Abstract
Background
Peroxisome proliferator-activated receptor (PPAR) is a versatile regulator of distinct biological processes and overexpression of PPAR in cancer may be partially related to its suppression of its own co-regulators.
Aims
To determine whether recruited suppressor proteins bind to and regulate PPAR expression, activity and PPAR -dependent cholangiocarcinoma proliferation.
Methods
Yeast two-hybrid assays were done using murine PPAR as bait. PPAR mRNA expression was determined by qPCR. Protein expression was measured by western blot. Immunohistochemistry and fluorescence microscopy were used to determine PPAR expression and co-localization with NDP Kinase alpha (NM23-H2). Cell proliferation assays were performed to determine cell numbers.
Results
Yeast two-hybrid screening identified NM23-H2 as a PPAR binding protein and their interaction was confirmed. Overexpressed PPAR or treatment with the agonist GW501516 resulted in increased cell proliferation. NM23-H2 siRNA activated PPAR luciferase promoter activity, upregulated PPAR RNA and protein expression and increased GW501516-stimulated CCA growth. Overexpression of NM23-H2 inhibited PPAR luciferase promoter activity, downregulated PPAR expression and AKT phosphorylation and reduced GW501516-stimulated CCA growth.
Conclusions
We report the novel association of NM23-H2 with PPAR and the negative regulation of PPAR expression by NM23-H2 binding to the C-terminal region of PPAR. These findings provide evidence that the metastasis suppressor NM23-H2 is involved in the regulation of PPAR -mediated proliferation.
Keywords: Cholangiocarcinoma, Nucleoside diphosphate kinase alpha, Peroxisome proliferator-activated receptor
1. Introduction
Cholangiocarcinomas (CCA) are malignant epithelial tumours and are among the most life threatening of cancers originating from the bile duct system due to the late diagnosis and poor outcome associated with the disease [1]. CCA can be classified as intrahepatic, extrahepatic or hilar based upon its location within the hepatobiliary system [2]. The incidence of intrahepatic CCA is increasing in the USA and other western countries, although this pattern shows regional variation [3,4]. The histological diagnosis of most CCA tumours is that of a well-differentiated adenocarcinoma and most patients present with locally advanced and/or intra- or extrahepatic metastatic disease [5,6]. Advanced CCA is resistant to therapy and optimized treatment of CCA patients who are not amenable to surgical extirpation or liver transplantation continues to be a significant molecular challenge [7,8].
The nuclear receptor peroxisome proliferator-activated receptor (PPAR) has been linked to colorectal, breast, hepatocellular and prostate cancers [9,10]; however, the mechanism for PPAR overexpression in cancer remains unknown. PPAR was one of the orphan nuclear hormone receptors which were first identified as proteins that control the size and numbers of peroxisomes by binding to the peroxisome proliferator response elements. PPAR expression has been detected in 12 human tissues, including spleen, thymus, brain, spinal cord, heart, skeletal cord, liver, pancreas, prostate, kidney and lung [11,13]. Our prior studies showed that PPAR was expressed in cholangiocytes [12]. Here, our studies showed PPAR recruited a metastasis-suppressor protein NM23-H2 as a corepressor. The functional relevance of the NM23-H2 gene was validated with an overexpression and knockdown approach. Deregulation of NM23-H2 led to PPAR overexpression in CCA and NM23-H2 re-expression decreased invasive behaviour of CCA cells in vitro. Therefore, we believe that PPAR binding partners, such as NM23-H2, may be involved in the regulation of PPAR expression in cholangiocytes and the pathogenesis of CCA. Reactivation of metastasis-suppressor NM23-H2 gene expression in CCA patients could halt metastatic colonization and may yield a potential therapeutic intervention for patients with advance stage or metastatic disease.
2. Materials and methods
2.1. Cells and reagents
Normal Rat Cholangiocytes (NRC) were isolated and established in culture as described [13]. Human CCA cell lines (KWCH, KWBC-1, Mz-ChA-1) and normal human cholangiocyte H69 cells were gifted from Dr. Gregory Gores. Cells were cultured in DMEM with 10% FBS. PPAR agonist GW501516, Akt inhibitor LY294002, fatty acid-free BSA, c-myc, Flag, GST and other chemicals were from Sigma (St. Louis, MO). All cell-culture reagents were from Invitrogen (Carlsbad, CA). PPAR, Akt, p-AKT, CDK2, NM23-H2 and -actin monoclonal antibodies were purchased from Santa Cruz (Santa Cruz, CA). All of the secondary antibodies were from Cell Signaling Technology (Danvers, MA).
2.2. Yeast two-hybrid assays
The mouse coding sequence was cloned to a bait plasmid mPPAR and used to screen a custom-generated mouse kidney cDNA library. The yeast two-hybrid (y2h) screening was performed according to the manufacturer’s instructions (Clontech Laboratories). The positive clones were sequenced and analyzed with NCBI’s BLAST database.
2.3. Western blotting and co-immunoprecipitations
Whole-cell protein preparation and western blotting was performed using standard procedures. Equal amounts of total protein were loaded and anti- -actin antibody was used as a loading control. For co-immunoprecipitations, HEK293 cells were co-transfected with full length myc-tagged NM23-H2 and flag-tagged PPAR constructs. To detect endogenous PPAR and NM23-H2 interaction, co-immunoprecipitation was performed in NRC. Experiments were performed according to standard procedures.
2.4. Immunofluorescence verification of co-localized proteins
For immunofluorescence analysis, HEK293 cells were transfected with plasmids encoding GFP-tagged PPAR or RFP-tagged NM23-H2 by a liposomal-based method (Lipofectamine Plus, Invitrogen) and subsequent immunofluorescence procedures for simultaneous detection of GFP or RFP expression. Mz-ChA-1 cells were immunostained with anti-PPAR antibody (green) and anti-NM23-H2 antibody (red). Nuclei of Mz-ChA-1 cells were counterstained with DAPI. Two-dimensional images were collected and saved using a Nikon A1 scanning confocal imaging system.
2.5. Analysis of PPARı gene expression by real-time PCR
Total RNA was extracted from KWCH, KWBC-1, Mz-ChA-1 and H69 cells by using an miRNeasy mini kit (Qiagen), following the manufacturer’s protocol. RNA was reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kits according to manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). Real time PCR was carried out using a 7500 sequence detection System (Applied Biosystems). Gene expression profiling was achieved using the comparative cycle threshold (ct) method of relative quantification using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the endogenous gene. PPAR gene primers were as follows: Forward: CACATCTACAATGCCTACCT; Reverse: CTTCTCTGCCTGCCACAATGTCT.
2.6. Tissue preparation and immunohistochemistry
For immunohistochemistry (IHC) analysis, paraffin embedded sections (4 m) were dewaxed and antigens were retrieved. After blocking, slides were incubated with primary antibodies. The EnVision Kit (Dako) avidin–biotin complex method was used to visualize the signals [14].
2.7. RNA interference
NM23-H2 siRNA was purchased from Dharmacon (Chicago, IL). Cells with fifty percent confluence were transfected with NM23-H2 siRNA or a 21-nucleotide irrelevant RNA duplex as a control using Lipofectamine™ 2000. Depletion of NM23-H2 was confirmed by immunoblotting.
2.8. Luciferase assay
Mz-ChA-1 cells (2 × 107) were transfected with the reporter construct, PPAR response element (PPRE-Luc) and NM23-H2 or NM23-H2 RNAi using Lipofectamine 2000 (Invitrogen) and incubated for 48 h. Luciferase activity was measured 24 h later using the Dual-Luciferase kit according to manufacturer’s instructions (Promega, Fitchburg, WI) and reported as a ratio of firefly luciferase to Renilla luciferase.
2.9. Proliferation assays
Proliferation assays were performed by using the Rapid Cell Viability assay (Oncogene Research Products, San Diego) according to the manufacturer’s protocol.
2.10. Statistical analyses
The values reported were the mean ± SD. Student’s t-test was used, where applicable, and an ANOVA analysis was applied to assess interactions between groups and differences between means. P < 0.05 was accepted as statistically significant. Data were representative of three independent experiments performed in triplicate.
3. Results
3.1. PPARı overexpression in human CCA
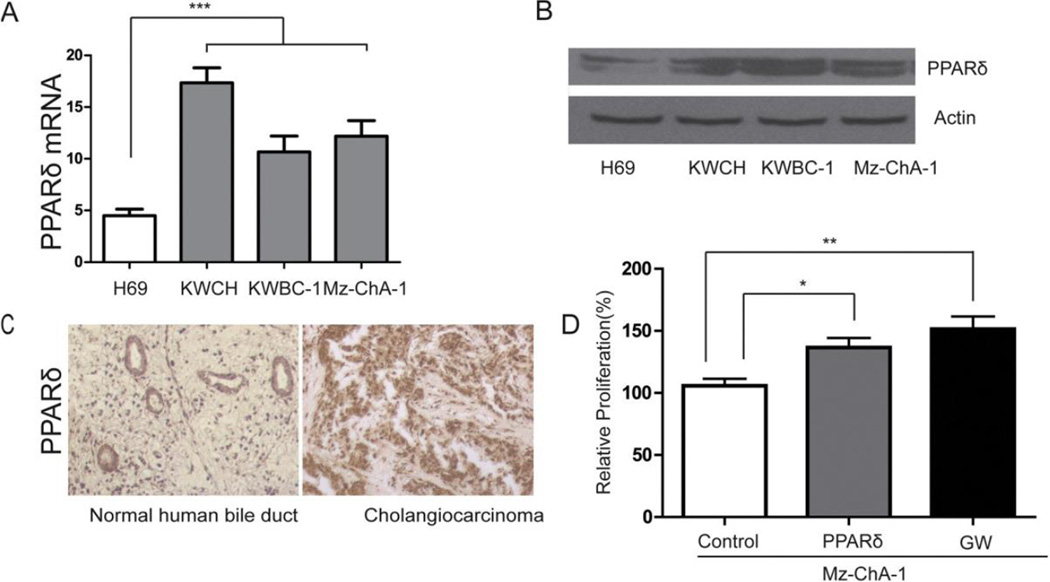
Although our previous studies showed PPAR was expressed in cholangiocytes, whether PPAR was overexpressed in CCA and involved in the pathogenesis of CCA was still unknown. To answer this question, we used human CCA, three human CCA cell lines (KWCH, KWBC-1, Mz-ChA-1) and the normal human cholangiocyte cell line H69 to detect mRNA and protein levels of PPAR. The experimental results showed that PPAR mRNA levels in CCA cell lines were greater compared to normal cholangiocytes by real-time PCR (Fig. 1A) and by western blotting (Fig. 1B). Using immunohistochemistry, PPAR protein expression was higher in CCA compared to normal bile ducts (Fig. 1C). Overexpressed PPAR and PPAR ligand GW501516 significantly stimulated the proliferation of MZ-ChA-1 cells (Fig. 1D).
Fig. 1.
PPAR over-expression in CCA cell lines. (A) The steady-state levels of mRNA encoding PPAR in a panel of human normal cholangiocytes (H69) and human CCA cell lines were detected by quantitative real-time PCR. (B) PPAR protein levels in human normal cholangiocytes (H69) and human CCA cell lines were determined by western blotting. (C) PPAR protein levels in human normal bile duct and CCA (20×). (D) Overexpressed PPAR and PPAR ligand GW501516 (GW) stimulated the proliferation of MZ-ChA-1 cells. Data represent mean ± SD of each group. (A, ***P < 0.001 compared to all other groups; D, *P < 0.05 between selected groups, **P < 0.01 between selected groups.) PPAR, peroxisome proliferator-activated receptor; CCA, cholangiocarcinoma.
3.2. NM23-H2 interacts with PPARı
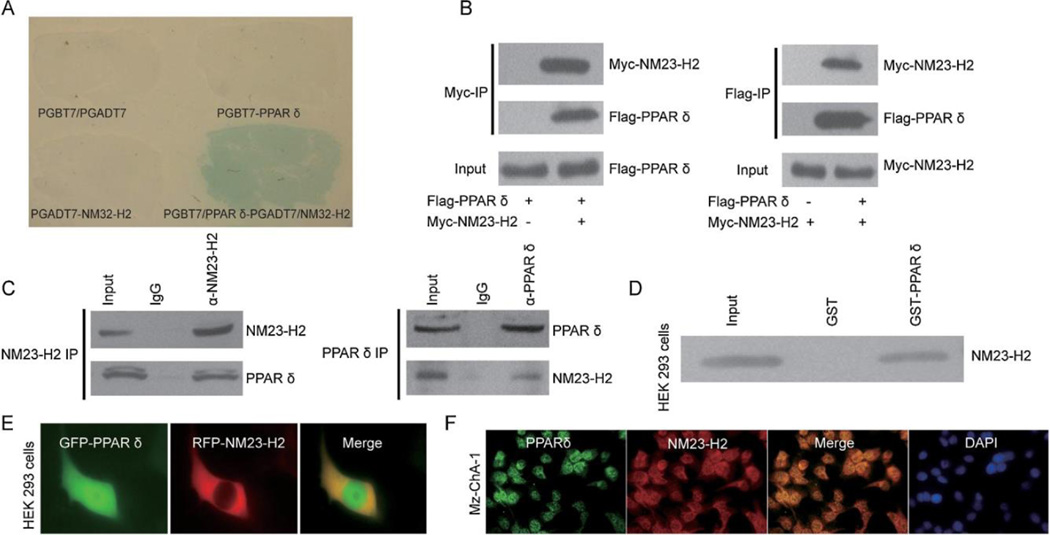
To better understand the underlying molecular mechanism responsible for elevated PPAR expression we used yeast two-hybrid assays designed to identify genes encoding PPAR regulatory proteins. The yeast two-hybrid assays with mPPAR bait vector transformed with a mouse kidney cDNA library showed that NM23-H2 interacts with PPAR (Fig. 2A). To confirm our yeast two-hybrid findings, we used co-immunoprecipitation (IP) assays to examine whether NM23-H2 interacted directly with PPAR in cells. Results showed that transfected myc-tagged NM23-H2 was associated with transfected Flag-tagged PPAR when anti-myc antibody was used in the immunoprecipitation followed by western blot analysis using anti-Flag antibody. In cells without transfected NM23-H2, no PPAR was immunoprecipated. In Flag-tagged PPAR expressing cells, transfected myc-tagged NM23-H2 was detected in anti-Flag immunoprecipations (Fig. 2B). Co-immunoprecipitation studies showed that endogenous PPAR and NM23-H2 were also coimmunoprecipitated (Fig. 2C). An in vitro binding assay using purified PPAR fused to GST in HEK293 cell extracts expressing NM23-H2 showed that NM23-H2 bound to GST-PPAR and not to GST (Fig. 2D). To investigate the intracellular distribution of NM23-H2 and PPAR, NM23-H2-RFP and PPAR -GFP fusion constructs were overexpressed in HEK293 cells. Both proteins were detected in the cytoplasm and shared the same distribution pattern (Fig. 2E). Furthermore Mz-ChA-1 cells were immunostained with PPAR and NM23-H2 antibodies. Overlap of PPAR (green) and NM23-H2 (red) staining was shown and nuclei were labelled with DAPI (blue). Images obtained with confocal microscopy (40×). The subcellular distribution of endogenous NM23-H2 and PPAR in Mz-ChA-1 cells were similar with HEK293 cells (Fig. 2F).
Fig. 2.
PPAR interacts with NM23-H2. (A) Yeast two-hybrid screening was performed using PPAR and a mouse kidney cDNA library. NM23-H2 was identified as a novel molecular partner that interacted with PPAR. (B) Co-immunoprecipitation experiments were performed in HEK293 cells transfected with pcDNA3-Myc-NM23-H2 and pcDNA3-Flag-PPAR. (C) Co-immunoprecipitation experiments were performed in normal rat cholangiocytes (NRC) to detect endogenous PPAR and NM23-H2 interaction. (D) A binding assay was performed using purified recombinant GST-PPAR protein and Myc-NM23-H2-expressing HEK293 cell extracts. (E) Co-localization of GFP-tagged PPAR and RFP-tagged NM23-H2 were tested in HEK293 cells (40×) transfected with plasmids encoding PPAR or NM23-H2. (F) Mz-ChA-1 cells were immunostained with PPAR and NM23-H2 antibodies. Overlap of PPAR (green) and NM23-H2 (red) staining was shown and nuclei were labelled with DAPI (blue). Images obtained with confocal microscopy (40×). PPAR, peroxisome proliferator-activated receptor; NM23-H2, CCA nucleoside diphosphate kinase alpha; NRC, cholangiocarcinoma. normal rat cholangiocytes.
3.3. NM23-H2 downregulated PPARı expression
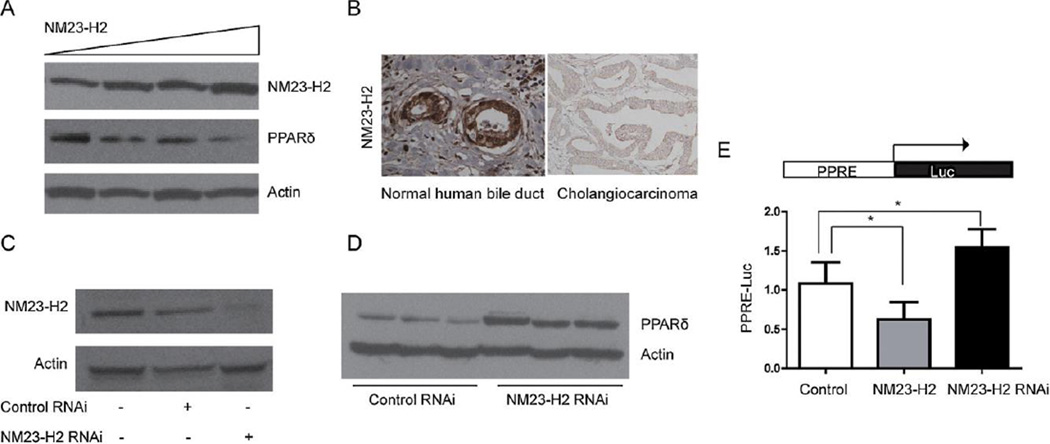
To investigate whether NM23-H2 regulated PPAR expression, Mz-ChA-1 cells were transfected with increasing concentrations of NM23-H2 expression plasmid. Immunoblotting showed that increasing NM23-H2 expression increased the expression of NM23-H2 protein and attenuated the expression of PPAR protein (Fig. 3A). Furthermore, the expression of PPAR was higher in contrast with the lower expression of NM23-H2 in human CCA tissues (Figs. 1C and 3B).
Fig. 3.
NM23-H2 down-regulates PPAR expression. (A) Transfection of increasing concentrations of NM23-H2 expression plasmid elevated the expression of NM23-H2 protein and attenuated the expression of PPAR protein as determined by immunoblotting. (B) NM23-H2 protein levels in human normal bile duct and CCA (20×). (C) Silencing of NM23-H2 was demonstrated by western blot analysis. (D) The expression of PPAR protein was increased in NM23-H2 silenced cells. (E) MZ-ChA-1 cells were transiently transfected with the reporter construct, PPAR response element (PPRE-Luc) and NM23-H2 or NM23-H2 RNAi. NM23-H2 significantly reduced PPRE luciferase reporter activity and NM23-H2 RNAi increased PPRE reporter activity. Data represent mean ± SD of each group. (D, *P < 0.05 between selected groups.) PPAR, peroxisome proliferator-activated receptor; NM23-H2, CCA nucleoside diphosphate kinase alpha; CCA, cholangiocarcinoma.
To study the inhibition of NM23-H2 expression in Mz-ChA-1 cells, siRNA technology was used. Fig. 3C shows that NM23-H2 siRNA but not control siRNA reduced NM23-H2 protein expression. PPAR proteins and promoter activities were increased in the NM23-H2-silenced Mz-ChA-1 cells (Fig. 3D and E).
3.4. PPARı stimulates the proliferation of human CCA cell lines while NM23-H2 inhibits CCA cell proliferation
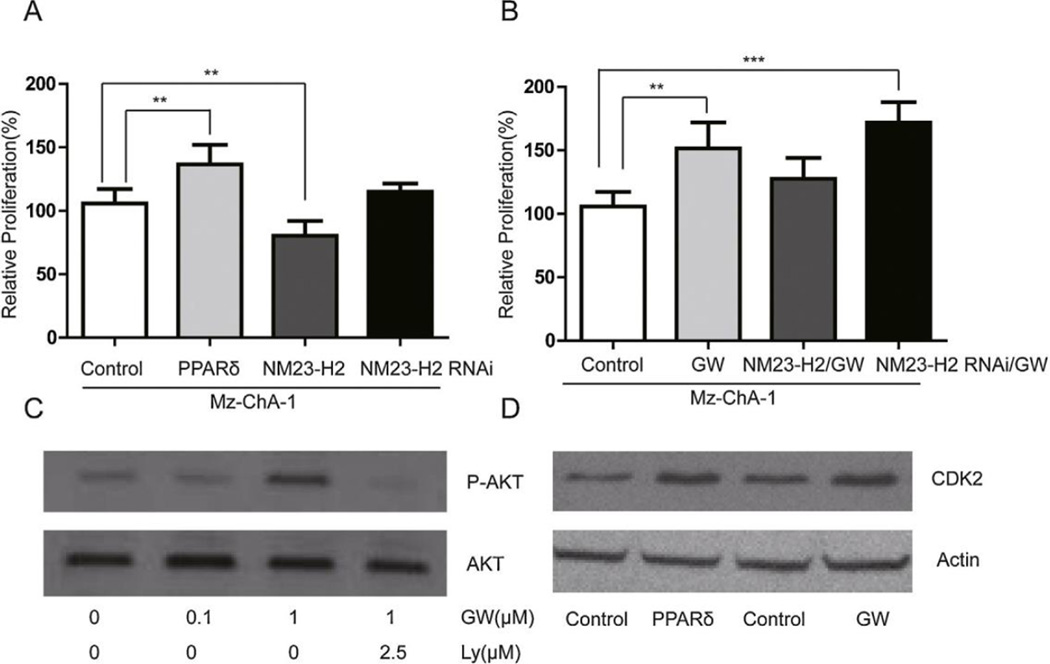
Mz-ChA-1 cells were transfected with PPAR/NM23-H2 or treated with PPAR ligand GW501516 or LY294002 overnight. Overexpression of PPAR or GW501516 treatment increased cell proliferation while overexpression of NM23-H2 decreased cell proliferation (Fig. 4A and B). NM23-H2 silencing increased cell proliferation (Fig. 4A and B). The PPAR agonist GW501516 induced Akt phosphorylation in a dose-dependent manner. The PI3K inhibitor LY294002 completely blocked the activity of Akt (Fig. 4C). Over-expression of PPAR or addition of the PPAR agonist GW501516 increased CDK2, a protein that drives cell proliferation (Fig. 4D).
Fig. 4.
PPAR stimulates the proliferation of Human CCA cell lines. (A, B) Overexpressed PPAR and PPAR ligand GW501516 (GW) stimulated the proliferation of MZ-ChA-1 cells. Overexpressed NM23-H2 reduced the proliferation of MZ-ChA-1 cells; additionally, NM23-H2 silencing increased GW501516 induced cell proliferation. (C) The PPAR agonist GW501516 induced Akt phosphorylation in a dose-dependent manner in MZ-ChA-1 cells and the activity of Akt was completely inhibited by the PI3K inhibitor LY294002. (D) Overexpressed PPAR and its ligand GW501516 increased CDK2 protein levels in MZ-ChA-1 cells, as measured by immunoblotting. Data represent mean ± SD of each group. (A, B **P < 0.01 between selected groups.) PPAR, peroxisome proliferator-activated receptor; CCA, cholangiocarcinoma; NM23-H2, nucleoside diphosphate kinase alpha; Akt, protein Kinase B; PI3K, phosphoinositide 3-kinase; CDK2, cyclin-dependent kinase 2.
4. Discussion
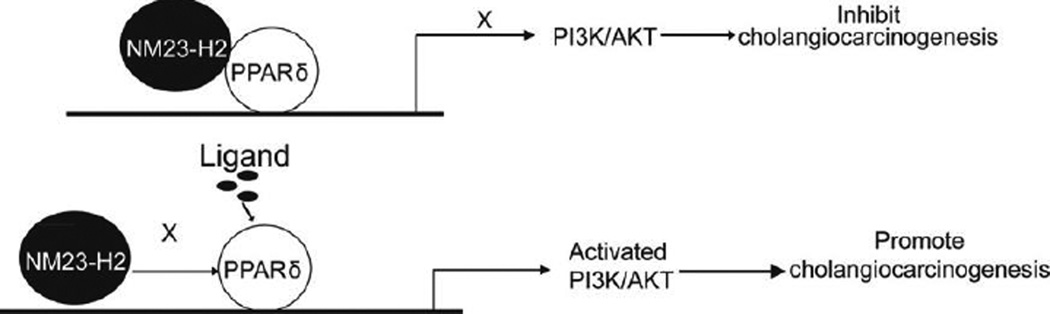
The results of this study provide a plausible mechanism of PPAR overexpression in CCA and a novel insight regarding the use of the NM23-H2 for the treatment of CCA. Our findings indicate that (1) PPAR interacts with NM23-H2, (2) PPAR expression is increased and NM23-H2 expression is reduced in human CCA and (3) NM23-H2 downregulates PPAR expression as well as PPAR stimulation of CCA proliferation and AKT phosphorylation (Fig. 5).
Fig. 5.
Working model. As shown in the upper panel, in the absence of ligand, PPAR recruits NM23-H2 as a corepressor to inhibit proliferation and carcinogenesis. As shown in the lower panel, corepressors are released in the presence of ligand. In the absence of NM23-H2, PPAR transcriptional activity is enhanced. Increased PPAR transcriptional activity activates the PI3K/AKT signalling pathway to promote cholangiocarcinogenesis. PPAR, peroxisome proliferator-activated receptor; NM23-H2, nucleoside diphosphate kinase alpha; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B.
PPARs (classified as PPAR, PPAR and) perform a number of functions and are implicated in diseases such as obesity, diabetes, cancer and atherosclerosis [15]. The role of PPAR in the development of colon cancer is not clear, with studies demonstrating both a tumorigenic and anti-tumorigenic role [16]. To date, only few target genes of PPAR are known [17]. Of particular interest with respect to tumorigenesis is the agonist-dependent PPAR mediated upregulation of the Akt pathway in keratinocytes through the transcriptional induction of PDK-1 and ILK and the repression of the phosphatidylinositol phosphate phosphatase and tumour suppressor gene PTEN [18]. In vascular smooth muscle cells, PPAR modulates the expression of several cell cycle regulators (cyclin A, cdk2, p57Kip) [19]. Preliminary studies from our laboratory have shown that PPAR is overexpressed in multiple CCA cell lines and human CCA tissues and that PPAR agonists increase CCA growth.
In recent years, a novel class of genes has been identified, the so-called ‘metastasis suppressor’ genes, which specifically regulate metastasis [20]. Metastasis suppressor genes inhibit the development and growth of tumour metastases without affecting primary tumour growth.
The first metastasis suppressor gene identified was NM23. NM23 proteins, also known as nucleoside diphosphate kinases (NDPK), are a family of highly conserved proteins found in eukaryotes [21]. The NM23 gene family has been demonstrated to suppress the development of lymph node, lung and liver metastases [22]. The biochemical mechanism of metastasis suppression is thought to involve attenuation of signals promoting tumour cell motility, invasion and colonization [23]. At least four classes of NM23 biochemical activities may contribute to metastasis suppression, including protein–protein interactions, regulation of GTP-binding protein function, DNA-associated activities and histidine-dependent protein phosphotransferase activity [24]. In humans, there are now nine genes encoding NM23 related proteins, but the two most abundantly expressed and studied genes are NM23-H1 and NM23-H2, also named NME1 and NME2. They encode, respectively, the A and B subunits of nucleoside diphosphate kinase (NDPK), which possess 88% identity in their amino acid sequences. The murine orthologs NM23-M1 and NM23-M2 encode proteins sharing, respectively, 94% and 98% identity with their human counterparts [25]. Clinical studies, mainly of NM23-H1, concerning NM23 expression at the protein or mRNA levels in different types of cancer have been widely reported since their discovery [26]. An inverse association between NM23 expression and metastatic potential was observed for some types of human solid tumours such as melanoma, breast cancer and colon and liver carcinoma. However, other studies into these tumour types failed to confirm this relationship [27]. Activity of NM23H1/M1 was confirmed in aggressive tumour cell lines forced to overexpress NM23-H1 or NM23-M1. Additionally, these tumour cell lines displayed reduced metastatic potential in experimental models [28]. Several studies point to a dual role for NM23-H1 in tumour progression, with early overexpression in the primary tumour and loss of expression associated with later metastatic stages [29]. Recently, contrary findings have been reported indicating that NM23-H2 may be pro-oncogenic in hepatocarcinogenesis [30]. The important difference between this study and our current work is that different endothelial cells were adopted, revealing the possibility of a dual function of NM23-H2 in endothelial cells. This implies potentially different context specific applications of NM23-H2 in the treatment of various diseases.
In the current study, we demonstrated that PPAR and its agonists effectively stimulated the proliferation of human CCA cells. We identified two mechanisms underlying this phenomenon: (1) treatment with PPAR agonists resulted in a dose-dependent activation of Akt phosphorylation in human CCA cells that could be completely inhibited by the PI3K inhibitor LY294002 and (2) over-expressed PPAR and its agonists increased proliferation marker CDK2 protein levels in human CCA cells. Pollock et al. demonstrated that PPAR activator GW501516 driving tumorigenesis is in part associated with AKT signalling through the enhancement of energy metabolism [31]. Furthermore, Stephen et al. observed that PPAR activation could result in increased Cdk2 protein levels in the epithelial cancer-derived cell lines T47D with treatment of PPAR agonist compound F [32]. Our work showed that PI3K/AKT-dependent mechanisms are likely to be one of the down-stream mechanisms for PPAR agonist promoted cell proliferation. Altered expression of cell cycle gene CDK2 was involved in this process.
In summary, PPAR was highly expressed in human CCA. Using the yeast two-hybrid screening system, we identified the novel PPAR -binding protein, NM23-H2. In CCA, activation of PPAR, stimulation of PI3K and cell proliferation was due to loss of NM23-H2 interaction with PPAR. Reactivation of the NM23-H2 gene may be a potential therapeutic intervention for CCA. Further work will be required to explore the metastasis-suppressive function of NM23-H2 in CCA.
Acknowledgments
We thank Dr. Gregory Gore for providing the Human CCA cell lines (KWCH, KWBC-1, Mz-ChA-1) and the normal human cholangiocyte cell line H69. This study was supported by the National Natural Science Foundation of China (No. 81270868, to X.X.) and National Institutes of Health (NIH/NCI 1R01CA180083-01 to L.Q).
Footnotes
Conflict of interest
None declared.
References
- 1.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;13:61903–61910. [Google Scholar]
- 2.de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. New England Journal of Medicine. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? Journal of Hepatology. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Burak K, Angulo P, Pasha TM, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. American Journal of Gastroenterology. 2004;99:523–526. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 5.Blechacz BR, Gores GJ. Cholangiocarcinoma. Clinics in Liver Disease. 2008;12:131–150. ix. doi: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Farley DR, Weaver AL, Nagorney DM. “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clinic Proceedings. 1995;70:425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 7.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. Journal of the National Cancer Institute. 2006;98:873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 9.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nature Medicine. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 10.Berger J, Moller DE. The mechanisms of action of PPARs. Annual Review of Medicine. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 11.Burdick AD, Kim DJ, Peraza MA, et al. The role of peroxisome proliferator- activated receptor-beta/delta in epithelial cell growth and differentiation. Cellular Signalling. 2006;18:9–20. doi: 10.1016/j.cellsig.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Xia X, Jung D, Webb P, et al. Liver X receptor beta and peroxisome proliferator-activated receptor delta regulate cholesterol transport in murine cholangiocytes. Hepatology. 2012;56:2288–2296. doi: 10.1002/hep.25919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alpini G, Phinizy JL, Glaser S, et al. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003;284:G1066–G1073. doi: 10.1152/ajpgi.00260.2002. [DOI] [PubMed] [Google Scholar]
- 14.Xia M, Hou M, Zhu H, et al. Anthocyanins induce cholesterol efflux from mouse peritoneal macrophages: the role of the peroxisome proliferator-activated receptor{gamma}-liver X receptor {alpha}-ABCA1 pathway. Journal of Biological Chemistry. 2005;280:36792–36801. doi: 10.1074/jbc.M505047200. [DOI] [PubMed] [Google Scholar]
- 15.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 16.Peters JM, Hollingshead HE, Gonzalez FJ. Role of peroxisome-proliferator-activated receptor beta/delta (PPARbeta/delta) in gastrointestinal tract function and disease. Clinical Science (London) 2008;115:107–127. doi: 10.1042/CS20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehrenborg E, Krook A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacological Reviews. 2009;61:373–393. doi: 10.1124/pr.109.001560. [DOI] [PubMed] [Google Scholar]
- 18.Di-Poi N, Ng CY, Tan NS, et al. Epithelium–mesenchyme interactions control the activity of peroxisome proliferator-activated receptor beta/delta during hair follicle development. Molecular and Cellular Biology. 2005;25:1696–1712. doi: 10.1128/MCB.25.5.1696-1712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim HJ, Lee S, Park JH, et al. PPAR delta agonist L-165041 inhibits rat vascular smooth muscle cell proliferation and migration via inhibition of cell cycle. Atherosclerosis. 2009;202:446–454. doi: 10.1016/j.atherosclerosis.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nature Clinical Practice Oncology. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosengard AM, Krutzsch HC, Shearn A, et al. Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature. 1989;342:177–180. doi: 10.1038/342177a0. [DOI] [PubMed] [Google Scholar]
- 22.Steeg PS, Palmieri D, Ouatas T, et al. Histidine kinases and histidine phosphorylated proteins in mammalian cell biology, signal transduction and cancer. Cancer Letters. 2003;190:1–12. doi: 10.1016/s0304-3835(02)00499-8. [DOI] [PubMed] [Google Scholar]
- 23.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nature Reviews Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald NJ, De la Rosa A, Benedict MA, et al. A serine phosphorylation of Nm23, and not its nucleoside diphosphate kinase activity, correlates with suppression of tumor metastatic potential. Journal of Biological Chemistry. 1993;268:25780–25789. [PubMed] [Google Scholar]
- 25.Wagner PD, Steeg PS, Vu ND. Two-component kinase-like activity of nm23 correlates with its motility-suppressing activity. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9000–9005. doi: 10.1073/pnas.94.17.9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garzia L, Roma C, Tata N, et al. H-prune-nm23-H1 protein complex and correlation to pathways in cancer metastasis. Journal of Bioenergetics and Biomembranes. 2006;38:205–213. doi: 10.1007/s10863-006-9036-z. [DOI] [PubMed] [Google Scholar]
- 27.Ouatas T, Salerno M, Palmieri D, et al. Basic and translational advances in cancer metastasis: Nm23. Journal of Bioenergetics and Biomembranes. 2003;35:73–79. doi: 10.1023/a:1023497924277. [DOI] [PubMed] [Google Scholar]
- 28.Ma D, Xing Z, Liu B, et al. NM23-H1 and NM23-H2 repress transcriptional activities of nuclease-hypersensitive elements in the platelet-derived growth factor-A promoter. Journal of Biological Chemistry. 2002;277:1560–1567. doi: 10.1074/jbc.M108359200. [DOI] [PubMed] [Google Scholar]
- 29.Chang CC, Shih JY, Jeng YM, et al. Connective tissue growth factor and its role in lung adenocarcinoma invasion and metastasis. Journal of the National Cancer Institute. 2004;96:364–375. doi: 10.1093/jnci/djh059. [DOI] [PubMed] [Google Scholar]
- 30.Lee MJ, Xu DY, Li H, et al. Pro-oncogenic potential of NM23-H2 in hepatocellular carcinoma. Experimental and Molecular Medicine. 2012;44:214–224. doi: 10.3858/emm.2012.44.3.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollock CB, Yin Y, Yuan H, et al. PPARdelta activation acts cooperatively with 3-phosphoinositide-dependent protein kinase-1 to enhance mammary tumorigenesis. PLoS ONE. 2011;6:e16215. doi: 10.1371/journal.pone.0016215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephen RL, Gustafsson MC, Jarvis M, et al. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Research. 2004;64:3162–3170. doi: 10.1158/0008-5472.can-03-2760. [DOI] [PubMed] [Google Scholar]