Abstract
Uveal melanoma (UM) is the most common intraocular tumor in adults and liver metastasis is the leading cause of death in UM patients. We have previously shown that NKT cell-deficient mice develop significantly fewer liver metastases from intraocular melanomas than do wild-type (WT) mice. Here, we examine the interplay between liver NKT cells and NK cells in resistance to liver metastases from intraocular melanomas. NKT cell-deficient CD1d−/− mice and WT C57BL/6 mice treated with anti-CD1d antibody developed significantly fewer liver metastases than WT mice following either intraocular or intrasplenic injection of B16LS9 melanoma cells. The increased number of metastases in WT mice was associated with reduced liver NK cytotoxicity and decreased production of IFN-γ. However, liver NK cell-mediated cytotoxic activity was identical in non-tumor bearing NKT cell-deficient mice and WT mice, indicating that liver metastases were crucial for the suppression of liver NK cells. Depressed liver NK cytotoxicity in WT mice was associated with production of IL-10 by bone marrow-derived liver cells that were neither Kupffer cells nor myeloid-derived suppressor cells and by increased IL-10 receptor expression on liver NK cells. IL-10−/− mice had significantly fewer liver metastases than WT mice, but were not significantly different from NKT cell-deficient mice. Thus, development of melanoma liver metastases is associated with upregulation of IL-10 in the liver and an elevated expression of IL-10 receptor on liver NK cells. This impairment of liver NK activity is NKT cell-dependent and only occurs in hosts with melanoma liver metastases.
Keywords: Eye, Ocular tumors, IL-10, Metastases, NKG2D, Uveal melanoma
Introduction
Uveal melanoma (UM) is the most common intraocular malignancy in adults1. Half of the patients with primary UM develop metastases, with the liver being the most frequently affected organ1. The current median survival time of patients with liver metastases is less than a year2. A significant body of research suggests that the liver’s unique immunoregulatory microenvironment might foster the growth of tumors that metastasize to the liver through the suppression of adaptive immunity3. The liver is endowed with immunosuppressive cytokines such as IL-10 and TGF-β, which are known to promote the induction of tolerogenic antigen presenting cells (APCs) and T-cells4. By contrast, the innate immune system has a strong presence in the liver and plays a vital protective role against pathogens and malignant tumors in the liver5.
Natural killer (NK) cells are effector lymphocytes of the innate immune system. They are more abundant in the liver than in any other organ3. The importance of NK cells in controlling metastases of UM has been shown in both humans and mice. In patients with primary UM, NK cells comprise up to 40% of tumor infiltrating lymphocytes6. Moreover, many melanoma cell lines isolated from primary UM are susceptible to NK cell-mediated cytolysis6. Studies in nude mice, which cannot mount a T cell-dependent adaptive immune response, but have an intact NK cell repertoire, have shown that the depletion of NK cells in vivo results in a significant increase in the number of liver metastases arising from human uveal melanoma cells transplanted into the eye7.
Natural killer T (NKT) cells are a distinct population of T cells with the characteristics of both innate and adaptive immunity8. Like NK cells, NKT cells are abundant in the liver and account for up to 25% and 40% of human and mouse liver lymphocytes, respectively9. Two populations of NKT cells have been described. Type I NKT cells are defined as invariant NKT (iNKT) cells and encompass 80% of total NKT cells10.
The role of NKT cells in the development of liver metastases that develop from uveal melanomas has not been sufficiently investigated. In murine models, it is widely believed that type I NKT cells have anti-tumor functions whereas type II NKT cells contribute to the suppression of anti-tumor immune responses8. We previously reported that mice deficient in NKT cells had a steep decrease in liver metastases arising from either intraocular melanomas or melanoma cells injected into the portal circulation and a significant elevation in the cytolytic activity of liver NK cells compared to mice with an intact NKT cell repertoire7. The depressed liver NK cell cytotoxicity activity in NKT cell-competent mice could be restored by in vivo neutralization with anti-IL-10 antibody suggesting that this cytokine was either produced by NKT cells or that NKT cells promoted IL-10 production by third-party cells. In the present study, we extended these investigations and examined the underlying mechanisms for reduced liver metastases and the coincidental enhanced cytolytic activity of liver NK cells in hosts depleted of NKT cells. Our results suggest that NKT cells simultaneously induce the expression of IL-10 in the liver by bone marrow-derived cells that are neither myeloid-derived suppressor cells (MDSC) nor Kupffer cells (KC), both of which are known to produce IL-1011, 12. Our results also indicate that the enhanced liver NK cytolytic activity in NKT cell-deprived mice correlates with an upregulation of the NK cell activation receptor NKG2D.
Materials and Methods
Cell lines
B16LS9 murine melanoma cell line was kindly provided by Hans E. Grossniklaus (Emory University School of Medicine, Atlanta, GA and preferentially metastasizes to the liver following intraocular transplantation7. Yac-1lymphoma cell line was obtained from American Type Culture Collection (Rockville, MD) and served as positive control cells for Mult1 and Rae1 expression.
Mice
Eight to twelve week old female C57BL/6 mice were obtained from the animal colony at the University of Texas Southwestern Medical Center (Dallas, TX). CD1d−/− mice (C57BL/6 background) which lack both type I and type II NKT cells, were kindly provided by Mark Exley (Beth Israel Deaconess Medical Center, Boston, MA). IL-10−/− mice (B6129P2-il10tm1Cgn/J) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Southwestern Medical Center and the Association for Research in Vision and Ophthalmology (ARVO) statement concerning the Use of Animals in Ophthalmic and Vision Research.
Tumor injections
Melanoma cells (5×104) were injected intravitreally into the posterior compartment (PC) of the eye as described previously13. Tumor-bearing eyes were enucleated when they reached 4.0 mm in diameter. Mice were euthanized two weeks after enucleation and their livers were collected for histological analysis. Melanoma cells (5×104) were injected beneath the spleen capsule as an ancillary method for producing liver metastases by facilitating the dissemination of tumor cells to the liver via the hepatic portal vein14, 15. Mice were euthanatized 14 days later and their livers collected for histological analysis. Tumor cells (5×104) were injected subcutaneously (SC) and the inoculation sites were palpated two times per week and measured with calipers to assess SC tumor growth. Subcutaneous tumors were surgically removed when they reached 4.0 mm in diameter in order to mimic the size of the ocular tumors at the time of enucleation. Mice were necropsied two weeks after tumor removal and the livers were collected for assessment of metastases7.
Isolation of liver NKT cells
Liver lymphocytes were isolated as previously described and were enriched for by cell sorting using a FACSAria II (BD Biosciences, San Jose CA)7. NKT cells were defined as either a NK1.1+ TCR-β+ preparation or a CD1d+ CD3+ CD19− CD1d tetramer+ (NIH Tetramer Core Facility, Atlanta, GA) lymphocyte population. The majority of the experiments were performed with CD1d+CD3+, CD19−, CD1d tetramer+ cells. The purity of both NKT cell preparations was consistently ≥ 95%.
In vitro NK cell-mediated cytotoxicity assay
Splenic and liver cells were isolated and enriched for NK cells with an EasySep Mouse NK Cell Enrichment Kit (Stemcell Technologies, Vancouver, BC, Canada) and tested for cytotoxicity against B16LS9 melanoma cells in vitro using an effector to target cell ratio of 25:1 for 18 hours7. Briefly, lactate dehydrogenase (LDH) is and was measured in a colorimetric coupled enzymatic assay in which color formation is proportional to the number of lysed cells. The wavelength absorbance data at 490 nm were collected using a standard 96-well plate reader (Biotek Instrument, Winooski, VT). NK cell-enriched suspensions were examined by flow cytometry for purity using anti-NK1.1 antibody (BD Biosciences, San Jose, CA) and anti-CD8 monoclonal antibody (BD Biosciences) and were found to be 74% NK1.1+ and <4% CD8+.
Generation of bone marrow chimera mice
C57BL/6 WT mice were lethally γ-irradiated (900 cGy) and reconstituted with bone marrow cells from C57BL/6 WT or C57BL/6 IL-10−/− mice. Bone marrow cells (1 × 106) were injected intravenously into each recipient. Chimeric animals were rested for eight weeks before intrasplenic injection of B16LS9 tumor cells.
Flow cytometry
Cells were stained using the following antibodies: Anti-mouse PE-NK1.1, APC-TCR-β, APC-Streptavidin and FITC-NKG2D (BD Biosciences, San Diego, CA), Biotin anti-mouse CD210 (IL-10RA), APC-conjugated anti-mouse Mult-1 (R&D Systems, Minneapolis, MN), PE-conjugated anti-mouse Rae-1 (R&D) and isotype control monoclonal antibodies. The cells were re-suspended in 100µl PBS with 1% FCS and incubated with 1µg BD Fc Block CD16/CD32 monoclonal antibody (BD Biosciences) at 4°C for 15 minutes. Cells were washed twice with PBS, treated with in intracellular cytokine kit according to the manufacturer’s instructions and analyzed on a FACScan flow cytometer (BD Biosciences). The results were analyzed with BD CellQuest analysis software (ver. 3.1f; BD Biosciences).
Depletion of Kupffer cells
Liposomes containing either clodronate (C12MDP; Sigma-Aldrich, St. Louis, MO) or PBS were prepared as previously described16. Kupffer cells (KC) were depleted as reported elsewhere17 by injecting clodronate-containing liposomes intravenously (200 µl) the day before intrasplenic tumor injection. Mice were euthanized and examined for liver metastases 14 days following intrasplenic tumor injection.
Depletion of MDSC
MDSC were depleted by injecting anti-Gr1 antibody (University of California at San Francisco Hybridoma Facility; Clone RB6-8C5) intraperitoneally every three days (250µg/injection) beginning on the day of tumor injection11, 18. Normal rat IgG (Sigma-Aldrich St. Louis, MO) was administered as an isotype control. Since anti-Gr1 antibody also depletes neutrophils, separate groups of mice were depleted of neutrophils using anti-Ly6G antibody (1A8; BD Biosciences), which depletes neutrophils but does not affect MDSC19. Anti-Ly6G antibody was injected intraperitoneally every three days (250µg/injection) beginning on the day of intrasplenic tumor injection and was found to deplete approximately 85% of liver neutrophils as determined by flow cytometry (data not shown).
Statistical analysis
The Student’s t-test or Rank-Sum t-test was used to assess the statistical significance of the differences between experimental and control groups. A P-value of < 0.05 was considered significant. Results were expressed as the mean +/− the standard deviation.
Results
Hepatic, but not splenic, NK cell-mediated cytotoxicity is elevated in NKT cell-deficient tumor-bearing mice
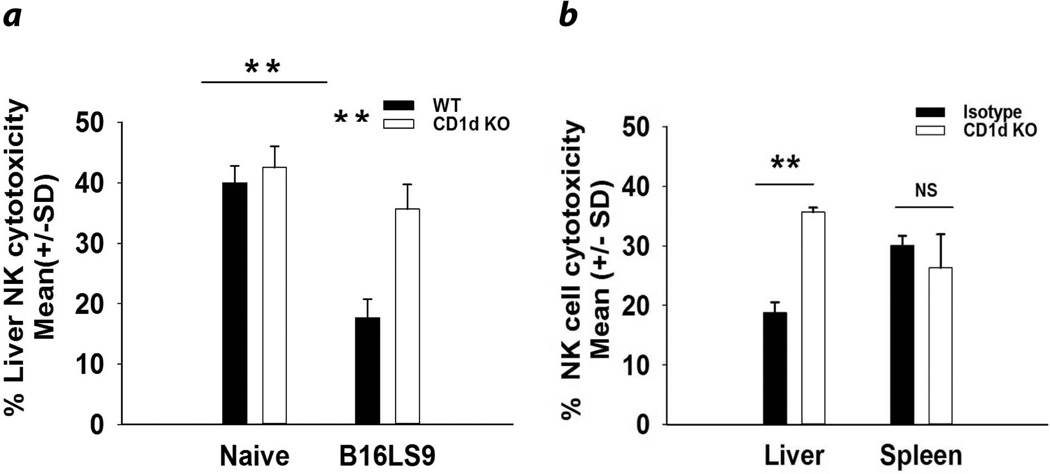
We hypothesized that NKT cells promote the development of liver metastases by suppressing NK cell anti-tumor immune responses in the liver, but not elsewhere. To test this, WT and CD1d−/− mice were injected intrasplenically with B16LS9 melanoma cells. The results confirmed our previously published findings7 that liver NK cells from WT mice bearing liver metastases arising from either intrasplenic tumor injection (Fig. 1a) or intraocular tumors (Fig. 1b) had steeply reduced cell-mediated cytotoxicity of B16LS9 melanoma cells. Regardless of the route of tumor inoculation, liver NK cells from WT mice had significantly reduced cytotoxicity against B16LS9 melanoma cells compared to CD1d−/− mice or WT mice treated with anti-CD1d antibody (Figs. 1a and 1b). Moreover, the NK cell-mediated cytotoxicity was reduced in the liver but was not altered in the spleens of tumor-bearing mice (Fig. 1b). Cultured B16LS9 melanoma cells and liver metastases were isolated and immediately examined by qPCR and flow cytometry for the expression of CD1d mRNA and protein respectively. Neither the original B16LS9 melanoma cell line nor liver metastases expressed either CD1d message or protein (data not shown).
Figure 1.
Hepatic, but not splenic, NK cell-mediated cytotoxicity is suppressed by NKT cells in mice with liver metastases. (a) Cytotoxicity of liver NK cells from non-tumor-bearing and tumor-bearing WT and NKT cell-deficient mice against B16LS9 melanoma target cells. Liver metastases were generated via intrasplenic tumor inoculation. (b) Liver and splenic NK cell cytotoxicity from tumor-bearing WT and NKT cell-deficient mice against B16LS9 melanoma target cells. Liver metastases were generated via PC tumor inoculation. Results are expressed as the Mean +/− SD and are representative of two independent experiments. **P<0.01 or NS= Not significant, P>0.05.
The increased cytotoxicity of liver NK cells in NKT cell-deficient mice was not simply due to an increase in the number of liver NK cells in CD1d−/− mice as the number of NK cells (as determined by flow cytometry) was the same in tumor-bearing WT and CD1d−/− mice (data not shown). Thus, NKT cells suppressed NK cell-mediated cytotoxicity in tumor-bearing mice and the enhanced liver NK cytotoxic activity in NKT cell-deficient mice were not simply due to increased numbers of liver NK cells.
In other experiments, WT and CD1d−/− mice were injected subcutaneously with melanoma cells. Subcutaneous tumors were extirpated when they reached 4.0 mm in diameter in order to mimic the experiments in which tumor-containing eyes were enucleated when they reached 4.0 mm in diameter. We found no microscopic evidence of liver metastases in WT mice or CD1d−/− mice with subcutaneous melanomas (data not shown). Moreover, the liver NK cell-mediated cytotoxicity in WT mice was not significantly different from that found in CD1d−/− mice injected subcutaneously with B16LS9 melanoma cells (data not shown). These data suggest that the suppression of liver NKT cell-induced NK cell-mediated cytotoxicity was unique to hosts with liver metastases and was not a systemic phenomenon related to the growth of melanoma cells at sites other than the liver.
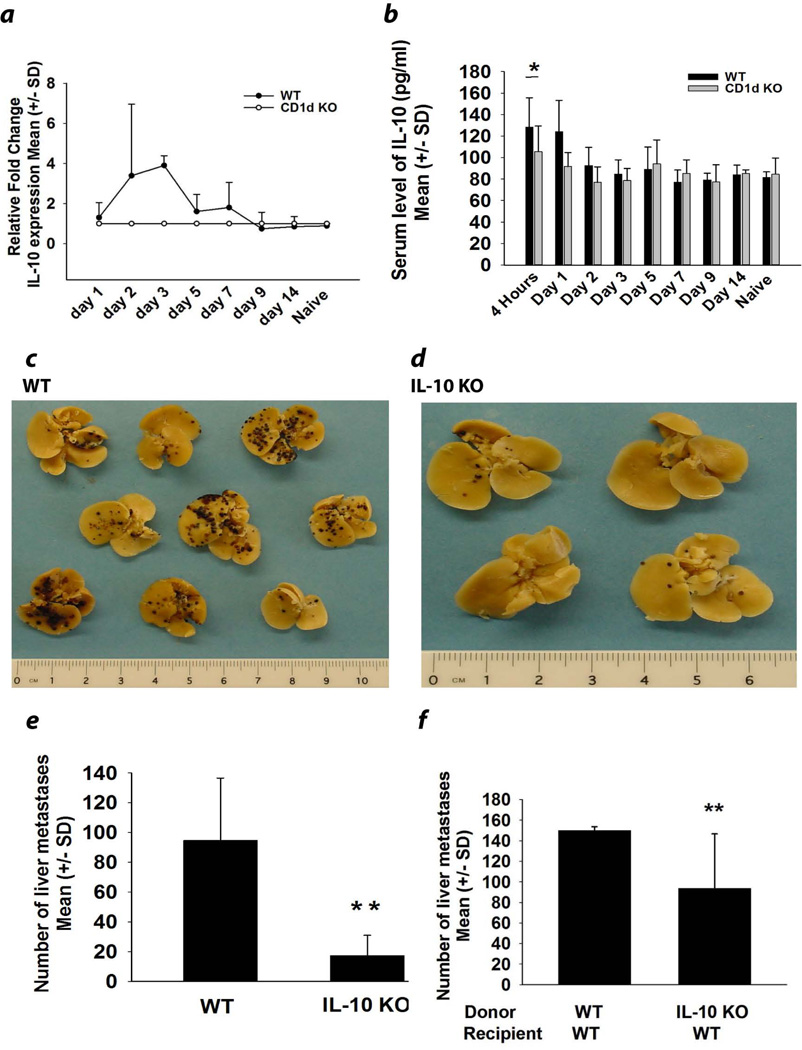
IL-10 promotes NKT cell-dependent exacerbation of liver metastases
We next sought to confirm our previous findings indicating that in vivo blockade of IL-10 through the administration of anti-IL-10 antibody reversed the depressed liver NK cell cytolytic activity, which ostensibly played a crucial role in the formation of liver metastases in B16LS9 melanoma-bearing mice7. We interrogated liver cell suspensions of tumor-bearing mice for the expression of IL-10 mRNA various times following intrasplenic tumor injection. IL-10 message was detected in WT mice within 48 hours of intrasplenic tumor injection and persisted for 7 days before returning to normal levels at day 9 (Fig. 2a). The increased production of IL-10 was restricted to the liver as the serum levels of IL-10 in NKT cell-deficient tumor-bearing mice were identical to tumor-bearing WT mice at all time points, with the exception of day one post tumor injection (Fig. 2b). To confirm the association between elevated IL-10 expression and increased liver metastases we assessed the number of liver metastases in IL-10−/− and WT mice (Figs. 2c–2e). There was a four-fold reduction in the number of liver metastases in IL-10−/− mice in comparison with WT mice indicating the important role of IL-10 in exacerbating liver metastases in melanoma-bearing mice (Fig. 2e). In order to examine whether IL-10 producing cells were derived from bone marrow cells or from liver parenchymal cells, we generated bone marrow chimera mice in which bone marrow-derived cells from either IL-10−/− or WT mice were adoptively transferred into irradiated WT recipient mice. Eight weeks post bone marrow reconstitution, mice were injected intrasplenically with B16LS9 melanoma cells and the formation of liver metastases was examined. Mice that received bone marrow cells from IL-10−/− mice developed significantly fewer liver metastases than mice receiving bone marrow from WT mice (Fig. 2f). These results indicate that bone marrow-derived cells were important producers of IL-10 in the melanoma-bearing mice. To confirm that melanoma liver metastases were not the source of IL-10, melanoma cells were isolated from liver metastases and immediately examined by qPCR and flow cytometry for the expression of IL-10 mRNA and protein respectively. Neither the original B16LS9 melanoma cell line nor liver metastases of B16LS9 melanoma expressed either IL-10 message or protein (data not shown).
Figure 2.
IL-10 produced by bone marrow-derived cells exacerbates liver metastasis. (a) IL-10 mRNA expression in whole liver extracts following intrasplenic tumor injection (N=5/group). (b) Serum levels of IL-10 in naïve or melanoma-bearing WT and CD1d−/− mice (N=5/group). (c) Liver metastases in WT and (d) IL-10−/− mice. (e) Liver metastases in WT and IL-10−/− mice (N=15/group) and (f) IL-10−/− bone marrow chimeric mice (N=15). The results are from one of two independent experiments that yielded similar results. Metastases were produced in all groups by intrasplenic injection of B16LS9 melanoma cells. Results were expressed as the Mean +/− SD and are representative of two independent experiments. *P<0.05 or **P<0.01
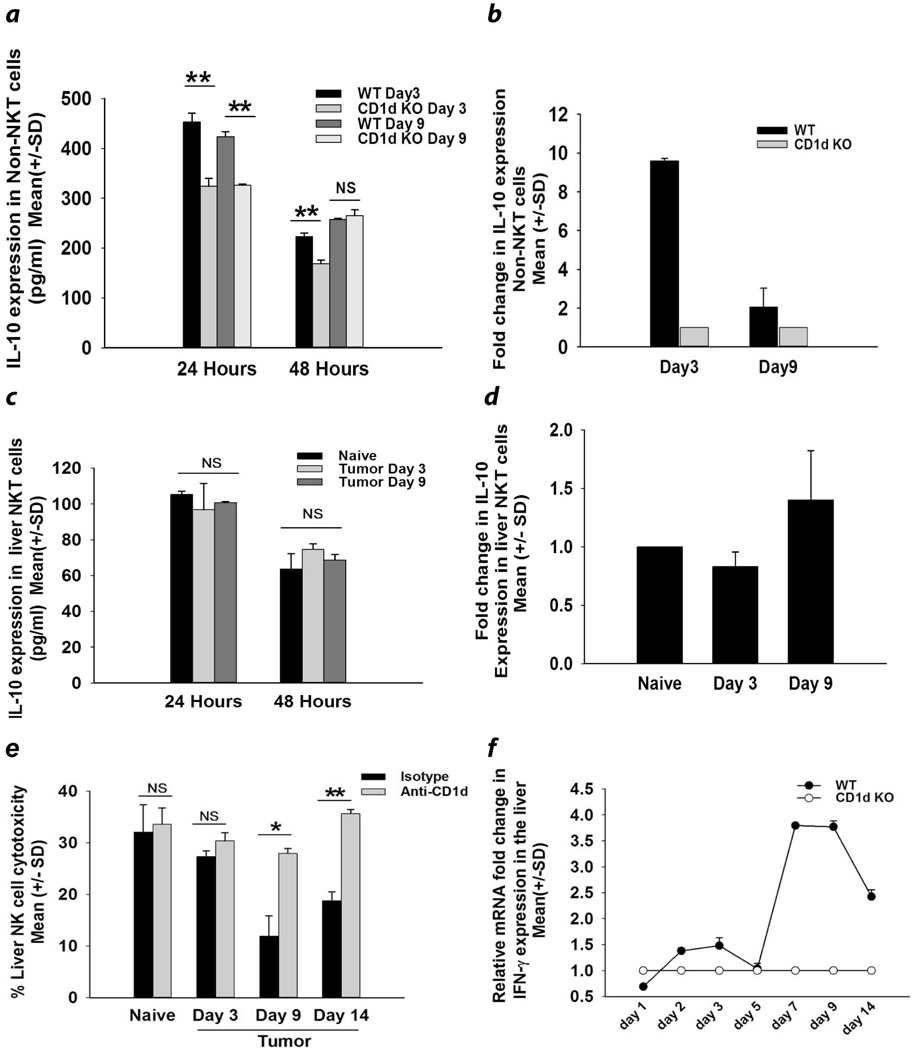
To determine the time course and peak production of IL-10 and if the maximum IL-10 production coincided with the suppression of liver NK activity, liver NKT cells and non-NKT cells were also isolated from tumor-bearing mice and were tested for IL10 production. Since liver expression of IL-10 peaked at day 3 and returned to baseline levels at day 9 post tumor injection (Fig. 2a), we selected these time points for assessing IL-10 expression by liver NKT cells and non-NKT cells. Although we did not observe elevated expression of IL-10 protein in NKT cells there was a pronounced increase in IL-10 protein by liver non-NKT cells on days 3 and 9 post tumor injection (Fig. 3a) and nine-fold higher amounts of IL-10 mRNA in WT mice on day 3 post tumor injection (Fig. 3b). By contrast, NKT cells from similar mice did not produce significantly higher quantities of IL-10 protein or increased expression of IL-10 mRNA (Figs. 3c and 3d). Interestingly, the maximum depression of liver NK cytotoxicity was detected on day 9 (Fig. 3e), which coincided with elevated expression of IL-10 protein by non-NKT cells in the livers of WT mice (Fig. 3a). Contrary to our expectations, WT mice displayed a spike in the liver expression of IFN-γ mRNA liver on days 7 and 9 (Fig. 3f), which coincided with maximal depression in liver NK cell activity (Fig. 3e).
Figure 3.
IL-10 levels in liver NKT cells and bone marrow-derived non-NKT cells in mice with liver metastases. CD45+ CD1d−, CD3− non-NKT cells were isolated from the livers of WT and CD1d KO mice 3 and 9 days after intrasplenic tumor injection. (a) IL-10 protein produced by non-NKT liver cells and detected by ELISA (N=10/group); (b) IL-10 mRNA levels assessed by qPCR (N=5/group). NKT cells were isolated from livers using CD1d tetramers and assessed for (c) IL-10 protein secreted by liver NKT cells as detected by ELISA (N=10/group), (d) IL-10 mRNA expression by liver NKT cells and detected by quantitative real time PCR (N=10/group non-NKT cells) and (e) Cytolytic activity of liver NK cells isolated from WT mice treated with either anti-CD1d antibody or an isotype control tested against B16LS9 melanoma target cells. Results are expressed as the Mean +/− SD and are representative of two independent experiments. *P<0.05 or **P<0.01.
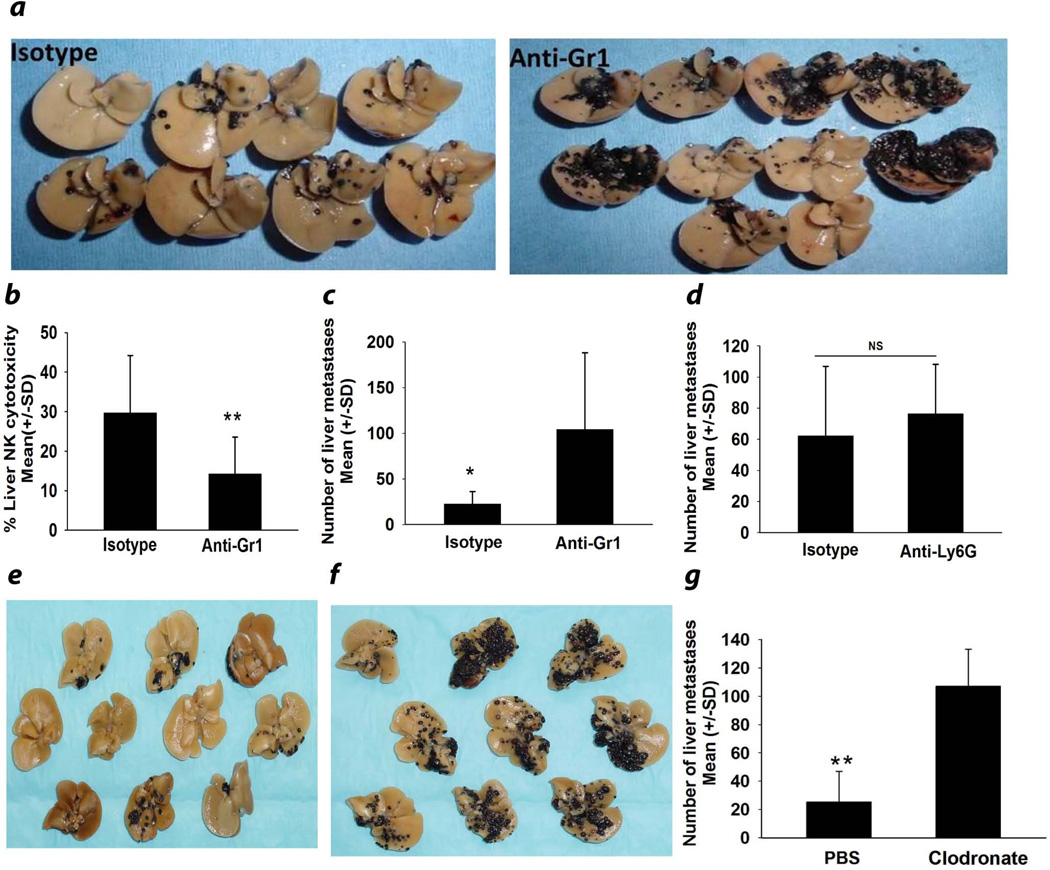
In the liver, two bone marrow-derived cell populations come to mind as potential sources of IL-10 production in tumor-bearing NKT cell-competent mice, MDSC and KC, both of which are known to produce IL-1012, 20. Accordingly, we employed a widely used protocol for depleting putative MDSC by administering anti-Gr1 antibody every 72 hr beginning on the day of intrasplenic tumor injection11. The results revealed >70% reduction of Gr1+CD11b+ putative MDSC (data not shown) but a sharp increase in the number of liver metastases and a commensurate reduction in liver NK cell cytolytic activity (Figs. 4a–4c). Although anti-Gr1 antibody also depletes neutrophils, parallel experiments were performed using anti-Ly6G antibody, which also depletes neutrophils and found that depletion of 85% of liver neutrophils (data not shown), did not affect the number of liver metastases (Fig. 4d). Thus, the increased number of liver metastases in mice treated with anti-Gr1 was most likely not due to depletion of neutrophils. We also depleted KC through the intravenous administration of clodronate-containing liposomes17 and found a steep increase, rather than decrease, in the number of liver metastases (Figs. 4e–4g). Thus, KC are not the third-party bone marrow-derived cells that produce IL-10, which leads to increased liver metastases and depression of liver NK cell activity in NKT cell-competent mice bearing liver metastases.
Figure 4.
Depletion of MDSC or Kupffer cells exacerbates liver metastasis. (a) Liver metastases and (b) Cytolytic activity of liver NK cells isolated from anti-Gr1 antibody-treated NKT cell-deficient mice and tested against B16LS9 melanoma target cells. (c) Liver metastases in anti-Gr1-treated mice and isotype control-treated mice. (d) Number of surface liver metastases following depletion of neutrophils with anti-Ly6G antibody (e) Liver metastases in mice treated with PBS-containing liposomes or (f) Clodronate-containing liposomes. (g) Number of liver metastases in mice treated with either clodronate-containing liposomes or PBS-containing liposomes. There were 10 mice/group. These experiments were performed twice with similar results. Results are expressed as Mean +/− SD and are representative of two independent experiments. ** P= 0.001.
Depressed liver NK activity coincides with upregulation of IL-10 receptor
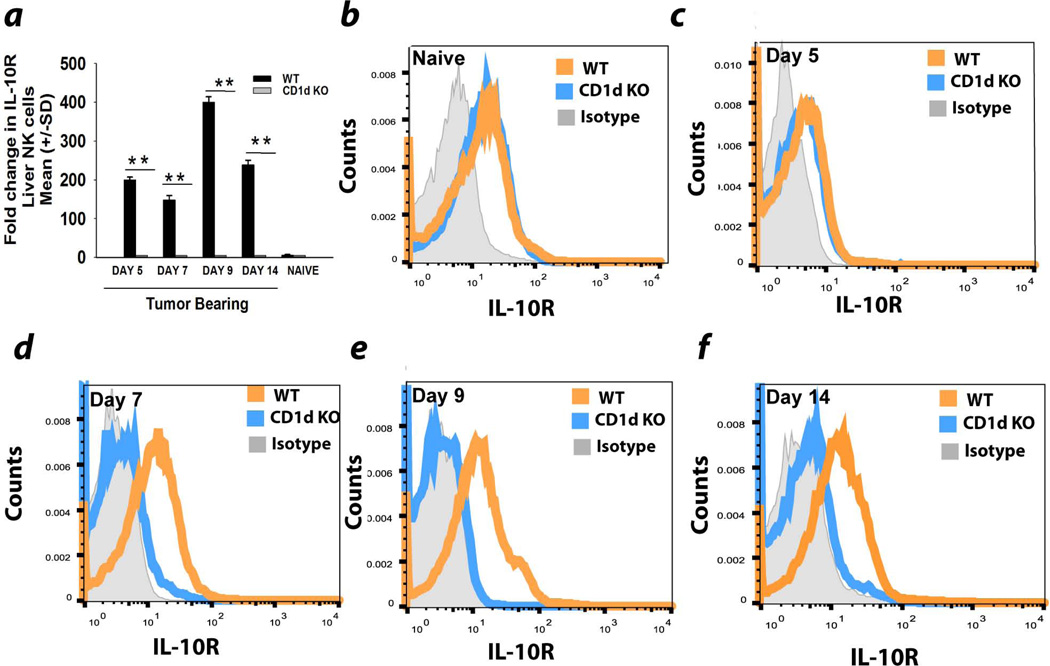
The regulatory functions of IL-10 are exerted through the IL-10 receptor21. Accordingly, we examined the expression of IL-10 receptor on liver NK cells in tumor-bearing WT and CD1d−/− mice. By day 9, IL-10R message was upregulated 400-fold in tumor-bearing WT mice compared to CD1d−/− mice (Fig. 5a). IL-10R protein was also elevated on liver NK cells from WT mice compared to CD1d−/− mice on days 5, 7, and 9 post tumor (Figs. 5b–5f). It bears noting that the maximal depression of liver NK cytotoxicity occurred at day 9 (Fig. 3e), which coincided with the maximum expression of IL-10R message on liver NK cells (Fig. 5e).
Figure 5.
Upregulation of IL-10 receptor on liver NK cells in WT mice harboring melanoma liver metastases. (a) Expression of IL-10 receptor mRNA in NK cells from the livers of naïve and melanoma-bearing WT and CD1d−/− mice following intrasplenic tumor injection. Liver NK cells from WT and CD1d−/− mice were assessed for IL-10R expression in (b) non-tumor bearing mice, or tumor-bearing mice (c) 5 days, (d) 7 days, (e) 9 days, or (f) 14 days following intrasplenic tumor injection. The results are representative of two independent experiments.
Elevated NK cell activity in NKT cell-deficient mice coincides with increased expression of NKG2D activation receptor
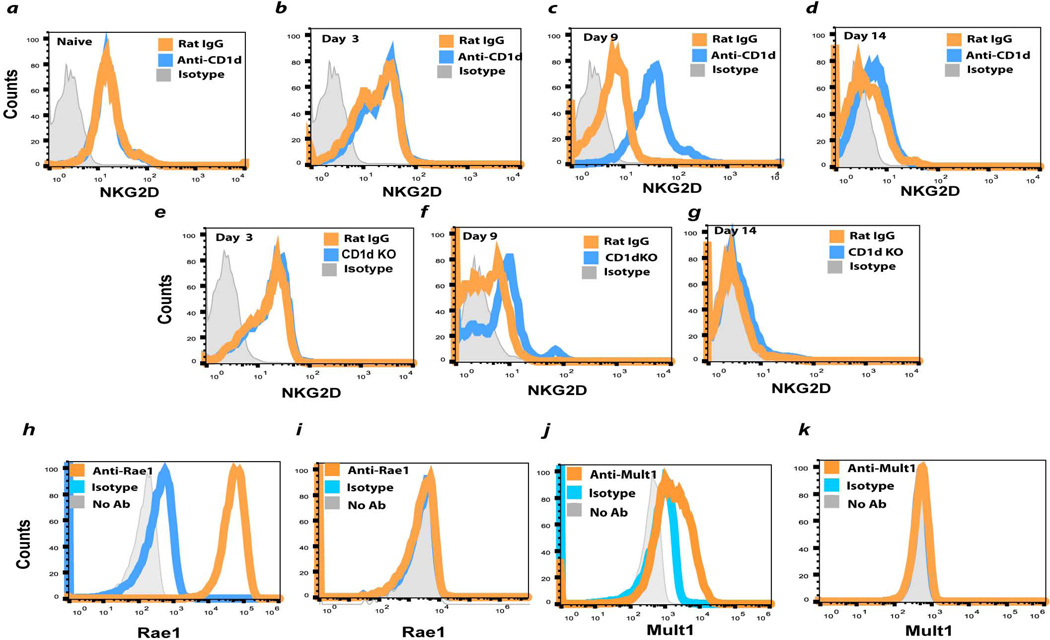
NKG2D is a C-type lectin-like receptor that is express on virtually all NK cells22–24 and is especially important in activating NK cells in the immune surveillance of tumors25–27. With this in mind, we examined the surface expression of NKG2D on liver NK cells from WT mice and CD1d−/− injection of B16LS9 melanoma cells. NKG2D expression was the same in WT mice, CD1d−/− mice, and WT mice treated with anti-CD1d antibody on days 0 and 14 post tumor injection, but was significantly increased in CD1d−/− and WT anti-CD1d antibody-treated mice on day 9 post tumor injection (Figs. 6a – 6h), however, which might explain the elevated liver NK activity in NKT cell-deficient mice. However, the two major ligands for NKG2D, Mult1 and Rae1, were not detected on either cultured B16LS9 cells (Figs. 6h – 6k) or liver metastases (data not shown).
Figure 6.
Expression of NK cell activating receptor NKG2D on liver NK cells and NKG2D ligands of B16LS9 melanoma cells. Liver NK cells were assessed for surface expression of the NK cell activating receptor NKG2D: (a) Naive WT mice and WT mice treated with anti-CD1d or isotype control antibody and tested (b) 3, (c) 9, or (d) 14 days after intrasplenic tumor injection or CD1d KO mice tested (e) 3(f) 9, or (g) 14 days after intrasplenic tumor injection. Tumor cells were examined by flow cytometry for the expression of the NKG2 activating ligand Rae1 (h) Yac-1 lymphoma cells (positive control) or (i) B16LS9 melanoma cells. NKG2D ligand Mult1 was examined on (j) Yac-1 lymphoma cells (positive control) or (k) B16LS9 melanoma cells. The results shown here are from one of two independent experiments that produced similar results.
Discussion
The general notion is that iNKT cells promote anti-tumor immunity and type II NKT cells impair it8. iNKT cells are also able to directly kill CD1d expressing tumor cells28 and it has been suggested that CD1d-dependent cross-presentation of endogenous tumor antigen by dendritic cells activates iNKT cell anti-tumor function29. However, most tumor cells, including B16LS9 melanoma cells, do not express CD1d30. Murine NKT cells can exert regulatory effects on tumor immune surveillance31–33 and it has been reported that patients with uveal melanoma have the highest number of hepatic iNKT cells compared with hepatocellular carcinoma patients34.
Studies have shown that the anti-tumor effector functions of NK and NKT cells are suppressed in the tumor microenvironment35. Here, we show that the presence of melanoma metastases in the livers of WT mice suppressed liver NK cell-mediated cytotoxicity. Interestingly, in the absence of NKT cells, the presence of liver metastases does not result in enhanced suppression of liver NK cell cytolytic activity. This, in turn, strongly suggests that NKT cells enhance, rather than inhibit, the development of liver metastases by suppressing NK cell activity in the liver. In addition, the comparable levels of NK cell mediated-cytotoxicity in non-tumor bearing WT and CD1d−/− mice suggest that the presence of liver metastases is required for the NKT cell-dependent suppression of liver NK cell anti-tumor function. The impaired NK cell activity in WT mice compared to NKT cell-deficient mice appears to be restricted to the liver, as splenic NK activity was the same in NKT cell-deficient mice and WT mice.
Several molecules can suppress NK cell activity. However, three cytokines in particular come to mind. IL-10, TGF-β, and macrophage migration inhibitory factor (MIF) have been shown to suppress NK cell activity under certain conditions36–40. Although we found no evidence for upregulation of either TGF-β or MIF in the livers of WT mice with liver metastases (data not shown), there was a significant elevation of liver IL-10 expression in WT mice compared to CD1d−/− mice during the first seven days following intrasplenic tumor injection. IL-10 is a pleiotropic immune-modulatory cytokine that is produced by variety of cells such as dendritic cells (DCs), Kupffer cells, NKT cells, hepatocytes and hepatic stellate cells41, 42. IL-10 mediates its biological effects by binding to a two-subunit cell surface IL-10 receptor21. However, the effect of IL-10 on NK cells is controversial. Some reports have shown that IL-10 down-regulates NK cell function, while others suggest that IL-10 activates NK cells43. However, our results indicate that IL-10 promotes the formation of liver metastases arising from intraocular melanomas in WT mice. This is in agreement with previous results showing that IL-10 suppresses NK cell anti-tumor function7. Moreover, the time of maximum liver NK cytolytic activity impairment, day 9, coincides with the maximum upregulation in IL-10R expression on liver NK cells in WT mice with liver metastases. Results from bone marrow chimera experiments revealed that bone marrow-derived cells are an important source of IL-10 that suppresses liver NK cell activity in mice with melanoma liver metastases44, 45. Our findings also indicated that the two major bone marrow-derived cells in the liver that are known to produce IL-10, KC and MDSC, were not involved in the exacerbation of liver metastases.
The crosstalk between NKT cells and NK cells has been reported by multiple laboratories. Subleski et al. found that the removal of immunosuppressive NKT cells along with activation of NK cells resulted in significant reduction in the number of liver metastases originating from renal carcinomas46. By contrast, it has also been reported that activated NKT cells stimulate cytolytic activity of NK cells47, 48. Given that NKT cells and IL-10 play an immunosuppressive role in the present model of intraocular melanoma, we hypothesized that NKT cells were responsible for the suppression of liver NK cell anti-tumor function by producing IL-10. However, we failed to detect increased production of IL-10 message or protein by liver NKT cells in WT mice harboring liver metastases. This is in sharp contrast to our previous findings in which we found increased expression of cytoplasmic IL-10 in liver NKT cells from mice with liver metastases7. We are at a loss to explain this discrepancy. However, our previous study examined cytoplasmic expression of IL-10 in NKT cells that were enriched by cell sorting on NK1.1+TCR-β+ liver cells, while the present study measured IL-10 that was secreted from NKT cells that had been isolated using CD1d tetramers. In spite of this discordance, the results demonstrate an upregulation of IL-10 production in the liver and a concomitant enhancement in IL-10 receptor expression on liver NK cells in WT, but not NKT cell-deficient mice with liver metastases.
The present findings revealed that in the absence of NKT cells, the activating receptor on NK cells, NKG2D, was upregulated on liver NK cells. The upregulation of liver NK cell-mediated cytotoxicity in NKT cell-deficient mice occurred at day 9 post tumor injection, which coincided with the peak upregulation of IL-10R on liver NK cells and the maximum down-regulation of liver NK cytolytic activity in WT mice. Thus, in WT mice, the combination of increased production of IL-10 in the liver along with upregulation of IL-10R on liver NK cells conspire to depress NK cell-mediated resistance to liver metastases. Although the NK cell activation receptor NKG2D was upregulated on NK cells in CD1d−/− mice on day 9 post tumor injection, we do not believe that this contributed to the enhanced NK cell mediated cytolysis in CD1d−/− mice as we did not detect the expression of the two major ligands for the NKG2D receptor, Rae1 and Mult1, on B16LS9 melanoma cells by flow cytometry. Moreover, the cytolytic activity of liver NK cells in NKT cell-deficient mice was not elevated at the time of maximal NKG2D expression compared to other time points.
In conclusion, the present findings extend our previous report7 on the mechanisms by which tumor-induced iNKT cells inhibit the effector functions of liver NK cells. In the present study we demonstrate that NKT cell suppression of liver NK activity: a) occurs only in hosts with liver metastases; b) depressed NK activity is restricted to the liver; c) impaired liver NK activity correlates with an upregulation of IL-10R on NK cells; d) depressed NK activity is largely due to IL-10, which is produced by bone marrow-derived cells; and e) neither KC nor MDSC appear to be the bone marrow-derived cell population that produces IL-10 and suppresses liver NK cells activity.
Acknowledgments
The excellent technical assistance of Ms. Jessamee Mellon and Ms. Tracy Gray is gratefully appreciated.
Grant support: NIH grant CA030276 and Research to Prevent Blindness.
References
- 1.Bakalian S, Marshall JC, Logan P, Faingold D, Maloney S, Di Cesare S, Martins C, Fernandes BF, Burnier MN., Jr Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res. 2008;14:951–956. doi: 10.1158/1078-0432.CCR-06-2630. [DOI] [PubMed] [Google Scholar]
- 2.Feldman ED, Pingpank JF, Alexander HR., Jr Regional treatment options for patients with ocular melanoma metastatic to the liver. Ann Surg Oncol. 2004;11:290–297. doi: 10.1245/aso.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 4.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nature reviews. Immunology. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 5.O'Farrelly C, Crispe IN. Prometheus through the looking glass: reflections on the hepatic immune system. Immunol Today. 1999;20:394–398. doi: 10.1016/s0167-5699(99)01518-2. [DOI] [PubMed] [Google Scholar]
- 6.Niederkorn JY. Immune escape mechanisms of intraocular tumors. Prog Retin Eye Res. 2009;28:329–347. doi: 10.1016/j.preteyeres.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W, Li H, Mayhew E, Mellon J, Chen PW, Niederkorn JY. NKT cell exacerbation of liver metastases arising from melanomas transplanted into either the eyes or spleens of mice. Investigative ophthalmology & visual science. 2011;52:3094–3102. doi: 10.1167/iovs.10-7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 11.Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58:941–953. doi: 10.1007/s00262-008-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of immunology. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alizadeh H, Howard K, Mellon J, Mayhew E, Rusciano D, Niederkorn JY. Reduction of liver metastasis of intraocular melanoma by interferon-beta gene transfer. Investigative ophthalmology & visual science. 2003;44:3042–3051. doi: 10.1167/iovs.02-1147. [DOI] [PubMed] [Google Scholar]
- 14.Reinmuth N, Liu W, Ahmad SA, Fan F, Stoeltzing O, Parikh AA, Bucana CD, Gallick GE, Nickols MA, Westlin WF, Ellis LM. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–2087. [PubMed] [Google Scholar]
- 15.Kuruppu D, Christophi C, Bertram JF, O'Brien PE. Characterization of an animal model of hepatic metastasis. J Gastroenterol Hepatol. 1996;11:26–32. doi: 10.1111/j.1440-1746.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Rooijen N. The liposome-mediated macrophage 'suicide' technique. J Immunol Methods. 1989;124:1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- 17.Traeger T, Mikulcak M, Eipel C, Abshagen K, Diedrich S, Heidecke CD, Maier S, Vollmar B. Kupffer cell depletion reduces hepatic inflammation and apoptosis but decreases survival in abdominal sepsis. Eur J Gastroenterol Hepatol. 2010;22:1039–1049. doi: 10.1097/MEG.0b013e32833847db. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, Sansom OJ. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. The Journal of clinical investigation. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of Leukocyte Biology. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 20.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 22.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 23.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zafirova B, Wensveen FM, Gulin M, Polic B. Regulation of immune cell function and differentiation by the NKG2D receptor. Cell Mol Life Sci. 2011;68:3519–3529. doi: 10.1007/s00018-011-0797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nature reviews. Immunology. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 27.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nature reviews. Immunology. 2009;9:568–580. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17:1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 29.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. The Journal of experimental medicine. 2003;198:173–181. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, Ara T, Silverman AM, DeClerck YA, Seeger RC, Metelitsa LS. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. The Journal of clinical investigation. 2009;119:1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nature immunology. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 32.Park JM, Terabe M, van den Broeke LT, Donaldson DD, Berzofsky JA. Unmasking immunosurveillance against a syngeneic colon cancer by elimination of CD4+ NKT regulatory cells and IL-13. Int J Cancer. 2005;114:80–87. doi: 10.1002/ijc.20669. [DOI] [PubMed] [Google Scholar]
- 33.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. The Journal of experimental medicine. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, Rufer N, Im JS, Alves PM, Martinet O, Halkic N, Cerottini JC, Romero P, et al. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. Journal of immunology. 2009;182:5140–5151. doi: 10.4049/jimmunol.0711086. [DOI] [PubMed] [Google Scholar]
- 35.Jewett A, Tseng HC. Tumor induced inactivation of natural killer cell cytotoxic function; implication in growth, expansion and differentiation of cancer stem cells. J Cancer. 2011;2:443–457. doi: 10.7150/jca.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apte RS, Niederkorn JY. Isolation and characterization of a unique natural killer cell inhibitory factor present in the anterior chamber of the eye. Journal of immunology. 1996;156:2667–2673. [PubMed] [Google Scholar]
- 37.Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. Journal of immunology. 1998;160:5693–5696. [PubMed] [Google Scholar]
- 38.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. Journal of immunology. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 39.Hsu DH, Moore KW, Spits H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. International immunology. 1992;4:563–569. doi: 10.1093/intimm/4.5.563. [DOI] [PubMed] [Google Scholar]
- 40.Rook AH, Kehrl JH, Wakefield LM, Roberts AB, Sporn MB, Burlington DB, Lane HC, Fauci AS. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. Journal of immunology. 1986;136:3916–3920. [PubMed] [Google Scholar]
- 41.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. The Journal of experimental medicine. 2005;202:1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang LJ, Wang XZ. Interleukin-10 and chronic liver disease. World J Gastroenterol. 2006;12:1681–1685. doi: 10.3748/wjg.v12.i11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. Journal of immunology. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 44.Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL-10. Trends Immunol. 2003;24:36–43. doi: 10.1016/s1471-4906(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 45.Zheng LM, Ojcius DM, Garaud F, Roth C, Maxwell E, Li Z, Rong H, Chen J, Wang XY, Catino JJ, King I. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. The Journal of experimental medicine. 1996;184:579–584. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subleski JJ, Hall VL, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 2006;66:11005–11012. doi: 10.1158/0008-5472.CAN-06-0811. [DOI] [PubMed] [Google Scholar]
- 47.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. Journal of immunology. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 48.Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. The Journal of experimental medicine. 2005;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]