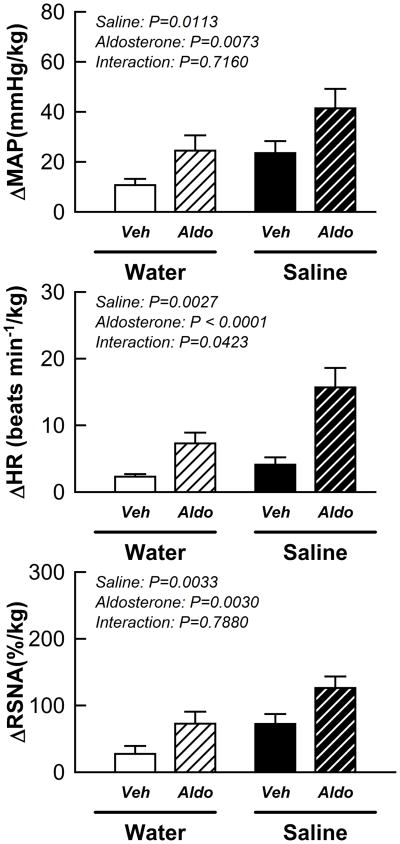
High salt intake alone as well as aldosterone alone significantly enhanced the changes in MAP, HR and RSNA in response to stimulation of the muscle mechanoreflex during passive muscle stretch (Fig. 3; aldosterone effect: P < 0.01 and saline effect: P < 0.05). The effects of aldosterone on the mechanoreflex-induced HR response, but not the MAP or RSNA responses, was potentiated by high sodium intake.
Figure 3.
Cardiovascular and sympathetic responses to activation of the mechanically sensitive component of the EPR in vehicle/water, vehicle/saline, aldosterone/water and aldosterone/saline rats. Veh: vehicle; Aldo: aldosterone. Saline: 0.9% NaCl.
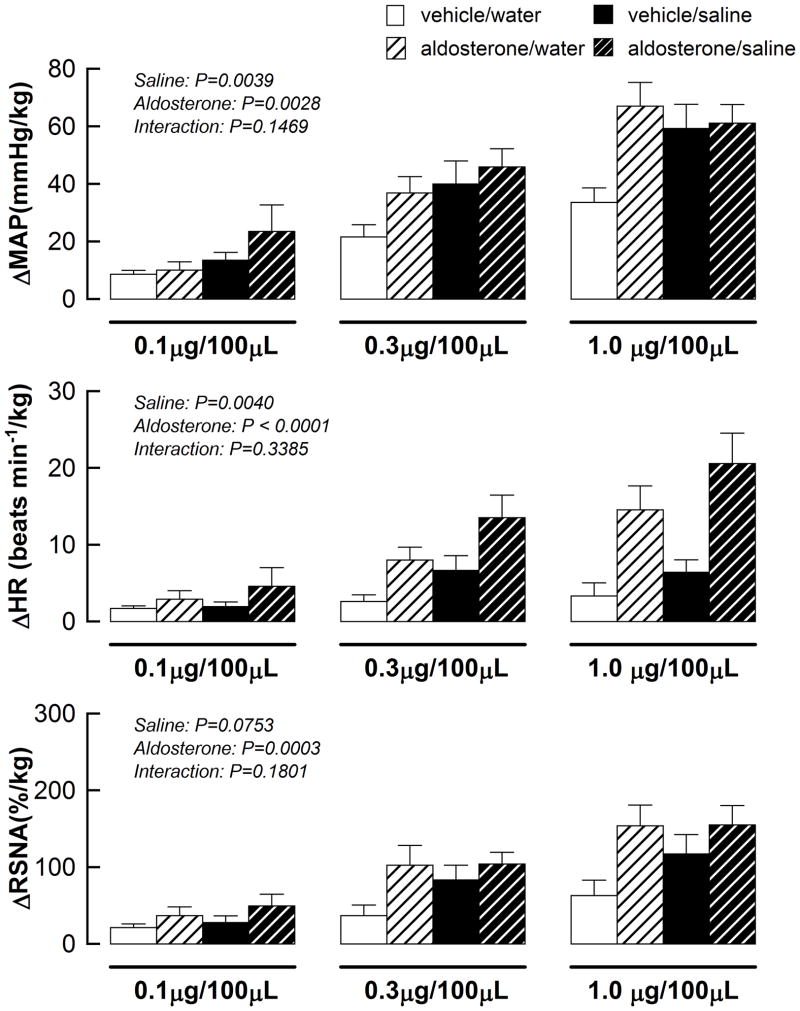
High salt intake alone and aldosterone alone exacerbated the cardiovascular response to stimulation of the muscle metaboreflex during capsaicin administration (Fig 4.). The effects of aldosterone administration on the metaboreflex-induced elevation in MAP, HR, and RSNA was not altered by concomitant sodium intake (all interaction p value > 0.1).
Figure 4.
Cardiovascular and sympathetic responses to activation of the metabolically sensitive component of the EPR in vehicle/water, vehicle/saline, aldosterone/water and aldosterone/saline rats. Saline: 0.9% NaCl.
Additional results are reported in the online-only Data Supplement.
DISCUSSION
The major new findings from this investigation were i) chronic aldosterone administration significantly augmented EPR activity; ii) aldosterone-induced EPR dysfunction was mediated by both the mechanically and chemically-sensitive components of the EPR; and iii) the deleterious actions of aldosterone on EPR activity were not appreciably amplified by concomitant high sodium intake. In agreement with a previous report, it was also demonstrated that salt loading alone significantly enhanced the sympathetic and cardiovascular responses to activation of the EPR. These findings establish that both excessive levels of aldosterone and high salt intake can each individually induce EPR overactivity. As such, each may contribute significantly to the exaggerated elevations in sympathetic activity and blood pressure generated during exercise in this disease.
Previous studies have demonstrated that aldosterone increases renal sympathetic nerve discharge and BP in the resting condition 15, 21. However, the influence of aldosterone on sympathetic regulation during exercise has not been investigated. To the best of our knowledge, this is the first report showing that aldosterone alters sympathetic and cardiovascular responses to activation of the EPR during physical stress. Interestingly, the EPR overactivity demonstrated was mediated by both functional components of the reflex: the muscle mechanoreflex and metaboreflex. The mechanisms underlying aldosterone-induced enhancements in mechano- and metaboreflex function remain unknown. However, It has been reported that aldosterone with salt loading for 4 weeks results in muscle atrophy 22. It has also been demonstrated that the pressor response to passive stretch is significantly greater in atrophied muscle compared to healthy muscle 23. In the current investigation, aldosterone administration with high salt intake significantly decreased body mass (Table 1) while likewise reducing the developed peak tension during muscle contraction (Table S2). The EPR overactivity generated in aldosterone treated animals might be explained by sensitization of the muscle reflexes as a result of saline/aldosterone induced-muscle atrophy. However temping to conclude, this would not explain the EPR dysfunction that manifested in rats treated only with aldosterone. Aldosterone alone is also known to induce cardiomyopathy 22, 24. Previous studies in rats and patients with congestive heart failure have demonstrated enhanced EPR activity via selective mechanoreflex sensitization 25–28. In our study, chronic aldosterone administration induced left ventricular hypertrophy as evidenced by increased heart weight to body weight ratios as well as heart weight to tibial length ratios. However, the lung weight in aldosterone treated rats was not increased suggesting that congestive heart failure had not developed. Thus, although the hypertrophic cardiomyopathy induced by aldosterone administration may contribute to the generation of EPR dysfunction to some extent it seems unlikely to be the primary cause. Other possibilities include aldosterone-induced impairments in baroreflex-mediated inhibition of SNA (chronic intracerebroventricular infusion of aldosterone has been shown to inhibit arterial baroreflex control of RSNA and HR) 20 and direct modulation by aldosterone of central sympathoexcitatory pathways 20.
In our study, high salt intake alone significantly augmented the MAP, HR and RSNA responses to activation of the EPR (Fig. 2). These findings are consistent with a recent report demonstrating that increased dietary salt intake enhances pressor and cardioaccelerator responses to muscle contraction in rats 19. Our data extended these previous findings by demonstrating that EPR dysfunction in saline treated rats is mediated by both mechanoreflex and metaboreflex overactivity. Accumulating reports indicate that salt intake does not alter vasoconstrictor responses to sympathetic stimulation in rats 19, 29–31. Thus, it is logical to postulate that salt-induced EPR overactivity results from the sensitization of brainstem circuits in the muscle reflex pathway. Sensitization of these circuits could cause the abnormally large elevations in sympathetic output observed during muscle reflex activation. However plausible, the exact downstream mechanisms underlying such changes remain undetermined. High salt intake has been shown to increase the concentration of sodium in cerebral spinal fluid and brain tissue which could evoke central sympathetic activation via stimulation of epithelial sodium channels (ENaC) 20, 32.33. Specifically, it has been suggested that sodium acts on neurons within the organum vasculosum of the lamina terminalis to enhance the responsiveness of sympathetic motor neurons emanating from the rostral ventrolateral medulla (RVLM) within the brainstem 34. Continued research in this area is needed to determine whether these pathways underlie high salt intake-induced EPR dysfunction.
In the current investigation, the excitatory effect of aldosterone on EPR activity was not dependent upon the level of salt intake. Although it has been suggested that the hypertensive and sympathoexcitatory action of aldosterone requires the presence of excess sodium, we found a strong independent action of aldosterone on EPR activity that was not appreciably affected by increased salt ingestion 15, 20. This suggests that aldosterone and salt loading may accentuate EPR function through similar pathways. The RVLM, an established cardiovascular regulatory nuclei within the brainstem, is a good candidate for the mediation of this EPR overactivity. Increased dietary salt intake enhances sympathetic responsiveness to stimulation of the RVLM 35. Furthermore, a neurophysiological study has clearly demonstrated that aldosterone activates RVLM neurons through mineralocorticoid receptors (MR) as well as ENaCs 36. Thus, it is plausible that aldosterone and salt loading alter neural activity in the RVLM through MR and/or ENaCs. The paraventricular nucleus (PVN) of the hypothalamus, known to be an important contributor to autonomic cardiovascular control, is another reasonable candidate. It comprises ~70% of the pre-ganglionic fibers by-passing the RVLM 37 and likewise expresses MR 38. Because the pre-collicular decerebration performed in the current study removes the hypothalamus, the PVN was unlikely to contribute substantially to the abnormal EPR function observed in the present investigation. However, we cannot exclude the possibility that chronic administration of aldosterone could have induced permanent alterations in the function of brainstem neurons prior to removal of the PVN contributing to the results reported. Although speculative in nature, it is tempting to suggest that sodium and aldosterone affect these cardiovascular regulatory regions of the brain and in this manner independently alter EPR function.
It should be noted, in the current study concomitant high salt intake did not further augment the increases in baseline SAP induced by aldosterone. This finding is inconsistent with a previous study using rats in which the effects of intracerebroventricular as well as subcutaneous aldosterone administration on blood pressure were examined 16. The discrepancy may be partly explained by the difference in experimental designs between studies. For example, in the current study a higher dose of aldosterone was utilized and the kidneys were not removed. Nevertheless, the findings clearly support the contention that aldosterone and high salt intake alter EPR function and may do so via similar mechanisms.
PERSPECTIVES
Excessive BP elevation during exercise has been shown to contribute to impaired exercise tolerance in hypertensive patients even in the absence of coronary artery disease or left ventricular dysfunction 39–42. Furthermore, numerous epidemiological studies have demonstrated that exercise blood pressure predicts development of left ventricular hypertrophy, stroke, myocardial infarction, and death 43–47 independent of resting BP. Our study in rodents suggests a potential role for both aldosterone and dietary salt intake in modulating the sympathetically mediated BP response to exercise via alterations in reflexes originating from the skeletal muscle. Based on these findings, further studies in humans are needed to confirm if salt restriction and MR blockade constitute two independent effective strategies in attenuating the augmented pressor response to exercise in hypertension.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
Although the sympathetically mediated cardiovascular response to physical activity in hypertensive patients is heightened as compared to healthy subjects, the underlying mechanisms are poorly understood. Accumulating evidence suggests that aldosterone and high dietary salt intake play a role in regulating baseline sympathetic outflow in hypertension. However, whether each contributes to the generation of the excessive sympathetic and cardiovascular responses to exercise in this disease remains unknown.
What Is Relevant?
The findings indicate that controlling salt intake and/or treating excessive levels of aldosterone may improve blood pressure regulation during exercise in hypertension.
Summary
The present findings demonstrate that systemic administration of aldosterone and high dietary salt intake independently potentiate the function of the skeletal muscle exercise pressor reflex; a reflex which regulates, in part, the rise in sympathetic nerve activity and blood pressure during physical exertion and is known to be overactive in hypertension.
Acknowledgments
The authors thank Martha Romero and Julius Lamar, Jr. for their expert technical assistance. In addition, we thank the UT Southwestern O’Brien Kidney Research Core Center for providing materials and support for the measurement of non-invasive blood pressure in conscious rats.
SOURCES OF FUNDING
This research was supported by a grant from the National Institutes of Health Heart, Lung and Blood Institute (HL-113738 to W.V.), the Lawson & Rogers Lacy Research Fund in Cardiovascular Disease (to J.H.M.), and the UT Southwestern O’Brien Kidney Research Core Center (NIH P30DK079328 to W.V.).
Footnotes
DISCLOSURES
None.
References
- 1.Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol. 2011;589:1209–1220. doi: 10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of myocardial infarction onset study investigators. N Engl J Med. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 3.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 5.Koba S, Watanabe R, Kano N, Watanabe T. Oxidative stress exaggerates skeletal muscle contraction-evoked reflex sympathoexcitation in rats with hypertension induced by angiotensin ii. Am J Physiol Heart Circ Physiol. 2013;304:H142–153. doi: 10.1152/ajpheart.00423.2012. [DOI] [PubMed] [Google Scholar]
- 6.Mizuno M, Siddique K, Baum M, Smith SA. Prenatal programming of hypertension induces sympathetic overactivity in response to physical stress. Hypertension. 2013;61:180–186. doi: 10.1161/HYPERTENSIONAHA.112.199356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SA, Williams MA, Leal AK, Mitchell JH, Garry MG. Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol. 2006;577:1009–1020. doi: 10.1113/jphysiol.2006.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005;288:E342–346. doi: 10.1152/ajpendo.00355.2004. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension. 2008;51:727–733. doi: 10.1161/HYPERTENSIONAHA.107.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felder RB. Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp Physiol. 2010;95:19–25. doi: 10.1113/expphysiol.2008.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Sanchez EP. The mammalian mineralocorticoid receptor: Tying down a promiscuous receptor. Exp Physiol. 2010;95:13–18. doi: 10.1113/expphysiol.2008.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis J, Beltz T, Johnson AK, Felder RB. Mineralocorticoids act centrally to regulate blood-borne tumor necrosis factor-alpha in normal rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1402–1409. doi: 10.1152/ajpregu.00027.2003. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Sanchez EP. Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology. 1986;118:819–823. doi: 10.1210/endo-118-2-819. [DOI] [PubMed] [Google Scholar]
- 14.Kageyama Y, Bravo EL. Hypertensive mechanisms associated with centrally administered aldosterone in dogs. Hypertension. 1988;11:750–753. doi: 10.1161/01.hyp.11.6.750. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Huang BS, Leenen FH. Brain sodium channels and ouabainlike compounds mediate central aldosterone-induced hypertension. Am J Physiol Heart Circ Physiol. 2003;285:H2516–2523. doi: 10.1152/ajpheart.00299.2003. [DOI] [PubMed] [Google Scholar]
- 16.Gomez Sanchez EP. Dose-response studies of intracerebroventricular infusion of aldosterone in sensitized and non-sensitized rats. J Hypertens. 1988;6:437–442. doi: 10.1097/00004872-198806000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Huang BS, Leenen FH. Mineralocorticoid actions in the brain and hypertension. Curr Hypertens Rep. 2011;13:214–220. doi: 10.1007/s11906-011-0192-0. [DOI] [PubMed] [Google Scholar]
- 18.Nakano M, Hirooka Y, Matsukawa R, Ito K, Sunagawa K. Mineralocorticoid receptors/epithelial na(+) channels in the choroid plexus are involved in hypertensive mechanisms in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2013;36:277–284. doi: 10.1038/hr.2012.174. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi K, Tsuchimochi H, Stone AJ, Stocker SD, Kaufman MP. Increased dietary salt intake enhances the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2014;306:H450–454. doi: 10.1152/ajpheart.00813.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang BS, Wang H, Leenen FH. Chronic central infusion of aldosterone leads to sympathetic hyperreactivity and hypertension in dahl s but not dahl r rats. Am J Physiol Heart Circ Physiol. 2005;288:H517–524. doi: 10.1152/ajpheart.00651.2004. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol. 2008;294:H1067–1074. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- 22.Cheema Y, Zhao W, Zhao T, Khan MU, Green KD, Ahokas RA, Gerling IC, Bhattacharya SK, Weber KT. Reverse remodeling and recovery from cachexia in rats with aldosteronism. Am J Physiol Heart Circ Physiol. 2012;303:H486–495. doi: 10.1152/ajpheart.00192.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi N, Koba S, Yoshida T. Disuse atrophy increases the muscle mechanoreflex in rats. J Appl Physiol (1985) 2005;99:1442–1445. doi: 10.1152/japplphysiol.00180.2005. [DOI] [PubMed] [Google Scholar]
- 24.Hattori T, Murase T, Sugiura Y, Nagasawa K, Takahashi K, Ohtake M, Ohtake M, Miyachi M, Murohara T, Nagata K. Effects of salt status and blockade of mineralocorticoid receptors on aldosterone-induced cardiac injury. Hypertens Res. 2014;37:125–133. doi: 10.1038/hr.2013.124. [DOI] [PubMed] [Google Scholar]
- 25.McClain J, Hardy C, Enders B, Smith M, Sinoway L. Limb congestion and sympathoexcitation during exercise. Implications for congestive heart failure. J Clin Invest. 1993;92:2353–2359. doi: 10.1172/JCI116840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middlekauff HR, Nitzsche EU, Hoh CK, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Exaggerated renal vasoconstriction during exercise in heart failure patients. Circulation. 2000;101:784–789. doi: 10.1161/01.cir.101.7.784. [DOI] [PubMed] [Google Scholar]
- 27.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- 28.Smith SA, Williams MA, Mitchell JH, Mammen PP, Garry MG. The capsaicin-sensitive afferent neuron in skeletal muscle is abnormal in heart failure. Circulation. 2005;111:2056–2065. doi: 10.1161/01.CIR.0000162473.10951.0A. [DOI] [PubMed] [Google Scholar]
- 29.Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol. 1999;276:R1600–1607. doi: 10.1152/ajpregu.1999.276.6.R1600. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman LJ, Vollmer RR. Low sodium diet augments plasma and tissue catecholamine levels in pithed rats. Clin Exp Hypertens A. 1984;6:1543–1558. doi: 10.3109/10641968409044068. [DOI] [PubMed] [Google Scholar]
- 31.Pawloski-Dahm CM, Gordon FJ. Increased dietary salt sensitizes vasomotor neurons of the rostral ventrolateral medulla. Hypertension. 1993;22:929–933. doi: 10.1161/01.hyp.22.6.929. [DOI] [PubMed] [Google Scholar]
- 32.Van Huysse JW, Amin MS, Yang B, Leenen FH. Salt-induced hypertension in a mouse model of liddle syndrome is mediated by epithelial sodium channels in the brain. Hypertension. 2012;60:691–696. doi: 10.1161/HYPERTENSIONAHA.112.193045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller RL, Wang MH, Gray PA, Salkoff LB, Loewy AD. Enac-expressing neurons in the sensory circumventricular organs become c-fos activated following systemic sodium changes. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1141–1152. doi: 10.1152/ajpregu.00242.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension. 2009;54:308–314. doi: 10.1161/HYPERTENSIONAHA.108.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension. 2007;50:354–359. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- 36.Oshima N, Onimaru H, Takechi H, Yamamoto K, Watanabe A, Uchida T, Nishida Y, Oda T, Kumagai H. Aldosterone is synthesized in and activates bulbospinal neurons through mineralocorticoid receptors and enacs in the rvlm. Hypertens Res. 2013;36:504–512. doi: 10.1038/hr.2012.224. [DOI] [PubMed] [Google Scholar]
- 37.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: Implications for cardiovascular regulation. Journal of chemical neuroanatomy. 2009;38:197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Gomez-Sanchez CE, Penman A, May PJ, Gomez-Sanchez E. Expression of mineralocorticoid and glucocorticoid receptors in preautonomic neurons of the rat paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2014;306:R328–340. doi: 10.1152/ajpregu.00506.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagard R, Staessen J, Amery A. Maximal aerobic power in essential hypertension. J Hypertens. 1988;6:859–865. doi: 10.1097/00004872-198811000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Marraccini P, Palombo C, Giaconi S, Michelassi C, Genovesi-Ebert A, Marabotti C, Fommei E, Ghione S, L’Abbate A. Reduced cardiovascular efficiency and increased reactivity during exercise in borderline and established hypertension. Am J Hypertens. 1989;2:913–916. doi: 10.1093/ajh/2.12.913. [DOI] [PubMed] [Google Scholar]
- 41.Olsen MH, Wachtell K, Hermann KL, Bella JN, Andersen UB, Dige-Petersen H, Rokkedal J, Ibsen H. Maximal exercise capacity is related to cardiovascular structure in patients with longstanding hypertension. A life substudy. Losartan intervention for endpoint-reduction in hypertension. Am J Hypertens. 2001;14:1205–1210. doi: 10.1016/s0895-7061(01)02223-3. [DOI] [PubMed] [Google Scholar]
- 42.Lim PO, MacFadyen RJ, Clarkson PB, MacDonald TM. Impaired exercise tolerance in hypertensive patients. Ann Intern Med. 1996;124:41–55. doi: 10.7326/0003-4819-124-1_part_1-199601010-00008. [DOI] [PubMed] [Google Scholar]
- 43.Filipovsky J, Ducimetiere P, Safar ME. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension. 1992;20:333–339. doi: 10.1161/01.hyp.20.3.333. [DOI] [PubMed] [Google Scholar]
- 44.Kjeldsen SE, Mundal R, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Supine and exercise systolic blood pressure predict cardiovascular death in middle-aged men. J Hypertens. 2001;19:1343–1348. doi: 10.1097/00004872-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O’Donnell CJ, Levy D, Vasan RS, Wang TJ. Exercise blood pressure and the risk of incident cardiovascular disease (from the framingham heart study) Am J Cardiol. 2008;101:1614–1620. doi: 10.1016/j.amjcard.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim PO, Donnan PT, MacDonald TM. Blood pressure determinants of left ventricular wall thickness and mass index in hypertension: Comparing office, ambulatory and exercise blood pressures. J Hum Hypertens. 2001;15:627–633. doi: 10.1038/sj.jhh.1001229. [DOI] [PubMed] [Google Scholar]
- 47.Sung J, Ouyang P, Silber HA, Bacher AC, Turner KL, DeRegis JR, Hees PS, Shapiro EP, Stewart KJ. Exercise blood pressure response is related to left ventricular mass. J Hum Hypertens. 2003;17:333–338. doi: 10.1038/sj.jhh.1001552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.