Abstract
Sex differences in human cognition are marked, but little is known regarding their neural origins. Here, in a sample of 674 human participants ages 9–22, we demonstrate that sex differences in cognitive profiles are related to multivariate patterns of resting-state functional connectivity MRI (rsfc-MRI). Males outperformed females on motor and spatial cognitive tasks; females were faster in tasks of emotion identification and nonverbal reasoning. Sex differences were also prominent in the rsfc-MRI data at multiple scales of analysis, with males displaying more between-module connectivity, while females demonstrated more within-module connectivity. Multivariate pattern analysis using support vector machines classified subject sex on the basis of their cognitive profile with 63% accuracy (P < 0.001), but was more accurate using functional connectivity data (71% accuracy; P < 0.001). Moreover, the degree to which a given participant's cognitive profile was “male” or “female” was significantly related to the masculinity or femininity of their pattern of brain connectivity (P = 2.3 × 10−7). This relationship was present even when considering males and female separately. Taken together, these results demonstrate for the first time that sex differences in patterns of cognition are in part represented on a neural level through divergent patterns of brain connectivity.
Keywords: adolescence, cognition, connectivity, connectome, development, fMRI, network, resting-state, sex differences
Introduction
Sex differences in human cognition are well documented (Halpern 2000). By adulthood, males are superior at visuospatial and motor tasks, whereas females are better in other domains such as social cognition and recognition memory (Gur et al. 2010b, 2012). Along with other groups (Szeszko et al. 2003; Schmithorst et al. 2008), we have previously reported substantial sex differences in brain structure, with females having a higher proportion of gray matter and men having more white matter (WM; Gur et al. 1999); such sex differences in brain structure are present even in childhood (Lenroot et al. 2007). In turn, brain structure has been shown to relate to cognition (Andreasen et al. 1993; Gur et al. 1999).
However, it is not known whether sex differences in brain structure translate into sex differences in functional brain networks. A powerful tool for the study of functional brain networks is resting-state functional connectivity MRI (rsfc-MRI; Biswal et al. 1995; Fox and Raichle 2007) which has been extensively used to delineate the functional neuroanatomy of the human brain both in health (Power et al. 2011b; Yeo et al. 2011), development (Fair et al. 2007; Dosenbach et al. 2010), and neuropsychiatric diseases (Bassett et al. 2008; Lynall et al. 2010). While several prior studies have presented evidence for sex differences in functional connectivity (Biswal et al. 2010; Tian et al. 2011; Zuo et al. 2010; Wang et al. 2012; Wu et al. 2013), it is unknown when such differences emerge during neurodevelopment, as most prior work has considered mainly adults. Furthermore, recent work from 3 independent samples has demonstrated that small amounts of in-scanner motion can have a marked confounding influence on rsfc-MRI (Power et al. 2012; van Dijk et al. 2011; Satterthwaite et al. 2012), which may have formed an unaccounted-for bias in prior studies of sex differences. Most importantly, no prior study has attempted to understand how sex differences in patterns of cognition may relate to sex differences in brain connectivity.
Here, in a large sample of children, adolescents, and young adults studied as part of the Philadelphia Neurodevelopmental Cohort (Satterthwaite et al. 2014), we investigated how sex differences in functional connectivity relate to sexually divergent patterns of cognition. Our hypothesis was that the extent to which a given subject demonstrated a stereotypically “male” or “female” pattern of brain connectivity would be related to the masculinity or femininity of their cognitive profile. To do this, we first establish how brain connectivity in males and females differs using graphical measures of network topology, mass-univariate analyses of network connections, and multivariate pattern analyses that classified a subject's sex by considering the complex, high-dimensional pattern of brain connectivity. As described below, sex differences in brain connectivity are prominent even during youth. Critically, multivariate, sexually divergent patterns of connectivity and cognition were found to be significantly correlated, suggesting that sex differences in profiles of human cognition may be related to fundamental differences in brain connectivity that are present early in life.
Materials and Methods
Participants
The PNC is a collaboration between the Center for Applied Genomics at Children's Hospital of Philadelphia (CHOP) and the Brain Behavior Laboratory at the University of Pennsylvania (Penn). Study procedures were approved by the Institutional Review Boards of both Penn and CHOP. The target population-based sample is of 10 000 youths who presented to the CHOP network for a pediatric visit and volunteered to participate in genomic studies of complex pediatric disorders (Gur et al. 2012). A subsample of 1445 participants, stratified by age and sex, were randomly selected for neuroimaging (Satterthwaite et al. 2014). Of these, 1275 had resting-state data acquired. Participants were excluded due to missing cognitive data, poor imaging data quality, or a history that suggested potential abnormalities of brain development. Data regarding medical history was gathered from both self-report at time of study entry as well as electronic medical records available from CHOP. Specifically, 234 participants were excluded due to a history of medical problems that might affect brain function, a history of inpatient psychiatric hospitalization, or current use of psychotropic medication. Additionally, 323 participants met exclusion criteria due to poor resting-state image quality (Satterthwaite et al. 2012, 2013a, 2013b), including if scan mean relative displacement exceeded 0.2 mm (see below), if there were >20 volumes with relative displacement >0.25 mm, or if gross between-run motion resulted in incomplete brain coverage. Finally, 58 participants were excluded due to missing cognitive data. These exclusion criteria resulted in a final eligible pool of 722 participants aged 8–22 years (312 males). Many participants were excluded due to multiple criteria.
In order to ensure that analyses of sex differences were not biased by developmental differences in connectivity or data quality, male and female groups were matched on subject age and in-scanner motion during the resting-state scan. As previously (Satterthwaite et al. 2012, 2013a) in-scanner motion was summarized using the mean relative displacement measure estimated during time series realignment (see below). Age- and motion-matched samples of males and females were constructed using a greedy matching algorithm (Carpenter 1977) written in-house and implemented in MATLAB. This group-matching algorithm recursively removed the single subject that minimized the sum of the absolute t-statistics from 2 t tests that separately evaluated the difference between male and female groups' age and motion. The algorithm stopped and samples were considered matched when both of the P-values of the t-statistic comparing the age and in-scanner motion of males and females were >0.9. This procedure produced groups of 312 males and 362 females where age and motion were tightly matched (see Table 1).
Table 1.
Demographics of age- and motion-matched samples of males and females
| Group | N | Age, years (SD) | Motion, mm (SD) | # Caucasian | Maternal education, years (SD) |
|---|---|---|---|---|---|
| Male | 312 | 15.66 (3.3) | 0.068 (0.04) | 166 | 14.45 (2.43) |
| Females | 362 | 15.67 (3.2) | 0.068 (0.04) | 157 | 14.27 (2.55) |
As noted in Table 1, the racial composition of the male and female samples was somewhat different. As we did not have specific hypotheses regarding race or ethnicity, this was not included in our final models. However, supplementary analyses indicated that inclusion of race as a covariate did not impact our results.
Computerized Neurocognitive Battery
As previously described (Gur et al. 2010a, 2012), the 1-h Penn computerized neurocognitive battery (Penn CNB) was administered to all participants, and consisted of 14 tests that evaluated a broad range of cognitive functions (Table 2). Except for the motor tests that only measured speed, each test provided measures of both accuracy and speed. For this adolescent sample, instructions and vocabulary for verbal stimuli were simplified from the adult CNB (Gur et al. 2010a, 2010b). Cognitive assessment was completed during a separate session from neuroimaging (average 3.4 [SD 5.4] months between sessions). As detailed in Gur et al. (2012), the assessment session was scheduled at home (68.8% of participants) or in the laboratory (31.2%), according to the family and subject preference. During task administration, potential interference was minimized, standard instructions were read aloud in addition to appearing on the screen, and a professional testing environment was maintained. Tests were administered in a fixed order; breaks were offered approximately every 15 min.
Table 2.
Penn computerized neurocognitive battery
| Test name | Psychological domain | Abbreviations |
|---|---|---|
| Penn Conditional Exclusion Test | Abstraction/flexibility | ABF |
| Penn Continuous Performance Test | Attention | ATT |
| Letter N-back | Working memory | WM |
| Penn Word Memory | Verbal memory | VME |
| Penn Face Memory | Face memory | FME |
| Visual Object Learning Test | Spatial memory | SME |
| Penn Verbal Reasoning Test | Language reasoning | LAN |
| Penn Line Orientation Test | Spatial ability | SPA |
| Penn Emotion Identification Test | Emotion identification | EMI |
| Penn Emotion Differentiation Test | Emotion differentiation | EMD |
| Penn Age Differentiation Test | Age differentiation | AGD |
| Motor Praxis | Sensorimotor speed | SM |
| Finger Tapping Test | Motor | MOT |
Computerized Neurocognitive Battery: Data Analysis
Raw accuracy and speed scores were normalized by age within the entire cohort of the PNC study (n = 9138 at time of analysis). Specifically, accuracy and speed for each test were z-transformed based on the mean and standard deviation of participants within a 2-year age bin. As prior (Gur et al. 2012), for ease of presentation, higher z-scores always reflect better performance; z-scores where higher numbers reflected poorer performance (i.e., response time) were multiplied by −1. The relationship between cognitive performance and subject sex was analyzed using two-sample t-tests; multiple comparisons were controlled using the false discovery rate (Q < 0.05).
Image Acquisition
All data were acquired on the same scanner (Siemens Tim Trio 3 Tesla, Erlangen, Germany; 32-channel head coil) using the same imaging sequences. Resting-state blood oxygen level–dependent (BOLD) fMRI was acquired using a whole-brain, single-shot, multislice, gradient-echo (GE) echoplanar sequence with the following parameters: 124 volumes, repitition time (TR) 3000 ms, echo time (TE) 32 ms, flip angle 90°, field of view (FOV) 192 × 192 mm, matrix 64 × 64, slice thickness/gap 3 mm/0 mm, effective voxel resolution 3.0 × 3.0 × 3.0 mm. Prior to functional time series acquisition, a magnetization-prepared, rapid acquisition GE T1-weighted image was acquired to aid spatial normalization to standard atlas space, using the following parameters: TR 1810 ms, TE 3.51 ms, FOV 180 × 240 mm, matrix 256 × 192, 160 slices, inversion time 1100 ms, flip angle 9°, effective voxel resolution of 0.9 × 0.9 × 1 mm. Additionally, a B0 field map was acquired for application of distortion correction procedures, using a double-echo gradient recall echo sequence: TR 1000 ms, TE1 2.69 ms, TE2 5.27 ms, 44 slices, slice thickness 4 mm, FOV = 240 mm, effective voxel resolution of 3.8 × 3.8 × 4 mm. Prior to scanning, in order to acclimate participants to the MRI environment and to help participants learn to remain still during the actual scanning session, a mock scanning session was conducted using a decommissioned MRI scanner and head coil. Mock scanning was accompanied by acoustic recordings of the noise produced by gradient coils for each scanning pulse sequence. During the mock scanning session, feedback regarding head movement was provided using the MoTrack motion tracking system (Psychology Software Tools, Inc., Sharpsburg, PA, USA). In order to further minimize motion, participants' heads were stabilized in the head coil using one foam pad over each ear and a third over the top of the head. During the resting-state scan, a fixation cross was displayed as images were acquired. Participants were instructed to stay awake, keep their eyes open, fixate on the displayed crosshair, and remain still.
Network Construction and Visualization
We examined sex differences in functional connectivity within a system of 264 nodes described by Power et al. (2011). In this network, nodes are 5-mm radius spheres in MNI space that were drawn from both meta-analysis of task fMRI studies and resting-state functional connectivity mapping techniques (Cohen et al. 2008; Nelson et al. 2010). Within this system of 264 nodes, there are 34 716 unique edges. Furthermore, Power et al. (2011) provides a parcellation scheme for these nodes that delineates 13 functional modules that correspond to known large-scale brain networks that are coherent during both task activity and at rest (Smith et al. 2009; Yeo et al. 2011). We used this node system due to 3 main advantages. First, Power et al. (2011a, 2011b) selected these nodes as being representative of the complete voxel-wise connectivity of the brain; these nodes therefore provide good coverage of the entire brain, but still affording a large amount of dimensionality reduction and resultant computational efficiency. Second, as the system has already been parsed into functional networks (Power et al. 2011a, 2011b), it provides an independent reference that allows examination of within- versus between-module connectivity, which is an important feature of neurodevelopment (Fair et al. 2007, 2008; Supekar et al. 2009; Dosenbach et al. 2010; Anderson et al. 2011; Satterthwaite et al. 2013b). Third, the relatively high-dimensional nature of the data (34 716 unique edges) is well suited for machine-learning approaches such as support vector machines, allowing us to investigate whether complex multivariate patterns of functional connectivity can be used to classify subject sex and examine if they relate to sex-specific profiles of cognitive performance.
In order to aid visualization of the overall network structure (Fig. 1), a mean connectivity matrix was created by averaging across all participants. This average connectivity matrix was displayed in graphical form using a spring-embedded force-based rendering using Gephi (Bastian et al. 2009). In this representation, nodes that are tightly connected are brought closer together, whereas nodes that are not connected are pushed farther apart on the graph. As negative connections cannot be visualized effectively in such a graph, only positive values are displayed, with graph edges thresholded at r > 0.2.
Figure 1.
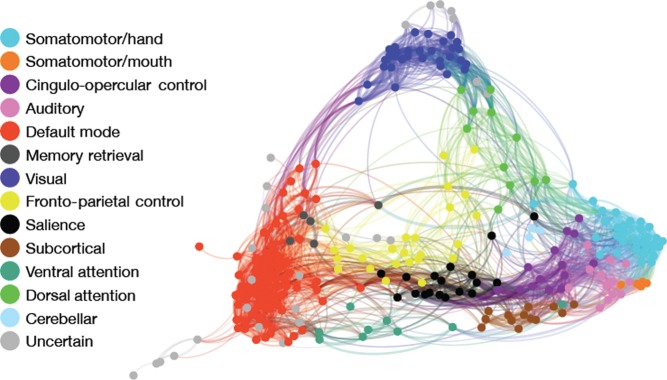
Network definition. Nodes in the network are defined according to the system established by Power et al. (2011a, 2011b), including 264 spheres (5 mm radius) comprising 13 functional brain modules. Nodes are colored according to module membership as indicated in the figure legend, and displayed using a spring-embedded rendering of the mean network connectivity matrix across all subjects. Although all analyses are conducted using fully connected networks with both positive and negative weights, for display graph edges are thresholded at r > 0.2. Graph edge thickness is scaled according to connection strength. As in Power et al. (2011a, 2011b), somatomotor, visual, and default mode modules are tightly integrated, show a predmoniance of intramodular connections, and are segregated from other functional modules. In contrast, the frontoparietal, salience, and attention (dorsal and ventral) systems are less segregated and show a greater number of intermodular connections.
Image Registration
Subject-level BOLD images were co-registered to the T1 image using boundary-based registration (Greve and Fischl 2009) with integrated distortion correction as implemented in FSL 5 (Jenkinson et al. 2012). Whole-head T1 images were registered to the Montreal Neurologic Institute 152 1-mm template using the diffeomorphic SyN registration that is part of ANTS (Avants et al. 2008, 2011; Klein et al. 2009). All registrations were inspected manually and also evaluated for accuracy using spatial correlations. Network nodes were registered to subject space for time series extraction by concatenating the co-registration, distortion correction, and normalization transformations so that only one interpolation was performed in the entire process.
Subject-Level Time Series Processing
A voxel-averaged time series was extracted from each of the 264 nodes in subject-space for every participant. Time series data were processed using a validated confound regression procedure that has been optimized to reduce the influence of subject motion (Satterthwaite et al. 2013a, 2013b). The first 4 volumes of the functional time series were removed to allow signal stabilization, leaving 120 volumes for subsequent analysis. Functional time series were band-pass filtered to retain frequencies between 0.01 and 0.08 Hz. Functional images were re-aligned using MCFLIRT (Jenkinson et al. 2002). Structural images were skull-stripped using BET (Smith 2002) and segmented using FAST (Zhang et al. 2001); mean WM and cerebrospinal fluid (CSF) signals were extracted from the tissue segments generated for each subject (Jakobs et al. 2012; Reetz et al. 2012). Improved confound regression (Satterthwaite et al. 2013a) included 9 standard confounding signals (6 motion parameters + global/WM/CSF) as well as the temporal derivative, quadratic term, and temporal derivative of the quadratic of each. Prior to confound regression, all motion parameters and confound time courses were band-pass filtered in an identical fashion as the time series data itself in order to prevent mismatch in the frequency domain and allow the confound parameters to best fit the retained signal frequencies (Hallquist et al. 2013). Furthermore, motion-related spike regressors were included in the model whenever a volume-to-volume displacement was >0.25 mm; for each such movement, a single regressor was included for each volume bounding the observed displacement (i.e., TR −1 and TR +1); these spike regressors effectively censor the influence of these volumes in subsequent analysis of residual time series (Lemieux et al. 2007). As 2 volumes are lost from analysis for each spike, participants with >20 spikes were excluded (see “Participants” above), ensuring that each subject had at least 4 min (80 volumes) of time series data for analysis (van Dijk et al. 2010).
Graph Construction
Using the time series extracted from each node, a symmetric connectivity matrix (264 × 264) was defined for each subject using pair-wise Pearson's correlations. While many graphical analyses of network topology threshold matrix values at an arbitrary level (or range of levels) to produce a sparse graph, such practices may be problematic when investigating individual or group differences (Zalesky et al. 2010; Rubinov and Sporns 2011). Specifically, this step may introduce a confound as a fixed threshold is likely to introduce different levels of sparsity across participants. Alternately, matching individuals on the level of sparsity requires use of a unique threshold for each individual, which may limit interpretability (Zalesky et al. 2010; Rubinov and Sporns 2011). In order to avoid the use of such arbitrary thresholds, all analyses were conducted on fully connected graphs including both positive and negative weights.
Graphical Analyses of Network Topology
Within this fully connected network, we next explored whether there were systematic differences in network topology based on a subject's sex. Specifically, we calculated 2 measures compatible with fully connected networks (Rubinov and Sporns 2011): strength and participation coefficient. Both measures were calculated on a node-wise basis and also summarized on a network-wise basis. At a given node, the strength is simply the total of the positive (or negative) connection weights for all edges connected to that node (Rubinov and Sporns 2010). Strength can be summarized on a network-wise basis by calculating a sum of all individual node strengths separately for positive and negative weights.
The participation coefficient is a summary measure of network topology that quantifies the degree to which a given node is connected to other nodes within a functional module or between functional modules (Rubinov and Sporns 2010). Thus, a node with a high participation coefficient has relatively more connections between defined network modules, contributing to network integration. In contrast, a node with a low participation coefficient has relatively more connections within the functional module to which it is assigned, denoting network segregation. As noted above, one advantage of the system of nodes defined by Power et al. (2011) is that nodes in this system were assigned to a functional network module based on parcellation in an independent dataset; this community structure was used to calculate the participation coefficient at each node for every subject. It should be noted that, in the present work, the participation coefficient reflects a single subject's pattern of connectivity relative to the static community assignments described by Power et al., not a community structure defined by that individual subject's connectivity matrix. To aid interpretability of the participation coefficient values compared with the sample as a whole, as for the cognitive data, all participation coefficient values were z-normed across the entire sample. In order to provide a summary measure across all network nodes, the mean positive participation coefficient was calculated for each subject. Sex differences in both strength and the positive participation coefficient were examined using a two-sample t-test at each node. Multiple comparisons were controlled using FDR (Q < 0.05; Genovese et al. 2002). Sex differences in network-wise total strength and mean positive participation coefficient were evaluated in an analogous fashion.
Sex Differences in Edgewise Functional Connectivity
While graphical analyses of network topology provide useful summary measures of network configuration, they do not provide detailed information regarding changes at individual network connections. Accordingly, a two-group t-test comparing males and females was run at each of the 34 716 unique connections in the network. Given the large number of comparisons present in this high-dimensional network data, correction for multiple testing is an important consideration. As above, for each edgewise analysis, the appropriate significance threshold was determined by the false discovery rate (Q < 0.05). Prior to performing edgewise statistical tests, all correlation values were z-transformed using a Fisher's r to z transformation.
As described in Results section, the graphical analysis of network topology revealed significant sex differences in the participation coefficient. In order to evaluate this observation analogously in the edgewise data, as in our prior work (Satterthwaite et al. 2013b) on developmental connectivity, we compared whether the proportion of connections that were significantly stronger in males than females were more likely to be within- or between-module using a χ2 test. For all edgewise analyses, connections that were significantly different between males and females were visualized in 3D using custom software written in-house with Mayavi (Ramachandran and Varoquaux 2011).
Examination of Sex Differences in Community Structure
As described in Results section, we found that males and females differed in the degree of network segregation across multiple networks scales. Potentially, such a finding could result from systematic differences in the community structure between males and females. In order to evaluate this, we calculated a mean connectivity matrix for both males and females, and then evaluated the community structure for each. As in Power et al. (2011), we utilized the InfoMap modularity detection algorithm (Rosvall and Bergstrom 2008). As this algorithm does not allow for negative weights, the values of such edges were set to zero. This returned the community structure for males and females, which was compared using normalized mutual information (NMI; as previously in Power et al. 2012; Satterthwaite et al. 2013a). We first examined the stability of male and female community assignments by running the InfoMap algorithm 1000 times; the same value was returned in 943 of 1000 runs. Next, we evaluated whether the community structure of these networks was statistically different using permutation testing, where the sex label of each subject was permuted. Therefore, instead of comparing the community structure resulting from the mean connectivity matrix of males and females, for each of 1000 permutations, 2 permuted groups (each containing a randomly determined proportion of males and females) were assembled, the mean connectivity matrix was calculated, and the resulting community structure was compared using NMI. This produced a null distribution of NMI values, allowing the significance of the real NMI value to be evaluated.
Examination of Sex Differences in Development
The above analyses examined the main effect of sex on both graphical measures of network topology as well as individual network edges. As described in Results section, sex differences were prominent at all network scales examined. However, given that subjects in this study spanned the critical developmental period of adolescence where we have previously demonstrated marked changes in functional connectivity (Satterthwaite et al. 2013b), we next examined whether observed sex differences varied by age (e.g., became more prominent with development). To do this, we tested for age by sex interactions on relevant outcome measures, including each CNB cognitive domain, mean network participation coefficient, participation coefficient at each node, and connectivity at each unique network edge. Linear age by sex interactions were first examined using terms including the main effects of age, sex, as well as an age by sex interaction; motion (summarized as mean relative displacement) was included as a covariate. As developmental trajectories are frequently nonlinear, nonlinear age by sex interactions were additionally examined using polynomial regression that also included quadratic and cubic terms for both age and age by sex interactions. As prior, multiple comparisons were controlled using FDR Q < 0.05.
Multivariate Pattern Analysis
Analyses described thus far examined whether males and females differed in their cognitive performance on individual tests or in their connectivity at individual graph nodes or edges. In contrast to such mass-univariate analyses, multivariate analyses have the potential to describe complex patterns of cognition or connectivity that discriminate between males and females, and additionally provide a quantitative summary of how masculine or feminine a given subject's pattern of cognition or connectivity is.
Notably, whereas the goal of a traditional mass-univariate analysis is to describe the relationship of a given set of brain measures to an outcome measure of interest on a regional basis, the goal of the multivariate analysis is to predict the outcome using the information contained in all regions jointly. It should be noted that while such multivariate models are ideally suited for classification problems (i.e., “prediction”), their “descriptive” utility is limited. One can easily identify which model features contribute are most heavily weighted within the multivariate model, but it is not possible to directly visualize their action within the model framework due to the extremely high-dimensional nature of the parameter space. As such, mass-univariate (descriptive) and multivariate (predictive) methods are complementary approaches, which is why they were used together in the current work.
We used a support vector machine as implemented in LIBSVM (Chang and Lin 2011) to construct multivariate models that classified participants as male or female. Models were constructed separately using either the cognitive performance domains or all unique edges in the functional connectivity matrices as input data. No feature selection process was used; as the number of connectivity features (>35k) was far greater than the number of participants in our sample, a linear kernel was chosen.
Classifications were generated using a 10-fold cross-validation procedure where the multivariate model was trained on 90% of the data, and then tested on 10% of the data. Such cross-validation provides an unbiased estimate of model predictive accuracy and prevents model overfitting to a specific training dataset. For each model, this procedure produced 3 important outcomes. First, the support vector machine (SVM) produced an overall classification accuracy score for classifying males or females on the basis of their cognitive or connectivity data. Second, each model yields a dimensional classification score for each subject that reflects the degree of masculinity or femininity in a given subject's pattern of connectivity or cognition. Dimensional classification scores from the cognitive and connectivity models were subsequently related to each other as described below. Third and finally, the SVM yields a vector of feature weights that describes how heavily weighted an individual cognitive or connectivity feature was within the multivariate model. However, as noted above, direct visualization of the action of a feature within the model beyond the feature weight is not possible given the high-dimensional nature of the parameter space.
The statistical significance of both the overall model classification and individual model features was tested with 1000 permutations. Permutation testing compared the actual classification accuracy and feature weights to predictive accuracy and weights assigned when subject sex labels were randomly reassigned on a subject-wise basis. Lastly, in order to evaluate the sensitivity of classification accuracy to the size of the training set, we re-calculated classification accuracy using split-half cross-validation within the SVM. The split between training/testing sets was chosen randomly; in order to reduce the impact of this assignment, split-half cross-validation was performed 10 times for each data type (i.e., cognitive scores and edgewise connectivity), and mean classification accuracy is reported.
Relationship Between Sex-Specific Multivariate Patterns of Cognition and Connectivity
The multivariate pattern analyses described above yielded a single score per subject describing the dimensional extent to which their specific pattern of cognition or connectivity was typically male or female. As a final step, we examined how these patterns related to each other, providing insight into whether masculine or feminine patterns of connectivity relate to male or female patterns of cognition. Specifically, dimensional classification scores from the CNB and connectivity SVM analyses were related to each other using a Pearson's correlation. In order to evaluate whether significant variation in patterns of cognition and connectivity could be discerned within a given sex, we repeated this procedure separately for males and females. Finally, we evaluated whether the masculinity (or femininity) of a given subject's connectivity profile related to performance on individual cognitive tests. To do this, the multivariate connectivity score was related to performance on each cognitive domain using Pearson's correlations. For every analysis performed, multiple comparisons were controlled using FDR (Q < 0.05).
Results
Males and Females Display Sex Differences in Cognition
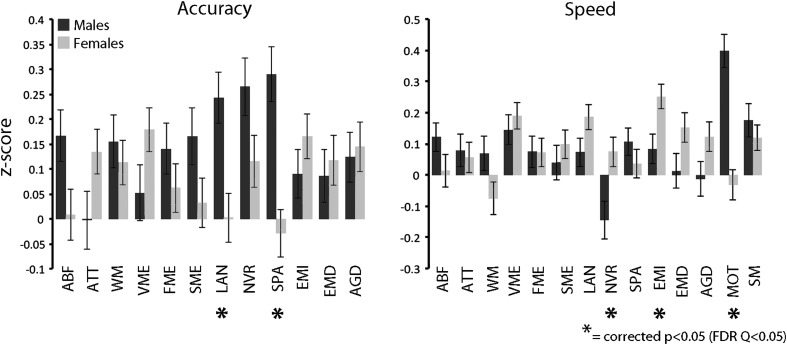
We have previously established that sex differences in cognition are prominent in the large PNC sample using the Penn CNB (Gur et al. 2012). In this subsample of PNC participants who were imaged, several previously reported sex differences are confirmed (Fig. 2). Specifically, males were more accurate on spatial tasks and faster on the motor task. Males in this subsample were also more accurate on language functioning; this was not found in the larger supraset of subjects but may be due to the enriched late-adolescent age range of the imaged sample compared with the overall sample, where males did perform better (Gur et al. 2012). In contrast, females were faster on nonverbal reasoning as well as emotion identification. The mean effect size of significant differences in cognition was small (d = 0.30).
Figure 2.
Sex differences in cognitive performance. In this sample of participants from the PNC who completed neuroimaging, expected sex differences in cognitive performance were confirmed using the Penn CNB. Males (black) were more accurate (FDR Q < 0.05) on spatial tests, as well as faster on the motor test. In contrast, females (gray) were faster on non-verbal reasoning as well as emotion identification. In this sample males were also more accurate on the verbal reasoning test.
Males Demonstrate More Between-Module Connectivity as Measured by the Participation Coefficient
As a first step, we compared the network organization of males and females using 2 graphical measures of network topology: strength and participation coefficient. Strength was not significantly different between males and females for either positive or negative connections either at the level of individual nodes or on a mean network-wise basis. Males had a significantly higher mean network positive participation coefficient than females (t(672) = 2.21, P = 0.027). As participation coefficient represents the balance of within- and between-module connectivity, this could reflect either greater within-module connectivity in females, greater between-module connectivity in males, or some combination thereof. However, the edgewise analysis (see below) allowed such possibilities to be disentangled. On a node-wise level, sex differences were found in a small minority of network nodes (2.3%). Males demonstrated a higher positive participation coefficient at 5 locations, whereas females had a higher participation coefficient at one node (Fig. 3). The mean absolute effect size of these effects was small (d = 0.33). Nodal sex differences were not concentrated in a single network; nodes with significant sex differences were located within the default mode network, ventral attention network, the auditory network, and the memory retrieval network. These results demonstrate that at both a network-wise and nodal level, males have a greater balance of between-module than within-module connectivity than females.
Figure 3.
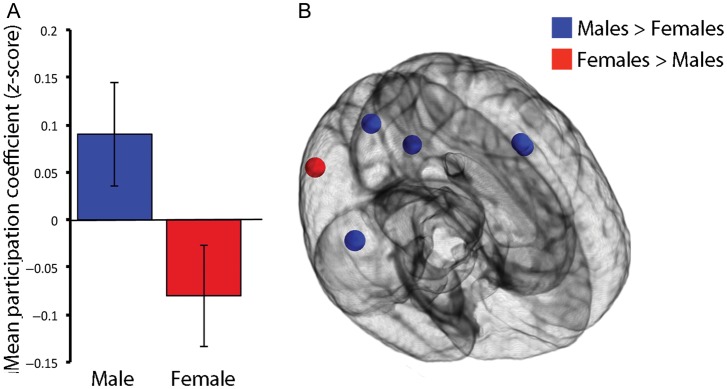
Males have a greater balance of between- versus within-module connectivity as summarized by the participation coefficient. (A) Network-wise summary. Males have a higher positive mean network participation coefficient than females, indicating that they have a greater balance toward between-module rather than within-module connectivity. (B) Node-wise analysis. At the level of a single network node, males had a greater positive participation coefficient than females at 5 nodes. In contrast, there was only one node where the participation coefficient was higher in females. All significant results corrected for multiple comparisons using the false discovery rate (Q < 0.05).
Sex Differences in Functional Connectivity Are Prominent and Vary by Within-Module Versus Between-Module Status
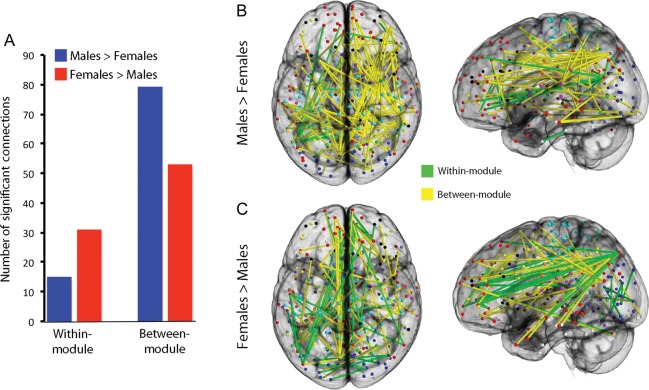
In order to further understand the differences in network structure identified by the participation coefficient, we compared connectivity of males and females at each of the network's 34 716 unique edges. As displayed in Figure 4, 178 connections (or 0.51% of total edges) showed a significant difference in functional connectivity above an FDR-corrected threshold (Q < 0.05). The mean effect size of these differences was small (d = 0.32). As suggested by the differences in the participation coefficient, the proportion of significantly different connections that were within-module versus between-module varied systematically by sex: Connections that were stronger in females were more likely to be within-module, whereas connections that were stronger in males were more likely to be between-modules ( χ2 = 10.16, P = 0.001; Fig. 5). Notably, such results were unlikely to be driven by gross differences in community structure between males and females. Although there was a trend towards a difference in community structures between males and females, this was not significant (NMI = 0.83; one-tailed permutation-based P = 0.075). Thus, sex differences in within- versus between-module connectivity are present across multiple network scales.
Figure 4.
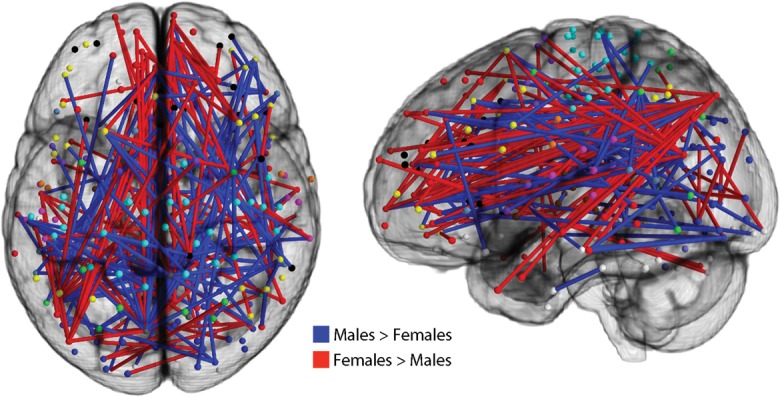
Sex differences in individual network connections are prominent. Connectivity at each of the network's 34 716 unique edges was compared between males and females. Significantly different levels of connectivity were seen in 179 individual edges (FDR Q < 0.05). Edges where connectivity was stronger in males are displayed in blue; edges where connectivity was stronger in females are displayed in red.
Figure 5.
Connections that are stronger in females are more likely to be within-module, whereas connections that are stronger in males are more likely to be between-module (A; χ2 = 10.16, P = 0.001). Significantly different edges (from Fig. 4) are here displayed separately for each sex; within-module connections are displayed in green, between-module connections are in yellow. Males have a greater predominance of between-module connections (B), whereas the proportion of within-module connections is relatively higher in females (C). As prior, all connections reported as significant are above an FDR-corrected threshold (Q < 0.05).
Sex Differences are Present and Stable Across the Adolescent Period
While the above results identify clear sex differences in cognition and connectivity between males and females, they do not describe whether such differences vary by development during youth. In the supraset of 3500 PNC participants that included many participants who did not undergo neuroimaging, age-by-sex interactions were detectable given the power of the much larger sample (Gur et al. 2012). However, in the current smaller imaged subsample, no interaction effects survived multiple comparison correction, suggesting that diverging trajectories of cognitive performance are more subtle than the main effects of sex that are present throughout the ages examined. Similarly, no linear or nonlinear age-by-sex interactions were found to be significant on any imaging measure examined, including mean network participation coefficient, nodal participation coefficient, or individual graph edges. It should be noted that this does not mean that developmental effects were not present; as we and others have reported, developmental effects in resting-state connectivity are prominent (Fair et al. 2012; Satterthwaite et al. 2013b) and, in general, network modules become more segregated with age. For example, the mean participation coefficient in this sample declined significantly with age (r = −0.15, P = 9.2 × 10−5). Despite the main effect of sex on this measure (see Fig. 3), the difference between males and females was quite stable across the ages studied, and age-related changes were similar in males and females (males: r = −0.14; females: r = −0.16).
Multivariate Pattern Analyses Classify Subject Sex Based on Cognition and Connectivity
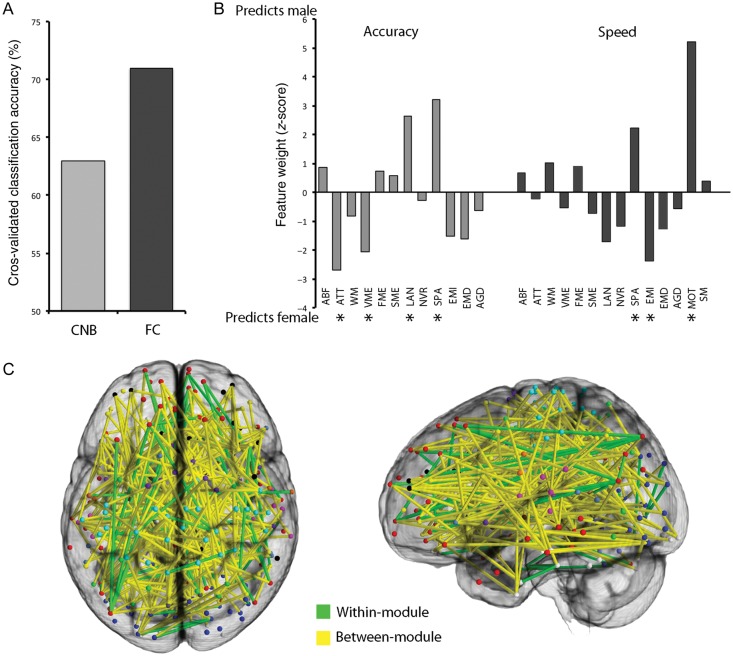
While the above analyses are instructive in that they identify aspects of cognition and network topology that are different among males and females, they are limited in that they cannot assess complex patterns of difference using the complete multivariate structure of the data. In order to determine if such complex multivariate patterns of cognition or connectivity differ by sex, we used support vector machines to classify males versus females subjects using the cognitive and connectivity data in turn. Classification using the cognitive data was significantly better than chance (permutation-based P < 0.001), with an overall accuracy of 63% (Fig. 6A). Significantly weighted features in this multivariate model identified by permutation testing included cognitive domains that were stronger in females (e.g., verbal memory accuracy, emotion identification speed) as well as those where males performed better (e.g., spatial accuracy and speed, motor speed; see Fig. 6B). Classification using connectivity data was more accurate (71%), and also highly significant (P < 0.001; Fig. 6A). Significantly weighted model features that discriminated males from females included a mix of within- and between-module connections (Fig. 6C). When split-half cross-validation was used, classification using connectivity remained more accurate than classification using cognitive scores, although the overall accuracy using split-half cross-validation was somewhat lower than the accuracy using 10-fold cross-validation (i.e., 68% for connectivity, 61% for cognition).
Figure 6.
Classification of subject sex using Penn CNB and functional connectivity. (A) Classification accuracy of subject sex is higher using functional connectivity data than using cognitive data. (B) Feature weights of cognitive tests in SVM classification of subject sex. Domains that were significantly weighted in the model (calculated using permutation testing) are starred. (C) Significantly weighted features in the SVM classification of sex using functional connectivity. As in Figure 5, significantly weighted within-module connections are displayed in green, whereas between-module connections are displayed in yellow.
Sex Differences in Multivariate Patterns of Cognition and Connectivity Are Related
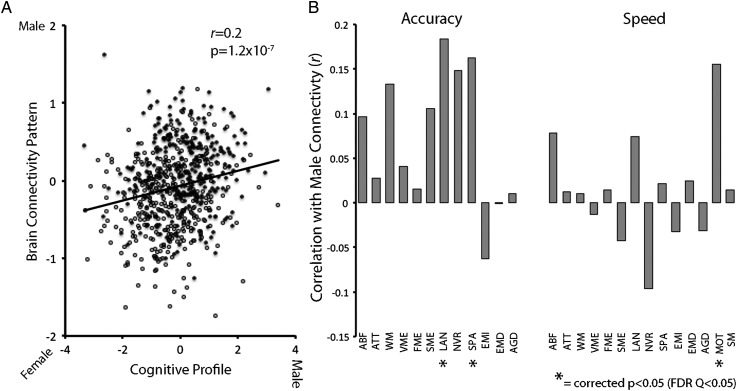
Multivariate pattern analysis using SVM established that both complex patterns of cognition and connectivity could discriminate males and females. Of course, the ground truth of an individual's sex is easily ascertained, so such classification is of relatively limited interest in and of itself. However, the SVM also provides a more interesting dimensional measure of the degree to which a given subject's pattern of cognition or brain connectivity is “male” or “female.” Critically, the degree of masculinity or femininity in participants' cognitive profile was significantly related to the masculinization (or feminization) of their brain connectivity (Fig. 7A; r = 0.20, P = 1.2 × 10−7). When males and females were examined separately, this relationship remained significant (males: r = 0.10, P = 0.046; females: r = 0.14, P = 4.6 × 10−3). Furthermore, participants with more “male” patterns of connectivity did significantly better on tests within cognitive domains that demonstrated a male performance advantage (Fig. 7B), including spatial accuracy, and motor speed, as well as language accuracy where this sample of males outperformed the females. Participants with a more “female” pattern of connectivity performed better on domains including nonverbal reasoning speed, although only at an uncorrected level of significance.
Figure 7.
Sex differences in multivariate patterns of cognition and functional connectivity are related. (A) Plot displaying the significant (P = 1.2 × 10−7) relationship between how “male” or “female” a given subject's connectivity pattern is and how “male” or “female” their cognitive profile is. This relationship remains significant when males and females are examined separately. Black data points indicate males, open circular data points indicate females. (B) Sex differences in pattern of functional connectivity relate to performance on specific cognitive domains. Individuals with more male patterns of connectivity are better at cognitive domains where males demonstrate superiority, including spatial accuracy and motor speed as well as language accuracy (FDR Q < 0.05). Subjects with more female patterns of connectivity are faster at non-verbal reasoning at an uncorrected level of significance.
Discussion
While sex differences in cognitive ability are well established, prior accounts have not described how differences in brain organization allow such divergent cognitive styles to occur. Our results demonstrate that sex differences in patterns of brain connectivity are present at an early age, with males having greater between-module connectivity and females having more within-module connectivity. Moreover, our results show that sex differences in patterns of brain connectivity are related to sex-specific profiles of cognitive performance, for the first time establishing a link between sex differences in cognition and the organization of the brain's functional connectome.
Convergent Sex Differences in Functional Connectivity Are Observed at Multiple Network Scales
There is a growing consensus within the neuroscience field that sex differences are underappreciated and merit further study (Gong et al. 2011; Giedd et al. 2012; Sacher et al. 2013). Indeed, sex differences in brain structure, brain metabolism, and cerebral blood flow have been reported. For example, females have a higher percent of intracranial volume consisting of gray matter while males have more WM (Gur et al. 1999; Lenroot et al. 2007). Females also have higher rates of cerebral blood flow (Gur et al. 1982), and higher metabolic rates in cortex as measured by fluorodeoxyglucose positron emission tomography (Baxter et al. 1987). However, despite nearly 30 years of evidence regarding sex differences in brain structure and perfusion, sex differences in brain connectivity have only recently been studied.
Our results demonstrate that sex differences in brain connectivity are present at an early age. Specifically, females have more within-module connectivity and males have more between-module connectivity. Importantly, this effect is present across a range of network scales, including the entire network, individual network nodes, and unique network connections. This convergent evidence emphasizes the degree to which sex differences are a feature of brain organization that is not dependent on the specific methodological approach.
While prior studies have reported sex differences in connectivity within certain networks (Biswal et al. 2010), or relating to hemispheric lateralization (Tian et al. 2011; Zuo et al. 2010), systematic differences in the balance of within-versus between-module connectivity have not previously been reported. We have previously demonstrated that this constitutes a valuable brain phenotype in the context of neurodevelopment, where within-module connectivity increases and between-module connectivity diminishes (Satterthwaite et al. 2013b). Such findings are consistent with prior accounts of functional segregation in network organization (Fair et al. 2007, 2008, 2009; Supekar et al. 2009; Dosenbach et al. 2010; Power et al. 2010; Anderson et al. 2011).
The present results suggest that female brains may be more functionally segregated than male brains, and that this difference is stable across late childhood, adolescence, and early adulthood. While one prior study of 51 subjects did report (uncorrected) age-by-sex interactions (Wu et al. 2013), we did not find any evidence of an age-by-sex interaction in connectivity at any scale of analysis, despite this being the largest sample to date examining subjects in this age range. This suggests that patterns of male and female connectivity have already diverged prior to adolescence, perhaps as a result of androgen-dependent in-utero programing or early environmental influences (MacLusky and Naftolin 1981; Berenbaum and Beltz 2011; McCarthy et al. 2012). As discussed below, our results additionally suggest that such stable differences in network segregation may relate to sex differences in cognition that are also already apparent during youth.
Additionally, it should be noted that no prior report controlled for the pernicious confounding influence of motion artifact on functional connectivity, which we and others have recently described (Power et al. 2012; van Dijk et al. 2011; Satterthwaite et al. 2012). In the present study, we used tight quality-control standards, controlled for motion artifact on the subject level using a validated confound regression procedure (Satterthwaite et al. 2013a; see also Yan et al. 2013), and also matched males and females on motion very closely. Overall, such procedures bolster confidence in our findings.
Sex Differences in Patterns of Functional Connectivity and Cognition Are Related
In addition to traditional mass-univariate statistics that allow localization of sex differences to a specific cognitive domain or network connection, we utilized multivariate pattern classifiers (support vector machines) to discover complex patterns of cognition or connectivity that discriminate between males and females. We found that while both data types classified subjects as males or females more accurately than chance alone, classification using connectivity data was more accurate than classification using the cognitive data. The fact that multivariate patterns provided moderate discriminative ability is particularly notable as mass-univariate differences were relatively sparse (i.e., 0.51% of edges showed a significant sex difference), and of a relatively small effect size (i.e., d = 0.32).
While classification of subject sex is of some interest, a subject's sex is a known ground truth that can be more simply ascertained without the use of functional MRI. However, multivariate pattern classification also provides a concise summary of a subject's masculine or feminine pattern of cognition or connectivity. We found that a given subject's pattern of cognition is significantly related to their pattern of connectivity: subjects who have a more “male” pattern of cognition also demonstrated a more “male” profile of connectivity. Notably, this relationship remained significant when males and females were examined separately, suggesting that, even within a single sex, there is meaningful variability in the masculinity or femininity of brain and cognitive patterns. Furthermore, the degree to which a given individual had a typically male or female pattern of connectivity related to their performance on specific cognitive domains. Though speculative, this suggests a link between sex differences in patterns of connectivity and cognitive style: The increased network segregation of female brains may allow better performance in domains (such as social cognition) where females often demonstrate superiority. Taken together, these data provide novel evidence that the divergent patterns of cognition seen in males and females are reflected on a neural level in differential patterns of brain connectivity.
Limitations, Future Directions, and Conclusions
Although this study capitalizes upon a large sample, uses advanced analytic techniques, and carefully controls for important confounds, certain limitations should be acknowledged. Most importantly, while the statistical relationship between patterns of cognition and connectivity suggests a biological relationship, such observed correlation is not a demonstration of causality. In future, systematic manipulation of masculinization or feminization in animal models (Sisk and Foster 2004), while measuring network organization and available cognition-related phenotypes could provide direct validation of the results presented here. Furthermore, future research using multimodal imaging is necessary to understand how observed differences in functional connectivity relate to known sex differences in brain structure (Lenroot et al. 2007), CBF (Gur et al. 1982), and brain metabolism (Baxter et al. 1987). Finally, it should be emphasized that the size of the effects detected was relatively small, and present at only small percentage of network nodes and edges. Thus, while sex differences in connectivity exist, on the whole connectivity patterns of male and female brains are more alike than different.
In conclusion, the present results provide novel data demonstrating that sex differences in patterns of brain connectivity relate to divergent profiles of cognitive performance in youth. As psychiatric disorders including depression, anxiety disorders, and schizophrenia often have strong gender disparities and often begin in adolescence (Goldstein 1988; Nolen-Hoeksema and Girgus 1994; Aleman et al. 2003; Slewa-Younan et al. 2004), description of sex differences in brain patterns is necessary to allow understanding of how such relative vulnerabilities arise. We have previously found evidence for sex differences in structural abnormalities associated with schizophrenia (Cowell et al. 1996; Gur et al. 2004). Moving forward, we will investigate whether sex differences in the patterns of brain connectivity described here bias risk for development of psychosis and mood disorders.
Funding
This work was supported by grants from the National Institute of Mental Health (MH089983 and MH089924). T.D.S. was supported by NIMH K23MH098130 and the Marc Rapport Family Investigator grant through the Brain & Behavior Research Foundation. D.H.W. was also supported by NIMH MH085096, APIRE, and the Sidney R. Baer, Jr. Foundation through the Brain & Behavior Research Foundation. D.R.R. was supported by NIMH T32 MH019112.
Notes
The authors thank the acquisition, assessment, and recruitment team: Monica E. Calkins, Karthik Prabhakaran, Marisa Riley, Jack Keefe, Nick DeLeo, Raphael Gerraty, Elliott Yodh, and Rosetta Chiavacci. Conflict of Interest: None declared.
References
- Aleman A, Kahn RS, Selten J-P. 2003. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 60:565–571. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. 2011. Connectivity gradients between the default mode and attention control networks. Brain Connect. 1:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze VD, O'Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT. 1993. Intelligence and brain structure in normal individuals. Am J Psychiatry. 150:130–134. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. 2011. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 54:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. 2008. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 28:9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian M, Heymann S, Jacomy M. 2009. Gephi: an open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media. [DOI] [PMC free article] [PubMed]
- Baxter LR, Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. 1987. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 21:237–245. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM. 2011. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol. 32:183–200. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34:537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. , 2010. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RG. 1977. Matching when covariables are normally distributed. Biometrika. 64:299–307. [Google Scholar]
- Chang CC, Lin CJ. 2011. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol. 2:27. [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NUF, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE. 2008. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage. 41:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell PE, Kostianovsky DJ, Gur RC, Turetsky BI, Gur RE. 1996. Sex differences in neuroanatomical and clinical correlations in schizophrenia. Am J Psychiatry. 153:799–805. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, et al. , 2010. Prediction of individual brain maturity using fMRI. Science. 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NUF, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. 2008. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 105:4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, Schlaggar BL, Petersen SE. 2009. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 5:e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. 2007. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 104:13507–13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, et al. , 2012. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 8:700–711. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. 2002. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 15:870–878. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills K, Lenroot RK. 2012. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM. 1988. Gender differences in the course of schizophrenia. Am J Psychiatry. 145:684–689. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC. 2011. Brain connectivity: gender makes a difference. Neuroscientist. 17:575–591. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. 2009. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 48:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M. 1982. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 217:659–661. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, et al. 2012. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 26:251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. 2010a. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 187:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. 2010b. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 187:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. 1999. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 19:4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Kohler C, Turetsky BI, Siegel SJ, Kanes SJ, Bilker WB, Brennan AR, Gur RC. 2004. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biol Psychiatry. 55:512–517. [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. 2013. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 82C:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern DF. (2000) Sex differences in cognitive abilities. New York: Psychology Press. [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Laird AR, Fox PT, Eickhoff SB. 2012. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus-context integration. Neuroimage. 60:2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. 2012. FSL. Neuroimage. 62:782–790. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, Christensen GE, Collins DL, Gee J, Hellier P, et al. , 2009. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 46:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L, Salek-Haddadi A, Lund TE, Laufs H, Carmichael D. 2007. Modelling large motion events in fMRI studies of patients with epilepsy. Magn Reson Imaging. 25:894–901. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. 2007. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 36:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall M-E, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. 2010. Functional connectivity and brain networks in schizophrenia. J Neurosci. 30:9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. 1981. Sexual differentiation of the central nervous system. Science. 211:1294–1302. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, Vries GJD. 2012. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 32:2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, et al. 2010. A parcellation scheme for human left lateral parietal cortex. Neuron. 67:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. 1994. The emergence of gender differences in depression during adolescence. Psychol Bull. 115:424–443. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. 2011. Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. 2010. The development of human functional brain networks. Neuron. 67:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P, Varoquaux G. 2011. Mayavi: 3D visualization of scientific data. Comput Sci Eng. 13:40–51. [Google Scholar]
- Reetz K, Dogan I, Rolfs A, Binkofski F, Schulz JB, Laird AR, Fox PT, Eickhoff SB. 2012. Investigating function and connectivity of morphometric findings—exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17). Neuroimage. 62:1354–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M, Bergstrom, CT. 2008. Maps of random walks on complex networks reveal community structure. Proc Natl Acad Sci USA. 105(4):1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2010. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2011. Weight-conserving characterization of complex functional brain networks. Neuroimage. 56:2068–2079. [DOI] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A. 2013. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn Reson Imaging. 31:366–375. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, et al. 2013a. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Hopson R, Jackson C, Keefe J, Riley M, et al. 2014. Neuroimaging of the Philadelphia Neurodevelopmental Cohort. Neuroimage. 86:544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. 2012. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, Gennatas ED, Jackson C, Prabhakaran K, Smith A, et al. 2013b. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 83C:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. 2008. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 29:696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. 2004. The neural basis of puberty and adolescence. Nat Neurosci. 7:1040–1047. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, Gordon E, Harris AW, Haig AR, Brown KJ, Flor-Henry P, Williams LM. 2004. Sex differences in functional connectivity in first-episode and chronic schizophrenia patients. Am J Psychiatry. 161:1595–1602. [DOI] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp. 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, et al. 2009. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA. 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. 2009. Development of large-scale functional brain networks in children. PLoS Biol. 7:e1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, Bilder RM, Frevert T, Lim K. 2003. Sex differences in frontal lobe white matter microstructure: a DTI study. Neuroreport. 14:2469–2473. [DOI] [PubMed] [Google Scholar]
- Tian L, Wang J, Yan C, He Y. 2011. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. Neuroimage 54(1):191–202. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL. 2011. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shen H, Tang F, Zang Y, Hu D. 2012. Combined structural and resting-state functional MRI analysis of sexual dimorphism in the young adult human brain: an MVPA approach. Neuroimage. 61:931–940. [DOI] [PubMed] [Google Scholar]
- Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, Thyreau B, He Y, Evans AC, Li X, et al. 2013. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS One. 8:e55347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo X-N, Castellanos FX, Milham MP. 2013. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 76C:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, et al. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. 2010. Network-based statistic: identifying differences in brain networks. Neuroimage. 53(4):1197–1207. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. 2001. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 20:45–57. [DOI] [PubMed] [Google Scholar]
- Zuo X-N, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang Y-F, Castellanos FX, et al. 2010. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 30:15034–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]