Abstract
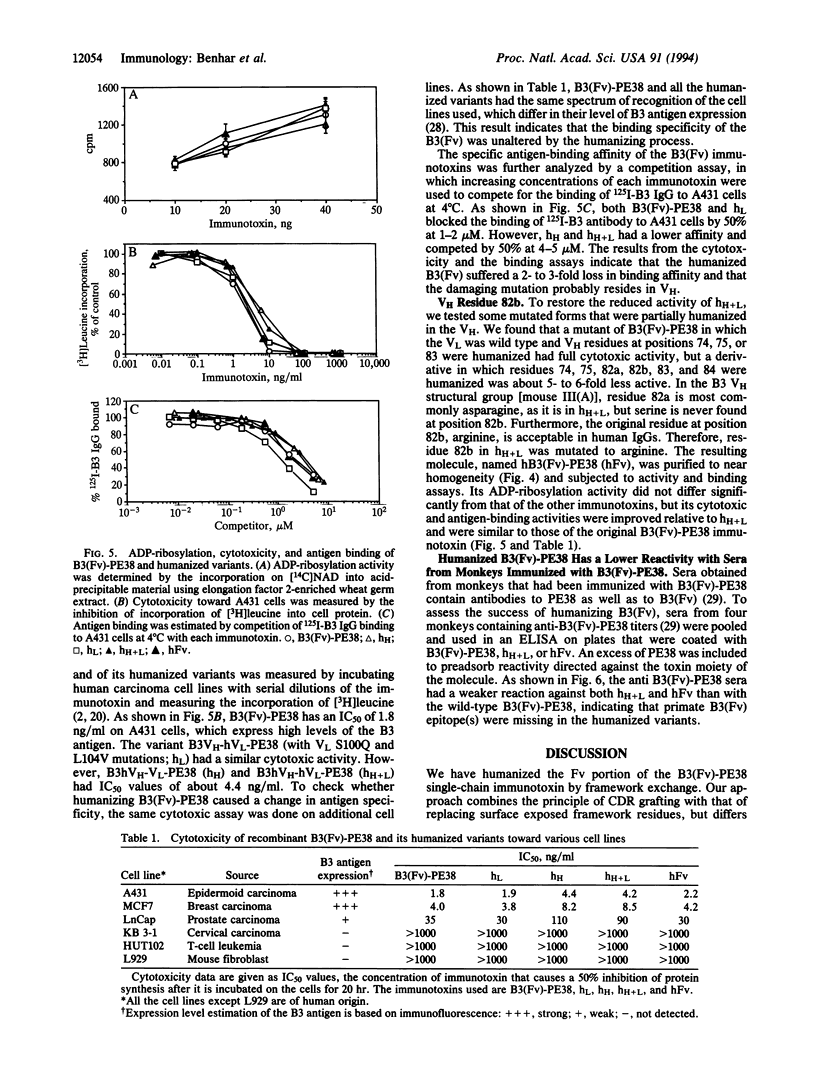
B3(Fv)-PE38 is a recombinant single-chain immunotoxin in which the Fv region of carcinoma-specific antibody B3 is fused to a truncated form of Pseudomonas exotoxin (PE). The efficacy of monoclonal antibody B3 and B3 immunotoxins in cancer therapy and diagnosis may be limited by the human anti-mouse response. Here we describe the humanization of the Fv of B3(Fv)-PE38 by "framework exchange." The variable domains of the heavy (VH) and light (VL) chains were aligned with their best human homologs to identify framework residues that differ. Initially, 11 framework residues in VH and six in VL were changed by site-specific mutagenesis to human residues and introduced simultaneously into a preassembled single-chain Fv expression cassette. Six VH and five VL residues that differ were not changed because they were buried, in the interdomain interface, or previously found to result in decreased affinity when mutated. This basic design resulted in some 20-fold loss of activity. Changing VL residues at the interdomain interfacial position 100 and at the buried position 104 to the human sequence increased the activity 8-fold. Changing VH residue at position 82b from the human sequence back to that of the mouse restored the activity 2- to 3-fold to the full binding and cytotoxic activity of the mouse sequence. Humanized B3(Fv)-PE38 lost immunogenic epitopes recognized by sera from monkeys that had been immunized with B3(Fv)-PE38.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benhar I., Brinkmann U., Webber K. O., Pastan I. Mutations of two lysine residues in the CDR loops of a recombinant immunotoxin that reduce its sensitivity to chemical derivatization. Bioconjug Chem. 1994 Jul-Aug;5(4):321–326. doi: 10.1021/bc00028a007. [DOI] [PubMed] [Google Scholar]
- Benhar I., Wang Q. C., FitzGerald D., Pastan I. Pseudomonas exotoxin A mutants. Replacement of surface-exposed residues in domain III with cysteine residues that can be modified with polyethylene glycol in a site-specific manner. J Biol Chem. 1994 May 6;269(18):13398–13404. [PubMed] [Google Scholar]
- Brinkmann U., Pai L. H., FitzGerald D. J., Willingham M., Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann U., Reiter Y., Jung S. H., Lee B., Pastan I. A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7538–7542. doi: 10.1073/pnas.90.16.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J., Pastan I., Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992 Sep;205(2):263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- Camera L., Kinuya S., Pai L. H., Garmestani K., Brechbiel M. W., Gansow O. A., Paik C. H., Pastan I., Carrasquillo J. A. Preclinical evaluation of 111In-labeled B3 monoclonal antibody: biodistribution and imaging studies in nude mice bearing human epidermoid carcinoma xenografts. Cancer Res. 1993 Jun 15;53(12):2834–2839. [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Gherardi E., Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. Structural repertoire of the human VH segments. J Mol Biol. 1992 Oct 5;227(3):799–817. doi: 10.1016/0022-2836(92)90224-8. [DOI] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Corti A., Barbanti E., Tempest P. R., Carr F. J., Marcucci F. Idiotope determining regions of a mouse monoclonal antibody and its humanized versions. Identification of framework residues that affect idiotype expression. J Mol Biol. 1994 Jan 7;235(1):53–60. doi: 10.1016/s0022-2836(05)80012-9. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. H., Moustafa Z. A., Harfeldt K. E., Isaacson C., Ostberg L. Potential of primate monoclonal antibodies to substitute for human antibodies: nucleotide sequence of chimpanzee Fab fragments. Hum Antibodies Hybridomas. 1990;1(1):23–26. [PubMed] [Google Scholar]
- Jones P. T., Dear P. H., Foote J., Neuberger M. S., Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. 1986 May 29-Jun 4Nature. 321(6069):522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Jung S. H., Pastan I., Lee B. Design of interchain disulfide bonds in the framework region of the Fv fragment of the monoclonal antibody B3. Proteins. 1994 May;19(1):35–47. doi: 10.1002/prot.340190106. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Solomon A., Zachau H. G. Contribution of human V kappa II germ-line genes to light-chain diversity. Nature. 1984 May 3;309(5963):73–76. doi: 10.1038/309073a0. [DOI] [PubMed] [Google Scholar]
- Kroemer G., Helmberg A., Bernot A., Auffray C., Kofler R. Evolutionary relationship between human and mouse immunoglobulin kappa light chain variable region genes. Immunogenetics. 1991;33(1):42–49. doi: 10.1007/BF00211694. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman R., Alberts J., Anderson D., Carner K., Heard C., Norton F., Raab R., Reff M., Shuey S., Hanna N. "Primatization" of recombinant antibodies for immunotherapy of human diseases: a macaque/human chimeric antibody against human CD4. Biotechnology (N Y) 1992 Nov;10(11):1455–1460. doi: 10.1038/nbt1192-1455. [DOI] [PubMed] [Google Scholar]
- Novotný J., Handschumacher M., Haber E., Bruccoleri R. E., Carlson W. B., Fanning D. W., Smith J. A., Rose G. D. Antigenic determinants in proteins coincide with surface regions accessible to large probes (antibody domains). Proc Natl Acad Sci U S A. 1986 Jan;83(2):226–230. doi: 10.1073/pnas.83.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padlan E. A. A possible procedure for reducing the immunogenicity of antibody variable domains while preserving their ligand-binding properties. Mol Immunol. 1991 Apr-May;28(4-5):489–498. doi: 10.1016/0161-5890(91)90163-e. [DOI] [PubMed] [Google Scholar]
- Parren P. W. Preparation of genetically engineered monoclonal antibodies for human immunotherapy. Hum Antibodies Hybridomas. 1992 Jul;3(3):137–145. [PubMed] [Google Scholar]
- Pastan I., Lovelace E. T., Gallo M. G., Rutherford A. V., Magnani J. L., Willingham M. C. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991 Jul 15;51(14):3781–3787. [PubMed] [Google Scholar]
- Pedersen J. T., Henry A. H., Searle S. J., Guild B. C., Roguska M., Rees A. R. Comparison of surface accessible residues in human and murine immunoglobulin Fv domains. Implication for humanization of murine antibodies. J Mol Biol. 1994 Jan 21;235(3):959–973. doi: 10.1006/jmbi.1994.1050. [DOI] [PubMed] [Google Scholar]
- Queen C., Schneider W. P., Selick H. E., Payne P. W., Landolfi N. F., Duncan J. F., Avdalovic N. M., Levitt M., Junghans R. P., Waldmann T. A. A humanized antibody that binds to the interleukin 2 receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10029–10033. doi: 10.1073/pnas.86.24.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1988 Mar 24;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- Roguska M. A., Pedersen J. T., Keddy C. A., Henry A. H., Searle S. J., Lambert J. M., Goldmacher V. S., Blättler W. A., Rees A. R., Guild B. C. Humanization of murine monoclonal antibodies through variable domain resurfacing. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):969–973. doi: 10.1073/pnas.91.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Verhoeyen M., Milstein C., Winter G. Reshaping human antibodies: grafting an antilysozyme activity. Science. 1988 Mar 25;239(4847):1534–1536. doi: 10.1126/science.2451287. [DOI] [PubMed] [Google Scholar]
- Williams S. C., Winter G. Cloning and sequencing of human immunoglobulin V lambda gene segments. Eur J Immunol. 1993 Jul;23(7):1456–1461. doi: 10.1002/eji.1830230709. [DOI] [PubMed] [Google Scholar]